Abstract
We recently reported involvement of oxidative stress in anxiety-like behavior of rats. Others in separate studies have demonstrated a link between oxidative stress and hypertension as well as with type 2 diabetes/insulin resistance. In the present study, we have tested a putative role of oxidative stress in anxiety-like behavior, hypertension and insulin resistance using a rat model of oxidative stress. Oxidative stress in rats was produced by xanthine (0.1%; drinking water) and xanthine oxidase (5U/kg; i.p.). X+XO-treated rats had increased plasma and urinary 8-isoprostane levels (a marker of oxidative stress) and increased malondialdehyde (MDA) levels in the hippocampus and amygdala as compared to control rats. Serum corticosterone (a systemic marker of stress and anxiety) levels also increased with X+XO treatment. Moreover, anxiety-like behavior measured via open-field and light-dark exploration behavior tests significantly increased in X+XO-treated rats. Mean arterial blood pressure measured in anesthetized rats increased in X+XO-treated compared to control rats. Furthermore, plasma insulin but not glucose levels together with homeostasis model assessment (HOMA), an index of insulin resistance, were higher in X+XO-treated rats. Our studies suggest that oxidative stress is a common factor that link anxiety-like behavior, hypertension and insulin resistance in rats.
Keywords: Oxidative stress, Anxiety, Antioxidants, Hypertension, Insulin resistance, Diabetes, Antioxidants
1. Introduction
An increased risk of psychiatric impairment is widely believed to be accompanied with a chronic illness (Simon et al., 1995). Substantial literature supports this clinically important association (Roy-Byrne et al., 2008). In particular, comorbidity of anxiety disorders with chronic medical conditions like hypertension and type-2 diabetes mellitus is a topic of both clinical and policy interest (Roy-Byrne et al., 2008; Thomas et al., 2003). Interestingly, presence of anxiety is reported to contribute to an additional time of hospitalization due to the complications resulting from anxiety (Ball et al., 2002). Given the implications of comorbidity between anxiety, hypertension and type-2 diabetes mellitus, it is imperative to investigate whether there is a shared relationship underlying these conditions. Our studies point towards a common factor, oxidative stress that more than likely is a contributing factor in the occurrence of comorbidity between anxiety disorders, hypertension and insulin resistance/type-2 diabetes mellitus.
In general, evidence for the involvement of oxidative stress in human disease is frequently cited (Salustri et al., 2010; Dean et al., 2009; Ko et al., 2010; Liu et al., 2008). Increased oxidative damage is likely to occur in most if not all human diseases, although it probably plays a significant pathologic role only in few. Recent studies including our own have shown direct involvement of oxidative stress with anxiety-like behavior in rodents (Salim et al., 2010; Hovatta et al., 2005; Gingrich 2005; Masood et al., 2008; Souza et al., 2007; Bouayed et al., 2007; de Oliveira et al., 2007). Recent work also has suggested correlation of oxidative stress with hypertension and type-2 diabetes mellitus (Ko et al., 2010; Liu et al., 2008). Occurrence of oxidative stress, anxiety-like behavior, hypertension and type-2 diabetes mellitus have never been examined in the same study. In this study, using Sprague Dawley rats, we induced oxidative stress by xanthine plus xanthine oxidase treatment for one week. This treatment increased oxidative stress markers, 8-isoprostane (in serum and urine) and malondialdehyde in blood plasma, hippocampus and amygdala brain regions of rats. Moreover, X+XO treatment increased anxiety-like behavior of rats examined via light-dark and open-field anxiety tests, when compared to the vehicle treated control rats. Furthermore, X+XO treatment increased blood pressure, insulin resistance and corticosterone levels. This suggests that induction of oxidative stress results in elevated anxiety, hypertension and insulin resistance. It is likely that oxidative stress is the common link that is responsible for increased incidence of comorbidity in these three chronic illnesses. Even though, there is a large volume of evidence suggesting anxiety as a risk factor for hypertension and other cardiovascular diseases, as well as type-2 diabetes mellitus, there remains paucity of information linking oxidative stress, hypertension, type-2 diabetes mellitus and anxiety, all in the same model.
In this study, we have employed a rat model in which we have induced oxidative stress using X+XO to assess direct involvement of oxidative stress in anxiety-like behavior, hypertension and insulin resistance.
2. Results
Oxidative stress was induced in rats by xanthine supplementation (0.1% in drinking water) and intraperitoneal injections at non-toxic dose of xanthine oxidase for one week. This treatment increased oxidative stress markers, 8-isoprostane (in serum and urine) and malondialdehyde in hippocampus and amygdala. The treatment also increased serum corticosterone levels, a generally considered marker of stress and anxiety (Kobayashi et al., 2009; Arranz et al., 2007). Moreover, X+XO treatment increased anxiety-like behavior of rats examined via light-dark and open-field anxiety tests, when compared to the control vehicle treated rats. Furthermore, X+XO treatment increased blood pressure of rats and insulin resistance as compared to vehicle-treated control rats.
2.1. Effect of X+XO treatment on general body parameters
There were no significant changes observed in body weight, body temperature, food, or water intake habits in all groups of rats. All rats irrespective of treatment consumed similar amounts of rodent chow and tap water daily (Table 1).
Table 1. X+XO treatment has no adverse effect on body weight, food or water intake and body temperature.
