Abstract
Cell phenotype alteration by cell-derived vesicles presents a new aspect for consideration of cell fate. Accumulating data indicates that vesicles from many cells interact with or enter different target cells from other tissues, altering their phenotype toward that of the cell releasing the vesicles. Cells may be changed by direct interactions, transfer of cell surface receptors or epigenetic reprogramming via transcriptional regulators. Induced epigenetic changes appear to be stable and result in significant functional effects. These data force a reconsideration of the cellular context in which transcription regulates the proliferative and differentiative fate of tissues and suggests a highly plastic cellular system, which might underlay a relatively stable tissue system. The capacity of marrow to convert to non-hematopoietic cells related to vesicle cross-communication may underlie the phenomena of stem cell plasticity. Additionally, vesicles have promise in the clinical arenas of disease biomarkers, tissue restoration and control of neoplastic cell growth.
Keywords: Cell-derived vesicles, stem cell plasticity, epigenetic alteration, cellular injury, disease biomarker, tissue restoration
2. Introduction
Molecular control of cellular differentiation and phenotype is well established, as is the central role of transcription factors. However, the cellular context in which this regulation takes place is less well established and concepts of the stability of cellular phenotypes may have, in fact, confused the field. The capacity of microvesicle transfer to alter genetic cell phenotype alters our perceptions of phenotype regulation and will be explored in depth here.
3. Mechanisms of Cellular Conversion
The capacity of cells to change phenotype has been clearly demonstrated by studies of cell fusion and nuclear transfer. Formation of heterokaryons by cell fusion showed that dormant gene expression programs can be activated [1]. Cell fusion has also been invoked to explain the phenotype shifts seen in experiments where bone marrow stem cells were transplanted into lethally irradiated mice, so-called “stem cell plasticity” [2]. Nuclear transfer was also established as another mode of dramatic alteration of cell phenotype, which in the extreme, could lead to the formation of a whole animal [3–5]. Organ regeneration in newts and salamanders has also been cited as examples of cellular fate change, although in these instances, production of differentiated cells from rare stem cell populations was never ruled out [6,7]. Studies have shown that induced expression of specific transcription factors impacts function in different cell lineages as forced expression of MyoD results in myotube formation in a fibroblast cell line [8]. When GATA-1 was ectopically expressed in monocyte cell lines at high levels, erythroid and megakaryocytic lineage markers were expressed and expression of PU.1 in an erythroid-megakaryocyte cell line induced a monocytic phenotype [9]. Further work has shown that cross-antagonism between transcription factors induces differentiation in specific cell lineages [9,10]. The temporal sequence of transcription factor induction also has a clear effect on cellular differentiation [11]. In addition, it is clear that exogenous cytokines can be involved in determining the final transcriptional outcomes [12]. A lineage instructive function of cytokines has recently been indicated by work with time-lapse microscopy on bipotent progenitors indicating that myeloid cytokines are capable of influencing lineage choice [13]. Induced pluripotent stem cells (IPS) represent an induced conversion of somatic cells into cells with profound proliferative and differentiative capacity similar in many respects to embryonic stem cells. The combination of Sox2, Oct4, Klf4 and Myc induces a transition from fibroblasts into IPS-type cells, indicating a very impressive example of transcriptional transformation of differentiated cells into stem-like cells. These observations have been extended to differentiated cells, such as hepatic or islet β-cells. The reprogramming here occurs at a low frequency and over a relatively long duration, suggesting that cell division may be required for these conversions.
Thus, in summary, it would appear that conversion of one cell into another cell with a different phenotype can be orchestrated by a variety of transcription factor transfers. This is further modulated by the number of interacting transcription factors and the input of exogenous regulators.
4. Stem Cell Plasticity
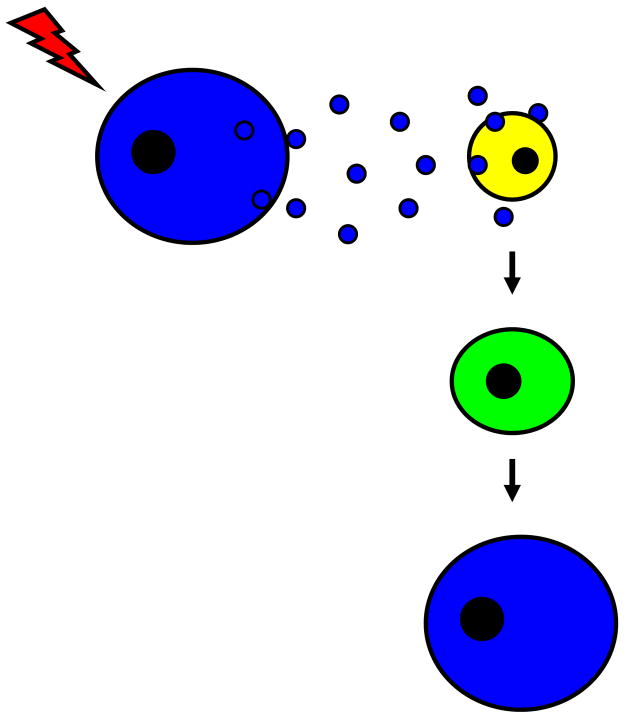
These concepts can be considered in the context of “stem cell plasticity.” This has been a controversial area with much discussion about the necessity for robustness, clonality and the role of cell fusion [2]. This area of study has led us to insights into the role of microvesicles in cell fate determination. Many studies have documented that after marrow transplantation in mice and, in some instances in humans, pulmonary epithelial cells are seen which carry markers of the donor marrow [14–33]. In many of the murine studies, donor marrow was marked with green-fluorescent protein (GFP). Alternatively, investigators have identified donor marrow cells by fluorescence in situ hybridization (FISH) for the Y chromosome in gender-mismatched transplant studies. Similar results have been seen with many other tissues. We found that there was a wide range of donor marrow-derived lung cells, from approximately 1–20 %, when GFP positive murine marrow was engrafted into GFP negative irradiated host mice [14]. Host irradiation was a prerequisite for conversion events and events were increased if the host mice were treated with granulocyte colony-stimulating factor [14]. Further studies showed that pulmonary epithelial conversion of marrow cells exposed to cytokines in vitro was markedly different