Abstract
Preclinical and correlative studies suggest reduced breast cancer with higher lignan intake or blood levels. We conducted a pilot study of modulation of risk biomarkers for breast cancer in premenopausal women after administration of the plant lignan secoisolariciresinol given as the diglycoside (SDG). Eligibility criteria included regular menstrual cycles, no oral contraceptives, a greater than 3-fold increase in 5 year risk, and baseline Ki-67 ≥2% in areas of hyperplasia in breast tissue sampled by random periareolar fine needle aspiration (RPFNA) during the follicular phase of the menstrual cycle. SDG 50 mg daily was given for 12 months, followed by repeat RPFNA. The primary endpoint was change in Ki-67. Secondary endpoints included change in cytomorphology, mammographic breast density, serum bioavailable estradiol, and testosterone IGF-I and IGFBP-3, and plasma lignan levels. Forty-five of 49 eligible women completed the study with excellent compliance (median = 96%) and few serious side effects (4% grade 3). Median plasma enterolactone increased ~ 9-fold, and total lignans 16 fold. Thirty-six (80%) of the 45 evaluable subjects demonstrated a decrease in Ki-67, from a median of 4% (range 2–16.8 %) to 2% (range 0–15.2%) (p<0.001 by Wilcoxon signed rank test). A decrease from baseline in the proportion of women with atypical cytology (p=0.035) was also observed. Based on favorable risk biomarker modulation and lack of adverse events, we are initiating a randomized trial of SDG vs. placebo in premenopausal women.
Introduction
Secoisolariciresinol diglycoside (SDG) is a polyphenolic plant lignan which when administered orally is hydrolyzed to secoisolariciresinol (SECO) and further metabolized by intestinal bacteria to the biologically active mammalian lignans, enterodiol (END) and enterolactone (ENL) [1–4]. In a high estrogen environment these lignans act as partial estrogen antagonists in a tissue specific manner [5–9]. SDG is found in highest concentration in flaxseed but is also present in other oil rich seeds, nuts, whole grains, legumes, and certain fruits, and vegetables [1,10,11]. The typical western diet provides < 10 mg/day of lignans [11,12,13]., Administration of flaxseed or SDG is associated with reduced ER- and ER+ mammary cancers in preclinical studies [14–17].
Some human studies show an inverse correlation between lignan intake or blood levels and breast cancer incidence, but others do not [18–32]. This inconsistency is not surprising given the inherent limitations of dietary recall, early use of intake questionnaires with incomplete validation for lignans, variation in lignan metabolism, single point blood collections, and different populations studied [33–37]. However, for premenopausal women, the preponderance of evidence suggests there is a reduced cancer incidence with higher lignan intakes or plasma ENL levels [19–22,24–26,30]. This is particularly in women with CYP17 A2 alleles that may result in higher endogenous estrogen levels [24,38,39]. Correlative studies indicate reduction in risk of ER- as well as ER+ breast cancer, including ER- cancer in premenopausal women [23,25,27–29].
Given the likelihood that lignans act as partial estrogen antagonists we undertook a pilot study of the plant lignan secoisolariciresinol diglycoside (SDG) in premenopausal women at increased risk for breast cancer. Our primary endpoint was change in the proliferation marker Ki-67 in hyperplastic benign breast tissue obtained by random periareolar fine needle aspiration (RPFNA). Proliferation plays a fundamental role in carcinogenesis [40] and higher proliferation (Ki-67) in hyperplastic and atypical hyperplastic specimens is associated with cancer development [41,42]. Reduction in Ki-67 is also associated with response in early cancer treatment trials [43,44]. We had previously demonstrated that cytomorphologic evidence of atypia in tissue obtained by RPFNA from high risk women is associated with a 5-fold increased risk of developing DCIS or invasive cancer [45] and that Ki-67 in cytology specimens obtained by RPFNA is positively associated [46] with evidence of cytologic atypia [47,48]. Secondary endpoints measured included cytomorphology, percent mammographic density, serum bioavailable estradiol and testosterone, and IGF-I and IGFBP-3 (Reviewed in [49]). We chose a commercial preparation (BrevailR) to avoid the marked variability in SDG content and bioavailability observed with different batches of raw or ground flaxseed [50]. Pharmacologic studies had shown that daily dosing with this formulation, which contains 50 mg of SDG, produced ENL levels (median 63 nmol/L) similar to those found in the highest quintiles associated with reduction in cancer incidence in case control studies [18,51].
Methods
Eligibility for Study
Premenopausal women with regular menstrual cycles, not on oral contraceptives for at least 6 months were eligible for tissue screening by RPFNA, provided they met risk criteria. Risk criteria included a 5 year Gail model risk of ≥1.7% or 3-fold higher than the average SEER risk for age group; a prior breast biopsy of atypical hyperplasia or lobular carcinoma in situ; or a prior contra-lateral treated breast cancer. A normal mammogram was required the day of or within 3 months prior to their RPFNA. Tissue eligibility required hyperplasia, with or without atypia, plus sufficient Ki-67 expression to enable detection of modulation. We selected a lower limit of Ki-67 as staining of 2% or more of epithelial cell nuclei assessed on a minimum of 500 hyperplastic epithelial cells. We had previously observed a median Ki-67 of 2% in high risk pre-menopausal women for whom Ki-67 could be assessed [46]. More recently, a Mayo Clinic study suggested that Ki-67 staining of 2% or higher in foci of atypia was associated with increased risk for development of breast cancer [42]. Entry onto the intervention protocol was required within 3 months of the RPFNA along with normal renal, hepatic, and hematologic function. Subjects were also asked not to take antibiotics or flaxseed supplements for 6 weeks prior to baseline sampling for blood lignan levels and entry into the study protocol Protocols for RPFNA and the flaxseed lignan intervention were approved by the KUMC Human Subjects Committee.
Gail Risk Calculation
The 5 year projected probability of developing invasive cancer was calculated at the time of RPFNA according to the Gail risk Model at http://bcra.nci.nih.gov/brc/ [52].
Biomarker Assessments and Assays
All biomarker assessments were performed at baseline and 12 months. Additional plasma for lignan measurements were also obtained at 6 months. Sera and plasma were stored in aliquots at −80°C after processing.
Tissue Acquisition and Specimen Processing
RPFNA was performed day 1–10 (follicular phase) of the menstrual cycle to reduce Ki-67 variability and minimize bleeding. Two sites per breast were aspirated under local anaesthesia as previously described [49]. The needle tip was preferentially guided to areas of increased resistance. Material from all aspiration sites was pooled in a single 15 cc tube with 9 mL of CytoLyt® and 1 mL of 10% formalin. After 48 hours cells were pelleted, washed in CytoLyt® and transferred to PreservCyt®. Aliquots were then transferred to slides via ThinPrep® methodology for pap staining for cytomorphology or Ki-67 (see below).
