Abstract
Objective:
The intrinsic radioresistance of pancreatic cancer (PaCa) is due to multiple oncogenic signaling pathways. In contrast to combining radiation therapy (RT) with targeted therapeutic agent(s) whose blockade can be circumvented by redundant signaling pathways, we evaluated the combination of RT with a broad-spectrum histone deacetylase inhibitor, vorinostat.
Methods:
Radiosensitization by vorinostat was analyzed using clonogenic survival assays. Apoptosis was evaluated using flow cytometry and immunoblotting. DNA repair was evaluated using immunofluorescence assessment of histone2AX phosphorylation and immunoblotting for DNA repair proteins. Pro-survival pathway proteins were measured by immunoblotting and electrophoretic mobility shift assays.
Results:
Vorinostat significantly sensitized PaCa cells to radiation, but vorinostat-induced apoptosis did not contribute significantly to the observed radiosensitization. However, vorinostat inhibited DNA damage repair by targeting key DNA repair proteins and also abrogated pro-survival pathways responsible for PaCa aggressiveness and radioresistance. Specifically, the constitutively overexpressed epidermal growth factor receptor and nuclear factor kappaB pathways were shown to be induced by radiation and inhibited by vorinostat.
Conclusions:
Vorinostat augments the anti-tumor effects of RT by abrogating radioresistance responses of PaCa cells mediated by pro-survival and DNA repair pathways, and promises to be a clinically relevant adjunct to RT for treatment of PaCa.
Keywords: Vorinostat, Pancreatic Cancer, Radiation, NF-κB, EGFR, Radiosensitization
INTRODUCTION
Due to its aggressive tumor biology and lack of specific symptoms during early stages, pancreatic cancer (PaCa) frequently presents as an incurable disease with about two-thirds of patients showing radiographically detectable metastasis at the time of diagnosis.1 This poor prognosis makes PaCa the fourth leading cause of cancer death in the US, with a mortality rate almost equaling its incidence.2 For patients with no metastatic spread, the only potentially curative treatment is surgery. However, even among the 10% of patients with resectable disease, only approximately 20% survive for 5 years,3 indicating a persisting need for good adjuvant therapies. About 40% of the patients diagnosed have locally advanced, unresectable, non-metastatic disease called locally advanced pancreatic cancer (LAPC). These patients are treated with chemoradiation therapy in the US. Nevertheless, due to the inherent chemo- and radioresistance of PaCa, this approach has had little therapeutic benefit.4 To improve outcomes in this cohort of patients, many targeted therapeutic approaches have been explored singly and in combination with chemotherapy and/or radiation therapy with universally disappointing results.5, 6 One explanation for this phenomenon is the ubiquitous prevalence of multiple redundant signaling pathways in PaCa cells that confer a survival advantage against a targeted therapeutic assault.
Recently, employing broad-spectrum agents that simultaneously inhibit multiple oncogenic/pro-survival pathways that confer chemo- and radioresistance to tumors has emerged as an exciting prospect. In this regard, inhibition of histone deacetylases (HDACs), the chromatin remodeling enzymes that silence tumor suppressor genes by transcriptional repression, has emerged as a promising therapeutic strategy. Several compounds that are described as HDAC inhibitors (HDACi), demonstrate potent anticancer activities in preclinical settings7 and are undergoing rigorous clinical trials as single agents and/or in combination with other therapeutic modalities.8 Suberoylanilide hydroxamic acid (vorinostat; Zolinza™ by Merck Inc.) is the prototype agent of the synthetic hybrid polar hydroxamic acid drugs. The in vitro and in vivo anticancer effects of vorinostat are mediated via several mechanisms including, but not limited to, induction of cell cycle arrest, apoptosis, and differentiation.9 In addition to its cytotoxicity, vorinostat has been shown to have additive and synergistic anticancer effects with cytotoxic modalities such as radiation therapy and selected chemotherapeutics. Based on promising preclinical activities, vorinostat has undergone several Phase I and Phase II clinical trials and is the first FDA approved HDAC inhibitor for clinical use.9 The active in vitro micromolar concentrations of vorinostat are pharmacologically attainable,10 making it a good candidate to explore in newer anticancer therapeutic approaches. We hypothesized that the combination of vorinostat with radiation therapy may enhance the radiosensitivity of pancreatic cancers and improve the efficacy of treatment.
The present study investigates the role of vorinostat in modulating the radioresponse of PaCa cells in vitro. Pre-irradiation exposure of human PaCa cell lines to sub-therapeutic doses of vorinostat profoundly inhibited subsequent clonogenic survival in vitro. However, direct cytotoxicity by vorinostat did not contribute significantly to the observed radiosensitization. Instead, vorinostat was shown to inhibit both- DNA damage repair and prominent pro-survival pathways that are both constitutively active in PaCa as well as transiently induced by radiation. Specifically, the epidermal growth factor receptor (EGFR) and nuclear factor kappa B (NF-κB) pathways, that mediate PaCa aggressiveness and radioresistance, were shown to be inhibited by vorinostat. Our studies elucidate a pro-survival and DNA repair signaling response of PaCa cells to irradiation that can be simultaneously abrogated by vorinostat to sensitize tumors to radiotherapy.
