Abstract
Major pelvic ganglia (MPG) neurons innervate urogenital organs and components of the lower bowel. Immunoreactivity for vasoactive intestinal polypeptide (VIP) has previously been observed in the MPG and VIP knockout animals have impaired micturition reflexes suggesting a role for this neuropeptide in urogenital function. Here we investigate the presence and action of VIP and a related neuropeptide, pituitary adenylate cyclase activating polypeptide (PACAP), in the pelvic ganglia of male mice. An abundance of VIP-immunoreactive (IR) neurons and nerve fibers were observed in the ganglion, whereas, PACAP-immunoreactivity was not seen. Extracts from acutely isolated MPG contained transcripts for the VPAC1, VPAC2 and PAC1 receptors. Local application of VIP, PACAP or maxadilan to isolated pelvic ganglion neurons shortened the duration of the afterhyperpolarization (AHP) of action potentials elicited by brief intracellular depolarization. All three peptides also increased neuronal excitability within a subpopulation of the sampled neurons. Bath application of apamin, a peptide antagonist of SK-channels, shortened the duration of the AHP indicating that AHP duration in pelvic neurons is determined principally by SK-channel activity. The results suggest VIP has a role in the neural control of pelvic organ function and activation of VPAC and/or PAC1 receptors can modulate the activity of the autonomic neurons innervating pelvic organs.
Index Entries: autonomic neurons, neuropeptide, excitability, afterhyperpolarization, intracellular recording
Introduction
Neurons of the major pelvic ganglia (MPG) innervate urogenital organs and components of the lower bowel. The MPG are unique autonomic ganglia in that they contain both sympathetic and parasympathetic postganglionic neurons and receive input from the lumbar sympathetic and sacral parasympathetic nerves. Classically the ganglia have been perceived as relay stations for the central neurochemical drive to the peripheral organs. However, the presence of a diversity of neurotransmitter and neuromodulator substances indicates neurotransmission through the ganglion might be more complex than originally perceived (Keast, 2006). The purpose of this study was to determine the presence and actions of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) in the MPG of the male mouse.
VIP and PACAP belong to the glucagon/secretin superfamily of hormones and mediate their effects through high affinity G-protein coupled receptors (Harmar et al., 1998; Dickson and Finlayson, 2009; Vaudry et al., 2009). VIP effects are mediated by VPAC1 and VPAC2 receptors. PACAP can activate both the PAC1 and VPAC receptors. VIP and PACAP are often co-localized (Fahrenkrug and Hannibal, 2004) and while the MPG have been shown to express VIP (Wanigasekara et al., 2003), no prior studies have determined whether PACAP is also present within the ganglia. Thus, one goal of this study was to determine whether PACAP-immunoreactivity is also present in the MPG.
While immunoreactivity to VIP is evident in neurons and nerve fibers of the MPG, it is not known whether VPAC1, VPAC2 or PAC1 receptors are also expressed. Consequently, we determined the presence of VPAC and PAC1 transcripts by polymerase chain reaction. To establish the functional expression of these receptors, intracellular recordings were made from the pelvic neurons to measure responses produced by local ‘puffer’ application of VIP, PACAP and maxadilan, a PAC1 selective agonist, onto pelvic neurons.
Our results confirm that many MPG neurons exhibit VIP-IR, but indicate the absence of PACAP-IR in the ganglia. The electrophysiological recordings demonstrate that VPAC and PAC1 receptors are functionally expressed by pelvic neurons and can modulate the membrane properties of these cells. This is the first study to demonstrate the effect of these peptides on MPG neurons. The physiological implications of these findings are discussed.
Materials and Methods
Preparation
Experiments were performed in vitro on whole-mount preparations of the MPG from male C57BL6 mice (3 to 5 months old). Protocols for use of mice were approved by the University of Vermont IACUC and followed NIH guidelines.