at different phases of cell cycle of the infused cells [34]. These studies led to studies of the mechanisms underlying marrow cell conversions to pulmonary epithelial cell phenotypes. We incubated normal or irradiated lung fragments opposite murine marrow cells, but separated from them by a cell-impermeable (0.4 micron) membrane and then assessed the marrow cells for expression of pulmonary epithelial cell-specific mRNA (surfactants A–D, clara cell specific protein and Aquaporin-5) after 2 or 7 days of co-culture [35]. We found reproducible and marked elevations of pulmonary cell-specific mRNA in marrow cells under these conditions. If cell-free lung conditioned media was incubated with marrow cells, similar results were obtained. When the conditioned media was ultracentrifuged (28,000 or 100,000 g), the inducing activity was found in the ultracentrifuged pellet, which contained large numbers of vesicles as demonstrated by electron microscopy. These vesicles were capable of entering marrow cells in culture and induced expression of lung-specific mRNA and protein in these marrow cells. Further work showed cells exposed to lung-derived vesicles in cultured were superior to non-co-cultured cells in their ability to convert to pulmonary epithelial cells after transplantation. These initial studies indicated that lung-derived vesicles could induce genetic and functional pulmonary epithelial characteristics in murine marrow cells and might underlie the phenomena of “stem cell plasticity” (figure 1).
Figure 1. Cell-derived vesicles and “stem cell plasticity”.
Vesicle release from a pulmonary epithelial cell (blue cell) is augmented by cellular injury. Vesicles are consumed by a marrow-derived stem cell (yellow cell) which later assumes phenotypic and functional characteristics of a pulmonary epithelial cell.
5. Cell-Derived Vesicles
5.1 Basic Definitions and Characteristics
Cell-derived vesicles are spherical structures bound by a lipid bilayer which is similar in composition to the cell membrane from which the vesicle was derived. Their contents include a variety of cytoplasmic elements which is also a reflection of their cell of origin. Consequently, as vesicles are released into the extracellular compartment, other cells are exposed to these membrane and cytoplasmic elements. Vesicles were first described to be present in the human circulatory system over 40 years ago [36] and subsequent reports have helped to elucidate the biological significance of these intriguing particles.
In the literature, generic terms have often been used to describe cell-derived vesicles, including “microparticles”. However, it is clear that they represent a heterogeneous population of discrete entities which include exosomes [37], microvesicles [38], ectosomes [39], membrane particles [40], exosome-like vesicles [41] and apoptotic vesicles [42]. Each population has its own panel of phenotypic and functional characteristics and is generated by different mechanisms. Microvesicles are created by direct budding of the plasma membrane into the extracellular space [43]. Conversely, exosomes are formed via endocytosis, resulting in the sequestration of plasma membrane proteins and ligands. As endocytic vesicles fuse to form early endosomes and invaginate to form multivesicular bodies, cytoplasmic components are incorporated into exosomes. Exosomes are eventually released into the extracellular space by fusion of multivesicular bodies to the plasma membrane [38]. Vesicles populations have been described to be of different size ranges, with exosome-like vesicles are (20–50nm in diameter) on one end of the spectrum [44] to microvesicles (up to 1um in diameter) on the other end. Different classes of proteins can be found in different populations of vesicles including histones in apoptotic vesicles [45] and tetraspanins, which include CD9, CD63 and CD81 in exosomes [46]. As the density of vesicles can differ, sedimentation of vesicles by ultracentrifugation of cell-free media or plasma is achieved at different speeds; ectosomes [47], membrane particles [48], exosomes [49] and exosome-like vesicles [44] all require 100,000g or greater whereas apoptotic vesicles [50] and microvesicles [45] can be isolated by ultracentrifugation at 10,000g. Although different fixatives can alter the appearance of vesicles, exosomes have been described to have a cup-shaped appearance by transmission electron microscopy, while other vesicle populations have different morphologies [45].
An ever-expanding list of cells has been described to be sources of vesicles. Numerous types of hematopoietic cells, including platelets [51], megakaryocytes [52], monocytes [53], neutrophils, B and T lymphocytes [54,55], reticulocytes [56] and mast cells [57]. Various stem/progenitor cell populations create vesicles, including embryonic stem cells [58] and endothelial progenitor cells [59,60] as do tumors, both as cell lines in vitro [51] and in vivo [61,62]. More recent reports describe vesicles being generated by epithelial and endothelial [63] surfaces of non-hematopoietic organs, including the pulmonary epithelium [35]. Vesicle release is mediated by a variety of different mechanisms. For example, microvesicles and ectosomes are released in response to increases in intracellular calcium, which may be cell surface receptor-mediated [40,53,64] and results in plasma membrane remodeling and vesicle shedding. Exosomes may be released by processes that are independent of changes in intracellular calcium but are still in response to activation of various cell surface receptors. Exosomes have also been described to be released spontaneously under certain circumstances, as is the case with many tumor cell lines [51], and Epstein Barr Virus (EBV)-infected B lymphocytes [65]. Cellular stress and injury, including radiation [35,66], can also stimulate the release of many different types of vesicles.
