Abstract
Because the choroid plexus normally controls the production and composition of cerebrospinal fluid and as such, its many functions of the central nervous system, we investigated whether ligand-mediated targeting could deliver genes to its secretory epithelium. We show here that when bacteriophage are targeted with epidermal growth factor, they acquire the ability to enter choroid epithelial cells grown in vitro as cell cultures, ex vivo as tissue explants or in vivo by intra-cerebro-ventricular injection. The binding and internalization of these particles activates EGF receptors on targeted cells and the dose-, and time- dependant internalization of particles is inhibited by the presence of excess ligand. When the phage genome is further re-engineered to contain like green fluorescent protein or firefly luciferase under control of the cytomegalovirus promoter, gene expression is detectable in the choroid plexus and ependymal epithelium by immunohistochemistry or by non-invasive imaging respectively. Taken together, these data support the hypothesis that re-engineered ligand-mediated gene delivery should be considered a viable strategy to increase the specificity of gene delivery to the central nervous system and bypass the blood brain barrier so as to exploit the biological effectiveness of the choroid plexus as a portal of entry into the brain.
Keywords: Cerebrospinal fluid, epithelial cell, epidermal growth factor, phage, endocytosis, gene delivery
1. INTRODUCTION
Of the many strategies to defeat the blood brain barrier (Johanson et al., 2005; Misra et al., 2003; Neuwelt et al., 2008; Pardridge, 2007a; Pardridge, 2007b; Patel et al., 2009; Schlachetzki et al., 2004; Soderquist and Mahoney, 2010), few have focused on the unique biological and biophysical features of the choroid plexus (CP), its intrinsic ability to produce cerebrospinal fluid (CSF) and its capacity to deliver bioactive proteins. Over the last several years however, there are a number of isolated reports that have begun to investigate the prediction that it could be an important window for drug delivery into the CNS (Johanson et al., 2005; Stopa et al., 2001). Herenu et al (Herenu et al., 2007), exploited an early observation by Bajocchi et al (Bajocchi et al., 1993) describing how adenoviral icv injections transduce ependymal and CP epithelial cells. Because an ependymal route for insulin like growth factor-1 (IGF-1) gene delivery circumvents the need to transport IGF-1 from blood and eliminates the need for repeated IGF-1 injections icv (Carro et al., 2000; Carro et al., 2005), an ependymal route of IGF-1 gene delivery could be an effective approach for therapeutics delivery.
While the mechanisms through which the biologically active molecules in CSF arrive at their targets in parenchyma is remains controversial (Bickel et al., 2001; Brown et al., 2004; Patel et al., 2009; Soderquist and Mahoney, 2010; Vigh and Vigh-Teichmann, 1998; Vigh et al., 2004), there are unequivocal data showing that CSF is rich in biologically active peptides (Veening and Barendregt, 2010). The introduction of biotherapeutics into CSF can have dramatic biological effects on CNS function (Guan et al., 1996; Guan et al., 2001) but there remain significant hurdles to delivering drugs into CSF. In as much as neuroactive peptides regulate tissue function through the CNS, they can be widely distributed if they can access CSF (Veening and Barendregt, 2010). Accordingly, CNS active drugs might use the choroid plexus as a direct entry into CSF, biotherapeutic proteins might target the CP to alter its function or alternatively genes might be targeted to CP so that the CP now produces factors into CSF to alter CNS function. In all three cases, CP epithelial cell targeting is required and in the latter, a capacity to transduce CP epithelial cells.
To this end, we and several other investigators have been using phage display to identify and characterize ligands capable of cell-targeting for the purpose of specific gene delivery(Harbottle et al., 1998; Larocca et al., 1998; Larocca et al., 2002; Li et al., 2001; Poul and Marks, 1999). To date, naturally occurring ligands like epidermal growth factor (EGF), fibroblast growth factor (FGF) and integrin-related peptides (RGD) have been shown capable of delivering genes to cells using phage-based vectors.
In as much as ependymal and CP epithelia express EGF receptors (Danilov et al., 2009; Hall et al., 1990; Kuhn and Miller, 1996), we explored whether EGF might be a suitable agent to increase targeting specificity to choroid epithelium for drug delivery to CSF. We demonstrate that (1) EGF phosphorylated receptors can be localized to epithelial cells of the choroid plexus in vitro, ex vivo and in vivo, that (2) EGF-targeted phage show specific and selective targeting of the choroid plexus epithelium in vitro, ex vivo and in vivo and that (3) when these phage are engineered to also contain reporter genes like GFP and firefly luciferase, then the EGF-targeted phage can transduce the CNS epithelia in ependyma and choroid plexus in vivo. The possibility that these targeted phage might themselves be candidates to re-engineer specific CNS-gene delivery vectors is discussed.
2. RESULTS
EGF targeting of choroid plexus in vitro
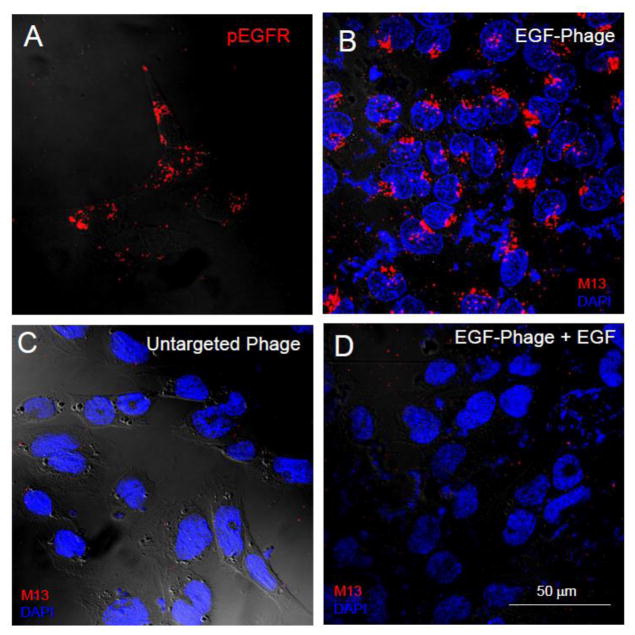
Because of the epithelial nature of the blood-CSF barrier, it seemed reasonable to evaluate the possibility that EGF could target particles to epithelial cells of the mouse and rat CP. Accordingly, epithelial cells of a rat CP cell line (Hosoya et al., 2004; Kitazawa et al., 2001) were grown in culture as described in the Materials and Methods, and an anti-phosphorylated EGF receptor (EGFR) antibody used to confirm that these cells, when stimulated by EGF, express an activated EGFR. As shown in Figure 1A, the EGF receptor is activated by the EGF-targeted phage and targeted cells appear immunopositive for phospho-EGF receptor thereby supporting our hypothesis that the EGFR is the portal of entry for EGF displaying phage in the CP. When the EGF-targeted phage were incubated with these cells, the phage were internalized by the cells (Figure 1B) and importantly, untargeted phage did not internalize when they were incubated with CP cells (Figure 1C). Finally, when cells were pre-incubated with the EGF-ligand so that the EGFR were occupied and down regulated (Figure 1D), the targeted phage failed to enter cells and no internalization is observed.
Figure 1. Internalization of EGF-targeted phage is specific in CP cells in vitro.