Immunohistochemistry
MPG whole mount preparations were fixed in 2% paraformaldehyde containing 0.2% picric acid for 2 hours at 4°C. When mouse anti-PACAP primary antibodies were used, the petri dish containing the pinned MPG whole mount was placed on a wave platform shaker and the preparations rinsed for 4 hours in culture media consisting of DMEM-F12 (1:1) containing 10% horse serum, gentamicin (10 g/ml), amphotericin B (3.75 g/ml), penicillin (100 U/ml), and streptomycin (100 g/ml; Sigma, St. Louis, MO) prior to fixation. The fixed whole mounts were washed in phosphate-buffered saline, permeabilized with 0.5% Triton X-100, and incubated at 4°C overnight with combinations of the primary antiserum. The primary antisera were then removed and the whole mounts were incubated for 2 hours at room temperature with fluorescein isothiocyanate (FITC)-conjugated or indocarbocyanine (Cy3)-conjugated secondary antiserum (Jackson Immunoresearch Laboratories, West Grove, PA), washed again, and mounted with Citifluor (UKA Chemical Laboratory, Canterbury, England) on microscope slides. The whole-mount preparations were viewed with an Olympus AX70 fluorescence microscope equipped with HBO 100-W UV light source and filters for FITC and Cy3. Digital images were obtained with a CCD camera (MagnaFire SP; Optronics; Optical Analysis Corp., Nashua, NH) and imported into Adobe Photoshop CS3 (Adobe Corporation, Mountain View, CA) to assemble figures, which were minimally adjusted for contrast and brightness.
Antibodies
Primary antisera used in this study included a mouse monoclonal anti-PACAP 1:10, from Dr. Jan Fahrenkrug, Copenhagen, Denmark and a rabbit anti-VIP 1:2000, from Dr. John Walsh, UCLA School of Medicine, USA. All antisera used are well characterized and have been used in our prior studies (Calupca et al., 2000; Girard et al., 2007). In addition, no staining was observed when whole mounts were treated only with primary or secondary antiserum. No VIP immunolabeling was seen in MPG neurons from VIP knockout mice (Studeny et al., 2008).
Reverse transcription-polymerase chain reaction
MPG preparations were dissected under RNase-free conditions, and total RNA was extracted from individual preparations using Tri reagent (Sigma). The total RNA quantity for each whole-mount preparation was determined with a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). One microgram of RNA per sample was used to synthesize complementary DNA with the Omniscript reverse transcription (Qiagen, Valencia, CA) and a mix of oligo-dT and random hexamer primers. Amplified MPG DNA product from specific primers was ligated into pCR2.1 TOPO using TOPO TA cloning kit (Invitrogen, Carlsbad, CA) to generate plasmid standards. The nucleotide sequences of the inserts were verified by automated fluorescent dideoxy dye terminator sequencing (Vermont Cancer Center DNA Analysis Facility). Amplification of the mouse cDNA templates was performed using HotStart IT® SYBR® Green qPCR Master Mix (USB) with the following primers: PAC1 forward (AACGACCTGATGGGACTAAAC), reverse (CGGAAGCGGCACAAGATGACC); VPAC1 forward (AAGTGGCGGCGTTGGCAT), reverse (TCCGCTTGGAAGCTGGAGGA); VPAC2 forward (ATGACCAGTCACAGTACAAGA), reverse (TCACACTGTACCTCACTGTTCA).
Intracellular stimulation/recording techniques
For intracellular recordings, the MPG whole mount preparations were pinned in a 2.5 ml Sylgard-lined chamber and superfused continuously (2-3 ml/min) with a modified Krebs solution containing 10 mM HEPES buffer. All recordings were obtained with the temperature maintained between 30 and 32°C. Neurons were visualized with an inverted microscope equipped with Hoffman optics and impaled using high impedance borosilicate microelectrodes (2 M KCl-filled; 80-120 MΩ). Membrane potential and action potentials were recorded from the impaled neurons using an Axoclamp-2A amplifier coupled with a Digidata 1322A data acquisition system and pCLAMP 8 (Molecular Devises; Sunnyvale, CA). If required, hyperpolarizing current was injected through the recording electrode to electrotonically maintain the resting membrane potential between -55 and -65 mV. This ensured that action potential generation was tested at the same potential prior to and following peptide application.
