Abstract
The area postrema (AP) is a circumventricular organ located in the dorsal midline of the medulla. It functions as a chemosensor for blood-borne peptides and solutes, and converts this information into neural signals that are transmitted to the nucleus tractus solitarius (NTS) and parabrachial nucleus (PB). One of its NTS targets in the rat is the aldosterone-sensitive neurons which contain the enzyme 11 β-hydroxysteroid dehydrogenase type 2 (HSD2). The HSD2 neurons are part of a central network involved in sodium appetite regulation, and they innervate numerous brain sites including the pre-locus coeruleus (pre-LC) and PB external lateral-inner (PBel-inner) cell groups of the dorsolateral pons. Both pontine cell groups express the transcription factor FoxP2 and become c-Fos activated following sodium depletion. Because the AP is a component in this network, we wanted to determine whether it also projects to the same sites as the HSD2 neurons. By using a combination of anterograde axonal and retrograde cell body tract-tracing techniques in individual rats, we show that the AP projects to FoxP2 immunoreactive neurons in the pre-LC and PBel-inner. Thus, the AP sends a direct projection to both the first-order medullary (HSD2 neurons of the NTS) and the second-order dorsolateral pontine neurons (pre-LC and PB-el inner neurons). All three sites transmit information related to systemic sodium depletion to forebrain sites and are part of the central neural circuitry that regulates the complex behavior of sodium appetite.
Keywords: area postrema, nucleus tractus solitarius, parabrachial nucleus, pre-locus coeruleus, salt appetite, sodium intake
1. INTRODUCTION
The area postrema (AP) is a circumventricular organ that lies in the dorsal midline of the caudal medulla, immediately next to the nucleus of the solitarius (NTS) and it projects directly to both the NTS and parabrachial nucleus (PB) (Cunningham et al., 1994; Herbert et al., 1990; Shapiro and Miselis, 1985b; van der Kooy and Koda, 1983). The AP innervates different types of NTS cells, including catecholamine, neurotensin, and aldosterone-sensitive neurons (Cunningham et al., 1994; Kachidian and Pickel, 1993; Sequeira et al., 2006). The latter group of neurons contains the enzyme 11 β-hydroxysteroid dehydrogenase type 2 (HSD2) as well as mineralocorticoid receptors, making them highly selective to aldosterone (Funder et al., 1988). These neurons also show an increase in c-Fos immunoreactivity (a marker for neuronal activation) during week-long periods of sodium deprivation, a response that is rapidly reversed by sodium chloride ingestion (Geerling et al., 2006).
The AP also projects to the PB and terminates mainly in its external lateral subnucleus (PBel) (Cunningham et al., 1994; Herbert et al., 1990; Shapiro and Miselis, 1985a; van der Kooy and Koda, 1983) and in a second nearby region lying in the pontine periventricular gray matter (Shapiro and Miselis, 1985b) which corresponds to the pre-locus coeruleus nucleus (pre-LC) (Geerling et al., 2010). These two regions are also innervated by the aldosterone-sensitive HSD2 neurons (Geerling and Loewy, 2006). Just like the HSD2 neurons, the FoxP2 immunoreactive (ir) neurons of both the pre-LC and the PB external lateral-inner division (PBel-inner) express elevated c-Fos levels one week after sodium depletion (Geerling et al., 2009), suggesting that these three cell groups may be part of the same central network that senses sodium deprivation (Geerling et al., 2006).
Further studies implicate the AP in sodium appetite regulation. For example, rats with AP lesions consume considerably more NaCl than normal rats (Contreras and Stetson, 1981; Curtis et al., 1999; Edwards et al., 1993; Hyde and Miselis, 1984; Wang and Edwards, 1997; Watson, 1985), suggesting that the AP provides a strong inhibitory control over sodium intake. Additionally, sodium-deprived rats show little c-Fos labeling in the AP, but once they are allowed to consume saline after long deprivation periods, the lateral AP neurons express elevated levels of c-Fos activity (Geerling and Loewy, 2007). (Note: Throughout the rest of this report the term “c-Fos activated” will be used in place of the more lengthy description presented in the previous sentence.) Finally, even more extensive c-Fos activity was observed throughout the AP after intragastric infusion of hypertonic saline (Kobashi et al., 1993).
In an effort to distinguish cytochemically between specific PB subnuclei, we extended the work of Gray and associates (Gray, 2008; Gray et al., 2004) who reported that subpopulations of PB neurons express the transcription factor- Forkhead box protein P2 (FoxP2). They observed a collection of FoxP2 neurons in the region of the pre-LC (see Figure 3F in Gray et al, 2008). In a preliminary study, our laboratory found that the FoxP2-ir neurons of the pre-LC and PBel-inner become Fos activated after one week of sodium deprivation (Geerling et al., 2009), indicating that FoxP2 antibodies may be a useful neuroanatomical tool for detecting these two subsets of dorsolateral pontine neurons that have been implicated in signaling systemic sodium depletion.
The present study was designed to investigate the projection of the AP to individual neurons in both the pre-LC and PBel-inner. By utilizing a combination of neuroanatomical tracing techniques along with FoxP2 immunohistochemistry, we report that the AP projects directly to the FoxP2-ir neurons of the pre-LC and PB-el inner.
2. RESULTS
2.1 Localization of the transcription factor FoxP2 in neurons of the dorsolateral pons
FoxP2-ir neurons were localized in the pre-LC and the PBel-inner, as well as several other PB regions, making antibodies against this transcription factor a useful reagent for defining some of the PB subnuclei.
Dense clusters of FoxP2-ir neurons were localized in the PBel-inner (Fig. 1A) and pre-LC (Fig. 1B). The PBel-inner neurons shown in Figure 1A (see insert) are from the caudalmost part of this subnucleus. The pre-LC lies just rostral to the locus coeruleus (see Fig. 6A in Geerling et al, 2010) and does not contain tyrosine hydroxylase or cholinergic immunoreactive neurons (Geerling et al., 2009). It resides in the lateral part of the periventricular gray matter of the dorsolateral pons, immediately medial to the mesencephalic trigeminal nucleus (MeV), and some of its neurons intermix with the MeV (Fig. 1B- see insert).
Figure 1.