Vesicle content varies depending on the vesicle type and cell of origin and has been demonstrated to be delivered to target cells both in vitro and in vivo. Contents include genetic material such as DNA [67], mRNA [35,51,59] and, in more recent reports, microRNA [35,68]. In some instances, investigators have reported that the genetic information carried by vesicles represents specific subsets rather than a random assortment. Deregibus and colleagues [59] identified that mRNA transcripts associated with the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway were contained within endothelial progenitor cell-derived microvesicles. We have shown that murine brain, liver and heart tissue induce tissue specific expression of mRNAs in target marrow cells (35). Similarly, specific classes of proteins have been found in certain vesicle populations. Among the hundreds of proteins found within exosomes derived from various cells include cytoskeletal, membrane trafficking, chaperone and signaling and T cell-stimulating proteins [45].
Jin and colleagues [69] determined the whole proteomic profile of plasma-derived microvesicles from healthy human donors. After isolation by ultracentrifugation at 250,000xg and flow cytometry analysis, proteins from microvesicles were separated by 2D-electrphoresis and identified by MALDI TOF/TOF, they identified 83 proteins out of 169 spots. Banfi and colleagues [70] analyzed the proteome of endothelial cell-derived procoagulant microvesicles. They identified approximately 80 cellular proteins, including cytoskeletal proteins, chaperonins, nucleosomal proteins, enzymes, annexins and proteins involved in folding and signaling. Annexins had previously been described as a category of proteins associated with microvesicles [71]. The microvesicle proteome also reflects the mechanism of particle formation, which explains the presence of cytoplasmic metabolic enzymes. Garcia and colleagues [72] analyzed the platelet-derived microvesicle proteome. They identified 578 proteins, 10% of which were plasma membrane proteins. However, the majority of proteins (48%) are normally located in the cytoplasm. Miguet and colleagues [71] reported on a thorough proteomic analysis of malignant lymphocyte membrane microparticles. They identified 390 proteins, 131 (34%) belonging to the plasma membrane proteins and 216 (54%) being cytoplasmic proteins. The composition of vesicles is dependent on the cell from which they originate and proteomic studies may allow the determination of different protein expression in different cell types [71]. In the case of tumor or other malignancies, they may carry their proteomic “signature” from the affected site into the blood stream or other body fluids [73,74]. In a recent study, Cho and colleagues [75] demonstrated that –MS/MS combined with isotope-coded affinity tag (ICAT) labeling technique enables quantitative analysis of paired microvesicle samples. This method was applied to investigate differences in proteome between microvesicles generated from platelets and those isolated from plasma.
Vesicle-based surface receptors, including CXCR4 [76] and epidermal growth factor receptor (EGFR) [77], have also been shown to be found in vesicles and can be transferred to target cells. In addition, vesicles may function as vehicles for the transfer of infectious agents like the Human Immunodeficiency Virus (HIV) [78] and prions [79]. Vesicle uptake into target cells is not well-established for all populations of vesicles. Mechanisms may include direct fusion with the recipient plasma membrane, leading to incorporation of transferred membrane elements into the recipient plasma membrane and deposition of vesicle contents into the recipient cytoplasm. Vesicles may be consumed by non-receptor-mediated internalization and fusion with a recipient cell endosome. Alternatively, vesicles by be internalized as a result of specific receptor-ligand interactions. These include target cell binding with vesicle-based phosphatidylserine [80], milk fat globule epidermal growth factor 8 protein (MFGE8) [46], β1 and β2 integrins [81] and ICAM-1 [82].
Several different vesicle isolation techniques have been described in the literature. Most begin with centrifugation to create cell free-media or cell and platelet-free serum. Supernatants are then ultracentrifuged at variable speeds creating a pellet which is enriched with vesicles. This pellet is then studied without any further processing or used as starting material for generating more purified populations of vesicles. In our lab, pellets generated by ultracentrifugation at 100,000g are labeled with the red-fluorescent cell membrane dye PKH26 and the green-fluorescent dye cell cytoplasm dye CFSE. All red/green fluorescent events, representing entities with both cell membrane and cytoplasm, are collected by flow cytometry [35]. Protocols as such are likely to result in preparations enriched with different vesicle populations, including exosomes and microvesicles. Specific vesicle populations, such as exosomes, have been isolated by others using sucrose gradient separations [83]. Investigators have also studied vesicle preparations enriched with certain surface epitopes using various fluorescent antibodies and selection by flow cytometry [84]. Others have used magnetic activated cell sorting (MACS) procedures to isolate specific populations of vesicles also based on the presence of certain surface epitiopes [61]. Depending on the speed of ultracentrifugation, these techniques are likely yield preparations with different vesicle populations that share a common surface epitope. In addition, storage of vesicles may further modify the nature of isolated populations. In one study, endothelial cell-derived vesicles isolated from human peripheral blood were stored at different temperatures. Here, investigators found that storage at −80°C increased the number of CD31+/CD42b+ and CD62E+ vesicles whereas CD144+ decreased in number under these conditions [85].
It is clear that cell-derived vesicle populations are inherently heterogeneous, as the literature is replete with studies using different vesicles populations, isolated and quantified using different protocols and that have been enriched with different phenotypic characteristics. Vesicles have been isolated from many different cell types and their functional impact has been studied under a variety of experimental conditions using different target cell populations. These observations highlight the importance of careful interpretation and avoiding generalizations when evaluating the biological relevance of certain vesicles.