Fifty grams of rodent chow (diet pellets) and 50 ml of tap water per rat was provided to the rats every day. The amount of food and water consumed was measured by weighing the left-over diet pellets and drinking water 24 hours later. Data collected were averaged for each treatment group of rats on the last day of the experiment before behavior testing. Rectal temperature was manually recorded using animal rectal probe every day after 30 min of X+XO-treatment/vehicle injections. N=8-12 rats
| General Parameters | CONTROL | X+XO |
|---|---|---|
| Body Weight (g) | 225 ± 1.0 | 223 ± 2.1 |
| Food Intake (g) | 22.4 ± 4.0 | 21.6 ± 3.8 |
| Water Intake (ml) | 24 ± 2.1 | 23 ± 2.0 |
| Body Temperature (°C) | 37.84 ± 0.22 | 36.6 ± 0.32 |
2.2. Effect of X+XO treatment on indices of oxidative stress and corticosterone levels
Treatment with X+XO for 7 days caused significant increase in plasma (166%) and urinary (129%) 8-isoprostane as compared to vehicle-treated control rats (Fig. 2A,B). Also, treatment with X+XO for 7 days caused significant increase in markers of lipid peroxidation (MDA assay) in hippocampus (MDA: 153%) and amygdala (MDA: 101%) tissues (Fig. 2C). Serum corticosterone levels were examined for control (27.1ng/ml) and X+XO treated (66.6ng/ml) rats. X+XO treatment increased serum corticosterone levels by 143% as compared to vehicle treated control rats (Fig. 2D).
Fig. 2. X+XO treatment increases oxidative stress and serum corticosterone levels.
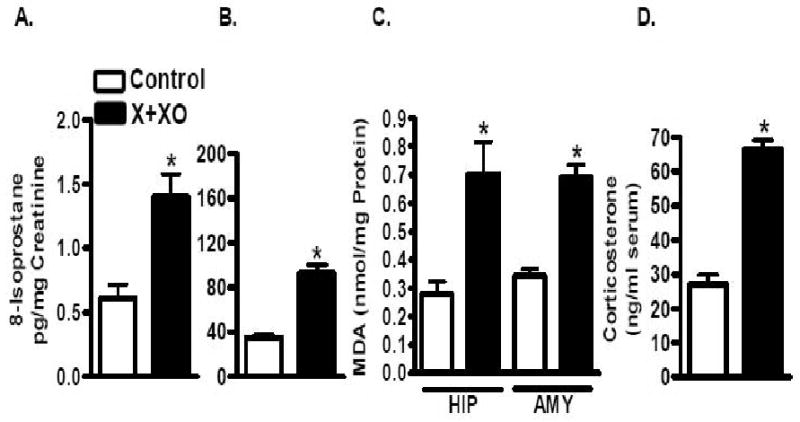
X+XO-treated rats had increased plasma and urinary 8-isoprostane levels (a marker of oxidative stress). X+XO treatment increased MDA levels in the hippocampus and amygdala as compared to control rats. Corticosterone levels measured by EIA (cat#500651, Cayman Chem. Co., Ann Arbor, MI) increased in X+XO treated rats as compared to control rats. *significantly different from control, p<0.05, ANOVA, N=8-12 rats.
2.3. Effect of X+XO treatment on anxiety-like behavior of rats
Light-dark exploration and open-field activity tests were conducted to test for anxiety-like behavior. Open-field test demonstrated that X+XO treatment reduced center time (28.7 s) and rearing (22) (Fig 3A,B) of treated rats as compared to center time (58.6 s) and rearing (37) exhibited by vehicle treated control rats. The light-dark exploration test results suggest that X+XO treated rats spent 75 seconds in the light compartment and control rats spent 127 seconds in the light compartment suggesting that X+XO treatment significantly decreased the time X+XO treated rats spent in lit area (Fig. 3C). After behavior assessments were made, rats were quickly sacrificed, blood (for serum preparation) and urine was collected and brains harvested to conduct oxidative stress marker measurements in serum, urine, hippocampus and amygdala regions of the brain.
Fig. 3. X+XO treatment increases anxiety-like behavior of rats as compared to controls.
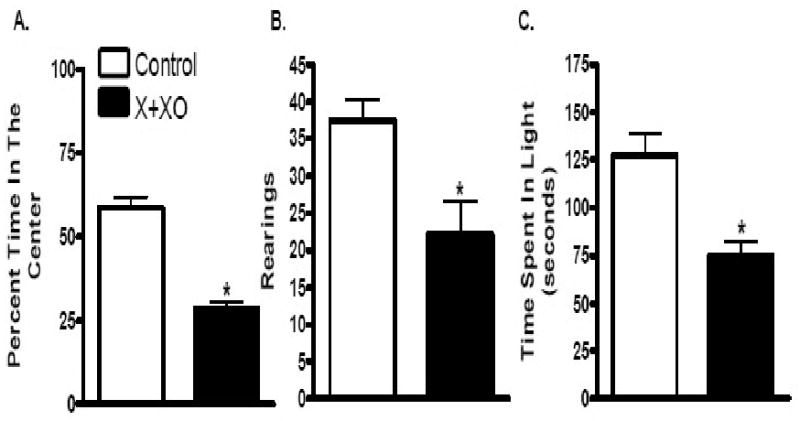
X+XO-treated rats displayed reduced center time (A), and rearing (B) in the open field-test and spent less time in lit areas than control (C). The total test time for light-dark test was 5 min and open-field test was 15 min. *significantly different from control treated rats (p<0.01), using ANOVA Tukey's post-hoc analysis, N=12 rats.