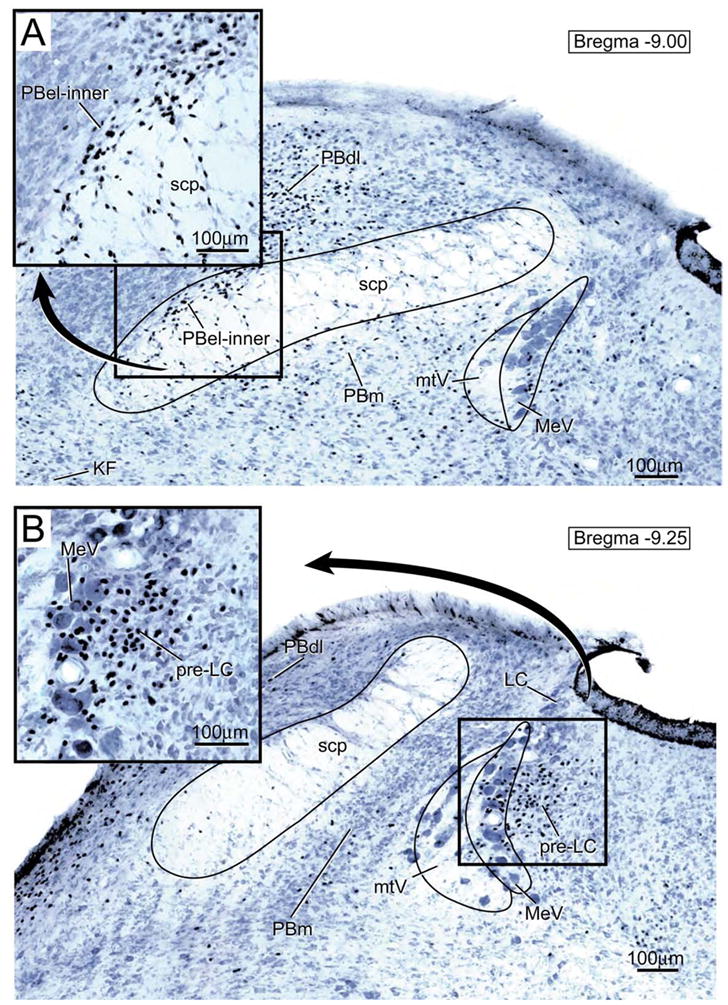
Transverse brainstem section showing the distribution of FoxP2-ir neurons in the parabrachial nucleus (PB). A. The insert shown in the upper left hand corner presents an enlargement of the FoxP2-ir neurons in the external lateral parabrachial subnucleus- inner division (PBel-inner). Other abbreviations: KF= Kölliker-Fuse nucleus; PBdl= dorsal lateral parabrachial subnucleus; PBm= medial parabrachial subnucleus; MeV = mesencephalic nucleus of the trigeminal nerve; mtV = tract of the mesencephalic trigeminal nucleus; scp= superior cerebellar peduncle.
B. Transverse brainstem section showing the pre-locus coeruleus nucleus (pre-LC). This nucleus lies immediately rostral to the locus coeruleus within the lateral part of the periventricular gray matter. As shown in the enlargement in the upper left hand corner, the FoxP2-ir neurons are concentrated in the zone medial to the mesencephalic nucleus of the trigeminal nerve (MeV), but some are interspersed among the MeV neurons..
The other PB regions were found to contain FoxP2 immunoreactive neurons; these results can be summarized as follows. Large collections of FoxP2-ir neurons were distributed in the dorsal lateral PB subdivision, moderate numbers were found in the Kölliker-Fuse nucleus, as well as in the ventral lateral and medial PB subnuclei. Very few FoxP2-ir neurons were seen in the central lateral, external medial, and lateral crescent PB regions, and none were observed in the PB waist area or PB internal lateral subnucleus. Since the focus of this report deals with the pre-LC and PB-el inner, further details regarding these PB subnuclei are not presented here.
2.2 AP projection to the pre-LC and PBel-inner nuclei
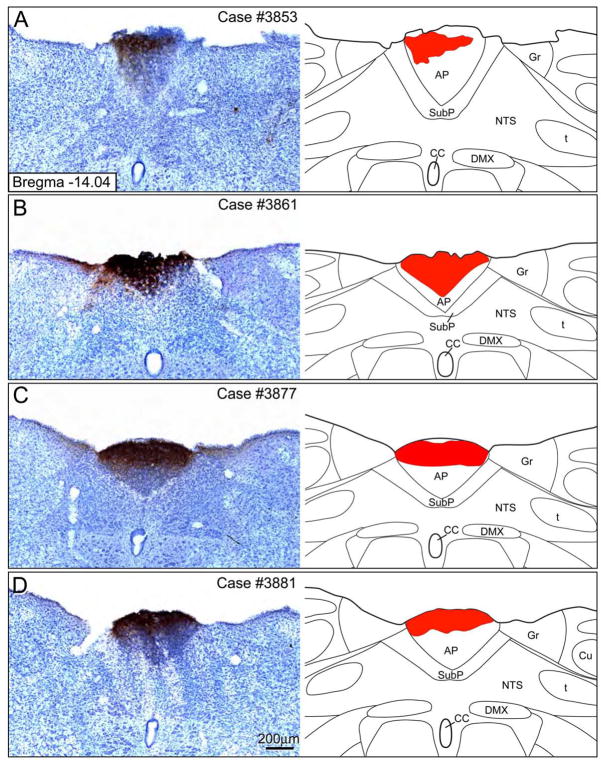
Four cases were available that had Phaseolus vulgaris leucoagglutinin (PHAL) injections totally confined to the AP (Figure 2). There were slight variations in the rostral-caudal extent of the PHAL injection sites. Similarly, there was variability in the overall density of the PHAL injection sites as well. All four cases resulted in similar findings in terms of the anterograde axonal labeling in the dorsolateral pontine region.
Figure 2.
A–D: PHAL injection sites in the area postrema along with accompanying line drawings. All cases presented here had injections confined to the area postrema without any cell body labeling in the subpostremal or medial NTS. Drawings were modified from Paxinos and Watson (2005). Abbreviations: AP = area postrema; CC = central canal; DMX = dorsal vagal nucleus; Gr = gracile nucleus; NTS = nucleus tractus solitarius; t = solitary tract; SubP = subpostremal NTS region.
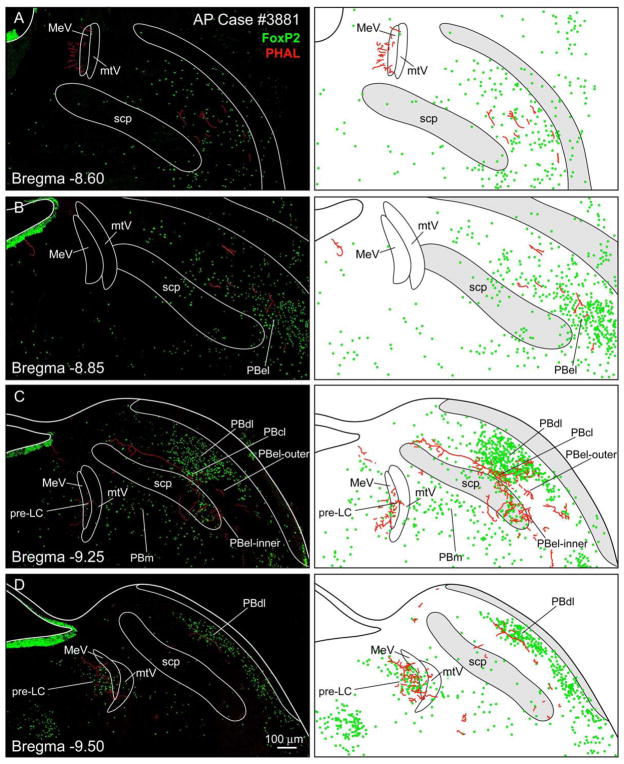
Figure 3 presents case #3881 which had a medium-sized PHAL injection in the AP (Fig. 2D). Moderate amounts of axonal labeling were identified in the pre-LC (Fig. 3D) and the PBel-inner (Fig. 3C). Other PB regions contained slight amounts of axonal labeling, including external lateral-outer, central lateral, dorsal lateral, and ventral lateral subnuclei. In addition, there was a periventricular area shown in Figure 3A at the rostral level of the MeV that had moderate amount of axonal labeling which was independent of the FoxP2 neurons. No axonal labeling was found in the locus coeruleus.