Cytomorphology
Cytomorphology was assessed by a single cytopathologist (CMZ) and classified by both a categorical method [48], and a semiquantitative index score. Index scores of 11–14 generally correlate with hyperplasia without atypia, 15–18 with hyperplasia with atypia, and 19–24 as suspicious for malignancy [47]. Cytomorphologic assessments were made without knowledge of the results of the Ki-67 assessment.
Ki-67
A categorical estimate of the number of ductal epithelial cells present was made as 500–1000, 1000–5000, or >5000 and only slides containing >500 cells were stained for Ki-67. Antigen retrieval was performed with 10 mmol/L citrate buffer, pH 6, in a BioCare decloaking chamber (Walnut Creek, CA) for 2 minutes at 120°C. Slides were then stained with MIB-1 monoclonal antibody (M7240; Dako Cytomation, Carpinteria, CA) at a 1:20 dilution in a Dako Autostainer. At baseline, only hyperplastic cell clusters were assessed, but at the 12 month follow-up, if no hyperplastic clusters were present, clusters containing the highest proportion of cells staining for Ki-67 were preferentially evaluated. The number of cells with unequivocal nuclear staining out of 500 cells assessed was recorded for each of two independent readers. In case of a difference between the two readers, the scores were averaged.
Hormone and Growth Factors
Assays were performed using commercially available kits. Each subject’s pre-and post-treatment samples were run together in duplicate on the same 96-well plate, along with a pooled sera control, plus the kit’s standards and controls. Estradiol (and SHBG performed with estradiol) assays were to be performed on blood samples collected at the time of the initial screening and final post-study RPFNAs during the follicular phase of the menstrual cycle as this phase is associated with the least fluctuations in estradiol levels. . However, due to oversight, blood for baseline estradiol was collected on only half of the subjects. IGF-I, IGFBP-3, progesterone, testosterone, and sex hormone binding globulin (SHBG) assays were performed on sera collected from all eligible subjects immediately prior to starting study agent and then after 12 months of study agent, between day 21 and 24 of the menstrual cycle, Levels of IGF-1 and IGFBP-3 are highest during the luteal phase [53] and progesterone and testosterone are most reproducible in the mid-luteal phase [54]. Specimens were thawed once for estradiol, IGF-I and IGFBP-3 and twice for progesterone and testosterone. Bioavailable estradiol and testosterone results were computed using values for estradiol, testosterone and SHBG according to standard formulas [55,56]. Estradiol (CAN-E-430), progesterone (CAN-P-305), testosterone (CAN-TE-250), and SHBG (CAN-SHBG-4010) assay kits were purchased from Diagnostics Biochem Canada Inc. (London, ONT, Canada). All were competitive enzyme immunoassays performed except for SHBG which was a direct capture ELISA. Limits of detection for each assay were: estradiol, 10 pg/mL; progesterone, 0.1 ng/mL; testosterone, 0.022 ng/mL SHBG, 0.1 nmol/L. IGF-I (DSL-10-2800) and IGFBP-3 (DSL-10-6600) assay kits were purchased from Diagnostic Systems Laboratories, Inc. (Webster, TX). Limits of detection for IGF-I was 0.01 ng/mL; for IGFBP-3, 0.04 ng/mL.
Mammographic Breast Density
Baseline and month 12 mammograms were performed between days 1 and 10 of the menstrual cycle, similar to that for RPFNA and generally on the same day as RPFNA. Images were digitally scanned or downloaded from a PACS system and were cropped to remove any identifying information. Digital images were assembled in batched sets for an assessment of percent density using the Cumulus software program [57]. The single reader (CJF) knew which two files were from the same subject, but did not know the sequence. Because there was a hospital conversion midway through the study from analog to digital mammography, a large number of subjects had pre-study and post-study mammograms acquired using different technology and a secondary analysis was also performed for the 25 subjects who had the same type of mammogram at baseline and 12 months.
Lignans
Baseline, 6 month, and 12 month plasma samples from the same subject were assessed together for analysis of lignans (SECO, END, and ENL). Samples were run in 2 batches approximately 2 months apart. Samples were thawed once, underwent solid phase extraction, hydrolysis [58], and HPLC analysis with Waters Quattro Micro UPLC system coupled to electrospray tandem mass spectrometry (ESI-MS/MS) [59]. The lower limit of quantification (LLOQ) of lignans was 1 ng/mL (1 ng/ml is ~ 2.5 nmol/L). For analysis, samples classified as non-detectable for lignans were coded as 0, while samples with detectable signal but below the LLOQ were coded as 0.5 ng/ml. The within batch reproducibility for the assay of ENL, END, and SECO based on repeat measures of a quality assurance plasma sample over 6 batches was 11.9%, 5.8% and 10.1%, respectively, expressed as coefficient of variation.
Data Capture and Statistical methods
The study design called for accrual of 50 subjects, with 40 evaluable for the primary endpoint biomarker. Based on assumptions of a mean baseline Ki-67 of 4% and a standard deviation of the change of 0.9%, the study had 86% power with a two-sided alpha of 0.05 to detect a 50% effect size for change in Ki-67 using a paired t-test. Because change in Ki-67 was not normally distributed, the nonparametric Wilcoxon’s Signed Rank test was used. Changes in plasma SECO, END and ENL were correlated with changes in Ki-67, percent mammographic breast density, progesterone and plasma IGF-I:IGFBP-3, SHBG, bioavailable estradiol, bioavailable testosterone. Since the data were highly skewed, a nonparametric Spearman’s correlation was used. For secondary endpoint markers for which the study was not specifically powered, no corrections for multiple comparisons were formally employed; however, this was taken into consideration in the interpretation of the results.
Results
Baseline Characteristics
Between December of 2005 and April of 2008, 78 women were screened by RPFNA for consideration of participation on the study. A total of 49 subjects were enrolled between February of 2006 and June of 2008. The last subject completed study in June of 2009. Characteristics of both the 49 enrolled and the 45 evaluable subjects are given in table 1 and were not different. Median age was 43, 73% had one or more first degree relatives with breast cancer and 22% had a prior biopsy with atypical ductal hyperplasia or lobular carcinoma in situ. Baseline RPFNA indicated hyperplasia with atypia in 59% of subjects (62% of evaluable) and a median Ki-67 of 4%. Baseline median mammographic density was 40.9%.
Table 1.
