Abstract
Obesity is associated with an increased incidence of many cancers, including leukemia, though it is unknown whether leukemia incidence is increased directly by obesity, or rather by associated genetic, lifestyle, health, or socio-economic factors. We developed animal models of obesity and leukemia to test whether obesity could directly accelerate acute lymphoblastic leukemia (ALL) using BCR/ABL transgenic and AKR/J mice weaned onto a high-fat diet. Mice were observed until development of progressive ALL. Although obese and control BCR/ABL mice had similar median survival, older obese mice had accelerated ALL onset, implying a time-dependent effect of obesity on ALL. Obese AKR mice developed ALL significantly earlier than controls. The effect of obesity was not explained by WBC count, thymus/spleen weight, or ALL phenotype. However, obese AKR mice had higher leptin, insulin, and IL-6 levels than controls, and these obesity-related hormones all have potential roles in leukemia pathogenesis. In conclusion, obesity directly accelerates presentation of ALL, likely by increasing the risk of an early event in leukemogenesis. This is the first study to demonstrate that obesity can directly accelerate the progression of ALL. Thus, the observed associations between obesity and leukemia incidence are likely to be directly related to biological effects of obesity.
Keywords: Obesity, leukemia, mouse models, BCR/ABL, AKR/J, high-fat diet
Introduction
Obesity is associated with increased incidence and mortality from several types of cancer, including leukemia (1, 2). The association between acute lymphoblastic leukemia (ALL) risk and caloric intake (3) and obesity (4, 5) is evident across many populations, though why this occurs is unknown. Because obesity is associated with numerous physiological effects and potential confounding lifestyle, socioeconomic, genetic, and health factors, it is difficult to investigate this association in human studies. Also, there are currently no obese mouse models that develop ALL. Given the high prevalence of obesity, it is critical to determine whether obesity directly increases cancer incidence, or is merely a marker of some other associated exposure.
The present study was designed to test whether diet-induced obesity would directly increase the rate of progression of ALL in two distinct mouse models.
Materials and Methods
Mouse model
We used a high-fat diet (60% calories from fat, Research Diets, New Brunswick, NJ) (6) to induce obesity in two mouse models of ALL. Male BCR/ABL mice on a C57Bl background, which develop pro-B ALL (7), were randomized at weaning to a high-fat diet (n=40) or standard laboratory chow (n=41, 13.5% calories from fat, Labdiet, Richmond, IN). Male AKR/J are a strain of inbred mice which develop T-cell ALL starting at 5–6 months of age due to recombinant retroviruses that target thymocytes (8). These mice were randomized to high-fat (n=12) or control diet (n=12, 10% calories from fat, Research Diets) at 5 weeks of age. Although it was not possible to define the precise onset of ALL in these experiments, mice were removed from the study when they developed signs of progressive ALL (palpable mass >1 cm, hindlimb paralysis, moribund state, weight loss, or respiratory distress). ALL was verified by necropsy on all mice.
In additional experiments, fat-fed and control male AKR mice (n=12 per group) were sacrificed at various time points prior to ALL onset. To test whether obesity alters T-cell maturation, thymocytes were isolated and lymphocytes selected by forward and side scatter on a BD FACScan, and gated populations analyzed for CD4/CD8 and TCR expression (TCR-αβ and TCR-γδ, BD Pharmingen, San Diego, CA).
All animal experiments were approved by the CHLA Institutional Animal Care and Use Committee and performed in accordance with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Assays
Plasma samples from the AKR mice which were sacrificed at various timepoints prior to ALL onset were analyzed for adiponectin concentration using an ELISA (Millipore). Leptin, IL-6, and insulin were measured using a Milliplex mouse adipokine kit (Millipore, Billerica, MA) in the Beckman Center for Immune Monitoring at the University of Southern California Norris Cancer Center, and analyzed with the Bio-Plex Suspension Array System (BIO-RAD, Hercules, CA).