Figure 3.
A–D. Photoimages and accompanying line drawings showing the distribution of PHAL labeled axons (red) among the FoxP2-ir neurons in the parabrachial nucleus (PB) and pre-locus coeruleus nucleus (pre-LC) after a PHAL injection in the area postrema. Sections are arranged from rostral (A) to caudal (D). Panel A shows additional axonal labeling that was medial to the mesencephalic nucleus of the trigeminal nerve (MeV); this area was independent of the pre-LC. Panel B shows very weak axonal labeling in the PB external lateral subnucleus. Panel C shows a dense cluster of labeled axons in the PB external lateral-inner division (PB-el inner). An occasional labeled axon was found in other parabrachial subnuclei including the PB central lateral (PBcl), PB dorsal lateral subnucleus (PBdl), and outer division of the PB external lateral subnucleus (C and D). Panel D shows a dense collection of labeled axons surrounding the FoxP2-ir neurons of the pre-LC. Additional abbreviations: mtV= tract of the mesencephalic nucleus of the trigeminal nerve; PBm = medial PB subnucleus; scp = superior cerebellar peduncle. Scale bar = 100 μm.
2.3 Combined anterograde and retrograde labeling experiments: AP input to the FoxP2-ir neurons of the pre-LC and PBel-inner
Combined anterograde-retrograde neural tracing experiments were used to determine whether the AP projects directly to the perikarya or primary dendrites of the FoxP2-ir neurons of the pre-LC and PBel-inner. These experiments are illustrated in schematic form in Figure 4. Unlike the experiments shown in Figure 3 which showed PHAL axonal labeling was present in the vicinity of the FoxP2 neurons, this set of experiments demonstrated with the aid of confocal microscopy that the AP projects directly to these neurons.
Figure 4.
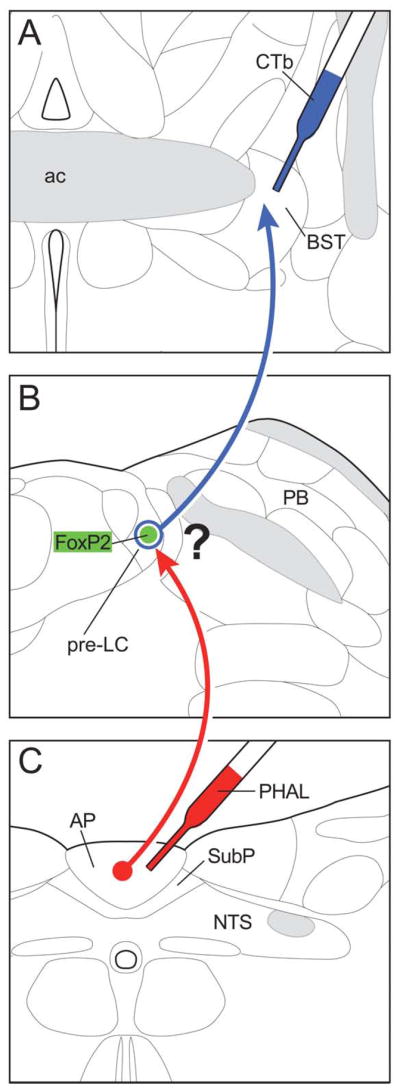
The experimental design of the double tract-tracing experiments used to examine whether the AP projection neurons make close contacts with the FoxP2-ir neurons in the pre-LC. The anterograde axonal tracer PHAL (red) was injected into the area postrema, and 3 days later, the retrograde cell body marker CTb (blue) was injected into one of several forebrain sites, including the bed nucleus of the stria terminalis (as shown here) or the dorsomedial, paraventricular, and perifornical areas of the hypothalamus. Sections through the dorsolateral pons were processed by a triple immunofluorescence method and examined by confocal microscopy.
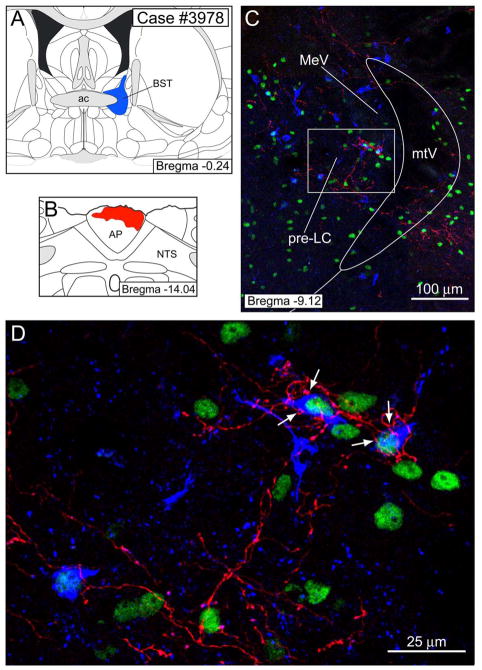
Figure 5 presents our best example of a case in which cholera toxin β-subunit (CTb) was injected into the bed nucleus of the stria terminalis (BST) and PHAL was injected into the AP. PHAL labeling was found in the pre-LC. Two examples of CTb-labeled pre-LC neurons which contained FoxP2-ir are shown in Figure 5. PHAL labeled fibers made close contacts with these cells (Fig. 5C- see arrows). In this case, a total of 16 FoxP2-ir cells were retrogradely labeled in the pre-LC.
Figure 5.
Double tract-tracing experiment to demonstrate that AP neurons project to FoxP2-ir neurons of the pre-LC that send efferent connections to the bed nucleus of the stria terminalis (BST). A–B. A CTb injection (blue) was made in the BST andPHAL injection (red) into the area postrema (AP). C. 20× confocal image of the pre-LC region showing PHAL axons in relationship to FoxP2-ir (green) and CTb labeled neurons. D. 40× confocal image showing PHAL axons making close contacts (arrows) with several CTb labeled FoxP2-ir neurons.
Axonal labeling was also found near the retrogradely labeled neurons in the PBel-inner, but in this experiment we could not find unequivocal examples in which PHAL labeled axonal terminals made close contact with these neurons. Perhaps, this was because of the limited sample size of CTb-labeled FoxP2-ir PBel-inner neurons found in this case (n = 7 neurons). Alternatively, this particular CTb injection may have been placed in a region of the BST that avoids the major site where PBel-inner neurons project. The CTb injection illustrated in Figure 5 involves dorsomedial and dorsolateral parts of the BST and excludes the lateral BST (see Discussion). A total of two cases showed these findings.