Baseline key variables of all 49 subjects enrolled and of the 45 subjects evaluable for the primary endpoint.
| Variable | N=49 | N=45 | |
|---|---|---|---|
| Race (non-white) | 2% | 2% | |
| Ethnicity (Hispanic/Latino) | 2% | 2% | |
| Age, years | median | 43 | 43 |
| mean ± SD | 41.8 ± 6.5 | 42.3 ± 6.3 | |
| range | 27 – 51 | 29–51 | |
| Education | High School/Voc. | 8 (16%) | 7 (15%) |
| College graduate | 27 (55%) | 25 (56%) | |
| Post-Graduate | 14 (29%) | 13 (29%) | |
| Height (in) | median | 66 | 66 |
| mean ± SD | 65.2 ± 2.8 | 65.3 ± 2.9 | |
| range | 56 – 70 | 56 – 70 | |
| Weight (lb) | median | 138 | 137 |
| mean ± SD | 148 ± 33 | 148 ± 34 | |
| range | 101 – 234 | 101 – 234 | |
| BMI (kg/m2) | median | 23.2 | 22.8 |
| mean ± SD | 24.6 ± 5.2 | 24.5 ± 5.3 | |
| range | 17.4 – 37.2 | 17.4 – 37.2 | |
| 5-Year Gail Risk, % | median | 1.6 | 1.6 |
| mean ± SD | 2.0 ± 1.5 | 2.0 ± 1.3 | |
| range | 0.1 – 6.5 | 0.1 – 5.7 | |
| Age at Menarche, years | median | 13 | 13 |
| mean ± SD | 12.8 ± 1.5 | 12.8 ± 1.5 | |
| range | 10 –16 | 10 –16 | |
| Age First Live Birth, years (42 parous = 86%) | median | 29 | 29 |
| mean ± SD | 28.6 ± 4.3 | 28.7 ± 4.2 | |
| range | 19 – 43 | 19 – 43 | |
| Prior Biopsy w/ ADH or LCIS | 11 (22%) (20 w/ any biopsy, 41%) | 8 (18%) (17 w/ any biopsy, 38%) | |
| Number of first degree relatives w/ BrCa | 0 | 13 (27%) | 11 (24%) |
| 1 | 30 (61%) | 29 (64%) | |
| ≥2 | 6 (12%) | 5 (11%) | |
| Family History Consistent with hereditary Breast Cancer | 16 (33%) | 16 (36%) | |
Change in Biomarkers over the Course of the Study
Ki-67
Our primary endpoint was change in Ki-67 over the 12 month study. Median Ki-67 was 4% at baseline (range 2–16.8 %) and 2% (range 0–15.2%) at 12 months in the 45 women who completed the trial (median decrease was 2.4%, range −13.0% to +8.2%). Thirty-six (80%) of the 45 women demonstrated a decrease in Ki-67 with a mean relative reduction of 0.67 (p<0.001 by Wilcoxon signed ranks test) (see Figure 1).
Figure 1.
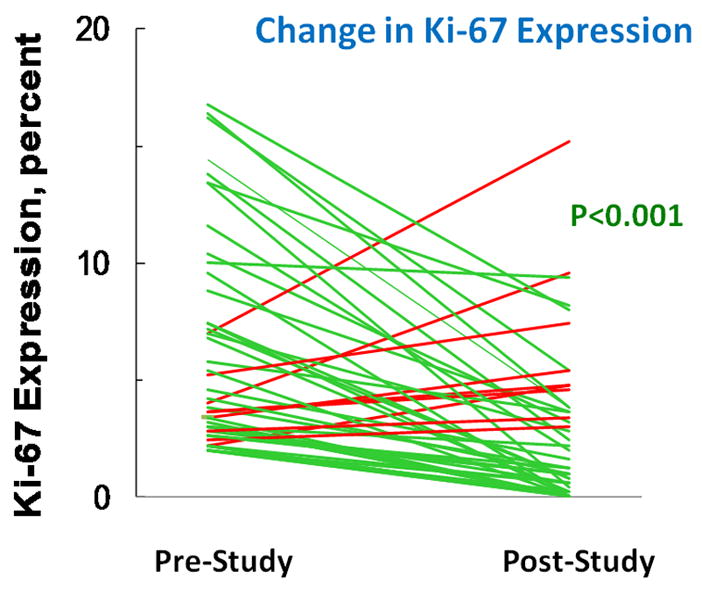
Change in expression of Ki-67 assessed by immunocytochemistry over the course of the 12 month study. The difference in expression is statistically significant by Wilcoxon’s sign rank test.
Cytomorphology
The proportion of evaluable women with atypical cytomorphology was greater at baseline (62%) than at the conclusion (42%) of the study (p=0.035, 2-sided McNemar’s test) although there was not a significant change in median semiquantitative Masood score. Consistent with the reduction in Ki-67, a significant shift in cell number category was also observed (See table 2).
Table 2.
Assessment of change in cell number and cytomorphology.
| Variable and Time/Change | Frequency | P-value | |
|---|---|---|---|
| Change in Cell Number per slide | |||
| Increase | 6 (13%) | 0.002 | |
| No Change | 19 (42%) | ||
| Decrease | 20 (44%) | ||
| Change in Categorical Descriptor | |||
| Worsen | 9 (20%) | 0.16 | |
| No Change | 19 (42%) | ||
| Improve | 17 (38%) | ||
| Change in Presence of Cytologic Atypia | |||
| Worsen (No Atypia → Atypia) | 3 (7%) | 0.013 | |
| No Change (Same at both RPFNAs) | 30 (67%) | ||
| Improve (Atypia → No Atypia) | 12 (27%) | ||
| Masood Score | Median (range) | ||
| Baseline | 15 11 – 17 |
0.13 | |
| 12-month | 14 10 – 20 |
||
| Change from baseline | 0 −5 to +5 |
||
| Change in Masood Score by ≥2 points | Frequency | ||
| Worsen | 5 (11%) | 0.090 | |
| No Change | 28 (62%) | ||
| Improve | 12 (27%) | ||
Mammographic Breast Density
Mammographic density declined over the 12 month period by a non-significant 6.3% (median). During the trial our hospital switched from analog to digital mammography, and digital mammography is generally associated with less density than analog. Restricting the analysis to the 25 individuals who had the same type of mammogram on and off study, there was no change either in median density or the proportion of individuals having increases or decreases in density (Figure 2).
Figure 2.