2.4. Effect of X+XO treatment on blood pressure of rats
The mean arterial blood pressure of X+XO rats showed a significant increase as compared to vehicle treated control rats, with X+XO rats exhibiting 124 mmHg blood pressure while control rats exhibiting 109 mmHg, with ∼14% increase in mean arterial blood pressure observed with induction of oxidative stress (Fig.4).
Fig. 4. X+XO treatment increases blood pressure of rats as compared to controls.
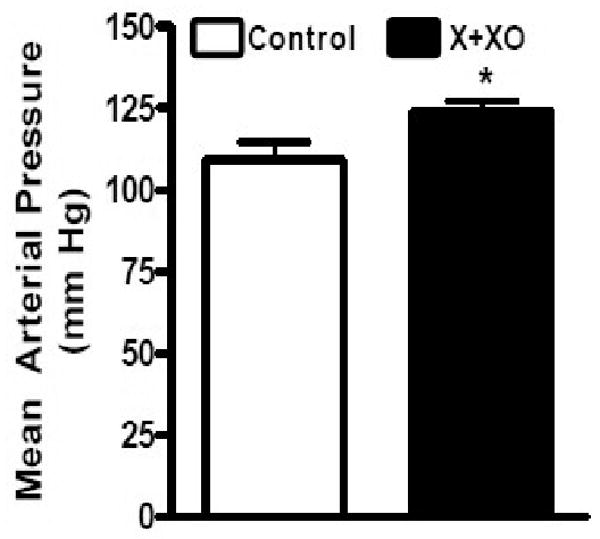
On the day of the experiment, the rats (fasted for 8-10 hrs) were anesthetized with Inactin (100 mg /kg; i.p.) followed by catheteriztion of carotid artery for blood pressure measurement. *significantly different from control, p<0.05, ANOVA, N=8-12 rats.
2.5. Effect of X+XO treatment on general body parameters, indices of oxidative stress and corticosterone levels of rats
There were no significant changes observed in body weight, body temperature, food or water intake habits in control or X+XO treated groups of rats. All rats irrespective of treatment consumed similar amounts of rodent chow and tap water daily (Table 2). Treatment with X+XO for 7 days caused significant increases in markers of lipid peroxidation (MDA assay) with 80% increase in blood plasma, 103% increase in serum and 118% urinary 8-isoprostane levels as compared to vehicle treated controls (Table 2). X+XO treatment increased serum corticosterone by ∼185%.
Table 2. X+XO treatment has no adverse effect on body weight, food or water intake and body temperature and X+XO treatment increases oxidative stress and corticosterone levels.
Fifty grams of rodent chow (diet pellets) and 50 ml of tap water per rat was provided to the rats every day. The amount of food and water consumed was measured by weighing the left-over diet pellets and drinking water 24 hours later. Data collected were averaged for each treatment group of rats on the last day of the experiment before behavior testing. Rectal temperature was manually recorded using animal rectal probe every day after 30 min of X+XO-treatment/vehicle injections. X+XO-treated rats had increased plasma and urinary 8-isoprostane levels (a marker of oxidative stress). X+XO treatment increased MDA levels in blood plasma as compared to control rats. *significantly different from control, p<0.05, ANOVA, N=8-12 rats.
| General Parameters | CONTROL | X+XO |
|---|---|---|
| Body Weight (g) | 212 ± 2.0 | 215 ± 1.1 |
| Food Intake (g) | 20.8 ± 2.2 | 20.2 ± 3.5 |
| Water Intake (ml) | 23 ± 2.2 | 24.2 ±2.0 |
| Body Temperature (°C) | 36.5 ± 0.31 | 37.2 ± 0.33 |
| Indices of oxidative Stress | ||
| 8-isoprostane. urine pg/mg creatinine | 0.711 ± 0.01 | 1.552 ± 0.02* p<0.05 |
| 8-isoprostane. serum fg/mg creatinine | 40.22 ± 0.01 | 82.00 ± 0.03* p<0.05 |
| MDA. serum nmol/mg protein | 0.830 ± 0.055 | 1.495 ± 0.053* p<0.05 |
| Index of Stress/Anxiety | ||
| Serum Corticosterone (ng/ml) | 33.58 ± 0.22 | 96.02 ± 0.21 *p<0.05 |
2.6. Effect of X+XO treatment on insulin resistance in rats
Plasma insulin but glucose levels increased in X+XO-treated than in control rats. Homeostasis model assessment (HOMA), an index of insulin resistance, was ∼3.5-fold high in X/XO-treated rats (Fig.5).
Fig. 5. X+XO treatment increases insulin resistance in rats.
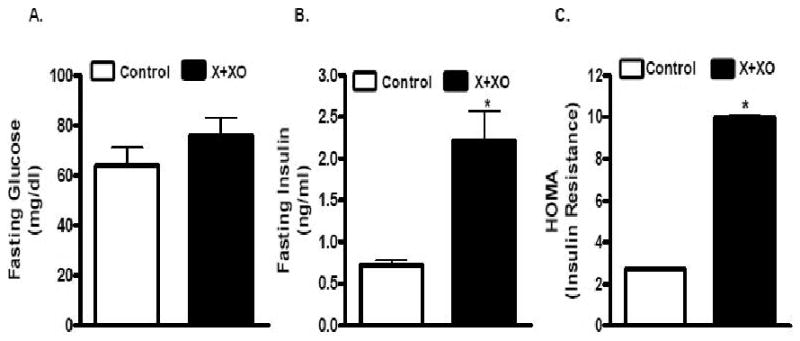
On the day of experiment, the rats (fasted for 8-10 hrs) were anesthetized with Inactin (100 mg /kg; i.p.) followed by catheteriztion of carotid artery for blood sample collection. Glucose levels were measured with automated glucose analyzer (AccuCheck). Insulin level was determined by using radioimmunoassay kit employing manufacturer's protocol (Linco Research, St. Charles, Missouri). HOMA (homeostasis model assessment), an index of insulin resistance was calculated by using the formula, [HOMA = glucose (mM) · insulin (μU ml−1)/22.5] as described by Pickavance et al. (1999).