In addition to the BST experiments, we performed another series of double tract-tracing experiments where CTb injections were made primarily into three sites of the hypothalamus, including the perifornical region (PeF), dorsomedial hypothalamic nucleus (DMH), and paraventricular hypothalamic nucleus (PVH).
Figure 6 presents a case in which CTb was injected into the caudal PVH and PHAL was injected into the AP. PHAL axonal labeling was found in both the pre-LC and PBel-inner. Figure 6D–F presents three examples of FoxP2-ir PBel-inner neurons that received a projection from the AP.
Figure 6.
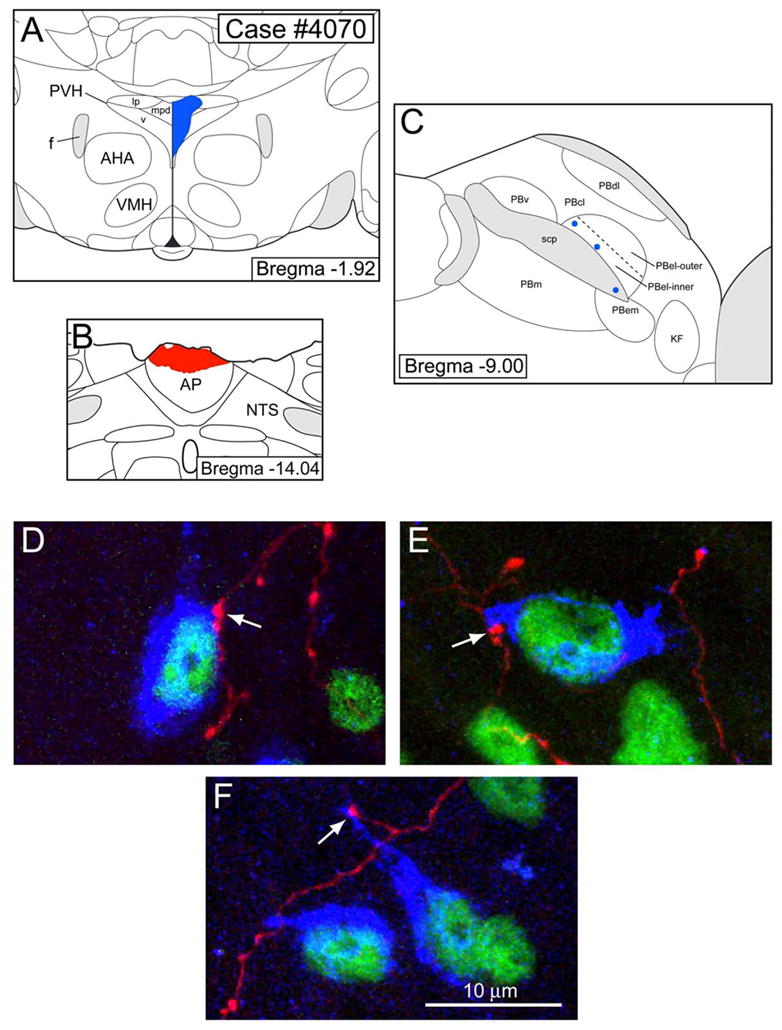
Double tract-tracing experiment to demonstrate that AP neurons project to FoxP2-ir neurons of the PBel-inner that send efferent connections to the paraventricular hypothalamic nucleus (PVH). A. A CTb injection (blue) was made into the caudal PVH, which spread into the lateral parvicellular, medial parvicellular, and ventral parts. B. PHAL was injected (red) into the area postrema (AP). C. A schematic line drawing of the parabrachial nucleus (PB) showing the relative positions of FoxP2-ir CTb-labeled PBel-inner neurons that project to the PVH as shown in D–F. The labeled neurons (blue dots) were positioned relative to the superior cerebellar peduncle (scp) using photoimages taken with both darkfield and fluorescence optics. D–F. 40× confocal images showing PHAL axons making close contacts (arrows) with three neurons in the PBel-inner. Other abbreviations: AHA= anterior hypothalamic area; f= fornix; KF= Kölliker-Fuse nucleus; PBcl= central lateral parabrachial subnucleus; PBdl= dorsal lateral parabrachial subnucleus; PBel-outer= external lateral parabrachial subnucleus- outer division; PBem= external medial parabrachial subnucleus; PBm= medial parabrachial subnucleus; PBv= ventral lateral parabrachial subnucleus; scp= superior cerebellar peduncle.
3. DISCUSSION
The key finding of this study shows that the AP provides a direct input to the FoxP2-ir neurons of both the pre-LC and PBel-inner. These studies are consistent with the notion that the AP is involved in the central neural network regulating sodium appetite and provide additional details regarding the neural circuitry underlying this regulation.
The neural pathways mediating sodium appetite are independent of those involved in the sodium taste system, as evidenced by their distinct c-Fos activation patterns in the brain. Specifically, activation of Na+ receptors on the tongue causes c-Fos activation of the rostral NTS as well as the medial, central lateral, and external lateral (outer division) PB subdivisions (Yamamoto et al., 1993), while sodium deprivation results in c-Fos activation of the HSD2 neurons in the AP level of the NTS, as well as neurons in both the pre-LC and PB-el inner (Geerling et al., 2006; Geerling and Loewy, 2007).
The AP participates in a range of visceral and autonomic functions that have been reviewed by Price et al. (2008). It has long been considered the chemoreceptor trigger zone for the emetic reflex (Borison and Brizzee, 1951). In addition, receptors for a variety of gastrointestinal-related peptide hormones are localized in the AP; these include amylin, cholecystokinin, ghrelin, and adiponectin (Price et al., 2008). Single-cell in vitro electrophysiological studies confirm that AP neurons are depolarized or hyperpolarized by these different gut-related peptide hormones (Price et al., 2008). Other AP functions include its modulatory role in cardiovascular regulation (Sun and Spyer, 1991). Two major cardiovascular-related peptide hormones- angiotensin II and vasopressin- modulate the baroreceptor reflex and are likely to act via the AP NTS connection (Price et al., 2008), possibly targeting the neurotensin NTS neurons (Higgins et al., 1984; Sequeira et al., 2006). Finally, the AP may influence other visceral functions, including fluid balance and immune responses (Price et al., 2008).