MATERIALS AND METHODS
Cells and chemicals
Human pancreatic cancer cell lines MiaPaCa-2 and AsPC-1 were from American Type Culture Collection (Manassas, VA) while Colo357FG was kindly provided by Dr. Arup Chakraborty (MD Anderson Cancer Center). MiaPaCa-2 and Colo357FG were maintained in DMEM and AsPC-1 was maintained in RPMI-1640. Both media were supplemented with 10% fetal bovine serum and 1% Pen-Step (Invitrogen, Grand Island, NY). Vorinostat was from Aton Pharma (Tarrytown, NY). A 200 mM stock in dimethyl sulfoxide (DMSO) was stored at −20°C in aliquots and freshly diluted prior to use.
In vitro cytotoxicity
The effect of vorinostat on tumor cell proliferation was assessed using the XTT cell proliferation kit (Roche Applied Science, Indianapolis, IN). Briefly, cells were seeded in 96-well plates (3 × 104/mL) and after overnight incubation, the medium was aspirated and cells were exposed to different concentrations of vorinostat. After further 48 h of culture, XTT labeling mixture was added to the cells and the cells were incubated for further 4 h. The resulting orange formazan product was then specrophotometrically quantified (490 nm) using an ELISA plate reader (Perkin Elmer). the Results are expressed as percent cell viability for each concentration of vorinostat with respect to controls (0.1% DMSO in medium).
Clonogenic survival
Cells were treated with vehicle (DMSO) or 2 μM vorinostat for 48 h and then irradiated with a 137Cs unit at room temperature. Cells were washed, trypsinized, and specific cell densities were re-plated in 60-mm petridishes and were incubated for colony formation for 10-14 days. Colonies were counted after staining with 0.5% alcoholic crystal violet. The fraction surviving a given treatment was calculated with respect to the survival of unirradiated controls (cells treated with DMSO or vorinostat alone).
Apoptosis assay
Cells were exposed to vorinostat for 48 h, administered a single radiation dose of 6 Gy and incubated for an additional 24 h. Apoptotic cells were labeled by TUNEL (terminal deoxynucleotidyl transferase mediated fluorescein-dUTP nick end labeling) reaction (Roche Applied Science, Indianapolis, IN) as per the manufacturer's instructions. Percent apoptotic cells (TUNEL positive) in each treatment group were quantified by flow cytometry (Beckman Coulter Altra, Beckman Coulter, Fullerton, CA).
Immunofluorescence
Cells grown on coverslips were pre-treated with 2 μM vorinostat for 48 h and then were irradiated (6 Gy). Samples were processed for phosphorylated histone 2AX (γ-H2AX) immunostaining at different time points post-irradiation. Briefly, cells were washed with PBS, fixed with 1% paraformaldehyde (15 minutes) and 70% ethanol (15 minutes) at room temperature. They were then treated with 1% NP-40 (30 minutes), blocked with 5% bovine serum albumin (BSA) (30 minutes) and incubated with anti- γ-H2AX antibody (Millipore Corporation, Billerica, MA) in 5% BSA for 2 h. Subsequently, cells were washed with PBS, labeled with Alexa-Fluor® 488 conjugated secondary antibody (Invitrogen Corporation, Carlsbad, CA) for 30 minutes and counterstained with 4′,6′-diamino-2-phenylindole (DAPI; 1 μg/mL in PBS) for 5 minutes. Coverslips were mounted with antifade agent (Molecular Probes, Eugene, OR), examined under fluorescent microscope (Leica, Bannockburn, IL) and images were captured. Nuclear γ-H2AX foci were then counted manually from at least 50 cells for each treatment condition by person blinded to treatment.
Electrophoretic mobility shift assay (EMSA)
NF-κB activation was assessed by EMSA as described previously.11 Briefly, nuclear extracts of treated MiaPaCa cells were incubated with 32P-end-labeled double-stranded NF-κB oligonucleotide (15 μg of protein with 16 fmol of DNA) from the human immunodeficiency virus long terminal repeat, 5′-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGG- 3′ (boldface indicates NF-κB binding sites) for 30 min at 37°C, and the DNA-protein complex was resolved on 6.6% native PAGE. The radioactive bands on dried gels were quantified by a PhosphorImager using ImageQuant software (v 5.1; Molecular Dynamics, Sunnyvale, CA)
Immunoblotting
Whole cell lysates were fractionated by SDS-PAGE, the proteins were electrotransferred to nitrocellulose membranes and probed with antibodies against the following: Ku70, Rad50, phospho-EGFRTyr1173, EGFR, poly-(ADP-ribose)-polymerase (PARP) (Santa Cruz Biotechnology, Santa Cruz, CA), anti-caspase-3, anti-caspase-9 [Cell Signaling technology (Danvers, MA)], DNA-dependent protein kinase-catalytic subunit (DNA-PKcs) (BD Biosciences, San Jose, CA) and Ku86 and anti-β actin [Sigma (St. Louis, MO)]. The blots were next probed with appropriate horseradish peroxidase conjugated secondary antibodies (Santa Cruz, CA) and developed using ECL™ (GE healthcare, Piscataway, NJ)
Statistics
All experiments were performed at least in triplicate. Representative data of one of the three independent experiments are shown. The differences between two groups were analyzed by Student's t test (GrpahPad Prism, v5.01) and a p value less than 0.05 was deemed statistically significant.