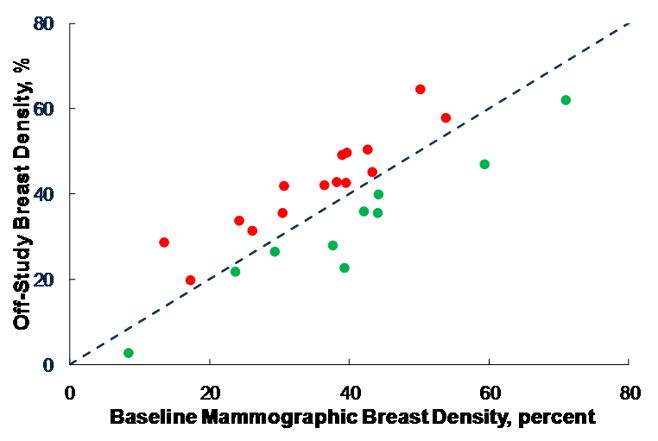
Change in mammographic breast density (expressed as percent of breast area that is considered to be at increased density) over the course of the 12 month study. The triangles indicate subjects where density increased; the squares indicate subjects where density decreased. The dashed line indicates equivalence, i.e., no change over the course of the study. The difference in density is not statistically significant by Wilcoxon’s sign rank test.
Hormone levels and Growth Factors
With the exception of a borderline decrease in IGFBP-3 and increase in bioavailable testosterone there were no changes in hormone or growth factor levels during the study (see Table 3). While there was a marginal decrease in SHBG when collected early in the menstrual cycle, no such effect was observed when collected at days 20–24 of the cycle.
Table 3.
Change in serum hormones and growth factors (median, mean, and std dev).
| Variable or Biomarker | N | Pre-Study | Post-Study | Difference | P-value (Wilcoxon) |
|---|---|---|---|---|---|
| Collected at time of RPFNA (day 1–10 of menstrual cycle) | |||||
| Estradiol, pg/mL | 22 | 97.5 154 ± 117 |
102 144 ± 90 |
1.29 −10.8 ± 53.4 |
0.55 |
| Estradiol, nmol/L | 22 | 0.36 0.57 ± 0.43 |
0.38 0.53 ± 0.33 |
0.00 −0.04 ± 0.20 |
|
| Bioavailable (Free) Estradiol, pmol/L | 22 | 4.6 6.3 ± 4.4 |
4.9 6.4 ± 4.1 |
0.32 0.12 ± 1.84 |
0.90 |
| SHBG (with E2), nmol/L | 22 | 80.2 81.2 ± 28.6 |
67.9 72.9 ± 26.3 |
−11.7 −8.3 ± 30.1 |
0.031 |
| Collected at day 20–24 of menstrual cycle | |||||
| IGF-I, ng/mL | 44 | 194 201 ± 78 |
180 196 ± 67 |
0.28 −5.1 ± 47.8 |
0.48 |
| IGF-I, nmol/L | 44 | 25.3 26.2 ± 10.1 |
23.4 25.5 ± 8.7 |
0.04 −0.66 ± 6.21 |
|
| IGFBP-3, ng/mL | 44 | 5854 5978±1009 |
5672 5729±1022 |
−220 −249 ± 790 |
0.029 |
| IGFBP-3, nmol/L | 44 | 205 209 ± 35 |
199 201 ± 36 |
−7.7 −8.7 ± 27.6 |
|
| IGF-I:IGFBP-3 Molar Ratio | 44 | 0.12 0.12 ± 0.04 |
0.12 0.13 ± 0.03 |
0.001 0.003±0.029 |
0.65 |
| Progesterone, ng/mL | 44 | 6.1 8.0 ± 8.2 |
6.4 8.8 ± 9.8 |
0.4 0.8 ± 8.5 |
0.58 |
| Progesterone, nmol/L | 44 | 19.3 25.5 ± 26.2 |
20.3 28.0 ± 31.2 |
1.3 2.5 ± 27.2 |
|
| Testosterone, ng/mL | 44 | 1.1 1.6 ± 2.4 |
1.1 1.6 ± 2.0 |
0.07 0.00 ± 1.32 |
0.11 |
| Testosterone, nmol/L | 44 | 3.7 5.7 ± 8.3 |
3.8 5.7 ± 7.0 |
0.25 0.00 ± 4.57 |
|
| Bioavailable (Free) Testosterone, pmol/L | 44 | 39.3 59.8 ± 63.3 |
43.3 73.9 ± 101.3 |
3.9 17.2 ± 74.8 |
0.061 |
| SHBG, nmol/L | 44 | 76.9 70.1 ± 25.5 |
70.9 72.2 ± 30.6 |
0.82 2.03 ± 23.5 |
0.99 |
| Secoisolariciresinol, nmol/L | 42 | 0.0 0.62 ± 1.15 |
5.7 33.7 ± 66.5 |
5.5 32.8 ± 66.5 |
< 0.001 |
| Enterodiol, nmol/L | 42 | 0.0 0.69 ± 1.29 |
23.3 84.3 ± 133.0 |
23.3 83.7 ± 133.0 |
< 0.001 |
| Enterolactone, nmol/L | 42 | 11.1 15.8 ± 17.4 |
99.2 132.7 ± 120.3 |
74.8 117.0 ± 117.7 |
< 0.001 |
| Total Lignans. nmol/L | 42 | 11.1 16.8 ± 17.9 |
183.3 248.8 ± 216.7 |
165.9 233.5 ± 213.9 |
< 0.001 |
Change in Lignan Blood levels over the Course of the Study
Forty-two subjects had plasma obtained for lignans at 0, 6, and 12 months. Plasma lignan levels were below the limit of quantification or undetectable at baseline for SECO in 94%, END 84%, and ENL in 16% of specimens. All three lignans demonstrated a statistically significant (P<0.001, Wilcoxon signed rank test) increase in levels between baseline and 6 months or 12 months (see Table 3), but no difference between 6 and 12 months. There was a 9-fold increase in median levels of ENL, the most biologically relevant lignan, and a 16-fold increase in median total lignan levels from baseline to 12 months. There was no significant relationship between reported compliance and change in ENL levels or ENL levels and change in Ki-67.
Compliance
Our preset criterion for compliance was ingestion of 70% of prescribed capsules and was met by 44/45 biomarker evaluable subjects. Median compliance as assessed by capsule count was 96% in biomarker evaluable subjects.