3.1 Brainstem pathways involved in sodium appetite regulation
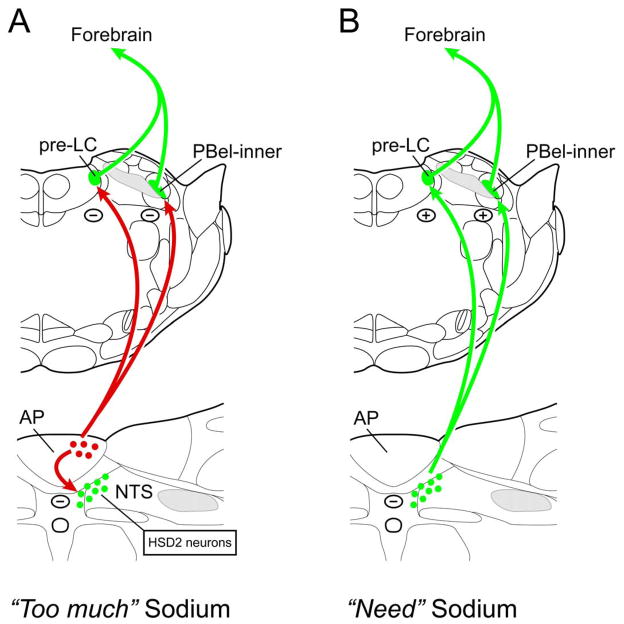
The pre-LC and PBel-inner both receive direct and indirect inputs from the AP, with the latter connection being relayed via the aldosterone-sensitive NTS neurons (Geerling and Loewy, 2006). These data are presented in summary diagrams presented in Figure 7.
Figure 7.
Summary drawings showing A) the AP projects to the HSD2 neurons of the NTS as well as to both the pre-LC and PBel-inner of the dorsolateral pontine region, and B) HSD2 neurons project directly to the pre-LC and PBel-inner. The AP is a circumventricular organ and functions as a chemosensor. Based on c-Fos studies in rats, HSD2 neurons have been implicated along with pre-LC and PBel-inner neurons as components in a neural system that detects peripheral sodium deprivation, signaling the “need” to find and ingest sodium to forebrain areas (Geerling et al., 2006; Geerling and Loewy, 2007). Once sodium ingestion has occurred and the need for sodium has been fulfilled, an inhibitory response that “too much” sodium has been consumed will likely occur. This may be mediated through the AP which provides an inhibitory effect on the HSD2 neurons as well as neurons in the pre-LC and PBel-inner. Lesions of the AP remove this inhibition, and as a consequence, rats consume considerably more amounts of sodium chloride than do normal rats (Curtis et al., 1999). In contrast, HSD2 neurons receive a convergence of both neural and chemosensitive inputs. The former originate from the stomach and duodenum (Shin and Loewy, 2009) and the latter comes from the area postrema (Sequeira et al., 2006). The HSD2 cells are the first central neurons in a pathway that regulates sodium appetite (Geerling et al., 2006), and their first ascending targets are the pre-LC and PBel-inner neurons of the dorsolateral pons. The HSD2 neurons are thought to be a glutamate-containing (Geerling et al., 2008). The efferent projections of the pre-LC and PBel-inner are unknown, except for two reports showing that the PBel projects to the lateral part of the central nucleus of the amygdala (Bernard et al., 1993) and to the dorsolateral and ventral parts of the bed nucleus of the stria terminalis (Alden et al., 1994). Although not defined in this drawing, the HSD2 neurons project to several forebrain sites including the parasubthalamic nucleus, lateral hypothalamic area, paraventricular hypothalamic area, central nucleus of the amygdala, and bed nucleus of the stria terminalis (Geerling and Loewy, 2006; Shin et al., 2008).
Three brainstem groups become c-Fos activated after one week of sodium deprivation: the HSD2 neurons of the NTS, FoxP2-ir neurons of the pre-LC, and FoxP2-ir neurons of the PBel-inner (See Introduction). In contrast, the AP is devoid of c-Fos labeling after a similar treatment (Geerling and Loewy, 2007), but 1–2 hours after consumption of NaCl, the lateral AP becomes c-Fos activated (Geerling and Loewy, 2007). Also, gastric infusion of hypertonic saline causes c-Fos activation in the AP (Kobashi et al., 1993). Thus, it appears the AP shows a different c-Fos activation profile as compared to the HSD2 and FoxP2-ir neurons of the pre-LC and PBel-inner.
The lateral AP receives a very weak vagal input from the stomach (Shapiro and Miselis, 1985b) and from the hepatic branch of the vagus nerve (Rogers and Hermann, 1983). The latter finding is difficult to interpret because this nerve carries sensory fibers from the liver, pyloric region of the stomach, pancreas, and proximal duodenum to the NTS (Berthoud and Neuhuber, 2000). Nevertheless, lateral AP neurons could receive two types of visceral information: neurally mediated gastrointestinal information carried via the vagus nerve and chemosensitive information regarding blood-borne agents in the plasma.
The AP plays an inhibitory role in regulating sodium appetite, and AP lesions cause large increases in sodium consumption (see Introduction). The mechanisms by which this inhibition occurs is unknown, but may be dependent on a serotonergic (5-HT) projection that originates in the AP (Lanca and van der Kooy, 1985). Whether the 5-HT containing AP neurons inhibit the aldosterone-sensitive NTS neurons or the FoxP2-ir neurons of the pre-LC and PBel-inner is unknown.
Bilateral injections of 5-HT drugs into the lateral PB affect sodium intake. For example, when the 5HT1/5-HT2 receptor antagonist - methysergide was injected bilaterally into the lateral PB, it caused an increase in sodium appetite (Menani et al., 1996). Likewise, bilateral activation of 5-HT1A receptors in the lateral PB caused an increase in sodium intake (De Gobbi et al., 2005), and activation of 5-HT2A and 5-HT2c receptors inhibited sodium ingestion (De Gobbi et al., 2007). Further work is needed to establish whether the 5-HT neurons of the AP are responsible for this response, and whether similar effects occur when 5-HT drug injections are confined to the pre-LC.
3.2 Methodological Considerations
This study establishes that the AP projects to the pre-LC and PBel-inner, although the density of these projections was relatively weak; the input to the pre-LC was approximately two times stronger than that observed in the PBel-inner. Several methodological points need to be considered.
Our selection of PHAL cases was biased, consisting of experiments that had injections confined to the dorsal AP. This was done because some subpostremal NTS neurons send their dendrites into the AP proper (Morest, 1960). If we had used AP cases where PHAL-labeled neurons were present in any part of the NTS, there would be uncertainty about the origin of the efferent projections to the dorsolateral pons. By mostly restricting our PHAL injections into the dorsal zone of the AP (see Figure 2 from Price et al. 2008), the amount of axonal labeling observed in the pre-LC and PB-el may have been considerably less than normally occurs. This could be attributed to the fact that our PHAL injections avoided the central and lateral zones of the AP.
Another issue was that relatively few FoxP2-ir pre-LC and PBel-inner neurons were retrogradely labeled after CTb injections in the forebrain. Even in the best cases, a total of 10–15 co-labeled pre-LC neurons for the BST cases and 15–20 neurons for the hypothalamic cases were observed. This was even more pronounced for the PBel-inner because this site appeared to contain an even smaller number of projection neurons than the pre-LC region, ranging from 3–15 FoxP2-ir CTb-labeled neurons in both BST and hypothalamic cases.