3. Discussion
Earlier, we reported that sub-chronic oxidative stress induced by pro-oxidant drug L-buthionine-(S,R)-sulfoximine (BSO) increases anxiety-like behavior in rats (Salim et al., 2010). Separate studies have indicated that induction of oxidative stress with chronic BSO treatment increases blood pressure in rats (Banday and Lokhandwala 2008a, 2008b, 2008c, 2008d). Other studies have shown increased plasma insulin levels and HOMA in a rodent aging model associated with oxidative stress (Asghar et al., 2007). In our study, we have systematically examined effect of inducing oxidative stress on anxiety-like behavior, blood pressure and insulin resistance, all in the same study, using the same oxidative stress inducer, X+XO.
X+XO treatment for 7 days increased anxiety-like behavior of rats assessed via two independent anxiety tests, open-field and light-dark tests. Display of increased anxiety-like behavior indicative from reduced time spent by the rats in the center versus the periphery of the open field arena and avoidance of lit area was not a result of sickness behavior that might be argued to have resulted from X+XO treatment as the body weight, food and water intake and body temperature of all groups of rats were similar. Fever, reduction of body weight and loss of appetite are common sickness responses to infection and inflammation (Skinner et al., 2009; Bluthé et al., 2006). Avoidance of lit areas by the rats was also not due to any sickness or eye inflammation as the eye reflexes were checked daily by placing a cotton swab close to ∼1 inch of the eyes. The rats blinked in response to the cotton swab and quickly moved aside. All rats responded to sounds and startle indicating that they were alert and active throughout the X+XO treatment period.
Our observation of increase in anxiety-like behavior with X+XO treatment is in agreement with our earlier findings of association of increased anxiety-like behavior with another oxidative stress inducer, BSO. These data suggest that two separate agents that cause oxidative stress, BSO and X+XO, both result in anxiety-like behavior of rats further supporting the involvement of oxidative stress in anxiety-like behavior. Moreover, consistent with previously reported oxidative stress-induced increase in serum corticosterones (Kobayashi et al., 2009), a known systemic marker of anxiety (Arranz et al., 2007), X+XO treatment increased serum corticosterone levels. Furthermore, X+XO treatment increased oxidative stress markers in serum, urine, and also increased oxidative stress in amygdala and hippocampus, brain regions implicated in anxiety response (Charney and Drevets 2002). This also is in agreement with our earlier observations in which BSO treatment resulted in increased MDA levels in these brain regions (Salim et al 2010). Therefore, rats with high levels of oxidative stress, exhibit higher anxiety evaluated via corticosterone levels and anxiety-like behavior.
As a second part of this study, we conducted blood pressure measurements on anesthetized rats, measured indices of oxidative stress, evaluated levels of corticosterones and plasma glucose and insulin levels (markers of insulin resistance) in rats that were treated with X+XO and compared our results with those of control rats. Body weight, food and water intake and body temperature was not adversely affected with X+XO treatment, suggesting that our observed increase in blood pressure is not a result of sickness but most likely a result of increase in oxidative stress. X+XO treatment for 7 days increased blood pressure and insulin resistance measured as HOMA in the same animals. Earlier studies conducted in rats have shown an increase in blood pressure with induction of oxidative stress with BSO treatment, however, insulin resistance parameters were not determined in those animals (Banday and Lokhandwala 2008a, 2008b, 2008c, 2008d). While one week X+XO treatment increased oxidative stress, insulin resistance and anxiety-like behavior, this treatment did not robustly elevate blood pressure. Probably some compensatory mechanisms come into play which restrict robust increase in blood pressure (for up to 7 days). Increasing the treatment period beyond 7 days with X+XO may further elevate blood pressure as has been shown with chronic BSO treatment (Banday et al, 2008).
Several limitations of our study deserve comment. Although we determined blood pressure and insulin resistance in the same rats, however, anxiety measurements were carried out in separate rats. The purpose of this approach was to harvest brains by quickly decapitating rats that underwent anxiety-like behavior measurements. The same rat could not be used for blood pressure measurements as blood pressure recording was done for 30 min under anesthesia, which would have delayed brain collection. Moreover, the risk of the involvement of non-specific effects due to longer exposure to anesthetic agent on biochemical parameters analyzed in this study was avoided as this would have complicated our results confounding accurate data interpretation. However, future studies are needed for a better and more accurate analysis of contribution of oxidative stress to anxiety-like behavior, insulin resistance and hypertension where both blood pressure measurement by telemetry approach and anxiety-like behavior measurements are carried out in conscious freely moving animals. Nevertheless, our present data are suggestive of the fact that oxidative stress is a likely contributing factor towards increasing blood pressure, insulin resistance and anxiety-like behavior.