TR-CSFB cells were incubated with EGF-targeted (Panels A, B & D) or non targeted phage (Panel C) (1×1012cfu/ml) for 2h at 37C. The cells were acid washed to remove any particles on the cell surface, fixed and then immuno-stained with antibodies against phosphorylated EGF-R (Panel A) or M13 phage (Panels B-D) as described in the text. Binding of primary antibodies was detected using 594 Alexa-donkey anti goat (Panel A) or 488 Alexa-goat anti rabbit antibodies (Panels B-D). Positive immunostaining for the phosphorylated form of the EGFR receptor is observed in TR-CSFB cells after incubation with specific antibodies (Panel A). Internalization of EGF-phage is observed in the cytoplasm of these cells (Panel B). In contrast, no internalization is observed when the cells are incubated with untargeted phage (Panel C). Internalization of EGF targeted phage is blocked in the presence of an excess of EGF (Panel D). Cells were counterstained with DAPI to visualize the cell nuclei. pEGFR: Red; M13 phage: Red; Cell nuclei: Blue.
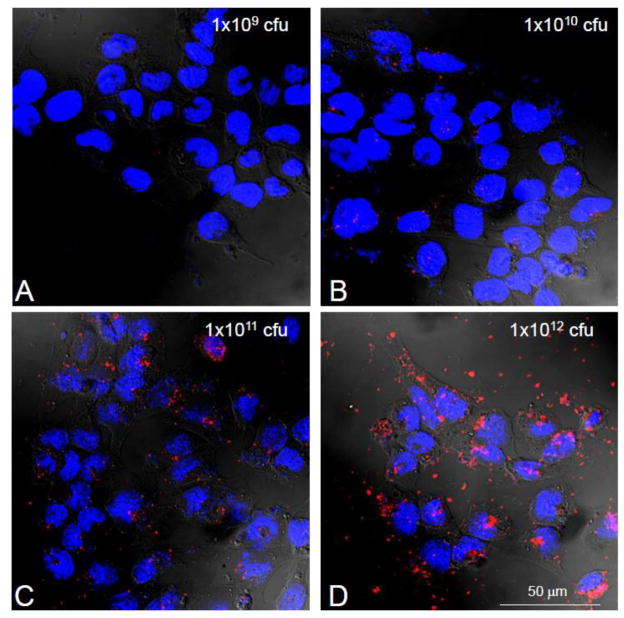
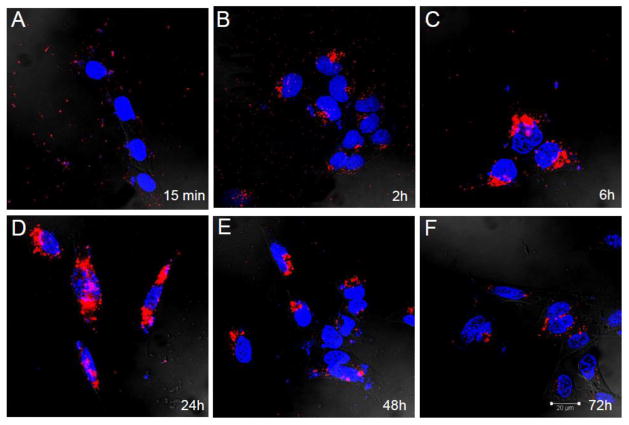
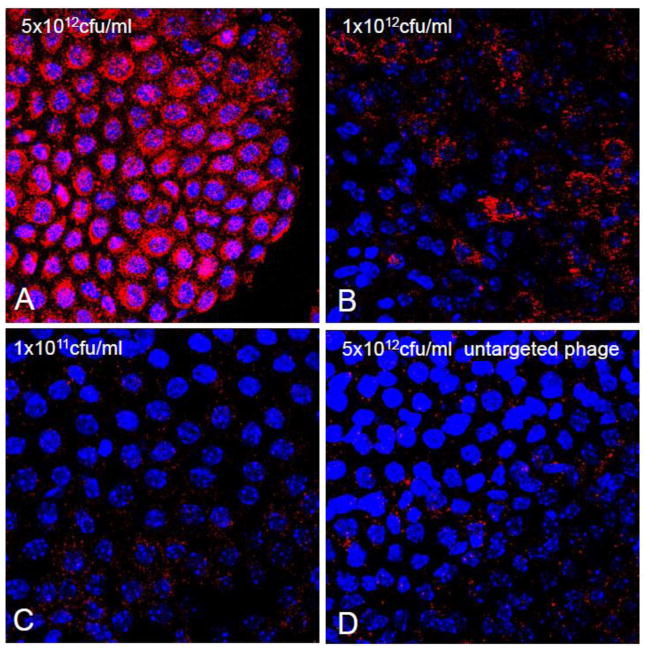
EGF-targeting of phage to the cultured CP cells is also dose (Figure 2) and time dependant (Figure 3). As compared to controls, as little as 109 phage particles are needed to visualize internalization. We also found that it was critical that contaminating endotoxins and lipopolysaccharide (LPS) be removed from the phage preparation as they causes cell ruffling of membranes in vitro and in vivo and as such an increase non-specific particle entry and increases background signal. For this reason all experiments were performed comparing internalization with the highest untargeted particle concentrations. The time course of internalization (Figure 3) reinforced the notion that binding was specific as cell surface labeling decreased as internalization signal increased. By 48 hours, phage immunoreactivity was undetectable inside cells due to degradation of intact phage particles.
Figure 2. Internalization of EGF-Phage is dose dependant in CP cells in vitro.
TR-CSFB cells were incubated with increasing concentrations of EGF-targeted phage for 2h, at 37C. Cells were immuno-stained with antibodies against M13 phage and detected using 488 Alexa goat labeled secondary antibodies. EGF-phage is internalized by TR-CSFB cells at high titers of phage (>1×1011 cfu/ml). Cells were counterstained with DAPI to visualize the cell nuclei. M13 phage: Red; Cell nuclei: Blue.
Figure 3. Internalization of EGF-Phage is time dependant in CP cells in vitro.
TR-CSFB cells were incubated with EGF-targeted phage for 2h at 37C, media replaced and cells returned to the 37C incubator. Cells were acid washed, immuno-stained with M13 antibodies and visualized using 488 Alexa labeled secondary antibodies. Cells were counterstained with DAPI to visualize the cell nuclei. By 2h EGF-targeted phage is already internalized and by 6h significant amounts of EGF-phage have translocated to the peri-nuclear area. By 48h, there is a reduction in intracellular M13 staining although phage particles are still detectable by 72h. M13 phage: Red; Cell nuclei: Blue.