These results can be attributed to at least three factors. First, the present study was based on systematic samples of the brainstem that were spaced at 250 μm intervals, which resulted in only three and two sections available for analysis that contained the pre-LC and PBel-inner, respectively. Thus, this limited the sample size of FoxP2-ir neurons.
Second, it must be noted that the efferent projections of both the pre-LC and PBel-inner have not been established. Our decision to select specific targets for the CTb injections was based on preliminary work being done in our laboratory (Loewy, AD, unpublished observations) in which the efferent projections of the pre-LC and PB-el inner are under study. As shown in this study (Figs. 5 & 6), both the pre-LC and PB-el inner project to the BST and hypothalamus, but it is possible that each of these two regions has its own distinct targets and do not necessarily innervate all common areas.
Figure 5 provides an example of this differential innervation of the BST. As shown in this figure, when CTb was injected into the dorsomedial and ventromedial BST it produced moderate numbers of retrogradely labeled neurons in the pre-LC, but relatively few in the PBel-inner. These two dorsolateral pontine cell groups appear to project to different regions within the BST. As shown by earlier work, the PBel projects heavily to the dorsolateral BST (equivalent to the oval subdivision of the BST described by Dong et al. (2001)) but weakly to the dorsomedial and ventromedial BST (Alden et al., 1994). Since the CTb injection in case #3978 excluded the dorsolateral BST (Fig. 5), very few retrogradely labeled PBel-inner neurons were seen.
Third, the relatively low cell counts of retrogradely-labeled neurons in the pre-LC and PBel-inner may be explained by the fact that these nuclei do not project en masse to all of their various forebrain targets, but are organized in a topographic fashion. This means that only select neurons in both cell groups project to various subnuclei of the BST while others project to select hypothalamic nuclei, such as the PeF, PVH, and DMH.
Even with the various technical considerations mentioned above, it is important emphasize that we still obtained positive results showing the AP targets the FoxP2-ir neurons in both the pre-LC and PBel-inner.
3.3 Conclusions
AP neurons project to select groups of FoxP2-ir neurons in the pre-LC and PBel-inner of the dorsolateral pons– two regions that are c-Fos activated after sodium deprivation. As illustrated in Figure 7, the AP provides a potential inhibitory channel that limits sodium intake via a pathway independent of the HSD2 neurons. These neuroanatomical findings support the interpretation that the AP is involved in sodium appetite regulation.
4. Experimental Procedures
All animal procedures were approved by the Washington University School of Medicine Animal Care Committee, conformed to NIH guidelines, and were performed on Sprague-Dawley rats (male and female; 250–350g; Charles River Labs, Wilmington, MA) under sodium pentobarbital (50 mg/kg, intraperitoneal) anesthesia. At the termination of each experiment, anesthetized rats were killed by transcardiac perfusion with 200 mL of saline, followed by 500 mL of 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH = 7.4). Brains were removed and stored in this fixative for several weeks.
4.1 FoxP2 transcription factor: immunohistochemistry
Brainstems (n=20) were cut either in the coronal, horizontal, or sagittal planes at 75 or 100 μm on a freezing microtome. Free-floating serial sections were processed for immunohistochemical detection of FoxP2 by the ABC-DAB method.
Sections were incubated overnight in rabbit antibodies to FoxP2 (1:8,000; ab6046, Abcam, Cambridge, MA) made in a solution containing 0.3% Triton-X (Sigma, St. Louis, MO) and 5% donkey serum in 0.1M sodium phosphate (pH = 7.4), washed in potassium phosphate buffered saline (KPBS; 0.01M, pH = 7.4), transferred to a biotinylated donkey anti-rabbit (1:250; Jackson ImmunoResearch, West Grove, PA) solution made in 5% donkey serum (as above), washed in KPBS, treated for 1 hour in the avidin-biotin complex (ABC, Vectastain kit, Vector Labs, Burlingame, CA) solution, washed in KPBS, and colorized in a cobalt-diaminobenzidine (Co-DAB) solution (D-0426, Sigma). A single Co-DAB tablet was dissolved in 20–30 ml of distilled water containing one tablet of urea. The sections were reacted for 15 min in this activated solution, washed three times in KPBS, mounted a gelatinized slides, and air dried. Some of the sections were stained with 0.1% thionin (pH = 4.6) and coverslipped, while other sections were coverslipped directly without any counterstaining.
The rabbit polyclonal antibody to FoxP2 was made against a synthetic peptide made from residues 700 to the C-terminus of human FoxP2 which was conjugated to keyhole limpet hemocyanin. The sheep antibody to FoxP2 (see below) was made against E. coli-derived, recombinant human FoxP2 isoform 1 (aa640–715). Both antibodies showed a single band in Western blots performed by the manufacturers and were specific to human and mouse FoxP2. Based on sequence homology, these antibodies were predicted by these vendors to be specific against rat FoxP2 as well. Both antibodies yielded similar immunohistochemical staining patterns in the parabrachial complex.
4.2 PHAL anterograde axonal tracing experiments
Anesthetized rats were placed in a stereotaxic frame, and their posterior fossa was surgically exposed so the AP could be visualized. The dorsal medulla was carefully packed with cotton patties to minimize the amount of cerebrospinal fluid being discharged over the medullary surface.
A solution of the anterograde axonal tracer Phaseolus vulgaris leucoagglutinin (PHAL: 2.5% solution made in 0.02 M potassium phosphate buffer, pH 8.0; Vector Labs, Burlingame, CA) was back-filled into a glass micropipette. The pipette was secured to a micromanipulator, and was advanced 0.15 mm deep into the central part of the AP. PHAL was iontophoresed for 30 minutes using 7 μA on/off positive pulses delivered from a Midguard precision current source (Stoelting, Wood Dale, IL). The pipette was left in place for 5 min, and then removed. The wound was closed in layers. Seven days later, the rats were re-anesthetized, perfused through the heart with saline followed by 4% buffered paraformaldehyde. Their brains were removed and stored in fixative for several weeks. Frozen sections through the AP level of the brainstem were cut in the transverse plane at 50 μm, and serial sections through the AP were stained with goat antibodies directed against PHAL (1:20,000; Product # AS-2226, Vector) using the ABC method. The sections were counter stained with 0.1% thionin, and coverslipped. The injection sites were examined microscopically to find PHAL cases in which the reaction product confined to the AP. Later, the rostral part of the brainstem from these cases was cut in the transverse plane at 50 μm, and a one-in-five series of sections was stained by a double-immunohistochemical procedure.
The double-immunofluorescence method was performed as follows: free-floating sections were incubated in a mixture of two antisera: rabbit anti-FoxP2 (1:5,000; ab16046; Abcam, Cambridge, MA) and goat anti-PHAL (1:7,500; Vector) overnight, washed in KPBS, and transferred to a solution containing both Cy2-donkey anti-rabbit and Cy3-donkey anti-goat (1:500 for each; Jackson). The sections were washed in KPBS, mounted on glass slides, dried at room temperature, and coverslipped using a fade-retardant glycerol mounting solution containing sodium azide and n-propyl gallate.