The question of whether oxidative stress precedes or follows anxiety, hypertension or insulin resistance is an interesting one. Recent studies have indicated that both hypertensive humans and animals have decreased antioxidant capacity and produce excessive amounts of reactive oxygen species and consequently have high oxidative stress (Banday and Lokhandwala 2008a, 2008b, 2008c, 2008d). The cause and effect relationship between oxidative stress and hypertension is not clear, although there is evidence of a causal role of oxidative stress in hypertension in animal models (Banday and Lokhandwala 2008a, 2008b, 2008c, 2008d). Furthermore, there are some studies which suggest that oxidative stress leads to insulin resistance, while others indicate a reverse relationship. For example, the most direct evidence for oxidative stress-induced insulin resistance comes from the studies using antioxidant α-lipoic acid, which was reported to improve insulin sensitivity in an animal model of diabetes (Packer et al., 2001). Also, in obese Zucker rats, a model of insulin resistance exhibiting increased oxidative stress, antioxidant (tempol) supplementation reduced oxidative stress and insulin resistance (Banday et al., 2005). Furthermore, several clinical trials demonstrated that vitamins E and C or glutathione (all antioxidants) improve insulin sensitivity in insulin-resistant individuals and/or patients with type 2 diabetes (Packer et al., 2009; Evans et al., 2003). Moreover, results of longitudinal studies suggest association of anxiety with an increased risk for the development of type 2 diabetes (Pouwer et al., 2010a, 2010b). Finally, direct involvement of oxidative stress in anxiety-like behavior in rodents also has been reported (Salim et al., 2010; Hovatta et al., 2005; Gingrich 2005; Masood et al., 2008; Souza et al., 2007; Bouayed et al., 2007; de Oliveira et al., 2007). These observations clearly indicate that there is a need to identify mechanisms that result in these consequences and determining a causal relationship between oxidative stress, anxiety, hypertension and insulin resistance is of paramount significance. For example, whether preventing anxiety-like behavior rescues high blood pressure or insulin resistance remains to be seen. Such information is important as it will help in the development of novel drugs that can reduce/prevent comorbidity of hypertension, diabetes and anxiety disorders.
4. Experimental Procedures
4.1. Animal model of oxidative stress
All experiments were conducted in accordance with the NIH guidelines using approved protocols from the University of Houston Animal Care Committee. Male Sprague-Dawley rats (200-250 g) between 7-8 weeks of age were acclimatized for one week before any treatment. Xanthine and xanthine oxidase, hereafter, referred as X+XO was administered to rats daily. Xanthine was supplied in drinking water ad libitum and xanthine oxidase (5 Units/kg) was injected intraperitonially once daily for 7 days. (Fig. 1). The control group received drinking water and saline injection as vehicle. After 24 h of last day of treatment, anxiety tests were conducted, following which rats were anesthetized with mild anesthesia and quickly decapitated as previously published by us (Salim et al. 2010). The abdomen was immediately opened and blood from aorta and urine from the bladder was collected. Brains were harvested at this time. Blood serum was collected after centrifugation at 3500 rpm at 4°C. Oxidative stress parameters were measured in serum, urine and brain tissue homogenates. In second set of experiment, the rats were similarly treated and anesthetized with Inactin (100 mg/kg) for blood pressure measurement.
Fig. 1. Schematic representation of the experimental plan.
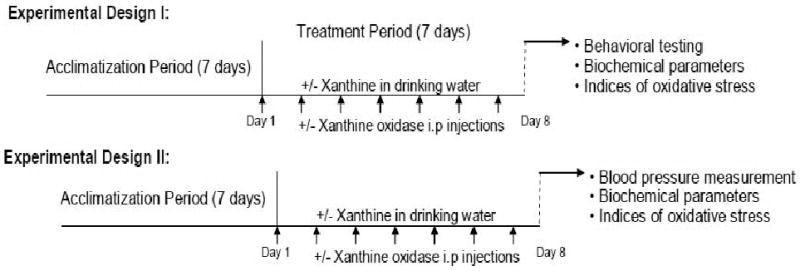
In experimental design I, the control group (n=8-12 rats) received vehicle injections (saline) and drinking water ad libitum for 7 days. Another group of rats (n=8-12 rats) received 0.2% xanthine supplementation in drinking water plus intraperitoneal injections at non-toxic dose of xanthine oxidase (5Units/kg) once daily for 7 days. After 24 h of the last X+XO treatment, anxiety tests were conducted, brains harvested and biochemical and oxidative parameters measured. In experimental design II, rats were randomly selected into two groups (control and treated) as before. After 24 h of the last X+XO treatment, rats were anesthetized with inaction and blood pressure measurements were made, biochemical and oxidative parameters measured were analyzed in serum and urine.
4.2. General body parameters
Body weight, food and water intake were recorded manually every day. Fifty grams of rodent chow (diet pellets) and 50 ml of tap water per rat was provided to the rats per day. The amount of food and water consumed was measured by weighing the left-over diet pellets and drinking water 24 hours later. Data collected were averaged for each group of rats on the last day of the experiment before behavior testing. Rectal temperature was determined in rats that had been gently restrained by wrapping in a towel using an animal rectal probe for adult rats (Physitemp-Clifton, NJ). The probe was held in place until the meter reading equilibrated which took less than 10 sec. An initial temperature was taken on all rats to allow them to acclimate to the probe. This initial temperature reading was not used in the data analysis. Temperature was manually recorded every day after 30 min of X+XO /vehicle treatment.
4.3. Anxiety Behavior Tests
Animals were housed in groups of three to five in plastic cages (14× 18×18 inches) and kept in a room on a 12 h light/dark schedule (lights on at 6:00 A.M.) at 23°C, 60% humidity, with food and tap water available ad libitum. On the day of behavior testing, the rats were brought to the behavior room at least an hour before testing time and left undisturbed in a quiet setting. After each behavior test, the apparatus was cleaned with 70% ethanol, wiped with hand towels and allowed to air dry in between animal testing. At first open-field test was conducted following a rest period of 1h, the same animals were tested in light-dark exploration test the same day.