Calculations
Body weights were compared using two-sided t-tests. Weight loss just prior to ALL onset was excluded from the weight curves. Kaplan Meier survival curves were generated on Prism 5.00 (GraphPad Software, Inc., La Jolla, CA) and compared by log-rank test. Due to the small number of AKR mice included in the survival analysis, p values for the difference in survival were confirmed using a permutation distribution of the log-rank test with 2,000 replicates. For the BCR/ABL mice, the survival curves diverged at later time-points, implying that obesity might have a late or cumulative effect to accelerate ALL. Thus, this data was reanalyzed (post-hoc) by Cox regression, using the day before the first death occurred as time 0. Test of Schoenfeld residuals demonstrated that the proportional hazard assumption was violated, so the interaction between obesity and time was evaluated and the hazard ratio of death of obese mice compared to controls was calculated at various time points. Organ weights and adipokines were compared between groups using ANOVA. When diet effects were significant by ANOVA, groups were compared at each timepoint by t-test. Multivariate regression analysis (9) was used to examine whether there was any difference in thymocyte expression patterns between diet groups. All p-values are two sided. Analyses were performed using Stata 9.2 (StataCorp, College Station, TX) and Prism 5.00.
Results
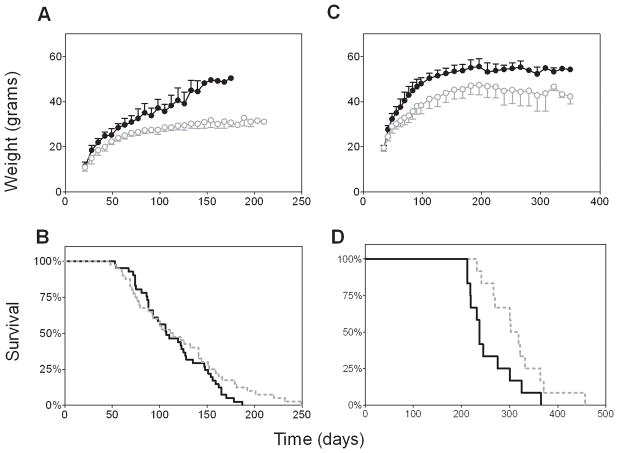
Both BCR/ABL and AKR mice were significantly heavier than controls after one week on the high-fat diet (p<0.001, Figures 1A and C). The BCR/ABL mice began presenting with signs of ALL at ~7 weeks of age. While fat-fed BCR/ABL mice had a similar median survival to the controls (107 vs. 113 days, Figure 1B, p=0.2, log rank), in post-hoc analysis, the hazard ratio of ALL significantly varied over time by diet group (p=0.047). At 60 days of age, the risk of ALL in obese mice was 0.61 times (95% CI: 0.24, 1.5) that of controls, while at 150 days, the risk was twice as high (95% CI: 1.1, 3.7) as controls. This was reflected by the decreased maximum lifespan in obese compared to control mice (175±10 vs. 226±26 days for the longest living 10% of each group, p<0.005). Thus, obesity increased the risk of ALL in older BCR/ABL transgenic mice.
Figure 1.
Weight and survival of two mouse models of leukemia in obesity. A. Weights of mice transgenic for human BCR/ABL randomized to a high-fat diet (60% of calories from fat, closed circles) or lab chow (13.5% calories from fat, open circles). B. Survival curves generated from Kaplan Meier life tables of obese (solid line) vs. control (dashed line) BCR/ABL mice. C. Weights and D. survival curves of AKR/J mice randomized to high fat (60% of calories from fat) vs. control (10% of calories from fat) diet. Weights were significantly higher in fat-fed mice in both models at all time-points after weaning (p<0.05), and survival significantly shorter (see methods, p<0.05 log rank).