4.3 Combined anterograde and retrograde tracing experiments: AP projection to the pre-locus coeruleus and parabrachial nuclei
Figure 5 summarizes the general design of the double tract-tracing experiments. Rats received PHAL injections in the AP as described above, and were allowed to survive 3 days before a second injection was made with the retrograde cell body tracer - cholera toxin β-subunit (CTb) - into various sites of the forebrain. In the example presented in Figure 5, a CTb injection was made in the bed nucleus of the stria terminalis (BST). Other forebrain targets included combinations of the dorsomedial hypothalamic nucleus (DMH), paraventricular hypothalamic nucleus (PVH), and perifornical hypothalamus (PeF). Seven days later, the rats were killed as described above, and their brains removed and stored in fixative for 1–2 weeks.
The CTb injections (Product #104; List Biological, Campbell, CA) were made in similar manner as the PHAL injections. A 0.1% solution of CTb was made in distilled water. Individual micropipettes were backfilled with the CTb solution under microscopic observation, and mounted on a micromanipulator. After a partial craniotomy, the CTb-filled micropipette was advanced into the forebrain target using stereotaxic coordinates taken from a rat brain atlas (Paxinos and Watson, 2005). The skull was held in the level position, and all measurements were made from the bregma.
CTb was iontophoresed for 30 minutes using 7 μA on/off positive pulses (as described above). The rats were allowed to survive for one week, and were anesthetized, killed by vascular perfusion, and their brains were removed, stored in fixative for one week.
Two sets of transverse frozen sections (50 μm) were cut. One was from the AP level of the medulla and the other was from the forebrain. The serial sections from the AP region were incubated overnight in a goat polyclonal antiserum against PHAL (1:20,000; Product #2224, Vector, Burlingame, CA). The forebrain sections (1-in-5 series) containing the injection site were incubated in a goat polyclonal antiserum to CTb (1:25,000; Product #703; List Biologicals, Campbell, CA) overnight. Both sets of sections were colorized by the ABC-DAB method, mounted on glass slides, air-dried, and counter-stained with 0.1% thionin.
The sections were examined microscopically to determine whether the respective injections had been successfully placed in both these two brain regions, and in those cases that were positive for both injections, sections from the pontomedullary brainstem were cut, and sections (1-in-5 series) containing the parabrachial nucleus were processed by a triple-color immunohistochemical procedure.
4.4 Triple-color immunohistochemical staining of PHAL axonal labeling in the pre-LC and PBel inner regions
Transverse, frozen sections (50 μm) of the dorsolateral pons which contained the pre-LC and PB were immunostained by a direct triple color method. Free-floating sections from a 1-in-5 series were incubated overnight at room temperature in a mixture of three antibodies: sheep anti-FoxP2 (1:5,000; Product # AF5647; R&D Systems, Minneapolis, MN), mouse anti-PHAL (1:700, V. Karpitskiy, St. Louis, MO) and rabbit anti-CTb (1:25,000; Product # B65927R; Biodesign International; Saco, Maine). The sections were washed in KPBS, and transferred to a mixture of three secondary antibodies: Cy5 donkey anti-sheep, Cy3 donkey anti-mouse, and Cy2 donkey anti-rabbit (all dilutions were 1:200) for 3 hours, washed, mounted glass slides, air-dried, and coverslipped with non-fade glycerol mountant.
4.5 Photoimages
Brightfield and darkfield images were taken on a Nikon microscope using a CCD camera and Nikon ACT-1 software (v2.62). Image cropping, resizing, and adjustments in brightness, contrast, sharpness, and color balance were performed using Adobe Photoshop CS (San Jose, CA).
Confocal imaging was performed with an Olympus Fluoview FV500b laser-scanning microscope. Images were acquired as multiple individual stacks using either a 20× (NA 1.17) or 40× (NA 1.35) oil objective lens. For the 20× and 40× oil lenses, images were taken in 1 μm and 0.4 μm z-steps, respectively, through the full thickness of the transverse tissue section of 50 μm. Next, 40–50 z-frames for 20× images and 80–90 z-frames for 40× images were collapsed into a two-dimensional, maximum-projection image to produce individual tiles (642 × 642 μm). Finally, individual two-dimensional projection images were aligned into photomontages using Adobe Photoshop, with adjustments in brightness and contrast as necessary.
All manipulations of confocal stacks, z-frame projections, and pseudocoloration were performed using MetaMorph software (Molecular Devices, Sunnyvale, CA, USA). Uniform brightness and contrast adjustments were performed in Photoshop. Line drawings of the cytoarchitectonic boundaries were added to all the photoimages. This was done in Adobe Illustrator (San Jose, CA) after the neuroanatomical landmarks had been obtained from a separate series of photoimages from dark field material. These images were superimposed on the photomontages of the fluorescence material, and outlines of the various relevant structures were made.
4.6 Terminology
Throughout this paper the term ‘close contacts’ has been used to describe PHAL labeled boutons (≈ 1–3μm in diameter) abutting on cell bodies or dendrites. These regions were visualized in one or more z-planes and no unlabeled pixels were found between the two structures. Nevertheless, it is important to note that a given contact seen with the confocal microscope is not necessarily a synapse. Documentation that a particular ‘close contact’ is a synapse requires electron microscopic evidence, and thus, the findings presented here need to be confirmed in the future at the ultrastructural level.
Research Highlights.
Area postrema projects to salt-sensitive central neurons.
FoxP2 neurons of parabrachial complex are involved in sodium appetite regulation
Acknowledgments
We thank Xay van Nguygen for excellent technical assistance, Dennis Oakley for help with the confocal microscopy.