Adverse Events
Reported adverse events were mild and for the most part probably unrelated to drug. There were no grade 4, grade 3 in only 4%, grade 2 in 47%, and grade 1 in 35%. Four subjects discontinued study prematurely, 1 with a grade 3 adverse event from pelvic pain at 3 months, 1 with pregnancy at 9 months, 1 with DCIS detected on her regularly scheduled 12 – month mammogram prior to RPFNA, and another failed to return for any follow-up visit. The majority of the grade 2 adverse events were considered to be probably unrelated to the study agent including minor teeth, sinus, and respiratory infections with GI symptoms such as nausea, flatulence, or diarrhea provided only 11% grade 2 events. Grade 1 AEs were predominately related to transient GI complaints and alteration of the menses. Table 4 gives frequency of reported AEs that might be expected from SDG. Half of the subjects reported some GI symptoms during the 12 months and 26% reported irregular menses. Only one subject reported becoming amenorheic, halfway through study, but then had a period 1 week prior to the post-study RPFNA. There was no correlation between side effects and ENL levels.
Table 4.
Frequency of anticipated adverse events.
| Adverse Event | Frequency – N (%) | ||
|---|---|---|---|
| Single Events | Per Subject | ||
| Diarrhea | Grade 1 | 4 (8%) | 2 (4%) |
| Grade 2 | 2 (4%) | 1 (2%) | |
| Flatulence | Grade 1 | 10 (20%) | 10 (20%) |
| Grade 2 | 2 (4%) | 2 (4%) | |
| GI symptom | Grade 1 | 22 (44%) | 18 (36%) |
| Grade 2 | 3 (6%) | 3 (6%) | |
| Irregular Menses | Grade 1 | 15 (30%) | 13 (26%) |
| Rash | Grade 1 | 1 (2%) | 1 (2%) |
| Grade 2 | 10 (20%) | 6 (12%) | |
| Hot Flashes | Grade 1 | 2 (4%) | 2 (4%) |
| Grade 2 | 1 (2%) | 1 (2%) | |
Breast Biopsies and Events
Six of the 49 high risk subjects underwent breast biopsy as a result of breast imaging (5, mostly new microcalcifications) or the RPFNA (1, suspicious cytomorphology) at the 12 month visit. Biopsies showed fibrocystic change or proliferative breast disease in four and LCIS in two. Four of the six women had baseline cytologic atypia and one of the two with LCIS has a prior history of LCIS. Subsequent to these biopsies shortly after going off-study, two women were diagnosed with DCIS and another with invasive cancer.
Discussion
To our knowledge this is the first report of a significant reduction in Ki-67 in benign breast tissue, with sufficient SDG to raise plasma lignan levels 10-fold. We also observed a reduction in the proportion of women with cytologic atypia. Our median ENL of 99 nmol/L following supplementation with 50 mg SDG per day was higher than the mean ENL in the highest quintile of the Finnish case-control study associated with a 62% breast cancer risk reduction when compared to the lowest quintile [18]. The mean level for the lowest quintile in the Finnish study (3 nmol/L) was similar to the baseline ENL (11 nmol/L) in our study. Our findings in premenopausal women at high risk for development of breast cancer parallel those of Thompson et al. who observed a reduction in Ki-67 in tumor tissue after ~ 30 days of muffins supplemented with 25 grams of flaxseed vs. muffins alone in a cohort of 32 pre- and post-menopausal women with newly diagnosed breast cancer [60].
Although SDG’s mechanism of action is not clear, several possibilities have emerged from pre-clinical studies and include:1) antioxidant effects [61]; 2) increase in BRCA1 protein and differentiation [62,63]; 3) reduced breast aromatase with reduction in tissue estrogen production and altered ER related signaling [64–66]; 4) activation of PPAR gamma with an increase in adiponectin and resulting suppression of AKT/mTOR activity [67,68]; 5) down-regulation of EGFR with resultant decrease in MAP kinase and reduction in IGF-I with down-regulation of PI3 kinase signaling [17,69,70]; and 6) reduced VEGF secretion and angiogenesis [3].
Lack of modulation of mammographic density [71] despite an increase in plasma lignans and a reduction in tissue Ki-67 is in line with findings of Steudal et al. who found no correlation of plasma ENL and mammographic density [72], and findings by ourselves and others indicating a lack of correlation between Ki-67 obtained from cytologic or histologic or specimens and mammographic density [73–75]. Since modulation of mammographic breast density has been observed with tamoxifen and other selective estrogen receptor modulators but not aromatase inhibitors [76–79], demonstration of modulation of mammographic density after a short term intervention may be drug class specific.
The primary limitation of our pilot study is the lack of a placebo control group. However, cytomorphology, and Ki-67 are reasonably stable over time when a stable hormonal milieu is maintained [80,81].
Given the favorable safety profile, prior studies indicating lignan associated reduction in breast pain and breast tumor cell proliferation [60,82,83], and the current study suggesting reduction in proliferation and atypia, SDG warrants further testing in pre-menopausal women in a Phase II placebo controlled trial.
Acknowledgments
Supported in part by grant R21 CA117847, from the National Cancer Institute, NIH.