RESULTS
Vorinostat decreased viability of pancreatic cancer cell lines
The cytotoxicity of vorinostat was determined using XTT assay. Vorinostat inhibited the proliferation of all the cell lines in a dose-dependent manner (Fig. 1). Among the three cell lines tested, Colo357FG cells were the most sensitive (IC50=2.2 μM) compared to both MiaPaCa-2 (IC50=4.1 μM) and AsPC-1 (IC50=4.7 μM).
Figure 1. Vorinostat decreases the viability of pancreatic cancer cells.
Colo357FG, AsPC-1 or MiaPaCa-2 cells (3 × 104/mL) were exposed to different concentrations of vorinostat in a 96-well plate for 48 h and the viability was assessed by XTT assay (Roche). The percent viability was calculated with respect to DMSO-treated controls. Data points = mean ± SE of quadruplicates for each concentration.
Vorinostat sensitized pancreatic cancer cells to radiation
The effect of vorinostat on intrinsic tumor cell radiosensitivity was assessed by clonogenic cell survival. The dose of vorinostat chosen was less than the IC50 of vorinostat in vitro. Pretreatment with vorinostat (48 hr) significantly suppressed the clonogenic survival of all three cell lines (Fig. 2). The radiation dose enhancement ratio (DER), calculated as the dose (Gy) for radiation alone divided by the dose (Gy) for radiation plus vorinostat for a surviving fraction of 0.2 were 1.5 (AsPC-1), 1.4 (MiaPaCa-2) and 1.6 (Colo357FG). MiaPaCa-2 was chosen for further experiments as it displayed the maximum resistance towards vorinostat-mediated radiosensitization (lowest DER).
Figure 2. Vorinostat pre-treatment sensitizes pancreatic cancer cells to ionizing radiation.
AsPC-1 (a), MiaPaCa-2 (b) or Colo357FG (c) cells were exposed to 2 μM vorinostat for 48 h, following which they were irradiated to indicated doses and re-plated for colony formation. The enhancement in radiosensitivity by vorinostat was assessed on the basis of clonogenic cell survival assays in comparison with the controls (cells irradiated in the absence of vorinostat). Points, mean of the sextuplicates, bars, SE.
Vorinostat and radiation induced apoptosis
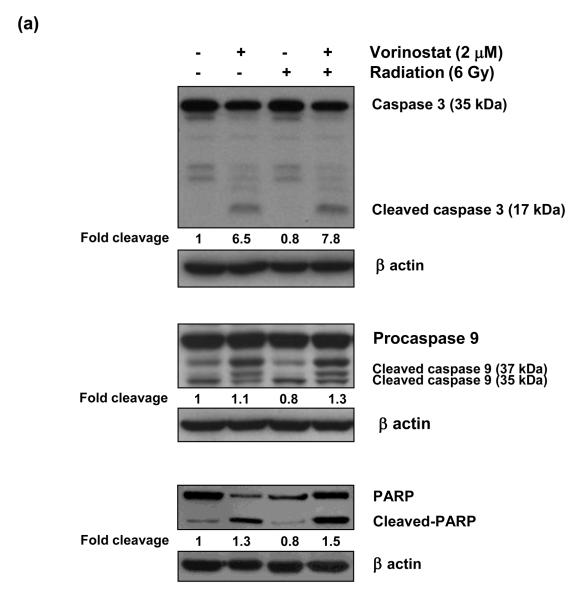
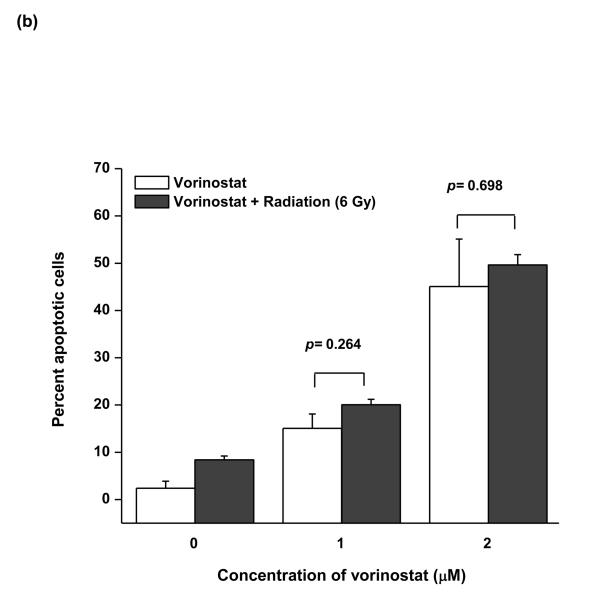
As vorinostat is a potent inducer of apoptosis, we investigated the role of apoptotic cell death in the observed radiosensitivity by vorinostat. We assessed the levels of key apoptotic proteins, namely cleaved caspase-9; caspase-3, and PARP, at an early time point (6 h) post-irradiation. Vorinostat induced cleavage of all these proteins (Fig. 3a), whereas radiation alone did not alter the endogenous levels of any of these proteins. Radiation, in combination with vorinostat also induced cleavage of all these proteins (Fig. 3a), however, these effects were not synergistic. We further confirmed these results by quantifying apoptosis by TUNEL assay. Vorinostat (1 μM and 2 μM) led to increased apoptotic cells (15.1% and 45.1% respectively) (Fig. 3b). Radiation itself did not induce apoptosis, and even its combination with vorinostat failed to show a significant increase in percent apoptotic over those with vorinostat alone at both 1 μM (20.1%; p = 0.26 compared to 1 μM vorinostat alone) and 2 μM vorinostat (49.7%; p = 0.69 compared to 2 μM vorinostat alone). These observations suggest that the apoptotic effects of vorinostat combined with radiation did not play a significant role in the observed radiosensitization.