Grant sponsor: National Heart, Lung, and Blood Institute of the National Institutes of Health – Grant number: HL025449, Bakewell Imaging Center Fund, and National Institutes of Health – Grant number: NS057105 Neuroscience Blueprint Core Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alden M, Besson JM, Bernard JF. Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: a PHA-L study in the rat. J Comp Neurol. 1994;341:289–314. doi: 10.1002/cne.903410302. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Borison HL, Brizzee KR. Morphology of emetic chemoreceptor trigger zone in cat medulla oblongata. Proc Soc Exp Biol Med. 1951;77:38–42. doi: 10.3181/00379727-77-18670. [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Stetson PW. Changes in salt intake lesions of the area postrema and the nucleus of the solitary tract in rats. Brain Res. 1981;211:355–366. doi: 10.1016/0006-8993(81)90707-1. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Miselis RR, Sawchenko PE. The relationship of efferent projections from the area postrema to vagal motor and brain stem catecholamine-containing cell groups: an axonal transport and immunohistochemical study in the rat. Neuroscience. 1994;58:635–648. doi: 10.1016/0306-4522(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Huang W, Sved AF, Verbalis JG, Stricker EM. Impaired osmoregulatory responses in rats with area postrema lesions. Am J Physiol. 1999;277(1 Pt 2):R209–219. doi: 10.1152/ajpregu.1999.277.1.R209. [DOI] [PubMed] [Google Scholar]
- De Gobbi JI, Barbosa SP, De Luca LA, Jr, Thunhorst RL, Johnson AK, Menani JV. Activation of serotonergic 5-HT(1A) receptors in the lateral parabrachial nucleus increases NaCl intake. Brain Res. 2005;1066:1–9. doi: 10.1016/j.brainres.2005.04.055. [DOI] [PubMed] [Google Scholar]
- De Gobbi JI, Martinez G, Barbosa SP, Beltz TG, De Luca LA, Jr, Thunhorst RL, Johnson AK, Menani JV. 5-HT2 and 5-HT3 receptors in the lateral parabrachial nucleus mediate opposite effects on sodium intake. Neuroscience. 2007;146:1453–1461. doi: 10.1016/j.neuroscience.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Edwards GL, Beltz TG, Power JD, Johnson AK. Rapid-onset “need-free” sodium appetite after lesions of the dorsomedial medulla. Am J Physiol. 1993;264(6 Pt 2):R1242–1247. doi: 10.1152/ajpregu.1993.264.6.R1242. [DOI] [PubMed] [Google Scholar]
- Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Chimenti PC, Loewy AD. Phox2b expression in the aldosterone-sensitive HSD2 neurons of the NTS. Brain Res. 2008;1226:82–88. doi: 10.1016/j.brainres.2008.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Engeland WC, Kawata M, Loewy AD. Aldosterone target neurons in the nucleus tractus solitarius drive sodium appetite. J Neurosci. 2006;26:411–417. doi: 10.1523/JNEUROSCI.3115-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary tract: efferent projections. J Comp Neurol. 2006;497:223–250. doi: 10.1002/cne.20993. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Sodium deprivation and salt intake activate separate neuronal subpopulations in the nucleus of the solitary tract and the parabrachial complex. J Comp Neurol. 2007;504:379–403. doi: 10.1002/cne.21452. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Shin JW, Loewy AD. Pre-locus coeruleus neurons are activated by sodium deprivation, express FoxP2, and project to the diencephalon. Society for Neuroscience Abstracts. 2009:573, 578. [Google Scholar]
- Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol. 2010;518:1460–1499. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA. Transcription factors and the genetic organization of brain stem respiratory neurons. J Appl Physiol. 2008;104:1513–1521. doi: 10.1152/japplphysiol.01383.2007. [DOI] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, Alberta JA, Cheng LP, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, Stiles CD, Ma Q. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Hoffman GE, Wray S, Schwaber JS. Distribution of neurotensin-immunoreactivity within baroreceptive portions of the nucleus of the tractus solitarius and the dorsal vagal nucleus of the rat. J Comp Neurol. 1984;226:155–164. doi: 10.1002/cne.902260202. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Miselis RR. Area postrema and adjacent nucleus of the solitary tract in water and sodium balance. Am J Physiol. 1984;247(1 Pt 2):R173–182. doi: 10.1152/ajpregu.1984.247.1.R173. [DOI] [PubMed] [Google Scholar]
- Kachidian P, Pickel VM. Localization of tyrosine hydroxylase in neuronal targets and efferents of the area postrema in the nucleus tractus solitarii of the rat. J Comp Neurol. 1993;329:337–353. doi: 10.1002/cne.903290305. [DOI] [PubMed] [Google Scholar]
- Kobashi M, Ichikawa H, Sugimoto T, Adachi A. Response of neurons in the solitary tract nucleus, area postrema and lateral parabrachial nucleus to gastric load of hypertonic saline. Neurosci Lett. 1993;158:47–50. doi: 10.1016/0304-3940(93)90609-o. [DOI] [PubMed] [Google Scholar]
- Lanca AJ, van der Kooy D. A serotonin-containing pathway from the area postrema to the parabrachial nucleus in the rat. Neuroscience. 1985;14:1117–1126. doi: 10.1016/0306-4522(85)90281-7. [DOI] [PubMed] [Google Scholar]
- Menani JV, Thunhorst RL, Johnson AK. Lateral parabrachial nucleus and serotonergic mechanisms in the control of salt appetite in rats. Am J Physiol. 1996;270(1 Pt 2):R162–168. doi: 10.1152/ajpregu.1996.270.1.R162. [DOI] [PubMed] [Google Scholar]
- Morest DK. A study of the structure of the area postrema with Golgi methods. Am J Anat. 1960;107:291–303. doi: 10.1002/aja.1001070307. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- Price CJ, Hoyda TD, Ferguson AV. The area postrema: a brain monitor and integrator of systemic autonomic state. Neuroscientist. 2008;14:182–194. doi: 10.1177/1073858407311100. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE. Central connections of the hepatic branch of the vagus nerve: a horseradish peroxidase histochemical study. J Auton Nerv Syst. 1983;7:165–174. doi: 10.1016/0165-1838(83)90044-9. [DOI] [PubMed] [Google Scholar]
- Sequeira SM, Geerling JC, Loewy AD. Local inputs to aldosterone–sensitive neurons of the nucleus tractus solitarius. Neuroscience. 2006;141:1995–2005. doi: 10.1016/j.neuroscience.2006.05.059. [DOI] [PubMed] [Google Scholar]
- Shapiro RE, Miselis RR. The central neural connections of the area postrema of the rat. J Comp Neurol. 1985a;234:344–364. doi: 10.1002/cne.902340306. [DOI] [PubMed] [Google Scholar]
- Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol. 1985b;238:473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- Shin JW, Geerling JC, Loewy AD. Inputs to the ventrolateral bed nucleus of the stria terminalis. J Comp Neurol. 2008;511:628–657. doi: 10.1002/cne.21870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JW, Loewy AD. Gastric afferents project to the aldosterone-sensitive HSD2 neurons of the NTS. Brain Res. 2009;1301:34–43. doi: 10.1016/j.brainres.2009.08.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MK, Spyer KM. GABA-mediated inhibition of medullary vasomotor neurones by area postrema stimulation in rats. J Physiol. 1991;436:669–684. doi: 10.1113/jphysiol.1991.sp018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY. Organization of the projections of a circumventricular organ: the area postrema in the rat. J Comp Neurol. 1983;219:328–338. doi: 10.1002/cne.902190307. [DOI] [PubMed] [Google Scholar]
- Wang T, Edwards GL. Differential effects of dorsomedial medulla lesion size on ingestive behavior in rats. Am J Physiol. 1997;273(4 Pt 2):R1299–1308. doi: 10.1152/ajpregu.1997.273.4.R1299. [DOI] [PubMed] [Google Scholar]
- Watson WE. The effect of removing area postrema on the sodium and potassium balances and consumptions in the rat. Brain Res. 1985;359:224–232. doi: 10.1016/0006-8993(85)91432-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Sakai N, Tanimizu T, Wakisaka S. c-Fos expression in the parabrachial nucleus after ingestion of sodium chloride in the rat. Neuroreport. 1993;4:1223–1226. doi: 10.1097/00001756-199309000-00003. [DOI] [PubMed] [Google Scholar]