VIP, PACAP(1-27) (hereafter referred to simply as PACAP) and maxadilan were applied by pressure pulse ejection (‘puffer’ application) from micropipettes placed within close proximity to the impaled cell (~ 50 μm). Micropipettes were forged from capillary tubing pulled to a fine tip (Flaming/Brown Micropipette Puller Model P-97; Sutter Instrument Co., CA) and fire polished (Narashige Micro Forge MF-830; Narashige, NY) to a tip diameter of ~5 μm. Brief pulses of pressure (3 psi, 500 ms), controlled and delivered by a Picospritzer (General Valve Corp.), were applied to the pipette for agonist delivery.
Neuronal excitability was characterized with long duration depolarizing current pulses (0.1-0.5 nA, 1 sec) given before and after peptide application. Increased excitability was classified as a three-fold increase in the total number of action potentials elicited throughout the range of depolarizations.
Only cells with stable resting membrane potentials, input resistances greater than 60 MΩ, and action potentials with positive overshoots are included in the results.
Peptide sources
VIP (guinea pig) and PACAP (human, ovine, rat) were purchased from American Peptide Company (Sunnyvale, CA). Recombinant maxadilan was provided by Dr. Ethan Lerner (Department of Dermatology, Massachusetts General Hospital, Boston, MA) (Lerner and Shoemaker, 1992). Apamin was from Sigma-Aldrich (St. Louis, MO).
Statistical analyses
Student’s t-test was used to evaluate differences among groups. Differences were considered statistically significant at P ≤ 0.05.
Results
MPG neurons exhibit immunoreactivity to VIP, but not PACAP
We confirmed that many MPG neurons exhibit immunoreactivity to VIP (Figure 1A) (Wanigasekara et al., 2003). In addition, numerous VIP-IR fibers were present within the ganglia in nerve trunks (Figure 1A, B) and blood vessels were covered with VIP-IR nerve fibers (Figure 1C). Although many VIP-IR neurons were present in freshly dissected and in 4-hour rinsed MPGs, there was no evidence of any PACAP-IR neurons in the 4-hour rinsed MPG (data not shown).
Figure 1. VIP-IR neurons and nerve fibers are present in the MPG whole mount.
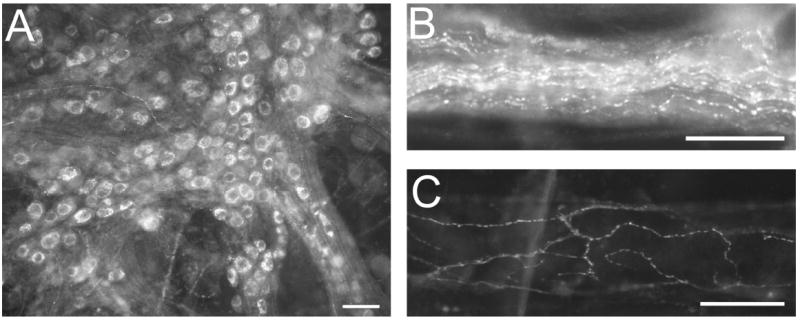
A: A low power micrograph showing the distribution of VIP-IR neurons. B: Many VIP-IR fibers are present in a nerve bundle. C: Blood vessels in the MPG whole mount are innervated by VIP-IR nerve fibers. Calibration equals 50 μm.
VPAC1, VPAC2 and PAC1 receptor transcripts are present in extracts of acutely isolated MPGs
Although many MPG neurons are VIP-IR, it is not known whether MPG neurons express receptors for VIP. Thus, we determined using PCR whether the MPG contains transcripts for any of the receptors that mediate VIP actions on neurons. VIP effectively activates VPAC1 and VPAC2 receptors, but is much less effective in activating the PACAP-selective PAC1 receptor (Harmar and Lutz, 1994). We found that transcripts for all three receptors were present in extracts of acutely isolated MPGs (Figure 2).
Figure 2. Presence of PAC1, VPAC1 and VPAC2 receptor transcripts in the mouse MPG.