Figure 3. Apoptosis induction by vorinostat does not play a significant role in radiosensitization.
(a) Assessment of apoptotic cell death by immunoblot analysis of caspase-3, caspase-9 and PARP cleavage. MiaPaCa-2 cells were treated with 2 μM vorinostat for 48 h, following which they were irradiated (6 Gy) and incubated for an additional 6 h. At the end of this treatment, cells were collected for immunoblot analysis. Quantification of the cleaved fragments for each protein was done by ImageQuant software. Fold cleavage with respect to the untreated control is indicated below each figure. (b) Assessment of vorinostat-induced apoptosis by TUNEL assay. Cells were treated with vorinostat for 48 hrs, irradiated (6 Gy) and DNA strand breaks were labeled with fluorescein-dUTP using TUNEL reaction (Roche) 24 h post-radiation. The apoptotic population was quantified by flow cytometry.
Vorinostat prolonged post-irradiation DNA repair
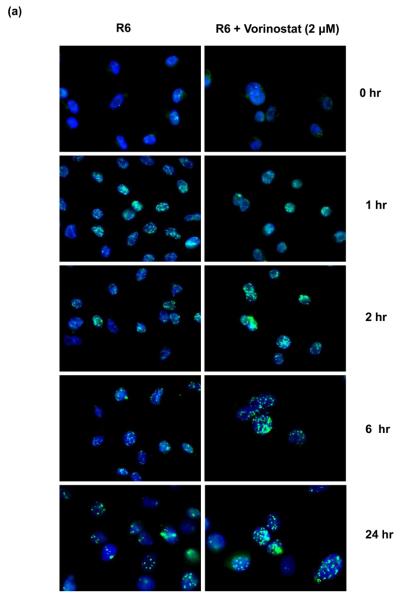
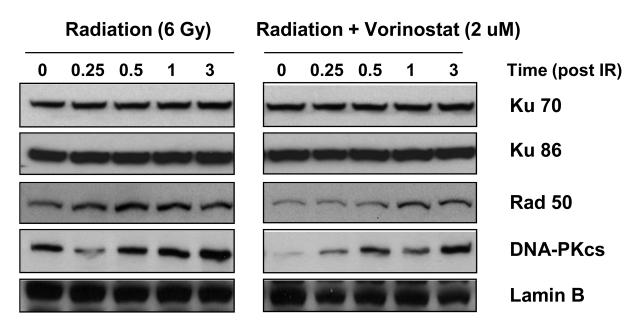
Repair of DNA damage between fractions of radiation is known to reduce the radiosensitivity of tumors. We investigated whether vorinostat affects post-radiation DNA repair. Expression of γ-H2AX foci was assessed as a measure of DNA double strand breaks (DSBs). Radiation induced the formation of γ-H2AX foci within 1 h post-radiation, peaked at 2 h and diminished to reach near-baseline levels by 24 h (Fig. 4). Pre-treatment with vorinostat resulted in a slight increase in the number of foci at 1 h post-radiation. However, the expression of γ-H2AX foci continued to remain elevated thereafter; even at 24 h with a significantly high number of nuclear foci (p < 0.0001) in the vorinostat pre-treated group compared to the radiation alone group (Fig. 4b). It is to be noted here that although vorinostat prolonged the radiation-induced expression γ-H2AX foci, it did not affect the baseline levels of γ-H2AX foci by itself (Fig. 4). We further assessed the effect of vorinostat on DNA repair proteins of two major pathways involved in radiation-induced DNA DSB repair - the non-homologous end-joining (NHEJ) pathway and homologous recombination (HR) pathway- DNA PKcs, Ku70 and Ku86 (NHEJ) and Rad50 (HR) were chosen as representative markers. Vorinostat did not affect the expression of Ku70 and Ku86 (Fig. 5), however, treatment with vorinostat resulted in decreased expression of Rad50 and DNA-PKcs. Together, these results demonstrated that vorinostat-mediated radiosensitization is at least partly mediated via prolongation of post-radiation DNA repair.