EGF targeting of choroid plexus ex vivo
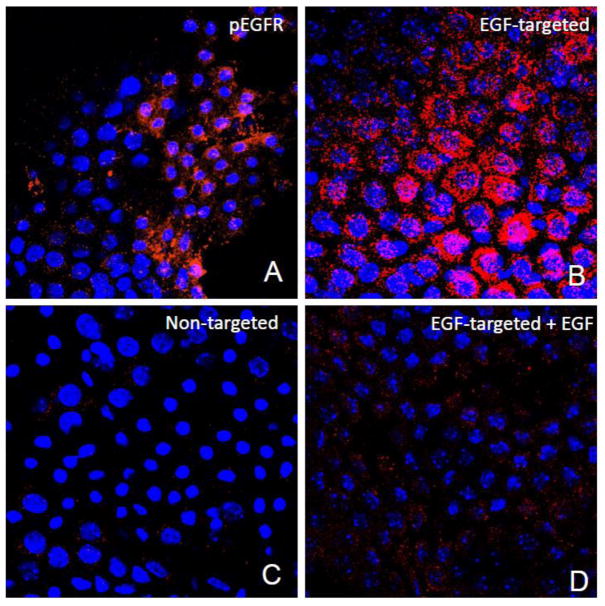
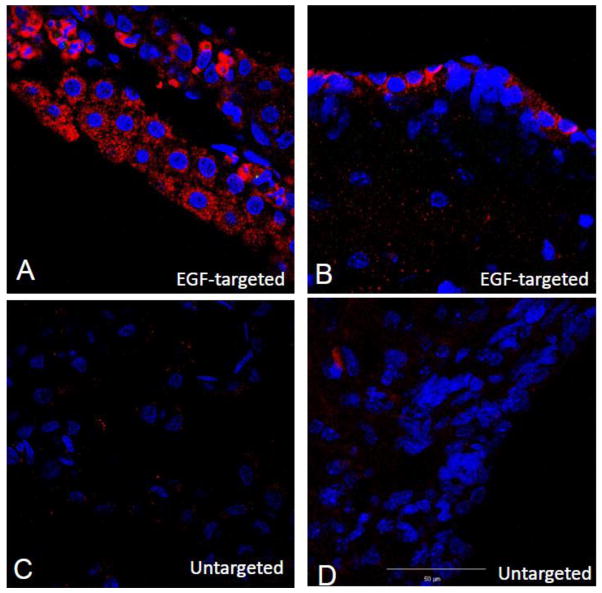
With the knowledge that EGF could target the choroid epithelial cells in vitro, it then seemed reasonable to evaluate the possibility that EGF could also target these epithelial cells ex vivo and ensure that EGFR expression was not an artifact of cell culture. CP were harvested as described in the Materials and Methods, and immunostaining with commercially available anti-phosphorylated EGFR antibodies used to generate immunohistochemical evidence that the EGF receptor is normally expressed in the rat choroid plexus. As shown in Figure 4A, many but not all cells in the CP explants appeared immuno-positive for phosphorylated EGFR supporting our hypothesis that the EGF receptor could be used as a portal of entry for particle targeting to the CP. As shown in Figure 4B, when the EGF-targeted phage were incubated with these explants over 2 hours, we obtained immunohistochemical evidence that they have the ability to specifically internalize the EGF-phage. Again, untargeted phage did not internalize when they were incubated with CP explants (Figure 4C). Furthermore, when explants were pre-incubated with EGF so that the EGFR are occupied by the ligand (Figure 4D), the EGF-targeted phage failed to enter cells and the immunoreactive signal was significantly decreased.
Figure 4. Internalization of EGF-targeted phage is specific in CP explants.
Choroid plexus were dissected from the lateral and 4th ventricle and incubated with EGF-targeted (Panels A, B &D) or non targeted phage (Panel C) at a concentration of 1×1012cfu/ml for 2h at 37C. To remove any particles on the cell surface, the explants were washed with PBS containing Tween 20, fixed and then immunostained with antibodies against phosphorylated EGFR (Panel A) or M13 phage (Panels B-D). Binding of primary antibodies was detected using 594 Alexa-donkey anti goat (Panel A) or 488 Alexa-goat anti rabbit antibodies (Panels B-D). Positive immunostaining for the phosphorylated form of the EGFR receptor was observed in the choroid epithelial cells after incubation with specific antibodies (Panel A). Internalization of EGF-phage is observed in the cytoplasm of the epithelial cells (Panel B). In contrast, no immunoreactivity is observed when the explants are incubated with untargeted phage (Panel C). Internalization of EGF-targeted phage is blocked in the presence of an excess of EGF (Panel D). Tissue explants were counterstained with DAPI to visualize the cell nuclei. pEGFR: Red; M13 phage: Red; Cell nuclei: Blue.
EGF targeting of particles to CP explants was also dose (Figure 5) dependant. As compared to controls, as little as 109particles were needed to visualize internalization into epithelial cells. As with TRCSF-B cells in culture however (Figure 2), when higher concentrations of phage were used, there was an increase in non specific entry of untargeted phage into cells of the explant. Similarly, we found that it was critical that LPS be removed from the phage preparation to avoid this non-specific background.
Figure 5. Internalization of EGF-targeted phage is dose dependant in CP explants.
Choroid plexus explants were incubated with increasing concentrations of EGF-targeted phage for 2h, at 37C and processed as described in the text. Cells were immunostained with antibodies against M13 phage and the binding detected using 488 Alexa goat labeled secondary antibodies. At low titers (1×1011 cfu/ml), the internalization EGF-targeted phage is almost undetectable but significant increase of immunoreactive M13 internalization is observed at 1×1012 cfu/ml. At a concentration of 5×1012 cfu/ml, almost all of choroid epithelial cells in the explants are immunopositive. Cells were counterstained with DAPI to visualize the cell nuclei. M13 phage: Red; Cell nuclei: Blue.
EGF targeting of choroid plexus in vivo
When EGF-targeted or untargeted phage were injected ICV into rat brain, we had the opportunity to evaluate target selectivity of the phage to determine the scope of cell targets in vivo. It was clear that an injection of EGF-targeted phage had the capacity to internalize into the CP (Figure 6A) but there was also significant signal detected in the ependymal cell lining of the ventricles (Figure 6B). In contrast, there was little, if any, signal in the CP (Figure 6C) or ependymal cells (Figure 6D) of animals treated with untargeted phage. Occasional cells showed phage immunoreactivity that appeared to correlate with the cilia-associated, macrophage-like cells in the CP (McMenamin, 1999). There was also a significant immunohistochemical signal at the site of injection and along the needle track, presumably due to injury-induced expression of EGFR. Little non-specific signal was detected at the site of injection that could not be attributed to injury induced inflammatory cells.
Figure 6. EGF-targeted phage is internalised 24h after ICV injection.
EGF-targeted phage (Panels A & B) or untargeted phage (Panels C & D) were injected into the lateral ventricle and 24h later rats were killed and brain sections immunostained with anti-M13 antibodies. M13 immunoreactivity is observed in epithelial cells of the choroid plexus (Panel A) and the ependyma (Panel B). No immunoreactivity is observed in these tissues when untargeted phage was injected ICV. (Red: M13; Blue: DAPI).