5.2 Transfer of Cellular Phenotype
The biological significance of cell-derived vesicles remained largely overlooked until recent years, but now there is growing evidence that these entities may be important mediators of cell-to cell communication. Their interactions with target cells appear to directly impact phenotypic and functional characteristics of these cells and may do so by a variety of mechanisms. This may be accomplished by direct stimulation of target cells with vesicle-based growth factors or bioactive lipids, the transfer of a variety of membrane surface receptors, epigenetic reprogramming by transfer of genetic material and/or protein based transcription factors or the transfer of infectious particles [86].
Embryonic stem cell-derived microvesicles have been reported to reprogram hematopoietic stem/progenitor cells via the horizontal transfer of mRNA and protein [43]. Similarly, tumor-derived microvesicles have been shown to carry several surface determinants and mRNA and to transfer some of these determinants to monocytes [42]. Apoptotic bodies from irradiated EBV-carrying cell lines have been seen to transfer DNA to a variety of co-cultured cells and integrated, but not episomal, copies of EBV resulted in expression of the EBV-encoded genes EBER and EBNAI in recipient cells at high copy number [52]. Extracts from T lymphocytes containing transcription factor complexes can induce fibroblasts to express lymphoid genes [55]. In addition, microvesicles derived from endothelial progenitor cells can induce a vascular phenotype both in vitro and in vivo through delivery of microvesicles [59]. Recently, Prokopi and colleagues have published that the previously-described endothelial progenitor cell may represent mononuclear cells which have consumed platelet-derived microvesicles [60]. Cell fate change by vesicle interactions are summarized in Table 1
Table 1.
Cell fate change induced by microvesicles
| Cell of origin | Target cell* |
|---|---|
| Lung | Marrow |
| Brain | Marrow |
| Heart | Marrow |
| T lymphocyte | Fibroblast |
| Platelet | Monocyte |
| Tumor cell | Monocyte |
| Embryonic stem cells | Hematopoietic progenitor cells |
Target cell with altered characteristics towards that of the cell of origin
Overall, cell-derived vesicles appear to play a significant role in determination of cellular phenotype especially in the context of cellular injury. The mechanism of the induced genetic phenotype switch in our lung-to-marrow experiments [35] was initially felt to be due to the direct transfer of lung-derived mRNA in microvesicles. However, it was determined that message expression lasted out to three weeks and degradation of the transferred mRNA would be considered likely over this interval of time. Experiments were carried out where rat lung was cultured opposite mouse marrow and Surfactant B and C mRNA determined using primers specific for either rat or mouse surfactant mRNA. We found that after 7 days of co-culture there were high and relatively equal levels of both rat and mouse surfactant mRNA, indicating that there had been both direct transfer of mRNA and also transfer of a transcriptional activator, presumably a protein (35). Furthermore, recent studies has indicated that in vitro expression of lung-specific mRNA persists out to at least 12 weeks on in vitro cytokine culture and out to 6 weeks in marrow after marrow transplantation, the furthest time point accessed. These data indicate that the microvesicle genetic phenotype shift is relatively stable over time speaking to the potential biologic significance of such cell fate modulations.
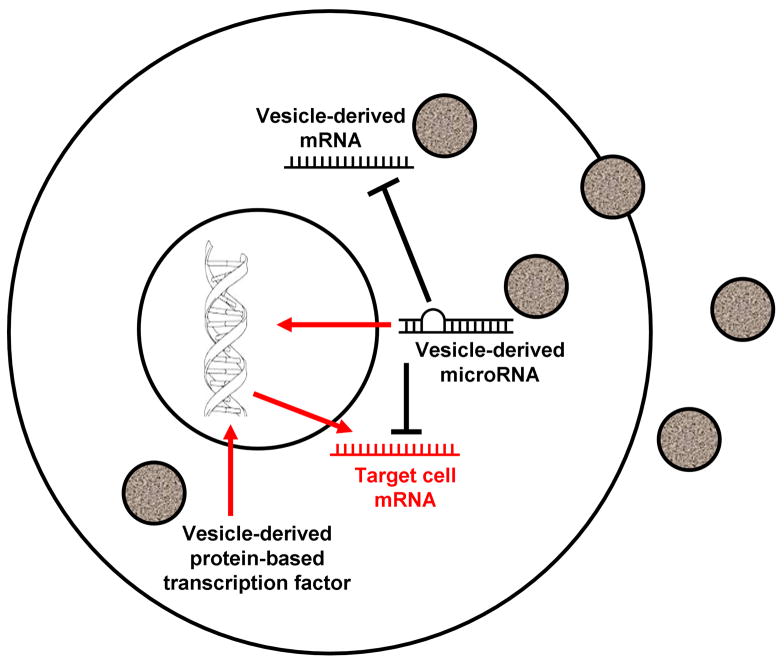
Cell-derived vesicles appear capable of transferring the essential genetic phenotype of one cell to another. This immediately suggests a cellular system in which the cellular transcriptional machinery is shuttled from one cell to another, prompted at least in past by cellular injury. Thus, while cellular populations may be relatively stable there may be a continuing flux of cellular phenotypes to maintain that stability. Transcriptional regulation is then a key determinant of the phenotype of a particular cell, but may shift between different cell types. This indicates that while the models of transcriptional regulation for certain cell lineages have been well defined, the context in which they operate is not a single stable cell population but rather a population of cells which continues to change fate and phenotype. This concept is present in figure 2.
Figure 2. Vesicle-induced epigenetic alteration.
Cell-derived vesicles appear capable of transferring the essential genetic phenotype of one cell to another. They may do so by a variety of mechanisms, including the transfer of mRNA, microRNA and protein-based transcription factors, which results in epigenetic alteration of target cells.