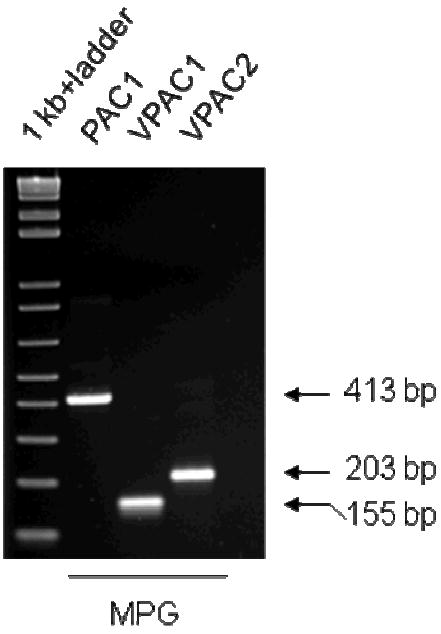
RT-PCR was used to assess the presence of PAC1, VPAC1 and VPAC2 receptor mRNA. The same MPG cDNA sample was used for each primer set. The presence of PAC1, VPAC1 and VPAC2 receptor transcripts is confirmed by the selective amplification of MPG cDNA using PAC1 and VPAC selective primers. The amplified products correspond to the expected base pair size.
VIP, PACAP and maxadilan can increase excitability and shorten the AHP in MPG neurons
The membrane responsiveness to VIP, PACAP and maxadilan was studied in separate preparations of the MPG using intracellular recoding technique and ‘puffer’ application of the peptide agonists (Methods). Resting membrane properties of the sampled cells were recorded prior to peptide application. For cells in which no holding current was used the resting membrane potential was -56 ± 1.5 mV (n = 12). The input resistance in tested cells was 131 ± 8 MΩ (n = 26). An AHP followed the action potential in all cells from which recordings were made. AHP durations were normally distributed (D’Agostino & Pearson omnibus normality test) but had a large degree of variability within the sampled population (mean = 298 ± 34 ms, n = 33). AHP amplitudes varied less and were grouped tightly around a mean of 12 ± 0.7 mV (n = 33).
Neurons of the MPG were classified into two groups, ‘phasic’ and ‘multiple firing’, based on the membrane response to multiple long duration (1 s) depolarizing current pulses of increasing amplitude (0.1 – 0.6 nA). Cells termed ‘phasic’ fired one to two action potentials and the frequency did not increase in response to increasing the stimulus intensity. ‘Multiple firing’ cells fired 3 or more spikes and typically increased action potential frequency with increasing stimulus intensity. Sixty percent of the MPG neurons sampled were phasic (21/35) and 40 percent were multiple firing (14/35).
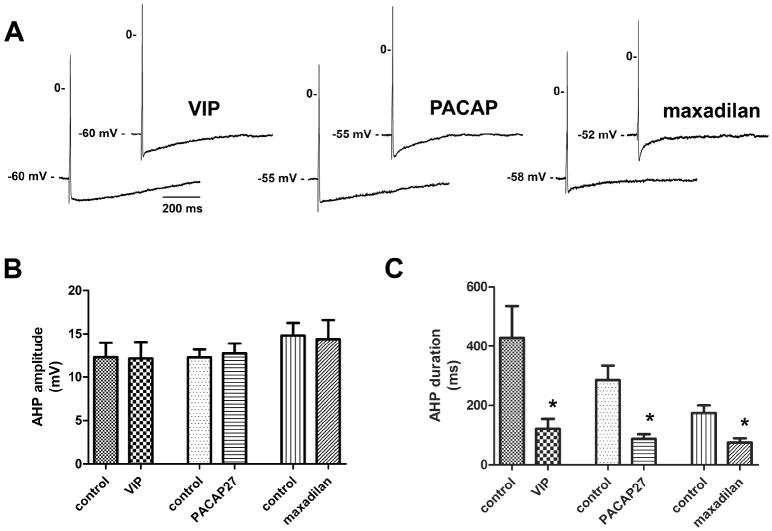
The amplitude and duration of the AHP, input resistance and excitability were all characterized before and after puffer application of the peptide agonists (VIP, PACAP, and maxadilan; all at 50 μM). Peak AHP amplitude was measured as the difference between the maximum hyperpolarized membrane potential value from the resting membrane potential. AHP duration was measured from the time the AHP crossed the resting membrane potential until it returned to 2/3rds of the peak AHP amplitude. All three agonists significantly reduced the duration of the AHPs while peak AHP amplitudes were unaffected (Fig. 3). Puffer application of the peptide vehicle had no effect on the AHP of MPG neurons (n=5).