EGF-mediated transduction of choroid plexus and ependyma in vivo
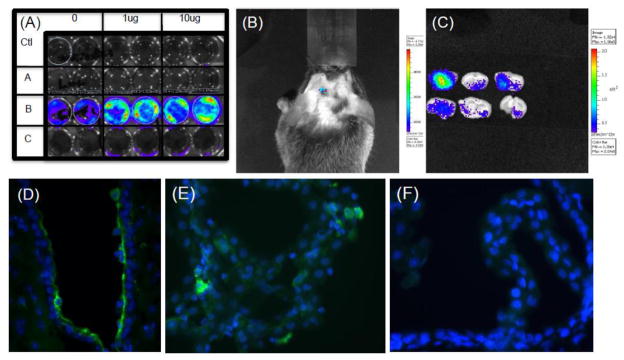
We exploited the fact that the constructs of phage and EGF-targeted phage were engineered to contain the green fluorescent protein (GFP) under control of the cytomegalovirus (CMV) promoter. Accordingly, we used anti-GFP antibodies to evaluate whether there was any evidence for transduction of cells targeted by the EGF-phage. As shown in Figure 7, GFP positive cells were detected in the CP epithelium (Fig 7A), the epithelial cells of the lateral (Figure 7B) and third (Figure 7C) ventricles. No GFP was detected in untargeted phage treated animals (Figure 7D). Although few cells are transduced, particularly when compared to the effects of adenovirus, we explored whether in vivo we might be able to detect transduction non-invasively using a method to detect bioluminescence induced by luciferase gene expression. To this end, the phage particle was re-engineered so that the GFP gene was removed and substituted by the firefly luciferase gene but still under control of the CMV promoter. When this phage is added to EGF target cells, there is was a significant light signal generated by luciferase transgene expression that is was not present in cells treated with untargeted phage. Pre-treating cells with camptothecin, an inhibitor of topisomerase increased transduction (Burg et al., 2002) also gave a dose dependant increase in light detection to cells in culture (Figure 7A). Accordingly, it was used to enhance signaling in vivo while cyclosporine used to decrease drug transport out of the brain. Using non-invasive imaging of luciferase activity, significant light signal was detected in the brains of EGF-targeted but not untargeted phage (Figure 7B/C). Although lower the signal generated by the positive control of the fluc gene delivered in adenovirus (Panel D), transduction was detectable and significant above a negative control background obtained with untargeted phage containing the fluc gene under control of the CMV promoter.
Figure 7. Gene expression after ICV injection.
Panel (A) In vitro transduction: The EGF target PC3 cells were incubated with 1× 1011 untargeted or EGF-targeted phage that were both re-engineered as described in the text to contain the firefly luciferase (f-luc) gene under the control of the CMV promoter. Duplicate wells of cells were treated with 0, 1ug or 10 ug of camptothecin and either incubated with no phage (Ctl), EGF-targeted phage containing GFP gene (A), EGF-targeted phage containing f-luc gene (B) or untargeted phage containing the f-luc gene (C). After incubating cells for 48 hrs, a 5 minute incubation with luciferin enabled a Lumina CCD Imaging system to visualize gene expression in cells treated with EGF-targeted phage (Row B) and, as expected the response was dose dependant on cells treated with camptothecin. No signal was observed in the absence of targeting (Row C) or when EGF-targeted particles do not contain the luciferase gene Row A.
Panel B-F: Effects of ICV injection of EGF-targeted phage in vivo. Mice were injected icv with 1×1012 untargeted or EGF-targeted phage-luc and in Panels B and D, transgene expression of luciferase monitored on 48 hours later by Lumina CCD Imaging either non invasively (Panel B) or after brain dissection (Panel C). To identify the cellular localization of this gene expression similar experiments were performed with EGF-targeted phage-gfp (Panel D and E) or untargeted phage-gfp (Panel F) which demonstrated cell expression of GFP in ependymal cells and in isolated cells in the choroid plexus. Whereas a 4-fold lower background signal could be quantified after luciferase activity imaging, immunostaining localized this signal to macrophages and monocytes at the site of injection and not in ependyma or choroid plexus (Panel F).
3. DISCUSSION
Several years ago, we proposed that it might be possible to target the CNS by exploiting the unique features of the choroid plexus which exists at the interface between blood and CSF (Johanson et al., 2005). If possible, we reasoned that drugs could either be designed to translocate across the CP into CSF or, alternatively, to modify CP function so that the composition of CSF was altered. After all, the regulation of different brain states can be modulated by neuroactive substances that distribute through the CNS via CSF (Veening and Barendregt, 2010). To this end, we show here that when phage are re-engineered to target EGF receptor on the CP epithelium, they internalize into epithelial cells and that the targeting is ligand specific, time and dose dependant and that it can lead to transduction of target cells in vivo. The findings raise the possibility of using combinatorial techniques to evolve a vector from these primordial particles that would be selectively and specifically engineered for drug delivery to the CNS via CSF.
In 1985, Smith (Smith and Petrenko, 1997) conceived of a method whereby short nucleic acid sequences of DNA could be inserted into the coding sequence of the M13 phage glll gene to generate particles that display a peptide-plll fusion protein. Reasoning that the displayed peptides would confer phage with new intrinsic activities, he proposed that it should be possible to introduce random and known sequences of DNA into the glll gene to create particles with new activities. Over the last twenty years, we and other investigators have been adapting this original phage display technique to identify novel peptides with different specificities and activities for example, peptides have that confer physical stability to particles in organic solvents like chloroform, decrease complement activation of macromolecules in blood, modify immunogenicity, alter viral tropism in vitro and in vivo, internalize particles into cells, transduce cells, promote transcytosis in vitro and in vivo and even promote transmigration of particles across cell barriers in vitro and in vivo (Muruganandam et al., 2002; Pasqualini and Ruoslahti, 1996b; Pasqualini and Ruoslahti, 1996c; Tanha et al., 2003; Vitiello et al., 2005; Yip et al., 1999). These biopanning approaches have been used to characterize organ and cell homing peptides that can target the vasculature and, parenchyma targeting peptides that can mediate transcytosis across epithelial cells in vitro and cell surface antibodies that transmigrate phage across the blood brain barrier. Our own laboratories’ focus has been to identify and exploit ligands that internalize into target cells. To this end, we and others previously re-engineered phage vectors for binding to mammalian cells and monitored their entry into cells by immunohistochemistry for internalization, transfection for drug delivery PCR for DNA delivery and transduction for gene expression (Barry et al., 1996; Burg et al., 2004; Harbottle et al., 1998; Ivanenkov and Menon, 2000; Kassner et al., 1999; Koivunen et al., 1999; Kolonin et al., 2004; Larocca et al., 1998; Mount et al., 2004; Pasqualini and Ruoslahti, 1996a; Pasqualini, 1999; Poul and Marks, 1999; Rajotte et al., 1998; Trepel et al., 2000).
Although we prove the concept of CP targeting with EGF, it is not clear that this ligand is ideally suited for CP targeting. First, EGF has intrinsic activity and is a naturally occurring growth factor. Second, EGF may be specific for epithelial cells in the brain as described here, but there are several alternative cell types that express EGF receptors including astrocytes, activated glia and endothelial cells. The specificity that we observe in vivo is likely due to the compartmentalization of phage in the ventricular space while the specificity in vitro due to the limited number of cell types available for targeting. Accordingly, it would be useful to take a more traditional approach of phage display to mine libraries and identify biologically inert targeting peptides capable of redirecting particles to the CP.