References
- 1.Setchell KDR, Lawson AM, Mitchell FL, et al. Lignans in man and in animal species. Nature. 1980;287:740–42. doi: 10.1038/287740a0. [DOI] [PubMed] [Google Scholar]
- 2.Setchell KDR, Lawson AM, Borriello SP, et al. Lignan formation in man--microbial involvement and possible roles in relation to cancer. Lancet. 1981;2:4–7. doi: 10.1016/s0140-6736(81)90250-6. [DOI] [PubMed] [Google Scholar]
- 3.Bergman Jungeström M, Thompson LU, Dabrosin C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res. 2007;13:1061–67. doi: 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- 4.Penttinen P, Jaehrling J, Damdimopoulos AE, et al. Diet-derived polyphenol metabolite enterolactone is a tissue-specific estrogen receptor activator. Endocrinology. 2007;148:4875–86. doi: 10.1210/en.2007-0289. [DOI] [PubMed] [Google Scholar]
- 5.Nesbitt PD, Lam Y, Thompson LU. Human metabolism of mammalian lignan precursors in raw and processed flaxseed. Am J Clin Nutr. 1999;69:549–55. doi: 10.1093/ajcn/69.3.549. [DOI] [PubMed] [Google Scholar]
- 6.Mousavi Y, Adlercreutz H. Enterolactone and estradiol inhibit each other's proliferative effect on MCF-7 breast cancer cells in culture. J Steroid Biochem Mol Biol. 1992;41:615–19. doi: 10.1016/0960-0760(92)90393-w. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Higuchi CM, Zhang R. Individual and combinatory effects of soy isoflavones on the in vitro potentiation of lymphocyte activation. Nutr Cancer. 1997;29:29–34. doi: 10.1080/01635589709514598. [DOI] [PubMed] [Google Scholar]
- 8.Mueller SO, Simon S, Chae K, et al. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci. 2004;80:14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- 9.Carreau C, Flouriot G, Bennetau-Pelissero C, et al. Enterodiol and enterolactone, two major diet-derived polyphenol metabolites have different impact on ER alpha transcriptional activation in human breast cancer cells. J Steroid Biochem Mol Biol. 2008;110:176–85. doi: 10.1016/j.jsbmb.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 10.Thompson LU, Robb P, Serraino M, et al. Mammalian lignan production from various foods. Nutr Cancer. 1991;16:43–52. doi: 10.1080/01635589109514139. [DOI] [PubMed] [Google Scholar]
- 11.Thompson LU, Boucher BA, Liu Z, et al. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer. 2006;54:184–01. doi: 10.1207/s15327914nc5402_5. [DOI] [PubMed] [Google Scholar]
- 12.de Kleijn MJ, van der Schouw YT, Wilson PW, et al. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: the Framingham study. J Nutr. 2001;131:1826–32. doi: 10.1093/jn/131.6.1826. [DOI] [PubMed] [Google Scholar]
- 13.Boker LK, Van der Schouw YT, De Kleijn MJ, et al. Intake of dietary phytoestrogens by Dutch women. J Nutr. 2002;132:1319–28. doi: 10.1093/jn/132.6.1319. [DOI] [PubMed] [Google Scholar]
- 14.Thompson LU, Seidl MM, Rickard SE, et al. Antitumorigenic effect of a mammalian lignan precursor from flaxseed. Nutr Cancer. 1996;26:159–65. doi: 10.1080/01635589609514472. [DOI] [PubMed] [Google Scholar]
- 15.Rickard SE, Yuan YV, Chen J, et al. Dose effects of flaxseed and its lignan on N-methyl-N-nitrosourea-induced mammary tumorigenesis in rats. Nutr Cancer. 1999;35:50–7. doi: 10.1207/S1532791450-57. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Wang L, Thompson LU. Flaxseed and its components reduce metastasis after surgical excision of solid human breast tumor in nude mice. Cancer Lett. 2006;234:168–75. doi: 10.1016/j.canlet.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Chen J, Thompson LU. The inhibitory effect of flaxseed on the growth and metastasis of estrogen receptor negative human breast cancer xenografts is attributed to both its lignan and oil components. Int J Cancer. 2005;116:793–8. doi: 10.1002/ijc.21067. [DOI] [PubMed] [Google Scholar]
- 18.Pietinen P, Stumpf K, Männistö S, et al. Serum enterolactone and risk of breast cancer: a case-control study in eastern Finland. Cancer Epidemiol Biomarkers Prev. 2001;10:339–44. [PubMed] [Google Scholar]
- 19.Linseisan J, Piller R, Hermann S, et al. Dietary phytoestrogen intake and premenopausal breast cancer risk in a German case-control study. Int J Cancer. 2004;110:284–90. doi: 10.1002/ijc.20119. [DOI] [PubMed] [Google Scholar]
- 20.McCann SE, Muti P, Vito D, et al. Dietary lignan intakes and risk of pre- and postmenopausal breast cancer. Int J Cancer. 2004;111:440–3. doi: 10.1002/ijc.20262. [DOI] [PubMed] [Google Scholar]
- 21.Boccardo F, Lunardi G, Guglielmini P, et al. Serum enterolactone levels and the risk of breast cancer in women with palpable cysts. Eur J Cancer. 2004;40:84–9. doi: 10.1016/s0959-8049(03)00576-8. [DOI] [PubMed] [Google Scholar]
- 22.Zeleniuch-Jacquotte A, Adlercreutz H, Shore RE, et al. Circulating enterolactone and risk of breast cancer: a prospective study in New York. Br J Cancer. 2004;91:99–105. doi: 10.1038/sj.bjc.6601893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen A, Knudsen KE, Thomsen BL, et al. Plasma enterolactone and breast cancer incidence by estrogen receptor status. Cancer Epidemiol Biomarkers Prev. 2004;13:2084–9. [PubMed] [Google Scholar]
- 24.Piller R, Verla-Tebit E, Wang-Gohrke S, et al. CYP17 genotype modifies the association between lignan supply and premenopausal breast cancer risk in humans. J Nutr. 2006;136:1596–603. doi: 10.1093/jn/136.6.1596. [DOI] [PubMed] [Google Scholar]
- 25.McCann SE, Kulkarni S, Trevisan M, et al. Dietary lignan intakes and risk of breast cancer by tumor estrogen receptor status. Breast Cancer Res Treat. 2006;99:309–11. doi: 10.1007/s10549-006-9196-x. [DOI] [PubMed] [Google Scholar]
- 26.Thanos J, Cotterchio M, Boucher BA, et al. Adolescent dietary phytoestrogen intake and breast cancer risk (Canada) Cancer Causes Control. 2006;17:1253–61. doi: 10.1007/s10552-006-0062-2. [DOI] [PubMed] [Google Scholar]