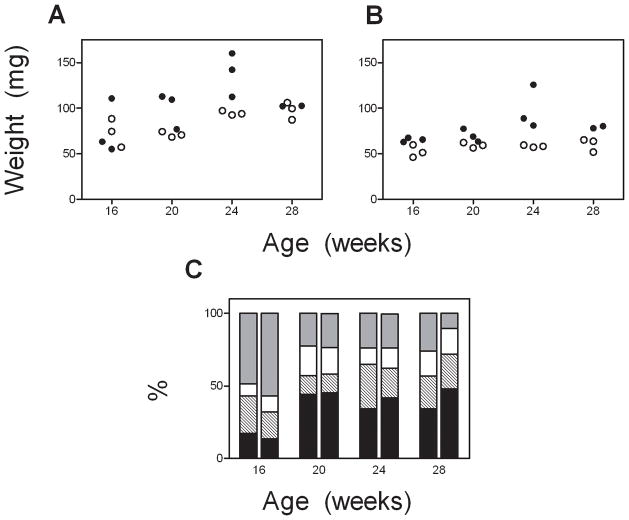
AKR mice began presenting with ALL at ~7 months of age. Cause of death could not be confirmed in one mouse in each diet group; otherwise, all mice presented with labored breathing and/or tumor. Obese AKR mice developed ALL earlier than control mice, resulting in a 2 month lower median survival (237 vs. 310 days, Figure 1D, p=0.035 log rank, p=0.039 permutation analysis). Thymus weights from AKR mice prior to ALL were increased by both obesity and age (p=0.005 and 0.003, respectively, ANOVA, n=3/group/time point, Figure 2A), but there was no interaction between these variables (p=0.59). A similar pattern was observed with spleen weight (diet: p<0.001, age: p=0.026, interaction: p=0.15, Figure 2B). When expressed as a percentage of body weight, there was no effect of diet on thymus size, and only a borderline significant effect of high fat diet to increase spleen size (p=0.066, data not shown). Also, age and diet did not appear to alter the thymocyte expression patterns of CD4/CD8 (Figure 2C) or the TCR subtype (α/β vs. γ/δ, not shown, p=0.26). In both obese and control AKR mice sacrificed at the time of progressive ALL, the thymus samples demonstrated a predominant outgrowth of cells with similar expression (most frequently CD4+/CD8+ or CD4−/CD8−, not shown), and this also did not differ between diet groups.
Figure 2.
Wet weights of thymuses (A) and spleens (B) from obese (solid) and control (open circles) AKR/J mice. C. CD4/8 expression of lymphocytes isolated from thymuses of AKR/J mice. Left bars are from obese mice, right from controls. Black – CD4+/CD8+; Hatched – CD4+/CD8−; White – CD4−/CD8+; Gray – CD4+/CD8+
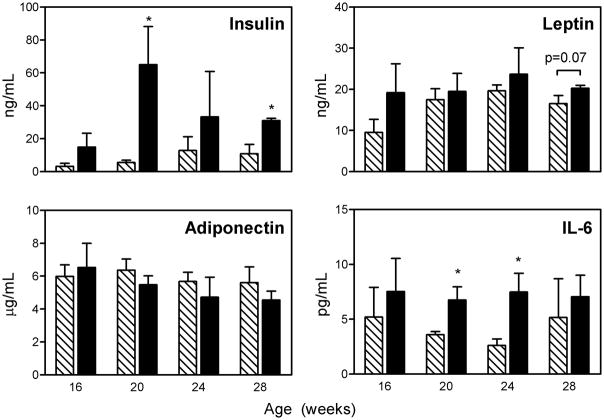
To investigate potential mediators of the obesity-leukemia link in this study, we measured various obesity-related hormones in plasma from the AKR mice sacrificed at various timepoints. High fat diet was associated with increased (non-fasting) levels of insulin (p<0.001), leptin (p=0.004), and IL-6 (p<0.001, ANOVA, Figure 3). Leptin also increased over time (p=0.041). There was no significant effect of diet or age on plasma adiponectin level.
Figure 3.
Plasma levels of insulin, leptin, adiponectin, and IL-6 from AKR/J mice sacrificed at various ages on low (hatched bars) or high-fat diet (solid bars). *p<0.05 compared to control mice. See text for ANOVA results.