Figure 4. Vorinostat prolongs DNA repair.
Vorinostat prolongs radiation-induced γ-H2AX foci. MiaPaCa-2 cells were treated with 2 μM vorinostat (48 h), irradiated (6 Gy), and fixed at indicated time intervals for immunofluorescence staining of nuclear γ-H2AX foci. (a) Representative images for each group; (b) Quantification of foci – columns = mean ±SE of nuclear foci counted in 50 cells.
Figure 5. Effect of vorinostat on expression of DNA repair proteins.
MiaPaCa-2 cells were exposed to indicated concentrations of vorinostat for 48 h, irradiated (6 Gy) and harvested at indicated times post-irradiation. Immunoblot analysis of DNA repair proteins was performed using the nuclear fraction (Lamin B) as loading control.
Vorinostat inhibited constitutive and inducible EGFR and NF-κB signaling
Radiation is known to activate pro-survival signaling pathways in many tumor cell types, a hallmark of tumor cell radioresistance and a major cause of treatment failure. We investigated the effects of vorinostat on these pro-survival pathways. EGFR and the NF-κB were particularly chosen targets for their known relevance to PaCa aggressiveness and treatment resistance.12 To examine whether vorinostat inhibits EGFR signaling, MiaPaCa-2 cells were pre-treated with vorinostat or DMSO (control) for 48 h and irradiated (6 Gy). Cells were harvested at indicated time points post-irradiation and the phosphorylation of EGFR (pEGFR) was examined by immunoblotting. The baseline pEGFR levels were high in MiaPaCa (Fig. 6a), which could only be slightly elevated by radiation. However, pre-treatment with vorinostat significantly inhibited this EGFR phosphorylation (Fig 6a).
Figure 6. Vorinostat inhibits radiation-induced pro-survival signaling pathways.
(a) Vorinostat inhibits radiation-induced EGFR phosphorylation. MiaPaCa-2 cells were exposed to vorinostat (2 μM; 48 hr), irradiated (6 Gy), and harvested at indicated time points post-irradiation for immunoblot analysis. (b) Vorinostat decreases radiation-induced expression of phospho (active) c-jun. Cells were treated with vorinostat (2 μM; 48 h), irradiated (6 Gy), and harvested at indicated time points post-irradiation for immunobloting. (c) Effect of vorinostat on NF-κB activation. Cells were treated with indicated concentrations of vorinostat for 48 h, irradiated (6 Gy), and harvested 3 h post-irradiation. NF-κB activity in the nuclear fraction was analyzed by EMSA.
The proliferative cascades downstream of EGFR can lead to activation of transcription factor AP-1 (activator protein-1) via MAP kinases.13 Protein c-jun plays a critical role in this loop by positively modulating AP-1 activity and thereby cell proliferation. Recently, c-jun and AP-1 have been shown to be critical mediators of PaCa cell proliferation.14 Hence, we evaluated the effects of vorinostat on expression of active (phoshorylated) c-jun. As evident in Fig. 6b, vorinostat effectively abrogated radiation-induced phospho c-jun expression. These results demonstrated that vorinostat inhibits PaCa cell proliferation by inhibiting both EGFR and c-jun activation.
To determine the effect of vorinostat on radiation-induced NF-κB activation, DMSO (control) or vorinostat-treated (48 h) MiaPaCa-2 cells were exposed to radiation (6 Gy) and harvested 3 h post-irradiation. NF-κB activation in the nuclear fraction was evaluated by EMSA. Radiation induced NF-κB activation in the untreated cells (Fig. 6c). However, in vorinostat treated cells, NF-κB activation was inhibited either partly (1 μM), or almost completely (2 μM) (Fig. 6c). Of note, 2 μM vorinostat also inhibited constitutive NF-κB activation, albeit to a lesser extent. Together, these results suggested that radiosensitizing effects of vorinostat are partly mediated through abrogation of constitutive and radiation-induced EGFR and NF-κB signaling.