There are at least three processes that could exploit the central role of the choroid plexus in the brain for drug delivery to the CNS (Figure 8). The first, more classical approach, is to target the CP epithelium for direct drug translocation into CSF. This involves exploiting cellular processes in the CP epithelia in the same way that has proposed exploiting receptors in the blood brain barrier for translocation across brain endothelial cells into brain parenchyma (Pardridge, 2007a; Pardridge, 2007b). Whereas the studies presented here support the concept of CP-epithelial cell targeting, they were not aimed to demonstrate or evaluate the translocation of EGF-particles across the barrier. The second approach to drug targeting for CNS delivery through the CP epithelium is to treat the CP directly so as to alter its function. Treatment of one cell could alter the function of its target and surrounding cells, for example in terms of CSF production and composition. The fact that EGF can target particles to CP epithelial cells certainly supports the concept that an EGF therapeutic might alter CP function, but this was beyond the scope of the work presented here. Instead, the mode of drug delivery we investigated is illustrated by the third approach. A gene is delivered to the CP epithelium, either via blood or CSF, to alter CP function. Here, we show that if the CP epithelium can be targeted (as with EGF in the experiments here), then the particles can be processed inside the cell to deliver, transcribe and transduce a gene (luciferase and green fluorescent protein in the experiments here) and potentially modify the epithelial cell’s function to make CSF and modify its composition. Because the studies here demonstrate the feasibility of targeting and transduction epithelial cells from the luminal (CSF) compartment, they support an initiative to achieve the same result via blood-borne delivery.
Figure 8. Drug Delivery to the Central Nervous System Via Choroid Plexus.
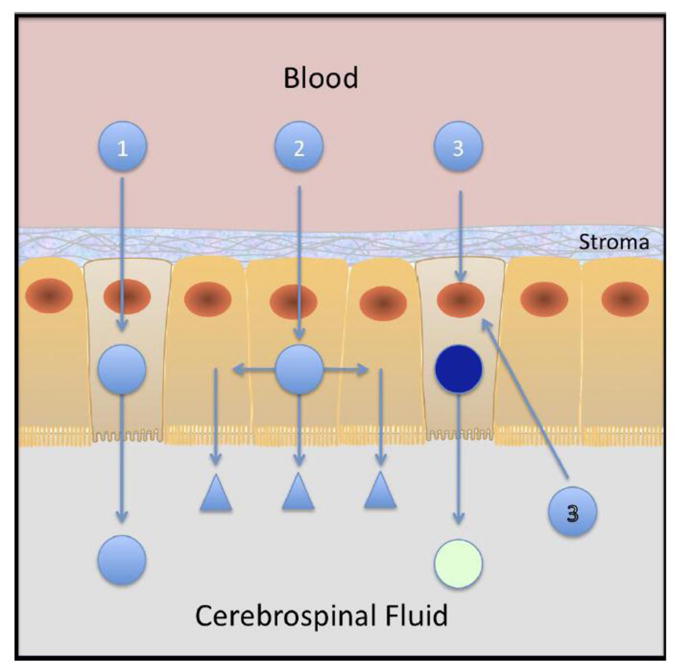
There are three modes of drug delivery to the CNS using the choroid plexus as a portal of entry. The first (1) is direct whereby molecules are targeted and directly translocated across the CP epithelial barrier to find their targets from within the CNS. A second (2) is illustrated by entering and trateing the CP epithelium so that its capacity the produce and composition of CSF is modified. These then find their targets in the CNS parenchyma. Finally the third strategy, in the example here using gene delivery, results in longer term changes of the choroid plexus, allows the epithelial cell to produce novel therapeutic factors itself and as such can confer the epithelium with an ability to change the production and composition of CSF. This latter process and the feasibility with luminal targeting (3) is demonstrated in the results presented here.
The possibility of using the CP as a doorway into the CNS has gained momentum with the difficulties in overcoming the blood brain barrier (Pardridge, 2007a; Pardridge, 2007b; Schlachetzki et al., 2004). Indeed, most investigators propose using brain’s endothelium as a target for CNS drug delivery because brain endothelial cells can be used to deliver bioactive agents into the brain parenchyma. While the effects are certainly localized to the CNS, the wide distribution of endothelium in the brain ensures wide distribution of drug if all endothelia is targeted. But CNS barriers also provide different areas of the CNS differential private milieus that open to the portal vasculature and CSF (Rodriguez et al., 2010). Herenu et al however (Camihort et al., 2010; Herenu et al., 2009), used a different approach and transduced ependyma so that cells now produce the growth factor IGF-1. In this case they exploited the capacity of adenovirus to infect epithelial cells to increase the levels of IGF1 in CSF and mimic the concentrations achieved by the injection of protein into CSF. Presumably, adenoviral retargeting strategies that modify its pharmacokinetics, biological activity and even potency could be used to improve its use as a gene delivery agent to the CNS. Alternatively, it is interesting to speculate that with an introduction of targeting ligands, the re-engineering of primordial particles might be possible so as to create de novo, CNS-specific vectors for gene delivery. The observation reported here that the simple targeting of a primordial particle like M13 bacteriophage with a ligand like EGF confers it with a capacity to penetrate and transduce CP epithelia supports this hypothesis.
4. EXPERIMENTAL PROCEDURES
EGF display on phage
Phagemid were engineered with the sequence human EGF fused onto the gene encoding the plll protein as described, prepared to titers of greater then 1013/ml with helper phage and purified by PEG precipitation (Larocca et al., 2002). LPS was removed from all preparations of phage using differential fractionation in detergent (Aida and Pabst, 1990). The resulting purified phage average 2–3 copies of EGF displayed per particle as estimated by differential immunoblotting with anti-plll and and anti-EGF antibodies. As indicated, the EGF displayed on phage was prepared by fusion to amino acid 198 of plll (puc198EGF), amino acid 250 of plll (puc250EGF) or there was no displayed ligand (puc198). No biological differences were observed between EGF-targeted phage although yields of puc250EGF are somewhat higher. Because both puc198 and puc198EGF plasmids also contain green fluorescent protein gene driven by the CMV promoter (Kassner et al., 1999), the genome of each was used as the starting material to substitute firefly luciferase (fluc) for GFP into the phage genome to prepare puc198-fluc and puc198EGF-fluc. Briefly, the fluc gene was removed from the pGL4.14-luc2 plasmid (Promega, Madison, WI) by Hindlll digestion, DNA polymerase I and Large Klenow Fragment (KF) treatment followed by Xbal/Stul digestion. The fluc gene was ligated into either the puc198 and puc198EGF plasmids that had their GFP gene excised by BssH II digestion, treatment with KF, Xba I digestion and CIP treatment. XL1Blue MRF’ bacteria were transformed and grown overnight using standard molecular techniques and the resulting plasmids were purified using the Qiagen plasmid DNA miniprep kit. Identity was confirmed by restriction enzyme analyses with Attlll and Xbal digestions. Renilla luciferase (rluc) was similarly ligated into the BssH II/KF treatment and Xbal excised puc198 and puc198EGF plasmids using Hind III/KF treatment and Xba I digestion of the rluc excised from a pGL4.76-rluc plasmid (Promega, Madison, WI). Only the findings with puc198fluc and puc198EGFfluc are reported here.
Culture of rat choroid plexus (CP) epithelial cells
We evaluated the effects of EGF targeting in vitro using an immortalized choroid plexus cell line (TRCSFB cells) developed from transgenic Wistar rats (Kitazawa et al., 2001). TRCSFB cells were plated on flasks coated with collagen type I (BD Biosciences) and cultured in DMEM + GlutaMAX (Invitrogen) supplemented with 4.5g/L glucose, 5ml Sodium Pyruvate (100mM, Invitrogen), 5ml NEAA (non-essential amino acids, x100, (Invitrogen), 5ml Penicillin/Streptomycin (10,000 units/ml Penicillin, 10,000 g/ml Streptomycin, Invitrogen), and 10% FBS (Sigma).