Discussion
The present results are the first to our knowledge to demonstrate that obesity can directly accelerate ALL progression. Obesity increased the risk of ALL in older, but not younger, BCR/ABL mice, while it had an even more significant effect on the AKR mice, which did not develop ALL until after 7 months of age. This is consistent with a time-dependent and/or cumulative effect of obesity, such as that observed for other exposure-related cancers, such as lung carcinoma (smoking) and breast cancer (estrogen). This is also consistent with other comorbidities of obesity which are related to a cumulative exposure, such as heart disease, diabetes, and arthritis. Additionally, a certain threshold of obesity may need to be reached before it begins to exert an effect on ALL progression. This is suggested by human cancer incidence and mortality data. Reeves et al. found that a modest degree of overweight (body mass index [BMI] 25–27.4 kg/m2) was not associated with increased leukemia or overall cancer incidence (2). However, leukemia and overall cancer incidence was increased in women with more substantial obesity. Calle et al. reported similar effects of obesity on leukemia and cancer mortality in men, though they did not detect an effect of obesity on leukemia mortality in women (1). Finally, it is possible that ALL which develops in young mice may be intrinsically more aggressive, and thus may be less influenced by environmental or physiological modulators of the disease such as obesity. In any case, it is plausible that the observed effects of obesity might be relevant in adolescents and adults, who may have been obese for years or decades.
In this study, we cannot differentiate between the effects of a high-fat diet and of obesity per se on ALL progression. However, it is interesting to note that a subset (11 out of 41) of the BCR/ABL mice were “obesity-resistant”, with weights within 2 SD of the control group (10). These mice had a median survival of 135 days, compared to 99 days in the remaining “obesity-prone” mice (p=0.10 in post-hoc analysis). Removal of the “obesity-resistant” mice from the survival analysis does not change the results (p=0.12, log rank control vs. “obesity-prone”). However, the tendency for the “obesity-resistant” mice to have prolonged survival, despite being on the high-fat diet, does imply that obesity itself is responsible for the accelerated appearance of leukemia. Further studies would be needed to formally test this hypothesis.
Although ALL presented earlier in both obese mouse models, we did not identify any factors that predicted this. White blood cell counts were similar between groups of BCR/ABL mice at 2 and 3 months of age (not shown). Although thymus weights were increased by obesity in 4 to 7 month old AKR mice, we identified no difference in TCR subtype or thymocyte expression pattern or TCR subtype. Interestingly, diet-induced obesity has recently been shown to increase adipocytes in the thymus, associated with decreased thymocyte counts and increased thymocyte apoptosis (11). Thus, obesity does not appear to increase risk of T-cell ALL simply by increasing leukocyte or thymocyte number or rate of turnover. Together, these results imply that obesity acts by increasing the risk of an early event in leukemogenesis, rather than accelerating ALL proliferation once it is started.
As has been shown in other mouse models of obesity, we found that obese AKR/J mice had higher plasma levels of insulin, leptin, and IL-6. Insulin is a potent growth factor, and has been shown to increase ALL cell proliferation in vivo (12). Interestingly, the common pre-B cell ALL translocation, t(1;19), may enhance insulin receptor signaling, implicating this pathway as a potential contributor to ALL pathogenesis (13). Leptin has been shown to stimulate hematopoietic progenitors and multiple leukemia cell types (14, 15), though a direct effect on ALL has not been demonstrated to our knowledge. However, ALL blasts can express leptin receptors, and so it is possible that this hormone could contribute to ALL proliferation and/or survival (16). IL-6 has been implicated in the pathogenesis of several types of cancer, and may play a role in bone metastasis and cancer-stromal cell interactions (17). As IL-6 is increased in obesity, it has been investigated as a potential link between obesity and several types of cancer (18), IL-6 induces B-cell proliferation (19) and enhances differentiation (20), and so could in theory alter ALL progression. Thus, these and other obesity-related hormones (e.g. IGF-1) could potentially be responsible for all or part of the accelerated ALL presentation in the present study. Further mechanistic work is necessary to tease apart these possibilities.