DISCUSSION
The inherent resistance of PaCa to conventional chemoradiation therapy4 is mediated largely by prevalence of multiple oncogenic signaling pathways12, many of these being triggered by radiation itself.15 In contrast to targeting single pathways with specific agents, we investigated the combination of radiation therapy with an agent that concurrently suppresses multiple oncogenic signaling pathways to achieve tumor radiosensitization. Our studies demonstrate that the HDACi vorinostat enhanced the efficacy of radiation therapy in PaCa via (a) inhibition of radiation-induced DNA strand break repair and (b) inhibition of constitutive and radiation-induced pro-survival signaling pathways.
A dose of vorinostat below the IC50 could significantly enhance the intrinsic radiosensitivity of all K-ras mutant PaCa cell lines, independent of their p53, Smad4 or EGFR status. The choice of the pre-treatment schedule of 48 h was predicated on the desire to achieve maximum histone acetylation status at the time of radiation.16
To understand the mechanism of radiosensitization, we evaluated a range of parameters determining classical radiosensitivity. First, we evaluated the role of direct cytotoxicity of vorinostat. Interestingly, vorinostat-mediated direct cytotoxicity was not synergistic, with radiation-induced apoptosis, which could not adequately explain the observed radiosensitization. Therefore, we explored whether vorinostat influenced post-irradiation DNA repair, a known mechanism of radioresistance.
Mammalian cells have multiple pathways for repair of radiation-induced DNA damage,17 and thus inhibition of post-radiation DNA repair is an attractive target for tumor radiosensitization.18 Radiation-induced DNA DSBs can be indirectly measured by quantification of the foci formed by phosphorylated histone H2AX (γ-H2AX) at DSB sites. The role of γ-H2AX foci in the repair of DNA DSBs and cellular radiosensitivity has been well established.19 After documentation a delay in post-irradiation resolution of γ-H2AX foci,19 we quantified the nuclear γ-H2AX foci at 1, 2, 6 and 24 h post-irradiation. Pre-treatment with vorinostat increased the radiation-induced expression of γ-H2AX foci at all these time points when compared to radiation alone suggesting that delayed repair of DNA DSBs contributes to the observed radiosensitization. Several HDACis, including vorinostat, have been reported to radiosensitize different tumor cell types at least in part by inhibiting radiation-induced DNA repair20, 21 and our findings are consistent with these observations. A plausible explanation for prolongation of DNA repair is altered expression of proteins involved in this process. Proteins participating in NHEJ and HR pathways of DNA repair play key roles in governing radiosensitivity.17 Amongst numerous other players, the Ku proteins and DNA-PKcs orchestrate NHEJ whereas MRE11, Rad50 and NBS1 (MRN complex) chiefly participate in HR.17 Vorinostat pre-treatment effectively suppressed both DNA-PKcs and Rad50, indicating that it targets both NHEJ and HR. Notably, vorinostat decreased the baseline levels of these proteins and also effectively inhibited their radiation-induced activation. However, vorinostat did not alter the levels of Ku proteins. The specificity of HDACi for either NHEJ or HR pathway appears to be both tumor cell type- and HDACi-specific as they have been shown to down-regulate the expression of proteins of either NHEJ22 or HR23 or both21, 24 pathways. Our observation of vorinostat inhibiting nuclear Rad50 expression in PaCa is noteworthy because Rad50 is the component of the multiprotein MRN complex, which is an essential component in many aspects of DNA end metabolism including, but not limited to, HR.25
Finally, we investigated the role of specific radiation-inducible pro-survival pathways vorinostat-mediated radiosensitization. A radiation-inducible pathway is specifically invoked in irradiated cells and serves as a mechanism to overcome the radiation-induced genotoxic insult. In this regard, signaling through EGFR15 and the transcription factor NF-κB26 are of particular importance.
The transmembrane glycoprotein receptor EGFR regulates signaling cascades governing cell proliferation, differentiation, and survival.27 Mutated or overexpressed EGFR is a hallmark of many solid tumors, including PaCa12 and a crucial determinant of tumor cells' resistance to chemotherapy and radiation therapy.27 Furthermore, radiation-induced activation of EGFR signaling mediates radioresistance by conferring cells with a survival advantage.15, 28 Owing to its dual role in governing radioresistance and its significance in PaCa biology, we investigated the effect of vorinostat on EGFR signaling. We examined the ability of vorinostat to inhibit the EGFR phosphorylation, the first and critical step in EGFR signaling.28 Vorinostat pre-treatment inhibited constitutive and radiation-induced EGFR phosphorylation (Tyr1173).
Receptor autophosphorylation is associated with enhanced cell proliferation via MAP kinase cascades27, which in turn lead to activation of AP-1, a critical regulator of cell proliferation.13 Of the many proteins constituting the AP-1 complex, c-jun is of particular importance as recently it has been shown to positively modulate EGFR expression 29 and also to be critical for PaCa cell proliferation,14 hence we evaluated the effect of vorinostat on expression of active (phosphorylated) c-jun. Vorinostat decreased the radiation-induced expression of phospho-c-jun. Collectively, these results indicate that vorinostat abrogates radiation-induced cell proliferation by down-regulating EGFR and c-jun.