4.3.1. Open Field activity
The open field test was conducted 24 h after the last X+XO injection. The open field task was carried out in 60×40 cm open field surrounded by 50 cm high walled Plexiglas chambers in standard room lighting conditions (Salim et al., 2010; Paylor et al., 1998). The animals were placed in the center of the compartment and were left free to explore the arena for 15 min. Activity was quantitated using a computer-operated Opto-Varimex Micro Activity Meter v2.00 system (Optomax, Columbus Instruments; OH) that utilizes sensors containing 8 infrared light emitting diodes and 8 phototransistors that emit and detect modulated infrared light beams. Sensors were positioned to form two-dimensional cages each with rearing monitoring. Movement was detected by beam breaks and data from three test chambers were recorded simultaneously, one rat per chamber, collected in 3 min intervals over a 15-min test session. The program tabulated activity counts, zone entries, zone times, center time and the periphery time, distance travelled and rearing for every cage in the system. For center time analysis, an approximately 25 cm × 25 cm square in the center of the open field arena was defined as the center zone for data analysis. Total time spent in the center of the arena and rearings were calculated for each group.
4.3.2. Light-Dark exploration
The rats were subjected to light-dark exploration test. Rodents are nocturnal and prefer darker areas, so the decrease in the exploratory activity in a lighted area is believed to be indicative of increased anxiety-like behavior (Salim et al., 2010) and the time spent in the light is considered as a measure of anxiety-like behavior (Salim et al., 2010; Crawley and Goodwin 1980). The light-dark box consisted of a light compartment (27×27×27 cm) and a dark compartment (black colored surrounding walls and floor, 27×18×27 cm) separated by a partition with a single opening (7×7 cm) for passage from one compartment to the other as described in our previous publication (Salim et al., 2010) and as published by others (Ramos et al., 2008). The apparatus was situated within a screen enclosed area of behavior core facility room with only one experimenter/observer present in the room at the time of experiment under standard lighting conditions of approximately 700 lux as previously used by others (Salim et al., 2010; Paylor et al., 1998; Gangitano et al., 2009). A Microsoft Excel software program enabled the observer to record time by manually scoring the data as described in detail in our earlier publication (Salim et al., 2010). Thus, total time spent in the illuminated part was recorded by an observer blinded to treatment for 5 minutes. A rat was defined to have entered the lit or dark box when both front paws and shoulders were inside the respective compartment.
4.4. Blood Pressure Measurement
Blood pressure measurement was done as previously described (Asghar et al., 2009). Briefly, separate groups of rats (other than the ones used for anxiety-like behavior measurement) were anesthetized with Inactin (100 mg/kg ip). Tracheotomy was performed to facilitate breathing. To measure blood pressure and to collect blood samples, the left carotid artery was catheterized with PE-50 tubing. This tubing was connected to a pressure transducer, which was connected to an amplifier (GRASS, LP122). Blood pressure was continuously recorded for 30 min using GRASS PolyView Data Acquisition and Analysis Software systems (Astro-Med, GRASS Instrument Division, West Warwick, RI). After blood pressure measurement, aliquots of blood samples were withdrawn and plasma was isolated by centrifugation and kept frozen until further use.
4.5. Brain Dissections
Experimental and control rats were anesthetized using mild anesthesia (Isoflurane, #57319-479-06, Phoenix Pharmaceuticals) immediately after anxiety behavior tests. The brains were quickly removed and rapidly frozen over dry ice and stored at -80°C until analysis. Hippocampus and amygdala identified according to the atlas of Paxinos and Watson (1986) were grossly dissected out using a brain slicer (Zivic instruments, PA). Homogenates of the brain regions were prepared in buffer containing 20 mM Tris-HCl, 4mM EDTA plus protease inhibitors, 100 μg/ml PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin and 1 μg/ml pepstatin (Salim and Dessauer 2004). The lysates were examined for protein concentration using Pierce's protein detection kit (Pierce, Rockford, IL) (Smith et al., 1985) and used for analysis of oxidative stress marker measurements.
4.6. Indices of oxidative stress
Serum and urine 8-isoprostane levels were measured using EIA kit (Cayman, Ann Arbor, MI). Malondialdehyde (MDA) was measured as published (Salim et al., 2010; Schacter et al., 1994; Urchiyama and Mihara 1978) in serum and in brain homogenates prepared from amygdala and hippocampus. Briefly, protein was diluted to 1-2 mg/ml with 1.15% KCl and boiled with 2 ml of 15% trichloroacetic acid, 0.375% thiobarbituric acid, 0.25 N HCl for 15 min. The sample was cooled, centrifuged at 1,000g for 10 min and the color was read at 535 nm on a spectrophotometer. MDA was quantified using molar extinction coefficient, 1.56 · 105 M–1 cm–1.
4.7. Plasma glucose and insulin measurement
Insulin level was determined by using radioimmunoassay kit employing manufacturer's protocol (Linco Research, St. Charles, Missouri). HOMA (homeostasis model assessment), an index of insulin resistance was calculated by using the formula, [HOMA = glucose (mM) · insulin (μU ml−1)/22.5] as described by Pickavance et al. (1999).