These results complement a previous study by Shields et al., which showed that caloric restriction causes an increased lifespan in AKR mice (21). In that study, a ~25–30% lower body weight in the restricted mice caused a 50% longer median lifespan. In our study, a ~15% higher body weight in the obese mice was associated with a 25% shorter lifespan. Thus, body weight appears to have a continuous, bidirectional effect on lifespan in this model.
One weakness in the present study is that the decision to remove mice from the experiment was made by an unblinded investigator, and therefore subject to bias. This potential bias is minimal in the P190 mouse model, as these mice develop signs of ALL which are fairly obvious (swollen lymph nodes or tumor, hind limb paralysis, hunched posture, acute weight loss, and/or labored breathing). These symptoms progress fairly rapidly to moribund status and death if the mouse is not removed from the study. Furthermore, other causes of death are rare in the background C57Bl/6 strain of this age range (22). The AKR/J mice were significantly older when they were removed from the study. Thymoma and/or T-cell ALL was confirmed by necropsy, FACS analysis, and/or presentation with respiratory distress in all but one mouse per diet group. Exclusion of these 2 mice from the data analysis did not change the survival curve comparison. Thus, the effect of obesity to decrease survival in these two mouse models most likely reflects accelerated ALL.
The present study shows that obesity directly accelerates the development of both B-cell and T-cell derived ALL in mouse models. This supports the epidemiological data showing an increased incidence of leukemia in obese adults (4, 5), and argues that these observations are indeed due to obesity per se, and not confounding genetic, socio-economic, and/or lifestyle factors. This also adds ALL to the list of cancers that are accelerated by obesity in mice, including colon (23) and breast cancer (10). These results are particularly relevant in light of the evidence that obesity can directly impair ALL and AML treatment (24–26). It is important that future research be aimed at elucidating the mechanisms linking cancer and obesity so that the burdens from both of these diseases can be reduced.
Footnotes
This work was supported in part by funding from the NIH NICHD (5K12HD052954 to S.D.M.), NCI (TREC U54 CA 116848 and R01CA139060 to S.D.M.; R01CA090321 to N.H.; and R01CA137060 and R01CA139032 to M.M.), and the Children’s Cancer Research Fund (a California nonprofit organization) to S.D.M.
Reference List
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hursting SD, Margolin BH, Switzer BR. Diet and human leukemia: an analysis of international data. Prev Med. 1993;22:409–22. doi: 10.1006/pmed.1993.1034. [DOI] [PubMed] [Google Scholar]
- 4.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer. 2008;122:1418–21. doi: 10.1002/ijc.23176. [DOI] [PubMed] [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. The Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 6.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–7. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 7.Heisterkamp N, Jenster G, ten Hoeve J, Zovich D, Pattengale PK, Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature. 1990;344:251–3. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- 8.Cloyd MW, Hartley JW, Rowe WP. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980;151:542–52. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. Prentice Hall; Englewood Cliffs, NJ: 1992. [Google Scholar]