Transcription factor NF-κB plays a pivotal role in pleiotropic biological phenomena encompassing inflammation, tumorigenesis, cell proliferation and apoptosis and is constitutively active in PaCa.30 NF-κB is of considerable interest to radiobiologists as it can be activated by ionizing radiation,26 and inhibition of NF-κB activity leads to tumor radiosensitization.31, 32 Preliminary studies in our laboratory have shown radiation (6 Gy) to activate NF-κB in PaCa cells, which peaks at around 1-3 h post-irradiation and returns to baseline by 24 h (data not shown). This transient activation of NF-κB at 3 h post-irradiation (6 Gy), was considerably inhibited by vorinostat. Interestingly, vorinostat inhibited both constitutive and radiation-induced NF-κB activity- a feature that could increase its relevance in treating NF-κB-overexpressing PaCa12 that remain at a high risk for development of distant metastatic disease after radiation therapy and might benefit from maintenance therapy with vorinostat after combination with radiation therapy.
Vorinostat has been shown to inhibit growth of PaCa cells33 and sensitize PaCa cells to the proapoptotic effects of gemcitabine,34 however, these reports largely elucidate the potential of vorinostat to induce cell-cycle arrest and apoptosis. Our observations of augmentation of radioresponse in PaCa by vorinostat are noteworthy because not only does this indicate the potential of vorinostat to be combined with radiotherapy in the treatment of PaCa, but also for the first time vorinostat has been reported to target constitutive as well as inducible pro-survival EGFR and NF-κB pathways in PaCa as a means of overcoming treatment resistance. Taken together, these reports underscore the potential therapeutic benefits of combining vorinostat with radiation therapy for the treatment of PaCa. We are initiating a small phase 1 trial of vorinostat and radiation in patients with LAPC in anticipation of a larger phase 2 trial to assess the efficacy of this combination more comprehensively.
In summary, we have shown that the HDACi vorinostat profoundly enhances the radiosensitivity of pancreatic cancer cells via inhibition of DNA repair and abrogation EGFR and NF-κB signaling. These findings demonstrate for the first time that vorinostat targets principal pathways contributing to the inherent radioresistance of PaCa. Although the characterization of the molecular mechanism(s) underlying these effects needs further research, our findings certainly provide rationale for exploring the combination of vorinostat with radiation therapy in patients with LAPC.
Acknowledgments
Grant support: This work was supported, in part, by NIH CA16672 (CCSG to UT MDACC) grant for the use of core facilities.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Maheshwari V, Moser AJ. Current management of locally advanced pancreatic cancer. Nat Clin Pract Gastroenterol Hepatol. 2005;2(8):356–364. doi: 10.1038/ncpgasthep0240. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Schneider G, Siveke JT, Eckel F, et al. Pancreatic cancer: basic and clinical aspects. Gastroenterology. 2005;128(6):1606–1625. doi: 10.1053/j.gastro.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan S, Rana V, Janjan NA, et al. Prognostic factors in patients with unresectable locally advanced pancreatic adenocarcinoma treated with chemoradiation. Cancer. 2006;107(11):2589–2596. doi: 10.1002/cncr.22328. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, van de Velde H, Karasek P, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22(8):1430–1438. doi: 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 6.Von Hoff DD. What's new in pancreatic cancer treatment pipeline? Best Pract Res Clin Gastroenterol. 2006;20(2):315–326. doi: 10.1016/j.bpg.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5(9):769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 8.Rasheed WK, Johnstone RW, Prince HM. Histone deacetylase inhibitors in cancer therapy. Expert Opin Investig Drugs. 2007;16(5):659–678. doi: 10.1517/13543784.16.5.659. [DOI] [PubMed] [Google Scholar]
- 9.Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25(1):84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- 10.Ramalingam SS, Parise RA, Ramanathan RK, et al. Phase I and pharmacokinetic study of vorinostat, a histone deacetylase inhibitor, in combination with carboplatin and paclitaxel for advanced solid malignancies. Clin Cancer Res. 2007;13(12):3605–3610. doi: 10.1158/1078-0432.CCR-07-0162. [DOI] [PubMed] [Google Scholar]
- 11.Sandur SK, Deorukhkar A, Pandey MK, et al. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-κB activity. Int J Radiat Oncol Biol Phys. 2009;75(2):534–542. doi: 10.1016/j.ijrobp.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh JJ, Der CJ. Targeting signal transduction in pancreatic cancer treatment. Expert Opin Ther Targets. 2007;11(5):673–694. doi: 10.1517/14728222.11.5.673. [DOI] [PubMed] [Google Scholar]