4.8. Data Analysis
Data are expressed as mean ± SEM. Significance was determined by one way ANOVA applying Tukey's post-hoc test (GraphPad Software, Inc. San Diego, CA). A value of p< 0.05 was considered significant.
Acknowledgments
Funding for this research was provided by University of Houston GEAR grant awarded to S.S and NIH/NIA AG29904 to M.A. We would like to acknowledge Prof. Bhagwan S. Jhandyala for his valuable intellectual input and advice.
List of Abbreviations
- HOMA
homeostasis model assessment
- X+XO
xanthine and xanthine oxidase
- MDA
malondialdehyde
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- OF
open-field
- LD
light-dark
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arranz L, Guayerbas N, De la Fuente M. Impairment of several immune functions in anxious women. J Psychosom Res. 2007;62(1):1–8. doi: 10.1016/j.jpsychores.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Asghar M, Chugh G, Lokhandwala MF. Inflammation compromises renal dopamine D1 receptor function in rats. Am J Physiol Renal Physiol. 2009 Sep 30;297(6):F1543–9. doi: 10.1152/ajprenal.00366.2009. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar M, Monjok E, Kouamou G, Ohia SE, Bagchi D, Lokhandwala MF. Super CitriMax (HCA-SX) attenuates increases in oxidative stress, inflammation, insulin resistance, and body weight in developing obese Zucker rats. Mol Cell Biochem. 2007 May 15;304(1-2):93–9. doi: 10.1007/s11010-007-9489-3. Epub. [DOI] [PubMed] [Google Scholar]
- Ball S, Goddard A, Shekhar A. Evaluating and treating anxiety disorders in medical settings. Postgrad Med. 2002;48(4):317–21. [PubMed] [Google Scholar]
- Banday AA, Muhammad AB, Fazili FR, Lokhandwala MF. Mechanisms of oxidative stress-induced increase in salt sensitivity and development of hypertension in Sprague-Dawley rats. Hypertension. 2007 Jan 2;49(3):664–71. doi: 10.1161/01.HYP.0000255233.56410.20. Epub. [DOI] [PubMed] [Google Scholar]
- Banday AA, Lau YS, Lokhandwala MF. Oxidative stress causes renal dopamine D1 receptor dysfunction and salt-sensitive hypertension in Sprague-Dawley rats. Hypertension. 2008a;51(2):367–75. doi: 10.1161/HYPERTENSIONAHA.107.102111. [DOI] [PubMed] [Google Scholar]
- Banday AA, Lokhandwala MF. Oxidative stress-induced renal angiotensin AT1 receptor upregulation causes increased stimulation of sodium transporters and hypertension. Am J Physiol Renal Physiol. 2008b Jul 9;295(3):F698–706. doi: 10.1152/ajprenal.90308.2008. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banday AA, Lokhandwala MF. Loss of biphasic effect on Na/K-ATPase activity by angiotensin II involves defective angiotensin type 1 receptor-nitric oxide signaling. Hypertension. 2008c Oct 27;52(6):1099–105. doi: 10.1161/HYPERTENSIONAHA.108.117911. Epub. [DOI] [PubMed] [Google Scholar]
- Banday AA, Marwaha A, Tallam LS, Lokhandwala MF. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes. 2005;54(7):2219–26. doi: 10.2337/diabetes.54.7.2219. [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Kelley KW, Dantzer R. Effects of insulin-like growth factor-I on cytokine-induced sickness behavior in mice. Brain, Behavior, and Immunity. 2006;20:57–63. doi: 10.1016/j.bbi.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouayed J, Rammal H, Younos C, Soulimani R. Positive correlation between peripheral blood granulocyte oxidative status and level of anxiety in mice. Eur J Pharmacol. 2007;564:146–149. doi: 10.1016/j.ejphar.2007.02.055. [DOI] [PubMed] [Google Scholar]
- Charney DS, Drevets WC. The neurobiological basis of anxiety disorders. 2002:901–30. See Davis et al. [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–70. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- de Oliveira MR, Silvestrin RB, Mello E, Souza T, Moreira JC. Oxidative stress in the hippocampus, anxiety-like behavior and decreased locomotory and exploratory activity of adult rats: effects of sub acute vitamin A supplementation at therapeutic doses. Neurotoxicology. 2007 Jul 27;28(6):1191–9. doi: 10.1016/j.neuro.2007.07.008. Epub. [DOI] [PubMed] [Google Scholar]
- Dean OM, van den Buuse M, Bush AI, Copolov DL, Ng F, Dodd S, Berk M. A role for glutathione in the pathophysiology of bipolar disorder and schizophrenia? Animal models and relevance to clinical practice. Curr Med Chem. 2009;16(23):2965–76. doi: 10.2174/092986709788803060. [DOI] [PubMed] [Google Scholar]
- Evans M, Anderson RA, Smith JC, Khan N, Graham JM, Thomas AW, Morris K, Deely D, Frenneaux MP, Davies JS, Rees A. Effects of insulin lispro and chronic vitamin C therapy on postprandial lipaemia, oxidative stress and endothelial function in patients with type 2 diabetes mellitus. Eur J Clin Invest. 2003;33(3):231–8. doi: 10.1046/j.1365-2362.2003.01120.x. [DOI] [PubMed] [Google Scholar]
- Gangitano D, Salas R, Teng Y, Perez E, De Biasi M. Progesterone modulation of alpha5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav. 2009 Feb 11;8(4):398–406. doi: 10.1111/j.1601-183X.2009.00476.x. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich JA. Oxidative stress is the new stress. Nat Med Dec. 2005;11(12):1281–2. doi: 10.1038/nm1205-1281. [DOI] [PubMed] [Google Scholar]