5.3 Cell-Derived Vesicles and Human Disease
Cell-derived vesicles can be isolated from the peripheral blood of healthy humans. However, elevated levels of certain populations of vesicles have been detected in the setting of a variety of disease states [87]. For example, elevated levels of platelet-derived vesicles, which are believed to play a role in thrombosis, are found in patients with diseases associated with thrombosis, including cerebrovascular [88] and cardiovascular [89, 90] disease. They are also found in patients with risk factors for developing cardiovascular disease, including hypertension [91], diabetes mellitus [92], obesity [93] and hypercholesterolemia [94]. Although it is not known precisely how vesicles contribute to the pathogenesis of these diseases, they are believed to activate the coagulation cascade by providing a source of phosphatidyl serine. This is supported by the work of Nieuwland and colleagues [95], who reported that plasma-derived vesicles isolated from patients with meningococcal sepsis complicated by disseminated intravascular coagulation (DIC), induced thrombin formation in vitro. Tumor cells shed greater quantities of vesicles and this can be detected in patients with a variety of cancers, including ovarian [96, 97], prostate [98] and gastric cancers [99]. Elevated levels of vesicles are found in patients with chronic inflammatory diseases, including rheumatoid arthritis, where they may participate in the pathogenesis of this disease not only as a procoagulant but also as a chemoattractant molecule and inflammatory mediator [100].
One clinical application that has been suggested based on these observations is to use circulating vesicles as a disease biomarker. Amabile and colleagues [84] isolated peripheral blood vesicles from 44 patients with end stage renal disease and found that higher levels of endothelial cell-derived vesicles (CD31+/CD41− or CD144+) correlated with vascular dysfunction, as evidenced by greater impairment of brachial artery flow-mediated dilation. Circulating levels of platelet (CD31+/CD41+) and red blood cell (CD235a)-derived vesicles did not have the same correlation. Others have shown that in patients with pulmonary hypertension, circulating levels of endothelial cell (CD31+ or VE-cadherin+)-derived vesicles correlated with hemodynamic severity of the disease, whereas other populations (CD45+, E-cadherin+, annexin V+) did not [101]. Studies as such could be used as the basis for developing a non-invasive assay to stage a disease and provide prognostic information. Similarly, Taylor and colleagues [61] reported that there were higher numbers of circulating exosomes found in patients with ovarian cancer compared with patients with benign ovarian disease and healthy controls. Higher numbers of circulating exosomes also appeared to correlate with more advanced stages of ovarian cancer. In addition, eight microRNA species isolated from ovarian cancer tumor cells were also found in circulating exosomes of patients with ovarian cancer but not in patients with benign ovarian disease and healthy controls. One could envision the development of a screening assay to detect early stages of cancer in patients who are considered to be at high risk of developing certain cancers.
There is a limited but growing number reports in the literature demonstrating that certain vesicle populations can alter disease phenotype in clinically-relevant animal models. Endothelial apoptotic bodies isolated from human umbilical vein endothelial cells (HUVEC) and human atherosclerotic plaques were found to stimulate the recruitment of bone marrow-derived progenitor cells to atherosclerotic lesions in a mouse model which, in turn, participated in the attenuation of these lesions [102]. Progenitor cell recruitment was believed to be mediated by vesicle-mediated delivery of miR126 to vascular endothelial cells leading to the upregulation of CXCL12. Human platelet (CD41+)-derived vesicles not only promoted angiogenesis using bovine aortic endothelial cells in Matrigel, but also lead to an increase in the number of capillaries after direct injection into infracted rat myocardium [103]. These findings suggest potential cell-derived vesicle-based approaches for tissue repair. It is also important to consider that not all vesicle populations may impart a beneficial phenotype. Microvesicles produced from various human cancer cell lines, including A431, were found to contain epidermal growth factor receptor (EGFR) [104]. EGFR can be transferred to HUVECs in vitro, and elicit EGFR-dependent responses via activation of MAPK and Akt pathways. A431 xenografts in SCID mice treated diannexin, which blocks microvesicular production, lead to a decrease EGFR-dependent responses, and resulted in reduced tumor angiogenesis and growth. These findings suggest that impairing vesicle genesis may attenuate unwanted or harmful angiogenesis.
5.4 A New Stem Cell Biology
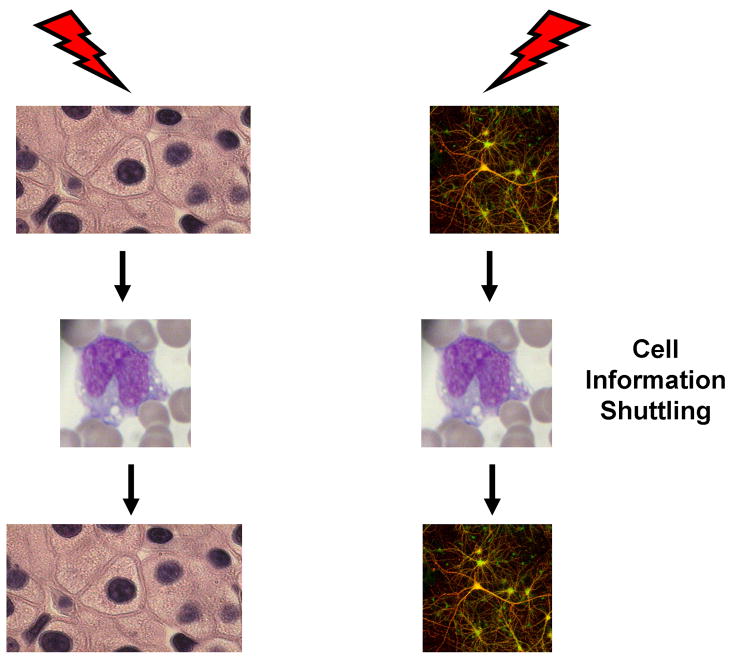
We have developed a model of stem cell biology in which marrow stem cells are on a continuum of change. Our data indicates that long-term renewal marrow stem cells are a cycling population of cells and thus of necessity are always changing. We have shown that marrow stem cell in vivo engraftment, homing in vitro differentiation, gene expression and in vivo conversion to lung cells all reversibly vary with cell cycle phase. Thus the potential of marrow stem cells is continually changing with cell cycle and will be further modulated by cell-derived vesicle interactions delivering transcriptional packages. This presents an extraordinarily flexible model of stem cell biology in which virtually any cell, given the appropriate phase of cell cycle and vesicle modulation, could become a stem cell. Obviously, such events would be further influenced by exogenous environmental stimuli. This model is summarized in figure 3
Figure 3. A model of stem cell biology.