Preparation of explants of mouse and rat choroid plexus
As indicated in each experiment, adult C/57 black and Balb/C mice, or Wistar and Sprague Dawley rats were killed with an intraperitoneral overdose of pentobarbital and brains immediately dissected and placed in wet ice. All studies, protocols and procedures using animals were approved by the Institutional Animal Care Use Committees. The CP was harvested from 4th ventricle by carefully separating the cerebellum from the brain stem and dissecting the choroid plexus from the roof of the cerebellum with tweezers. The lateral ventricle CP were dissected after removal of the 4th ventricle’s CP by immersing the brain in PBS and making two parallel sagital incisions 10 mm from the midline along the length of the brain to a depth of 4mm so as to cut through the corpus callosum. The cortex was then pulled away to the side exposing the lateral ventricles and choroid plexus. With a pair of tweezers, each end of the CP was gently pulled away and placed in RPMI media containing 10% fetal calf serum (FCS) and 5% normal horse serum (NHS). Experiments with CP explants were performed immediately after harvest.
Incubation with phage in vitro, ex vivo and in vivo
In vitro: TRCSF-B cells or primary cultures of PC3 cells were incubated at 37C in 95%CO2 controlled atmosphere with EGF-targeted or untargeted phage particles encoding the GFP or luciferase gene under the control of the CMV promoter as indicated in each of the experiments. Primary cultures of PC3 cells were treated with 1 or 10ug of camptothecin to increase transduction and at 48hrs, cells were incubated for 5 min with luciferin in order to visualize luciferase expression using a Lumina CCD Imaging system (Caliper Life Sciences, Hopkinton, MA). Ex vivo: CP explants were incubated under the same conditions as TRCSF-B cells but incubation time did not extended beyond 2 hours to ensure patency of the epithelial cells. At the end of the indicated incubations, the cells or tissue were rinsed first in 10 mM PBS containing Ca+2 and Mg+2, and then washed briefly in either 50mM Glycine buffer pH 2.8 (cells in culture) or 10 mM PBS pH 7.4 containing 0.3% Tween 20. The amount of phage bound and internalized was evaluated by immunohistochemistry.
To evaluate targeting in vivo, EGF-targeted and untargeted phage were injected ICV into mice and rat brains as specifically indicated. All procedures involving the use of rats followed strict adherence to Home Office guidelines, and with approval of the local ethics committee while all mice ICV injections were approved by the institutional animal care and use committee at the University of California San Diego. Thirty minutes prior to receiving surgery, adult male Wistar (Charles River) rats (200–250g) received analgesia through a subcutaneous injection of 0.03mg/kg buprenorphine. The rats were then anaesthetised using 5% isofluorane, delivered with O2 at 1.7L/min through a mask, and full anaesthesia was maintained throughout the surgery.
During surgery, the rat was immobilised in a stereotactic frame with the head elevated and parallel to the table surface. A sagital incision was made along the midline of the scalp and teased apart to expose the skull. The landmark at co-ordinates 0/0 that indicate Bregma crossing the midline was marked using a fine marker pen. A small hole was drilled into the skull 1.5mm lateral to the midline, 1mm posterior to Bregma. The needle of the Hamilton syringe was inserted 4mm deep from the brain surface and 20μl of phage solution was injected over 30 seconds. The syringe was left in the brain for one minute to prevent fluid reflux. Once the ICV injection was complete the syringe was removed, the skin sutured and the animal was taken off the anaesthetic and allowed to recover on a warm fleece bed. At specific times after injection (24–72 hours), the rats were humanely killed by delivering rising concentrations of CO2 and immediately perfused with 4% paraformaldehyde in PBS, pH 7.4. The brains were dissected and post-fixed for 4h at 4 °C. The tissues were then cryoprotected by placing them in raising concentrations of sucrose (10–30%) in PBS at 4 °C. Tissues were embedded in OCT (RA Lamb Labs) and stored at −80C until required. Twelve micron-thick coronal midbrain sections were mounted onto positive charged slides to perform immunohistochemistry.
Immunohistochemistry and detection of internalized phage
Prior to fixation, CP cells in culture and CP tissue explants were rinsed in 50mM glycine buffer pH 2.8 for 5 min or 10mM PBS pH 7.4 containing 0.3% Tween 20 (Sigma), respectively. Cells and tissue samples were then rinsed in PBS (3X) and fixed for 20 min at room temperature in 2.2% formaldehyde in 10mM PBS pH 7.4 containing 2% glucose and 0.02% sodium azide. Cells and explants were rinsed once with PBS, permeabilized in methanol for 6 min, and washed twice again with PBS. Immunofluorescence and immunoperoxidase staining techniques using antibodies to the coat protein M13 (fd) phage were used to detect internalized phage in either cultured epithelial cells in vitro, CP tissue explants or midbrain coronal brain sections after ICV injection of phage particles. To block non specific staining, cells and tissue samples were incubated with PBS containing 1% BSA (Jackson Immuno Research), 0.3% Tween 20 (Sigma), and 10% NGS (Jackson Immuno Research) for 20 minutes at room temperature. Excess blocker solution was drained and samples incubated with rabbit anti-M13 phage (1/200, Sigma) for 1h at room temperature. For immunofluorescence staining, samples were then incubated for 45 min at room temperature with Alexa 594 labelled goat anti-rabbit antibodies (Invitrogen). After washing with PBS, cell and tissue samples were mounted using Vectashield mounting medium containing DAPI (Vector Labs) and visualized under an epifluorescent microscope and confocal microscopy. In some instances peroxidase staining was used to evaluate the distribution of phage particles in the brain after ICV injection. In this instance, after incubation of the tissues with rabbit anti-M13 phage antibodies, tissue sections were rinsed and incubated with biotinylated goat anti-rabbit IgG (Vector) for 45 min at room temperature. The tissue sections were rinsed in PBS and endogenous peroxidase quenched by incubating the tissue sections in 0.3% hydrogen peroxide (Sigma) for 30 min, washed in PBS, and incubated with ABC solution (ABC Kit, Vector Labs) for 30 min. Sections were incubated in Diaminobenzidine (DAB, Vector Labs) for 5–7 min rinsed, dehydrated, cleared in Histoclear and mounted. Immunostaining was visualized under bright field microscopy and DIC optics (Zeiss Axioplan).
When specifically stated, we evaluated the co-localization of immunoreactive phage protein with phosphorylated EGF receptor to demonstrate activation by targeted phage upon internalization. Cells, explants or tissue sections were incubated with goat anti-phosphorylated EGFR (Santa Cruz Biotechnology) at a dilution of 1:50 and processed as described above for anti-M13 phage antibodies. Normal donkey serum was used for the blocking solution and Alexa 594 donkey anti-goat (Invitrogen, San Diego CA) was used as a secondary labeled antibody to visualize staining by fluorescence and confocal microscopy as indicated in the text. To localize GFP gene expression after ICV injection, brain sections were incubated with mouse anti-GFP antibodies (1:200, Invitrogen, San Diego CA) and the signal was detected using Alexa 488 goat anti mouse antibodies as described above.