- 10.Dogan S, Hu X, Zhang Y, Maihle NJ, Grande JP, Cleary MP. Effects of high-fat diet and/or body weight on mammary tumor leptin and apoptosis signaling pathways in MMTV-TGF-alpha mice. Breast Cancer Res. 2007;9:R91. doi: 10.1186/bcr1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, Dixit VD. Obesity accelerates thymic aging. Blood. 2009;114:3803–12. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neely EK, Rosenfeld RG, Illescas A, Smith SD. Mitogenic effects of human recombinant insulin on B-cell precursor acute lymphoblastic leukemia cells. Leukemia. 1992;6:1134–42. [PubMed] [Google Scholar]
- 13.Newman JD, Harrison LC, Eckardt GS, Jack I. Enhanced insulin-receptor tyrosine kinase activity associated with chromosomal translocation (1;19) in a pre-B-cell leukemia line. Int J Cancer. 1992;50:500–4. doi: 10.1002/ijc.2910500328. [DOI] [PubMed] [Google Scholar]
- 14.Umemoto Y, Tsuji K, Yang FC, Ebihara Y, Kaneko A, Furukawa S, Nakahata T. Leptin stimulates the proliferation of murine myelocytic and primitive hematopoietic progenitor cells. Blood. 1997;90:3438–43. [PubMed] [Google Scholar]
- 15.Mouzaki A, Panagoulias I, Dervilli Z, Zolota V, Spadidea P, Rodi M, Panitsas FP, Lagadinou E, de Lastic AL, Georgakopoulos T. Expression patterns of leptin receptor (OB-R) isoforms and direct in vitro effects of recombinant leptin on OB-R, leptin expression and cytokine secretion by human hematopoietic malignant cells. Cytokine. 2009;48:203–11. doi: 10.1016/j.cyto.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Nakao T, Hino M, Yamane T, Nishizawa Y, Morii H, Tatsumi N. Expression of the leptin receptor in human leukaemic blast cells. Br J Haematol. 1998;102:740–5. doi: 10.1046/j.1365-2141.1998.00843.x. [DOI] [PubMed] [Google Scholar]
- 17.Ara T, DeClerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer. 2010;46:1223–31. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Current Opinion in Pharmacology. 2009;9:351–69. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tosato G, Seamon KB, Goldman ND, Sehgal PB, May LT, Washington GC, Jones KD, Pike SE. Monocyte-derived human B-cell growth factor identified as interferon-beta 2 (BSF-2, IL-6) Science. 1988;239:502–4. doi: 10.1126/science.2829354. [DOI] [PubMed] [Google Scholar]
- 20.Yoshizaki K, Nakagawa T, Fukunaga K, Tseng LT, Yamamura Y, Kishimoto T. Isolation and characterization of B cell differentiation factor (BCDF) secreted from a human B lymphoblastoid cell line. J Immunol. 1984;132:2948–54. [PubMed] [Google Scholar]
- 21.Shields BA, Engelman RW, Fukaura Y, Good RA, Day NK. Calorie restriction suppresses subgenomic mink cytopathic focus-forming murine leukemia virus transcription and frequency of genomic expression while impairing lymphoma formation. Proc Natl Acad Sci U S A. 1991;88:11138–42. doi: 10.1073/pnas.88.24.11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17:690–2. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gravaghi C, Bo J, LaPerle KMD, Quimby F, Kucherlapati R, Edelmann W, Lamprecht SA. Obesity enhances gastrointestinal tumorigenesis in Apc-mutant mice. Int J Obes. 2008;32:1716–9. doi: 10.1038/ijo.2008.149. [DOI] [PubMed] [Google Scholar]
- 24.Behan JW, Yun JP, Proektor MP, Ehsanipour EA, Arutyunyan A, Moses AS, Avramis VI, Louie SG, Butturini A, Heisterkamp N, Mittelman SD. Adipocytes impair leukemia treatment in mice. Cancer Res. 2009;69:7867–74. doi: 10.1158/0008-5472.CAN-09-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butturini AM, Dorey FJ, Lange BJ, Henry DW, Gaynon PS, Fu C, Franklin J, Siegel SE, Seibel NL, Rogers PC, Sather H, Trigg M, Bleyer WA, Carroll WL. Obesity and outcome in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2007;25:2063–9. doi: 10.1200/JCO.2006.07.7792. [DOI] [PubMed] [Google Scholar]
- 26.Lange BJ, Gerbing RB, Feusner J, Skolnik J, Sacks N, Smith FO, Alonzo TA. Mortality in overweight and underweight children with acute myeloid leukemia. JAMA. 2005;293:203–11. doi: 10.1001/jama.293.2.203. [DOI] [PubMed] [Google Scholar]