In this model, virtually any cell, given the appropriate phase of cell cycle and external influence, including interactions with cell-derived vesicles, could become a stem cell.
6. Conclusions
Cell-derived vesicles have the capacity to alter the cell phenotype and fate of other different cell populations. This has been demonstrated with many different cell and tissue combinations and the evolution of vesicles is enhanced by different cellular injuries. A major type of phenotype change appears to be due to modulation of transcription with stable epigenetic changes. There is great potential for vesicle modulation in areas of tissue restoration or neoplastic cell growth. In addition, cell-derived vesicles may provide a relevant biomarker in different diseases.
Acknowledgments
Grant Information: National Institutes of Health, 5K08HL086868-03 (J.M.A) and 1P20 RR025179-01 (P.J.Q).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blau HM. How fixed is the differentiated state? Lessons from heterokaryons. Trends Genet. 1989;5:268–272. doi: 10.1016/0168-9525(89)90100-5. [DOI] [PubMed] [Google Scholar]
- 2.Quesenberry PJ, Dooner G, Dooner M, Abedi M. Developmental biology: Ignoratio elenchi: red herrings in stem cell research. Science 2005. 2005;308:1121–1122. doi: 10.1126/science.1104432. [DOI] [PubMed] [Google Scholar]
- 3.Gurdon JB, Byrne JA. The first half-century of nuclear transplantation. Proc Natl Acad Sci USA. 2003;100:8048–8052. doi: 10.1073/pnas.1337135100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 5.Gurdon JB, Melton DA. Nuclear reprogramming of cells. Science. 2008;322:1811–1815. doi: 10.1126/science.1160810. [DOI] [PubMed] [Google Scholar]
- 6.Slack JM. Metaplasia and transdifferentiation: from pure biology to the clinic. Nature Rev Mol Cell Biol. 2007;8:369–378. doi: 10.1038/nrm2146. [DOI] [PubMed] [Google Scholar]
- 7.Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–65. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- 8.Davis RL, Weintraub H, Lasser AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 9.Kulessa H, Frampton J, Graf T. GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts and erythroblasts. Genes Dev. 1995;9:1250–1262. doi: 10.1101/gad.9.10.1250. [DOI] [PubMed] [Google Scholar]
- 10.Visvader JE, Elefanty AG, Strasser A, Adams JM. GATA-1 but not SCL induces megakaryocyte differentiation in an early myeloid line. EMBO J. 1992;11:4557–4564. doi: 10.1002/j.1460-2075.1992.tb05557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwasaki H, Mizuno S, Arinobu Y, Ozawa H, Mori Y, Shigematsu H, Takatsu K, Tenen DG, Akashi K. The order of expression of transcription factors directs hierarchical specification of hematopoietic lineages. Genes Dev. 2006;20:3010–3021. doi: 10.1101/gad.1493506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-β-induced FoxP3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eilken HM, Nishikawa A, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 14.Aliotta JM, Keaney P, Passero M, Dooner MS, Pimentel J, Greer D, Demers D, Foster B, Peterson A, Dooner G, Theise ND, Abedi M, Colvin GA, Quesenberry PJ. Bone marrow production of lung cells: the impact of G-CSF, cardiotoxin, graded doses of irradiation and subpopulation phenotype. Exp Hematol. 2006;34:230–241. doi: 10.1016/j.exphem.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theise ND, Henegariu O, Grove J, Jagirdar J, Kao PN, Crawford JM, Badve S, Saxena R, Krause DS. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol. 2002;30:1333–1338. doi: 10.1016/s0301-472x(02)00931-1. [DOI] [PubMed] [Google Scholar]
- 16.Herzog EJ, Van Arnam J, Hu B, Krause DS. Threshold of lung injury required for the appearance of marrow-derived lung epithelia. Stem Cells. 2006;24:986–992. doi: 10.1634/stemcells.2005-0579. [DOI] [PubMed] [Google Scholar]
- 17.Abe S, Lauby G, Boyer C, Rennard SI, Sharp JG. Transplanted BM and BM side population cells contribute progeny to the lung and liver in irradiated mice. Cytotherapy. 2003;5:623–633. doi: 10.1080/14653240310003576. [DOI] [PubMed] [Google Scholar]
- 18.Grove JE, Lutzko C, Priller J, Henegariu O, Theise ND, Kohn DB, Krause DS. Marrow-derived cells as vehicles for delivery of gene therapy to pulmonary epithelium. Am J Respir Cell Mol Biol. 2002;27:645–651. doi: 10.1165/rcmb.2002-0056RC. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto N, Jiu H, Liu T. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett. 2004;566:249–252. doi: 10.1016/s0014-5793(03)01399-1. [DOI] [PubMed] [Google Scholar]
- 23.Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2006;291:H1378–H1383. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- 24.Beckett T, Loi R, Prenovitz P, Poynter M, Goncz KK, Suratt BT, Weiss DJ. Acute lung injury with endotoxin or NO2 does not enhance development of airway epithelium from bone marrow. Mol Ther. 2005;12:680–686. doi: 10.1016/j.ymthe.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang Y, Jahagirdar JN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 27.Adachi Y, Oyaizu H, Taketani S, et al. Treatment and transfer of emphysema by a new bone marrow transplantation method from normal mice to Tsk mice and vice versa. Stem Cells. 2004;24:2071–2077. doi: 10.1634/stemcells.2005-0575. [DOI] [PubMed] [Google Scholar]