Figure 3. Effect of VIP and related peptides on the AHP waveform.
A, action potentials elicited with brief (5 ms) depolarizing current pulses prior to and following peptide application. Each pair of tracings was obtained from a separate preparation. VIP, PACAP and maxadilan reduced the duration of the AHP. B, peak AHP amplitude was unaffected by the peptide agonists. C, AHP duration was reduced significantly (*, P ≤ 0.05) by all three agonists. Error bars indicate SEM. n = 7 for VIP, n = 11 for PACAP, and n = 9 for maxadilan).
Peptide application did not produce a consistent change of the membrane input resistance. VIP increased input resistance in 3 of 11 cells. In the remaining 8 cells a significant decrease was observed (129 ± 17 MΩ before vs 84 ± 10 MΩ after, P = 0.04). PACAP increased input resistance in 2 of 8 cells and the input resistance decreased insignificantly in the remaining 6 (137 ± 18 MΩ before vs 101 ± 24 MΩ after, P = 0.24). Maxadilan significantly decreased input resistance in 7 of 9 cells (154 ± 11 MΩ before vs. 106 ± 12 MΩ after, P = 0.01).
VIP increased excitability in 33% (4/12), PACAP in 44% (4/9) and maxadilan in 22% (2/9) of the tested cells (Fig. 4). An increased neuronal excitability was observed in both phasic and multiple firing cells.
Figure 4. VIP, PACAP and maxadilan increase MPG neuron excitability.
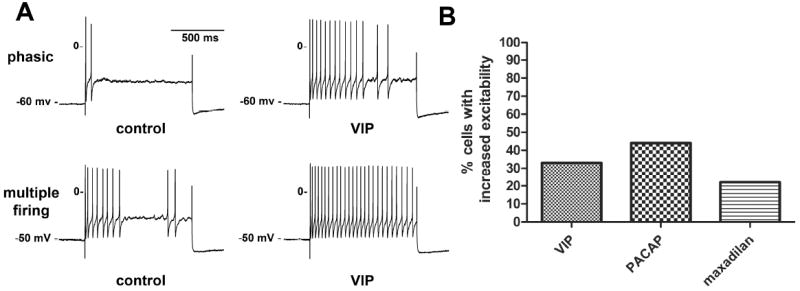
A, membrane potential recordings in response to long duration (1 s) depolarizing current pulses (500 pA) from cells exposed to VIP. The excitability of both the phasic (upper recordings) and the multiple firing cell (lower recordings) was increased following puffer application of VIP (similar responses were observed with PACAP and maxadilan). The enhanced excitability is evident by the increase in the frequency of action potentials after peptide application. B, shown is the percent of cells in which an increase in excitability was observed with application of each peptide (quantification of excitability explained in Methods).
Potassium currents initiate both the repolarizing phase of the action potential and the hyperpolarization following an action potential (AHP). Calcium activated small-conductance potassium channels (SK channels), are responsible for the slow phase of the AHP in many cell types (Pedarzani and Stocker, 2008). In the MPG, apamin (100 nM), a selective SK channel antagonist, reduced significantly the duration of the AHP (241 ± 57 mV before vs. 22 ± 3 mV after, P = 0.019), indicating that the duration of the AHP in MPG neurons is largely dependent on SK channel activity (Fig. 5). Apamin increased excitability in 75% (3/4) of the cells tested.
Figure 5. The SK channel inhibitor apamin significantly reduced the AHP amplitude of MPG neurons.
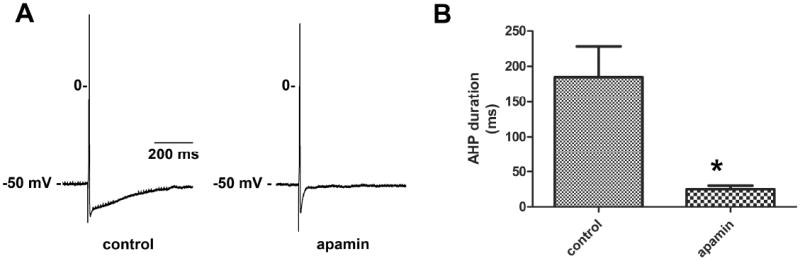
A, action potentials recorded in the same cell prior to and during apamin application. Apamin consistently shortened the AHP. B, the mean duration of the AHPs for all cells tested (n = 5) was significantly (*, P ≤ 0.05) reduced in the presence of apamin.