Evaluation of targeting specificity
To determine whether the internalization of EGF-targeted phage was ligand-receptor specific, we performed competition assays with synthetic EGF peptide. TRCSFB cells or tissue explants were incubated for 2hr at 37C under 5% CO2 with EGF-targeted phage (1012 particles/ml) ten minutes after having added 200 ng synthetic mouse EGF (Invitrogen).
Non-invasive in vivo imaging of transduction
Mice were anesthetized with isofluorane and given an intra-peritoneal injection with 1.5mg of the substrate D-luciferin (Caliper Life Sciences, Hopkinton, MA) in 150 μL in saline. After allowing 5 minutes to attain steady state distribution kinetics of the injected substrate, luciferase activity was measured in the still-anesthetized mice using a Lumina CCD Imaging System. Light emitting images were acquired and data analyzed with Living Image software (Version 3.0, Caliper Life Sciences, Hopkinton, MA). As necessary all animal images were exposure matched by matching color bar upper and lower limits in each panel. Regions of interest (ROI) showing bioluminescence in the head and matched sizes in the flank were used for image acquisition. Each image was quantified in units of photons/sec/cm2/steradian to obtain a fold change as defined by targeted over untargeted ROI signal.
RESEARCH HIGHLIGHTS.
Epidermal growth factor can target particles through its receptor and into the choroid plexus epithelium.
The choroid plexus can be exploited as a portal of entry into the brain.
When genes are re-engineered into targeted bacteriophage, they can transduce the brain epithelia and ependyma.
It may be possible to direct the evolution of biological nanoparticles for synthetic biology.
Acknowledgments
Research supported by the BBSRC grant BB/C50466X/1 (AMG), National Institutes of Health grants EY018479 (AB), GM078421(AB), and HL73396 (BE), the CDMRP BC073891(AB). The authors wish to thank Ms Emelie Amburn, Ms Tran Ngyuen and Ms Shuman Sun at UCSD for cell culture, phage preparations and phage qualification, respectively. The authors would also like to thank Drs David Larocca (Mandala Biosciences), Martin Berry (University of Birmingham, UK) and Ann Logan (University of Birmingham, UK) for their insight and helpful suggestions throughout the course of this work and the manuscript.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191–5. doi: 10.1016/0022-1759(90)90029-u. [DOI] [PubMed] [Google Scholar]
- Bajocchi G, Feldman SH, Crystal RG, Mastrangeli A. Direct in vivo gene transfer to ependymal cells in the central nervous system using recombinant adenovirus vectors. Nat Genet. 1993;3:229–34. doi: 10.1038/ng0393-229. [DOI] [PubMed] [Google Scholar]
- Barry MA, Dower WJ, Johnston SA. Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries. Nat Med. 1996;2:299–305. doi: 10.1038/nm0396-299. [DOI] [PubMed] [Google Scholar]
- Bickel U, Yoshikawa T, Pardridge WM. Delivery of peptides and proteins through the blood-brain barrier. Adv Drug Deliv Rev. 2001;46:247–79. doi: 10.1016/s0169-409x(00)00139-3. [DOI] [PubMed] [Google Scholar]
- Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957–70. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M, Ravey EP, Gonzales M, Amburn E, Faix PH, Baird A, Larocca D. Selection of internalizing ligand-display phage using rolling circle amplification for phage recovery. DNA Cell Biol. 2004;23:457–62. doi: 10.1089/1044549041474760. [DOI] [PubMed] [Google Scholar]
- Burg MA, Jensen-Pergakes K, Gonzalez AM, Ravey P, Baird A, Larocca D. Enhanced phagemid particle gene transfer in camptothecin-treated carcinoma cells. Cancer Res. 2002;62:977–81. [PubMed] [Google Scholar]
- Camihort GA, Herenu CB, Luna GC, Rodriguez SS, Bracamonte MI, Goya RG, Console GM. Morphological changes induced by insulin-like growth factor-I gene therapy in pituitary cell populations in experimental prolactinomas. Cells Tissues Organs. 2010;191:316–25. doi: 10.1159/000258701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–33. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J Neurosci. 2005;25:10884–93. doi: 10.1523/JNEUROSCI.2909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilov AI, Gomes-Leal W, Ahlenius H, Kokaia Z, Carlemalm E, Lindvall O. Ultrastructural and antigenic properties of neural stem cells and their progeny in adult rat subventricular zone. Glia. 2009;57:136–52. doi: 10.1002/glia.20741. [DOI] [PubMed] [Google Scholar]
- Guan J, Skinner SJ, Beilharz EJ, Hua KM, Hodgkinson S, Gluckman PD, Williams CE. The movement of IGF-I into the brain parenchyma after hypoxic-ischaemic injury. Neuroreport. 1996;7:632–6. doi: 10.1097/00001756-199601310-00061. [DOI] [PubMed] [Google Scholar]
- Guan J, Miller OT, Waugh KM, McCarthy DC, Gluckman PD. Insulin-like growth factor-1 improves somatosensory function and reduces the extent of cortical infarction and ongoing neuronal loss after hypoxia-ischemia in rats. Neuroscience. 2001;105:299–306. doi: 10.1016/s0306-4522(01)00145-2. [DOI] [PubMed] [Google Scholar]
- Hall WA, Merrill MJ, Walbridge S, Youle RJ. Epidermal growth factor receptors on ependymomas and other brain tumors. J Neurosurg. 1990;72:641–6. doi: 10.3171/jns.1990.72.4.0641. [DOI] [PubMed] [Google Scholar]
- Harbottle RP, Cooper RG, Hart SL, Ladhoff A, McKay T, Knight AM, Wagner E, Miller AD, Coutelle C. An RGD-oligolysine peptide: a prototype construct for integrin-mediated gene delivery. Hum Gene Ther. 1998;9:1037–47. doi: 10.1089/hum.1998.9.7-1037. [DOI] [PubMed] [Google Scholar]
- Herenu CB, Cristina C, Rimoldi OJ, Becu-Villalobos D, Cambiaggi V, Portiansky EL, Goya RG. Restorative effect of insulin-like growth factor-I gene therapy in the hypothalamus of senile rats with dopaminergic dysfunction. Gene Ther. 2007;14:237–45. doi: 10.1038/sj.gt.3302870. [DOI] [PubMed] [Google Scholar]
- Herenu CB, Sonntag WE, Morel GR, Portiansky EL, Goya RG. The ependymal route for insulin-like growth factor-1 gene therapy in the brain. Neuroscience. 2009;163:442–7. doi: 10.1016/j.neuroscience.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya K, Hori S, Ohtsuki S, Terasaki T. A new in vitro model for blood-cerebrospinal fluid barrier transport studies: an immortalized choroid plexus epithelial cell line derived from the tsA58 SV40 large T-antigen gene transgenic rat. Adv Drug Deliv Rev. 2004;56:1875–85. doi: 10.1016/j.addr.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Ivanenkov VV, Menon AG. Peptide-mediated transcytosis of phage display vectors in MDCK cells. Biochem Biophys Res Commun. 2000;276:251–7. doi: 10.1006/bbrc.2000.3358. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Duncan JA, Stopa EG, Baird A. Enhanced prospects for drug delivery and brain targeting by the choroid plexus-CSF route. Pharm Res. 2005;22:1011–37. doi: 10.1007/s11095-005-6039-0. [DOI] [PubMed] [Google Scholar]