- 28.Abe S, Boyer C, Liu X, Wen FQ, Kobayashi T, Fang Q, Wang X, Hashimoto M, Sharp JG, Rennard SI. Cells derived from the circulation contribute to the repair of lung injury. Am J Respir Crit Care Med. 2004;170:1158–1163. doi: 10.1164/rccm.200307-908OC. [DOI] [PubMed] [Google Scholar]
- 29.Bruscia EM, Ziegler EC, Price JE, Weiner S, Egan ME, Krause DS. Engraftment of donor-derived epithelial cells in multiple organs following bone marrow transplantation into newborn mice. Stem Cells. 2006;24:2299–2308. doi: 10.1634/stemcells.2006-0166. [DOI] [PubMed] [Google Scholar]
- 30.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 31.Macpherson H, Keir P, Webb S, Samuel K, Boyle S, Bickmore W, Forrester L, Dorin J. Bone marrow-derived SP cells can contribute to the respiratory tract of mice in vivo. J Cell Sci. 2005;118:2441–2450. doi: 10.1242/jcs.02375. [DOI] [PubMed] [Google Scholar]
- 32.Dooner M, Cerny J, Colvin G, Demers D, Pimentel J, Greer D, Abedi M, McAuliffe C, Quesenberry PJ. Homing and conversion of murine hematopoietic stem cells to lung. Blood Cells Mol Dis. 2004;32:47–51. doi: 10.1016/j.bcmd.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Suratt BT, Cool CD, Serls AE, Chen L, Varella-Garcia M, Shpall EJ, Brown KK, Worthen GS. Human pulmonary chimerism after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:318–322. doi: 10.1164/rccm.200301-145OC. [DOI] [PubMed] [Google Scholar]
- 34.Dooner MS, Aliotta JM, Pimentel J, Dooner GJ, Abedi M, Colvin G, Liu Q, Weier HU, Johnson KW, Quesenberry PJ. Conversion potential of marrow cells into lung cells fluctuates with cytokine-induced cell cycle. Stem Cells Dev. 2008;17:207–219. doi: 10.1089/scd.2007.0195. [DOI] [PubMed] [Google Scholar]
- 35.Aliotta JM, Pereira M, Johnson KW, de Paz N, Dooner MS, Puente N, Ayala C, Brilliant K, Berz D, Lee D, Ramratnam B, McMillan PN, Hixson DC, Josic D, Quesenberry PJ. Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp Hematol. 2010;38:233–245. doi: 10.1016/j.exphem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 37.Keller S, Sanderson MP, Stoeck A. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Fader CM, Colombo MI. Autophagy and multivesicular bodies: two closely related partners. Cell Death Differ. 2009;16:70–78. doi: 10.1038/cdd.2008.168. [DOI] [PubMed] [Google Scholar]
- 39.Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 2004;11:156–164. doi: 10.1097/01.moh.0000131441.10020.87. [DOI] [PubMed] [Google Scholar]
- 40.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 41.Nomura S, Nakamura T, Cone J, Tandon NN, Kambayashi J. Cytometric analysis of high shear-induced platelet microparticles and effect of cytokines on microparticle generation. Cytometry. 2000;40:173–181. [PubMed] [Google Scholar]
- 42.Janowska-Wieczorek A, Majka M, Kijowski J, Baj-Krzyworzeka M, Reca R, Turner AR, Ratajczak J, Emerson SG, Kowalska MA, Ratajczak MZ. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001;98:3143–3149. doi: 10.1182/blood.v98.10.3143. [DOI] [PubMed] [Google Scholar]
- 43.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 44.Hawari FI, Rouhani FN, Cui X, Yu ZX, Buckley C, Kaler M, Levine SJ. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci USA. 2004;101:1297–1302. doi: 10.1073/pnas.0307981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 46.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD, Jr, Thomson AW. Endocytosis, intracellular sorting and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 47.Gasser O, Hess C, Miot S, Deon C, Sanchez JC, Schifferli JA. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp Cell Res. 2003;285:243–257. doi: 10.1016/s0014-4827(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 48.Marzesco AM, Janich P, Wilsch-Bräuninger M, Dubreuil V, Langenfeld K, Corbeil D, Huttner WB. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci. 2005;118:2849–2858. doi: 10.1242/jcs.02439. [DOI] [PubMed] [Google Scholar]
- 49.Valadi H, Ekstrom K, Bossius A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNA and microRNA is a novel mechanism of genetic exchange. Nature Cell Biol. 2007;9:652–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 50.Thery C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 51.Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Urbanowicz B, Brański P, Ratajczak MZ, Zembala M. Tumor-derived microvesicles carry several surface determinants and mRNA of tumor cells and transfer some of these determinants to monocytes. Cancer Immunol Imunother. 2006;55:808–818. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rozmyslowicz T, Majka M, Kijowski J, Murphy SL, Conover DO, Poncz M, Ratajczak J, Gaulton GN, Ratajczak MZ. Platelet and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS. 2003;17:33–42. doi: 10.1097/00002030-200301030-00006. [DOI] [PubMed] [Google Scholar]