- 13.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4(5):E131–136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 14.Shin S, Asano T, Yao Y, et al. Activator protein-1 has an essential role in pancreatic cancer cells and is regulated by a novel Akt-mediated mechanism. Mol Cancer Res. 2009;7(5):745–754. doi: 10.1158/1541-7786.MCR-08-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dent P, Yacoub A, Contessa J, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003;159(3):283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Cerna D, Camphausen K, Tofilon PJ. Histone deacetylation as a target for radiosensitization. Curr Top Dev Biol. 2006;73:173–204. doi: 10.1016/S0070-2153(05)73006-4. [DOI] [PubMed] [Google Scholar]
- 17.O'Driscoll M, Jeggo PA. The role of double-strand break repair - insights from human genetics. Nat Rev Genet. 2006;7(1):45–54. doi: 10.1038/nrg1746. [DOI] [PubMed] [Google Scholar]
- 18.Schaue D, McBride WH. Counteracting tumor radioresistance by targeting DNA repair. Mol Cancer Ther. 2005;4(10):1548–1550. doi: 10.1158/1535-7163.MCT-05-CO1. [DOI] [PubMed] [Google Scholar]
- 19.Taneja N, Davis M, Choy JS, et al. Histone H2AX phosphorylation as a predictor of radiosensitivity and target for radiotherapy. J Biol Chem. 2004;279(3):2273–2280. doi: 10.1074/jbc.M310030200. [DOI] [PubMed] [Google Scholar]
- 20.Munshi A, Kurland JF, Nishikawa T, et al. Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin Cancer Res. 2005;11(13):4912–4922. doi: 10.1158/1078-0432.CCR-04-2088. [DOI] [PubMed] [Google Scholar]
- 21.Munshi A, Tanaka T, Hobbs ML, et al. Vorinostat, a histone deacetylase inhibitor, enhances the response of human tumor cells to ionizing radiation through prolongation of gamma-H2AX foci. Mol Cancer Ther. 2006;5(8):1967–1974. doi: 10.1158/1535-7163.MCT-06-0022. [DOI] [PubMed] [Google Scholar]
- 22.Chen CS, Wang YC, Yang HC, et al. Histone deacetylase inhibitors sensitize prostate cancer cells to agents that produce DNA double-strand breaks by targeting Ku70 acetylation. Cancer Res. 2007;67(11):5318–5327. doi: 10.1158/0008-5472.CAN-06-3996. [DOI] [PubMed] [Google Scholar]
- 23.Adimoolam S, Sirisawad M, Chen J, et al. HDAC inhibitor PCI-24781 decreases RAD51 expression and inhibits homologous recombination. Proc Natl Acad Sci U S A. 2007;104(49):19482–19487. doi: 10.1073/pnas.0707828104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chinnaiyan P, Vallabhaneni G, Armstrong E, et al. Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys. 2005;62(1):223–229. doi: 10.1016/j.ijrobp.2004.12.088. [DOI] [PubMed] [Google Scholar]
- 25.Assenmacher N, Hopfner KP. MRE11/RAD50/NBS1: complex activities. Chromosoma. 2004;113(4):157–166. doi: 10.1007/s00412-004-0306-4. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed KM, Li JJ. NF-κB-mediated adaptive resistance to ionizing radiation. Free Radic Biol Med. 2008;44(1):1–13. doi: 10.1016/j.freeradbiomed.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nyati MK, Morgan MA, Feng FY, et al. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer. 2006;6(11):876–885. doi: 10.1038/nrc1953. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt-Ullrich RK, Mikkelsen RB, Dent P, et al. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15(10):1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- 29.Zenz R, Scheuch H, Martin P, et al. c-Jun regulates eyelid closure and skin tumor development through EGFR signaling. Dev Cell. 2003;4(6):879–889. doi: 10.1016/s1534-5807(03)00161-8. [DOI] [PubMed] [Google Scholar]
- 30.Fujioka S, Sclabas GM, Schmidt C, et al. Function of nuclear factor κB in pancreatic cancer metastasis. Clin Cancer Res. 2003;9(1):346–354. [PubMed] [Google Scholar]
- 31.Sun Y, Clair DK, Fang F, et al. The radiosensitization effect of parthenolide in prostate cancer cells is mediated by nuclear factor-κB inhibition and enhanced by the presence of PTEN. Mol Cancer Ther. 2007;6(9):2477–2486. doi: 10.1158/1535-7163.MCT-07-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunnumakkara AB, Diagaradjane P, Guha S, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-κB-regulated gene products. Clin Cancer Res. 2008;14(7):2128–2136. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 33.Kumagai T, Wakimoto N, Yin D, et al. Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (Vorinostat, SAHA) profoundly inhibits the growth of human pancreatic cancer cells. Int J Cancer. 2007;121(3):656–665. doi: 10.1002/ijc.22558. [DOI] [PubMed] [Google Scholar]
- 34.Arnold NB, Arkus N, Gunn J, et al. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces growth inhibition and enhances gemcitabine-induced cell death in pancreatic cancer. Clin Cancer Res. 2007;13(1):18–26. doi: 10.1158/1078-0432.CCR-06-0914. [DOI] [PubMed] [Google Scholar]