- 27.Touillaud MS, Thiébaut AC, Fournier A, et al. Dietary lignan intake and postmenopausal breast cancer risk by estrogen and progesterone receptor status. J Natl Cancer Inst. 2007;99:475–86. doi: 10.1093/jnci/djk096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonestedt E, Ericson U, Gullberg B, et al. Variation in fasting and non-fasting serum enterolactone concentrations in women of the Malmö Diet and Cancer cohort. Eur J Clin Nutr. 2008;62:1005–9. doi: 10.1038/sj.ejcn.1602811. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki R, Rylander-Rudqvist T, Saji S, et al. Dietary lignans and postmenopausal breast cancer risk by oestrogen receptor status: a prospective cohort study of Swedish women. Br J Cancer. 2008;98:636–40. doi: 10.1038/sj.bjc.6604175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cotterchio M, Boucher BA, Kreiger N, et al. Dietary phytoestrogen intake--lignans and isoflavones--and breast cancer risk (Canada) Cancer Causes Control. 2008;19:259–72. doi: 10.1007/s10552-007-9089-2. [DOI] [PubMed] [Google Scholar]
- 31.Sonestedt E, Borgquist S, Ericson U, et al. Enterolactone is differently associated with estrogen receptor beta-negative and ER-positive breast cancer in a Swedish nested case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17:3241–51. doi: 10.1158/1055-9965.EPI-08-0393. [DOI] [PubMed] [Google Scholar]
- 32.Velentzis LS, Cantwell MM, Cardwell C, et al. Lignans and breast cancer risk in pre- and post-menopausal women: meta-analyses of observational studies. Br J Cancer. 2009;100:1492–8. doi: 10.1038/sj.bjc.6605003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilkkinen A, Stumpf K, Pietinen P, et al. Determinants of serum enterolactone concentration. Am J Clin Nutr. 2001;73:1094–100. doi: 10.1093/ajcn/73.6.1094. [DOI] [PubMed] [Google Scholar]
- 34.Kilkkinen A, Pietinen P, Klaukka T, et al. Use of oral antimicrobials decreases serum enterolactone concentration. Am J Epidemiol. 2002;155:472–77. doi: 10.1093/aje/155.5.472. [DOI] [PubMed] [Google Scholar]
- 35.Lampe JW. Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J Nutr. 2003;133(Suppl 3):956S–64S. doi: 10.1093/jn/133.3.956S. [DOI] [PubMed] [Google Scholar]
- 36.French MR, Thompson LU, Hawker GA. Validation of a phytoestrogen food frequency questionnaire with urinary concentrations of isoflavones and lignan metabolites in premenopausal women. J Am Coll Nutr. 2007;26:76–82. doi: 10.1080/07315724.2007.10719588. [DOI] [PubMed] [Google Scholar]
- 37.Morisset AS, Lemieux S, Veilleux A, et al. Impact of a lignan-rich diet on adiposity and insulin sensitivity in post-menpausal women. Br J Nutr. 2009;102:195–200. doi: 10.1017/S0007114508162092. [DOI] [PubMed] [Google Scholar]
- 38.McCann SE, Moysich KB, Freudenheim JL, et al. The risk of breast cancer associated with dietary lignans differs by CYP17 genotype in women. J Nutr. 2002;132:3036–41. doi: 10.1093/jn/131.10.3036. [DOI] [PubMed] [Google Scholar]
- 39.Feigelson HS, Shames LS, Pike MC, Coetzee GA, Stanczyk FZ, Henderson BE. Cytochrome P450c17alpha gene (CYP17) polymorphism is associated with serum estrogen and progesterone concentrations. Cancer Res. 1998;58:585–7. [PubMed] [Google Scholar]
- 40.Preston-Martin S, Pike MC, Ross RK, et al. Increased cell division as a cause of human cancer. Cancer Res. 1990;50:7415–21. [PubMed] [Google Scholar]
- 41.Shaaban AM, Sloane JP, West CR, et al. Breast cancer risk in usual ductal hyperplasia is defined by estrogen receptor-alpha and Ki-67 expression. Am J Pathol. 2002;160:597–604. doi: 10.1016/s0002-9440(10)64879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santisteban M, Reynolds C, Barr Fritcher EG, et al. Ki67: a time-varying biomarker of risk of breast cancer in atypical hyperplasia. Breast Cancer Res Treat. 2009 Sep 23; doi: 10.1007/s10549-009-0534-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–20. doi: 10.1200/JCO.2005.07.501. [DOI] [PubMed] [Google Scholar]
- 44.Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99:167–70. doi: 10.1093/jnci/djk020. [DOI] [PubMed] [Google Scholar]
- 45.Fabian CJ, Kimler BF, Zalles CM, et al. Short-term breast cancer prediction by random periareolar fine-needle aspiration cytology and the Gail risk model. J Natl Cancer Inst. 2000;92:1217–27. doi: 10.1093/jnci/92.15.1217. [DOI] [PubMed] [Google Scholar]
- 46.Khan QJ, Kimler BF, Clark J, et al. Ki-67 expression in benign breast ductal cells obtained by random periareolar fine needle aspiration. Cancer Epidemiol Biomarkers Prev. 2005;14:786–9. doi: 10.1158/1055-9965.EPI-04-0239. [DOI] [PubMed] [Google Scholar]
- 47.Masood S, Frykberg ER, McLellan GL, et al. Prospective evaluation of radiologically directed fine needle aspiration biopsy of nonpalpable breast lesions. Cancer. 1990;66:1480–7. doi: 10.1002/1097-0142(19901001)66:7<1480::aid-cncr2820660708>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 48.Zalles C, Kimler BF, Kamel S, et al. Cytologic patterns in random aspirates from women at high and low risk for breast cancer. Breast J. 1995;1:343–9. [Google Scholar]
- 49.Fabian CJ, Kimler BF, Mayo MS, et al. Breast-tissue sampling for risk assessment and prevention. Endocr Relat Cancer. 2005;12:185–213. doi: 10.1677/erc.1.01000. [DOI] [PubMed] [Google Scholar]
- 50.Eliasson C, Kamal-Eldin A, Andersson R, et al. High-performance liquid chromatographic analysis of secoisolariciresinol diglucoside and hydroxycinnamic acid glucosides in flaxseed by alkaline extraction. J Chromatogr A. 2003;1012:151–9. doi: 10.1016/s0021-9673(03)01136-1. [DOI] [PubMed] [Google Scholar]
- 51.Almada AL. SDG precision standardized Flaxseed Extract. Scientific Research Monograph. 2003 Website: www.lignan.com.