Discussion
In acutely isolated MPGs from male mice, many neurons are VIP-IR, but none show PACAP-immunoreactivity. We know the mouse anti-PACAP antibody is effective in this preparation because many PACAP-IR cells and fibers are present in the 72-hour explant cultured MPG (Girard et al., 2009).
The MPG neurons express both VPAC and PAC1 receptors. This was evident from the presence of transcripts for VPAC1, VPAC2 and PAC1 receptors in extracts from acutely isolated MPGs. In addition, application of VIP, PACAP and maxadilan produced similar effects on MPG neuron excitability and AHP. VIP and PACAP are equally effective at VPAC receptors. However, VIP is ~1000 fold less potent than PACAP at PAC1 receptors. This might suggest that only functional VPAC receptors are present on the membrane of neurons in acutely isolated MPGs. However, maxadilan, a PAC1-selective agonist, had comparable actions to VIP and PACAP. This latter result suggests that functional PAC1 receptors also are expressed by MPG neurons. Whether VPAC1 or VPAC2 receptors are preferentially expressed by MPG neurons cannot be determined from results obtained in the present study.
VIP, PACAP and maxadilan consistently shortened the duration of the AHP that follows an action potential. This change in duration occurred without a change in AHP amplitude. In many neurons the duration of the AHP is due to the activation of Ca2+-activated SK channels (Pedarzani and Stocker, 2008). This was true for the MPG neurons given that apamin, a potent blocker of SK channels, dramatically shortened the AHP.
During the action potential, voltage dependent calcium channels (VDCCs) are activated allowing Ca2+ influx and activation of Ca2+-sensitive SK channels to produce the sustained AHP (Pedarzani and Stocker, 2008). It is possible that the 3 agonists shortened the AHP by direct inhibition of SK channels. However, we consider it more likely that VIP, PACAP and maxadilan inhibited Ca2+ influx through VDCCs and reduced the number of SK channels activated. We propose this mechanism in light of prior whole cell voltage clamp studies in which VIP and PACAP have been shown to block Ca2+-currents (Zhu and Ikeda, 1994; Tompkins et al., 2006). Both VPAC and PAC1 receptors are G-protein coupled receptors, which can activate signaling cascades that block Ca2+ channel activation (Kamaishi et al., 2004).
MPG neurons from male mice showed a varied response to long depolarizing current steps. Some neurons exhibited a typical phasic firing pattern whereas others produced multiple firing that often increased with the extent of the depolarization. Neuronal excitability was enhanced in a subset of the MPG neurons; responsive neurons could be either phasic or multiply firing prior to agonist application. All three peptide agonists, PACAP, VIP and maxadilan, increased excitability in similar proportions of tested cells. Increases in excitability were also observed with apamin indicating that shortening of the AHP alone can increase action potential firing. It is unclear whether the increases in excitability observed with PACAP, VIP and maxadilan were due to shortening of the AHP alone or activation of intracellular signaling cascades that modulate neuronal excitability (Tompkins and Parsons, 2008).
Mice lacking VIP exhibit atypical micturition reflexes. This includes longer intercontraction intervals and increased void volumes (Studeny et al., 2008). While the MPG are only one component of the neural circuitry controlling bladder function, here we demonstrate potent effects of this peptide on neuronal activity within the ganglia. The membrane effects of VIP are consistent with increasing neuronal spike discharge. One can therefore speculate that absence of VIP would have reduced neuronal excitability at the ganglion which is consistent with the cystometry data above. We have not determined whether VIP is released constitutively within the ganglion. Additional work is necessary to determine the role of VIP in the MPG during micturition. It is clear however, that agonists targeting VIP receptors in the ganglion could be used to modulate autonomic control of pelvic organ function.