- Hovatta I, Tennant RS, Helton R, Marr RA, Singer O, Redwine JM, Ellison JA, Schadt EE, Verma IM, Lockhart DJ, Barlow C. Glyoxalase1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438:662–666. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- Ko SH, Cao W, Liu Z. Hypertension Management and Microvascular Insulin Resistance in Diabetes. Curr Hypertens Rep. 2010 Jun 29; doi: 10.1007/s11906-010-0114-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Machida T, Takahashi T, Takatsu H, Shinkai T, Abe K, Urano S. Elevation by oxidative stress and aging of hypothalamic-pituitary-adrenal activity in rats and its prevention by vitamin e. J Clin Biochem Nutr. 2009 Aug 28;45(2):207–13. doi: 10.3164/jcbn.09-33. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Yu LF, Lin CH, Lin SC. Effect of auricular pellet acupressure on antioxidative systems in high-risk diabetes mellitus. J Altern Complement Med. 2008;14(3):303–7. doi: 10.1089/acm.2006.6064. [DOI] [PubMed] [Google Scholar]
- Masood A, Nadeem A, Mustafa SJ, O'Donnell JM. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther. 2008;326(2):369–79. doi: 10.1124/jpet.108.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L, Kraemer K, Rimbach G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition. 2001;17(10):888–95. doi: 10.1016/s0899-9007(01)00658-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sixth. 1986. [DOI] [PubMed] [Google Scholar]
- Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7- deficient mice. Learn Mem. 1998;5:302–16. [PMC free article] [PubMed] [Google Scholar]
- Pikavance LC, Tadayyon M, Widdowson PS, Buckingham RE, Wilding JPH. Therapeutic index for rosiglitazone in dietary obese rats: separation of efficacy and haemodilution. Brit J Pharmacol. 1999;128:1570–1576. doi: 10.1038/sj.bjp.0702932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwer F, Kupper N, Adriaanse MC. Does emotional stress cause type 2 diabetes mellitus? A review from the European Depression in Diabetes (EDID) Research Consortium. Discov Med. 2010;9(45):112–8. [PubMed] [Google Scholar]
- Pouwer F, Geelhoed-Duijvestijn PH, Tack CJ, Bazelmans E, Beekman AJ, Heine RJ, Snoek FJ. Prevalence of comorbid depression is high in out-patients with Type 1 or Type 2 diabetes mellitus. Results from three out-patient clinics in the Netherlands. Diabet Med. 2010;27(2):217–24. doi: 10.1111/j.1464-5491.2009.02903.x. [DOI] [PubMed] [Google Scholar]
- Ramos A, Pereira E, Martins GC, Wehrmeister TD, Izidio GS. Integrating the open field, elevated plus maze and light/dark box to assess different types of emotional behaviors in one single trial. Behav Brain Res. 2008;193:277–88. doi: 10.1016/j.bbr.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne PP, Davidson KW, Kessler RC, Asmundson GJ, Goodwin RD, Kubzansky L, Lydiard RB, Massie MJ, Katon W, Laden SK, Stein MB. Anxiety disorders and comorbid medical illness. Gen Hosp Psychiatry. 2008;30(3):208–25. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh C. Moderate Treadmill exercise prevents oxidative stress-mediated anxiety-like behavior of rats. Behav Brain Res. 2010 Jan 8; doi: 10.1016/j.bbr.2009.12.039. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Salim S, Dessauer CW. Analysis of the interaction between RGS2 and adenylyl cyclase. Methods Enzymol. 2004;390:83–99. doi: 10.1016/S0076-6879(04)90006-7. [DOI] [PubMed] [Google Scholar]
- Salustri C, Squitti R, Zappasodi F, Ventriglia M, Bevacqua MG, Fontana M, Tecchio F. Oxidative stress and brain glutamate-mediated excitability in depressed patients. J Affect Disord. 2010 Jun 12; doi: 10.1016/j.jad.2010.05.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Schacter E, Williams JA, Lim M, Levine RL. Differential susceptibility of plasma proteins to oxidative modification. Examination by Western blot immunoassay. Free Rad Biol Med. 1994;17:429–437. doi: 10.1016/0891-5849(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Simon GE, VonKorff M, Barlow W. Health care costs of primary care patients with recognized depression. Arch Gen Psychiatry. 1995;52:850–856. doi: 10.1001/archpsyc.1995.03950220060012. [DOI] [PubMed] [Google Scholar]
- Skinner GW, Mitchell D, Harden LM. Avoidance of physical activity is a sensitive indicator of illness. Physiology & Behavior. 2009:421–427. doi: 10.1016/j.physbeh.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Souza CG, Moreira JD, Siqueira IR, Pereira AG, Rieger DK, Souza DO, Souza TM, Portela LV, Perry ML. Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life Sci. 2007 May 17;Jun 17;27(81)(3):198–203. doi: 10.1016/j.lfs.2007.05.001. Epub. [DOI] [PubMed] [Google Scholar]
- Thomas J, Jones G, Scarinci I, Brantley P. A descriptive and comparative study of the prevalence of depressive and anxiety disorders in low-income adults with type 2 diabetes and other chronic illnesses. Diabetes Care. 2003;26(8):2311–7. doi: 10.2337/diacare.26.8.2311. [DOI] [PubMed] [Google Scholar]
- Urchiyama M, Mihara M. Determination of malondialdehyde precursor in tissue by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]