- 52.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 53.Dabrosin C. Increase of free insulin-like growth factor-1 in normal human breast in vivo late in the menstrual cycle. Breast Cancer Res Treat. 2003;80:193–8. doi: 10.1023/A:1024575103524. [DOI] [PubMed] [Google Scholar]
- 54.Micheli A, Muti P, Secreto G, Krogh V, Memeghini E, Venturelli E, Sieri S, Pala V, Berrino F. Endogenous sex hormones and subsequent breast cancer in premenopausal women. Int J Cancer. 2004;112:312–18. doi: 10.1002/ijc.20403. [DOI] [PubMed] [Google Scholar]
- 55.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 56.Endogenous Hormones and Breast Cancer Collaborative Group. Free estradiol and breast cancer risk in postmenopausal women: comparison of measured and calculated values. Cancer Epidemiol Biomarkers Prev. 2003;12:1457–61. [PubMed] [Google Scholar]
- 57.Byng JW, Boyd NF, Fishell E, et al. The quantitative analysis of mammographic densities. Phys med Biol. 1994;39:1629–38. doi: 10.1088/0031-9155/39/10/008. [DOI] [PubMed] [Google Scholar]
- 58.Setchell KDR, Childress C, Zimmer-Nechemias L, et al. Method for measurement of dietary secoisolariciresinol using HPLC with multichannel electrochemical detection. J Medicinal Food. 1999;2:193–8. doi: 10.1089/jmf.1999.2.193. [DOI] [PubMed] [Google Scholar]
- 59.Setchell KD, Lawson AM, McLaughlin LM, et al. Measurement of enterolactone and enterodiol, the first mammalian lignans, using stable isotope dilution and gas chromatography mass spectrometry. Biomed Mass Spectrom. 1983;10:227–35. doi: 10.1002/bms.1200100321. [DOI] [PubMed] [Google Scholar]
- 60.Thompson LU, Chen JM, Li T, et al. Dietary flaxseed alters tumor biological markers in postmenopausal breast cancer. Clin Cancer Res. 2005;11:3828–35. doi: 10.1158/1078-0432.CCR-04-2326. [DOI] [PubMed] [Google Scholar]
- 61.Prasad K. Antioxidant activity of secoisolariciresinol diglucoside-derived metabolites, secoisolariciresinol, enterodiol, and enterolactone. Int J Angiol. 2000;9:220–5. doi: 10.1007/BF01623898. [DOI] [PubMed] [Google Scholar]
- 62.Tan KP, Chen J, Ward WE, et al. Mammary gland morphogenesis is enhanced by exposure to flaxseed or its major lignan during suckling in rats. Exp Biol Med. 2004;229:147–57. doi: 10.1177/153537020422900203. [DOI] [PubMed] [Google Scholar]
- 63.Vissac-Sabatier C, Coxam V, Déchelotte P, et al. Phytoestrogen-rich diets modulate expression of Brca1 and Brca2 tumor suppressor genes in mammary glands of female Wistar rats. Cancer Res. 2003;63:6607–12. [PubMed] [Google Scholar]
- 64.Adlercreutz H, Bannwart C, Wahala K, et al. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J Steroid Biochem Mol Biol. 1993;44:147–53. doi: 10.1016/0960-0760(93)90022-o. [DOI] [PubMed] [Google Scholar]
- 65.Brooks JD, Thompson LU. Mammalian lignans and genistein decrease the activities of aromatase and 17beta-hydroxysteroid dehydrogenase in MCF-7 cells. J Steroid Biochem Mol Biol. 2005;94:461–7. doi: 10.1016/j.jsbmb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Yokota T, Matsuzaki Y, Koyama M, et al. Sesamin, a lignan of sesame, down-regulates cyclin D1 protein expression in human tumor cells. Cancer Sci. 2007;98:1447–53. doi: 10.1111/j.1349-7006.2007.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukumitsu S, Aida K, Ueno N, et al. Flaxseed lignan attenuates high-fat diet- induced fat accumulation and induces adiponectin expression in mice. Br J Nutr. 2008;100:669–76. doi: 10.1017/S0007114508911570. [DOI] [PubMed] [Google Scholar]
- 68.Kim K, Baek A, Hwang J, et al. Adiponectin-activated AMPK stimulates dephosphorylation at AKT through protein phosphatase 2A activation. Cancer Res. 2009;69:4018–26. doi: 10.1158/0008-5472.CAN-08-2641. [DOI] [PubMed] [Google Scholar]
- 69.Power KA, Chen JM, Saarinen NM, et al. Changes in biomarkers of estrogen receptor and growth factor signaling pathways in MCF-7 tumors after short-and long-term treatment with soy and flaxseed. J Steroid Biochem Mol Biol. 2008;112:13–19. doi: 10.1016/j.jsbmb.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Youngren JF, Gable K, Penaranda C, et al. Nordihydroguaiaretic acid (NDGA) inhibits the IGF-1 and c-erbB2/HER2/neu receptors and suppresses growth in breast cancer cells. Breast Cancer Res Treat. 2005;94:37–46. doi: 10.1007/s10549-005-6939-z. [DOI] [PubMed] [Google Scholar]
- 71.Boyd NF, Byng JW, Jong RA, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670–5. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 72.Stuedal A, Gram IT, Bremnes Y, et al. Plasma levels of enterolactone and percentage mammographic density among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:2154–9. doi: 10.1158/1055-9965.EPI-05-0116. [DOI] [PubMed] [Google Scholar]
- 73.Khan QJ, Kimler BF, O'Dea AP, et al. Mammographic density does not correlate with Ki-67 expression or cytomorphology in benign breast cells obtained by random periareolar fine needle aspiration from women at high risk for breast cancer. Breast Cancer Res. 2007;9:R35. doi: 10.1186/bcr1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stomper PC, Penetrante RB, Edge SB, et al. Cellular proliferative activity of mammographic normal dense and fatty tissue determined by DNA S phase percentage. Breast Cancer Res Treat. 1996;37:229–36. doi: 10.1007/BF01806504. [DOI] [PubMed] [Google Scholar]
- 75.Hawes D, Downey S, Pearce CL, et al. Dense breast stromal tissue shows greatly increased concentration of breast epithelium but no increase in its proliferative activity. Breast Cancer Res. 2006;8:R24. doi: 10.1186/bcr1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cuzick J, Warwick J, Pinney E, et al. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96:621–8. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 77.Kimler BF, Ursin G, Fabian CJ, et al. Effect of the third generation selective estrogen receptor modulator arzoxifene on mammographic breast density. J Clin Oncol. 2006;24(Suppl 18S):18s. (abstract 562) [Google Scholar]
- 78.Cigler T, Yaffe MJ, Johnston D, et al. A placebo-controlled trial examining the effects of letrozole on mammographic breast density and bone and lipid metabolism. San Antonio Breast Cancer Symposium. 2007 Abstract 2082. [Google Scholar]
- 79.Fabian CJ, Kimler BF, Zalles CM, et al. Reduction in proliferation with six months of letrozole in women on hormone replacement therapy. Breast Cancer Res Treat. 2007;106:75–84. doi: 10.1007/s10549-006-9476-5. [DOI] [PubMed] [Google Scholar]
- 80.Kimler BF, Metheny T, Hughes J, et al. Validation of Ki-67 as a response biomarker in prevention trials of women at high risk for development of breast cancer. Proc 100th Annual Meeting of the American Association for Cancer Research; 2009 Apr 18–22; Denver, CO. Philadelphia (PA): AACR; 2009. pp. 723–724. (Abstract 2993) [Google Scholar]
- 81.Ibarra-Drendall C, Wilke LG, Zalles C, et al. Reproducibility of random periareolar fine needle aspiration in a multi-institutional Cancer and Leukemia Group B (CALGB) cross-sectional study. Cancer Epidemiol Biomarkers Prev. 2009;18:1379–85. doi: 10.1158/1055-9965.EPI-08-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goss PE, Li T, Theriault M, et al. Effects of dietary flaxseed in women with cyclical mastalgia. Breast Cancer Res Treat. 2000;64:49. [Google Scholar]
- 83.Rosolowich V, Saettler E, Szuck B, et al. Society of Obstetricians and Gynecologists of Canada (SOGC) Mastalgia J Obstet Gynaecol Can. 2006;28:49–71. doi: 10.1016/S1701-2163(16)32027-8. [DOI] [PubMed] [Google Scholar]