In summary, neurons and nerve fibers in the MPG of the male mouse exhibit VIP-immunoreactivity and have functional VPAC and PAC1 receptors.
Acknowledgments
The study was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants K01-DK081444] (to J.D.T.) [Grants R01-DK060481, R01-DK051369] (to M.A.V.), and the National Institutes of Health National Center for Research Resources [Grant P20-RR16435] (to R.L.P.). We thank Dr. Jan Fahrenkrug for kindly providing the PACAP antiserum and Dr. Ethan Lerner for the generous gift of maxadilan. Mr. Jonathan Galli’s assistance with the immunohistochemistry and creation of Figure 1 was greatly appreciated.
References
- Calupca MA, Vizzard MA, Parsons RL. Origin of pituitary adenylate cyclase-activating polypeptide (PACAP)-immunoreactive fibers innervating guinea pig parasympathetic cardiac ganglia. J Comp Neurol. 2000;423:26–39. [PubMed] [Google Scholar]
- Dickson L, Finlayson K. VPAC and PAC receptors: From ligands to function. Pharmacol Ther. 2009;121:294–316. doi: 10.1016/j.pharmthera.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Hannibal J. Neurotransmitters co-existing with VIP or PACAP. Peptides. 2004;25:393–401. doi: 10.1016/j.peptides.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Girard BM, Young BA, Buttolph TR, White SL, Parsons RL. Regulation of neuronal pituitary adenylate cyclase-activating polypeptide expression during culture of guinea-pig cardiac ganglia. Neuroscience. 2007;146:584–593. doi: 10.1016/j.neuroscience.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Galli JR, Young BA, Vizzard MA, Parsons RL. PACAP expression in explant cultured mouse major pelvic ganglia. J Mol Neurosci. 2010 doi: 10.1007/s12031-010-9359-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- Harmar T, Lutz E. Multiple receptors for PACAP and VIP. Trends Pharmacol Sci. 1994;15:97–99. doi: 10.1016/0165-6147(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Kamaishi H, Endoh T, Suzuki T. Multiple signal pathways coupling VIP and PACAP receptors to calcium channels in hamster submandibular ganglion neurons. Auton Neurosci. 2004;111:15–26. doi: 10.1016/j.autneu.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Keast JR. Plasticity of pelvic autonomic ganglia and urogenital innervation. Int Rev Cytol. 2006;248:141–208. doi: 10.1016/S0074-7696(06)48003-7. [DOI] [PubMed] [Google Scholar]
- Lerner EA, Shoemaker CB. Maxadilan. Cloning and functional expression of the gene encoding this potent vasodilator peptide. J Biol Chem. 1992;267:1062–1066. [PubMed] [Google Scholar]
- Pedarzani P, Stocker M. Molecular and cellular basis of small--and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell Mol Life Sci. 2008;65:3196–3217. doi: 10.1007/s00018-008-8216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studeny S, Cheppudira BP, Meyers S, et al. Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)-/- mice. J Mol Neurosci. 2008;36:175–187. doi: 10.1007/s12031-008-9100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins JD, Parsons RL. Identification of intracellular signaling cascades mediating the PACAP-induced increase in guinea pig cardiac neuron excitability. J Mol Neurosci. 2008;36:292–298. doi: 10.1007/s12031-008-9086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins JD, Hardwick JC, Locknar SA, Merriam LA, Parsons RL. Ca2+ influx, but not Ca2+ release from internal stores, is required for the PACAP-induced increase in excitability in guinea pig intracardiac neurons. J Neurophysiol. 2006;95:2134–2142. doi: 10.1152/jn.01077.2005. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Wanigasekara Y, Kepper ME, Keast JR. Immunohistochemical characterisation of pelvic autonomic ganglia in male mice. Cell Tissue Res. 2003;311:175–185. doi: 10.1007/s00441-002-0673-1. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ikeda SR. VIP inhibits N-type Ca2+ channels of sympathetic neurons via a pertussis toxin-insensitive but cholera toxin-sensitive pathway. Neuron. 1994;13:657–669. doi: 10.1016/0896-6273(94)90033-7. [DOI] [PubMed] [Google Scholar]