- Kassner PD, Burg MA, Baird A, Larocca D. Genetic selection of phage engineered for receptor-mediated gene transfer to mammalian cells. Biochem Biophys Res Commun. 1999;264:921–8. doi: 10.1006/bbrc.1999.1603. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Hosoya K, Watanabe M, Takashima T, Ohtsuki S, Takanaga H, Ueda M, Yanai N, Obinata M, Terasaki T. Characterization of the amino acid transport of new immortalized choroid plexus epithelial cell lines: a novel in vitro system for investigating transport functions at the blood-cerebrospinal fluid barrier. Pharm Res. 2001;18:16–22. doi: 10.1023/a:1011014424212. [DOI] [PubMed] [Google Scholar]
- Koivunen E, Arap W, Rajotte D, Lahdenranta J, Pasqualini R. Identification of receptor ligands with phage display peptide libraries. J Nucl Med. 1999;40:883–8. [PubMed] [Google Scholar]
- Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10:625–32. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- Kuhn PE, Miller MW. c-neu oncoprotein in developing rostral cerebral cortex: relationship to epidermal growth factor receptor. J Comp Neurol. 1996;372:189–203. doi: 10.1002/(SICI)1096-9861(19960819)372:2<189::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Larocca D, Witte A, Johnson W, Pierce GF, Baird A. Targeting bacteriophage to mammalian cell surface receptors for gene delivery. Hum Gene Ther. 1998;9:2393–9. doi: 10.1089/hum.1998.9.16-2393. [DOI] [PubMed] [Google Scholar]
- Larocca D, Jensen-Pergakes K, Burg MA, Baird A. Gene transfer using targeted filamentous bacteriophage. Methods Mol Biol. 2002;185:393–401. doi: 10.1385/1-59259-241-4:393. [DOI] [PubMed] [Google Scholar]
- Li X, Stuckert P, Bosch I, Marks JD, Marasco WA. Single-chain antibody-mediated gene delivery into ErbB2-positive human breast cancer cells. Cancer Gene Ther. 2001;8:555–65. doi: 10.1038/sj.cgt.7700337. [DOI] [PubMed] [Google Scholar]
- McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–62. [PubMed] [Google Scholar]
- Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci. 2003;6:252–73. [PubMed] [Google Scholar]
- Mount JD, Samoylova TI, Morrison NE, Cox NR, Baker HJ, Petrenko VA. Cell targeted phagemid rescued by preselected landscape phage. Gene. 2004;341:59–65. doi: 10.1016/j.gene.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Muruganandam A, Tanha J, Narang S, Stanimirovic D. Selection of phage-displayed llama single-domain antibodies that transmigrate across human blood-brain barrier endothelium. Faseb J. 2002;16:240–2. doi: 10.1096/fj.01-0343fje. [DOI] [PubMed] [Google Scholar]
- Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, Engelhardt B, Grammas P, Nedergaard M, Nutt J, Pardridge W, Rosenberg GA, Smith Q, Drewes LR. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Drug targeting to the brain. Pharm Res. 2007a;24:1733–44. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier delivery. Drug DiscovToday. 2007b;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Pasqualini R, Ruoslahti E. Tissue targeting with phage peptide libraries. Mol Psychiatry. 1996a;1:423. [PubMed] [Google Scholar]
- Pasqualini R, Ruoslahti E. Searching for a molecular address in the brain. Mol Psychiatry. 1996b;1:421–2. [PubMed] [Google Scholar]
- Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996c;380:364–6. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- Pasqualini R. Vascular targeting with phage peptide libraries. Q J Nucl Med. 1999;43:159–62. [PubMed] [Google Scholar]
- Patel MM, Goyal BR, Bhadada SV, Bhatt JS, Amin AF. Getting into the brain: approaches to enhance brain drug delivery. CNS Drugs. 2009;23:35–58. doi: 10.2165/0023210-200923010-00003. [DOI] [PubMed] [Google Scholar]
- Poul MA, Marks JD. Targeted gene delivery to mammalian cells by filamentous bacteriophage. J Mol Biol. 1999;288:203–11. doi: 10.1006/jmbi.1999.2678. [DOI] [PubMed] [Google Scholar]
- Rajotte D, Arap W, Hagedorn M, Koivunen E, Pasqualini R, Ruoslahti E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J Clin Invest. 1998;102:430–7. doi: 10.1172/JCI3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez EM, Blazquez JL, Guerra M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides. 2010;31:757–76. doi: 10.1016/j.peptides.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Schlachetzki F, Zhang Y, Boado RJ, Pardridge WM. Gene therapy of the brain: the trans-vascular approach. Neurology. 2004;62:1275–81. doi: 10.1212/01.wnl.0000120551.38463.d9. [DOI] [PubMed] [Google Scholar]
- Smith GP, Petrenko VA. Phage Display. Chem Rev. 1997;97:391–410. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- Soderquist RG, Mahoney MJ. Central nervous system delivery of large molecules: challenges and new frontiers for intrathecally administered therapeutics. Expert Opin Drug Deliv. 2010;7:285–93. doi: 10.1517/17425240903540205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopa EG, Berzin TM, Kim S, Song P, Kuo-LeBlanc V, Rodriguez-Wolf M, Baird A, Johanson CE. Human choroid plexus growth factors: What are the implications for CSF dynamics in Alzheimer’s disease? Exp Neurol. 2001;167:40–7. doi: 10.1006/exnr.2000.7545. [DOI] [PubMed] [Google Scholar]
- Tanha J, Muruganandam A, Stanimirovic D. Phage display technology for identifying specific antigens on brain endothelial cells. Methods Mol Med. 2003;89:435–49. doi: 10.1385/1-59259-419-0:435. [DOI] [PubMed] [Google Scholar]
- Trepel M, Arap W, Pasqualini R. Exploring vascular heterogeneity for gene therapy targeting. Gene Ther. 2000;7:2059–60. doi: 10.1038/sj.gt.3301361. [DOI] [PubMed] [Google Scholar]
- Veening JG, Barendregt HP. The regulation of brain states by neuroactive substances distributed via the cerebrospinal fluid; a review. Cerebrospinal Fluid Res. 2010;7:1. doi: 10.1186/1743-8454-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh B, Vigh-Teichmann I. Actual problems of the cerebrospinal fluid-contacting neurons. Microsc Res Tech. 1998;41:57–83. doi: 10.1002/(SICI)1097-0029(19980401)41:1<57::AID-JEMT6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Vigh B, Manzano e Silva MJ, Frank CL, Vincze C, Czirok SJ, Szabo A, Lukats A, Szel A. The system of cerebrospinal fluid-contacting neurons. Its supposed role in the nonsynaptic signal transmission of the brain. Histol Histopathol. 2004;19:607–28. doi: 10.14670/HH-19.607. [DOI] [PubMed] [Google Scholar]
- Vitiello CL, Merril CR, Adhya S. An amino acid substitution in a capsid protein enhances phage survival in mouse circulatory system more than a 1000-fold. Virus Res. 2005;114:101–3. doi: 10.1016/j.virusres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Yip YL, Hawkins NJ, Smith G, Ward RL. Biodistribution of filamentous phage-Fab in nude mice. J Immunol Methods. 1999;225:171–8. doi: 10.1016/s0022-1759(99)00044-7. [DOI] [PubMed] [Google Scholar]