- 53.MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 54.Rialland P, Lankar D, Raposo G, Bonnerot C, Hubert P. BCR-bound antigen is targeted to exosomes in human follicular lymphoma B-cells. Biol Cell. 2006;98:491–501. doi: 10.1042/BC20060027. [DOI] [PubMed] [Google Scholar]
- 55.Håkelien AM, Landsverk HB, Rob JM, Skålhegg BS, Collas P. Reprogramming fibroblasts to express T-cell functions using cell extracts. Nature Biotech. 2002;20:460–466. doi: 10.1038/nbt0502-460. [DOI] [PubMed] [Google Scholar]
- 56.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell. 1997;8:2631–2645. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 59.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 60.Prokopi M, Pula G, Mayr U, Devue C, Gallagher J, Xiao Q, Boulanger CM, Westwood N, Urbich C, Willeit J, Steiner M, Breuss J, Xu Q, Kiechl QS, Mayr M. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–732. doi: 10.1182/blood-2009-02-205930. [DOI] [PubMed] [Google Scholar]
- 61.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 62.Graves LE, Ariztia EV, Navari JR, Matzel HJ, Stack MS, Fishman DA. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res. 2004;64:7045–7049. doi: 10.1158/0008-5472.CAN-04-1800. [DOI] [PubMed] [Google Scholar]
- 63.Brodsky SV, Zhang F, Nasjletti A, Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol. 2004;286:H1910–H1915. doi: 10.1152/ajpheart.01172.2003. [DOI] [PubMed] [Google Scholar]
- 64.Obregon C, Rothen-Rutishauser B, Gitahi SK, Gehr P, Nicod LP. Exovesicles from human activated dendritic cells fuse with resting dendritic cells, allowing them to present alloantigens. Am J Pathol. 2006;169:2127–2136. doi: 10.2353/ajpath.2006.060453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raposo G. B lymphocytes secrete antigen presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 67.Holmgren L, Szeles A, Rajnavolgyi R, Folkman J, Klein G, Ernberg I, Falk KI. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood. 1999;93:3956–3963. [PubMed] [Google Scholar]
- 68.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Köppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;8:1–11. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 69.Jin M, Drwal G, Bourgeois T, Saltz G, Wu HM. Distinct proteome features of plasma microparticles. Proteomics. 2005;5:1940–1952. doi: 10.1002/pmic.200401057. [DOI] [PubMed] [Google Scholar]
- 70.Banfi C, Brioschi M, Wait R, Begum S, Gianazza E, Pirillo A, Mussoni L, Tremoli E. Proteome of endothelial cell-derived procoagulant microparticles. Proteomics. 2005;5:4443–4455. doi: 10.1002/pmic.200402017. [DOI] [PubMed] [Google Scholar]
- 71.Miguet L, Pacaud K, Felden C, Hugel B, Martinez MC, Freyssinet JM, Herbrecht R, Potier N, van Dorsselaer A, Mauvieux L. Proteomic analysis of malignant lymphocyte membrane microparticles using double ionization coverage optimization. Proteomics. 2006;6:153–171. doi: 10.1002/pmic.200500133. [DOI] [PubMed] [Google Scholar]
- 72.Garcia BA, Smalley DM, Cho H, Shabanowitz J. The platelet microparticle proteome. J Proteome Res. 2005;4:1516–1521. doi: 10.1021/pr0500760. [DOI] [PubMed] [Google Scholar]
- 73.Choi D, Lee J, Park GW, Lim H, Bang JY, Kim YK, Kwon KH, Kwon HJ, Kim KP, Gho YS. Proteomic analysis of microvesicles derived from human colorectal cancer cells. J Proteome Res. 2007;6:4646–4655. doi: 10.1021/pr070192y. [DOI] [PubMed] [Google Scholar]
- 74.Bagnato C, Thumar J, Mayya V, Hwang SI, Zebroski H, Claffey KP, Haudenschild C, Eng JK, Lundgren DH, Han DK. Proteomic analysis of human coronary atherosclerotic plaque: a feasibility study of direct tissue proteomics by liquid chromatography and tandem mass spectrometry. Mol Cell Proteomics. 2007;6:1088–1102. doi: 10.1074/mcp.M600259-MCP200. [DOI] [PubMed] [Google Scholar]
- 75.Cho HJ, Smalley DM, Theodorescu D, Ley K, Lee JK. Statistical identification of differentially labeled peptides from liquid chromatography tandem mass spectrometry. Proteomics. 2007;7:3681–3692. doi: 10.1002/pmic.200601034. [DOI] [PubMed] [Google Scholar]
- 76.Rozmyslowicz T, Majka M, Kijowski J, Murphy SL, Conover DO, Poncz M, Ratajczak J, Gaulton GN, Ratajczak MZ. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS. 2003;17:33–42. doi: 10.1097/00002030-200301030-00006. [DOI] [PubMed] [Google Scholar]
- 77.Al-Nedawia K, Meehana B, Kerbelb RS, Allison AC, Raka J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. PNAS. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fackler OT, Peterlin BM. Endocytic entry of HIV-1. Curr Biol. 2000;10:1005–1008. doi: 10.1016/s0960-9822(00)00654-0. [DOI] [PubMed] [Google Scholar]
- 79.Fevrier B, Vilette D, Archer F. Cells release prions in association with exosomes. Proc Natl Acad Sci USA. 2004;100:10592–10597. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 81.Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, Hallett MB. Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB J. 2004;18:977–979. doi: 10.1096/fj.03-1094fje. [DOI] [PubMed] [Google Scholar]
- 82.Nolte-‘t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T-cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–1981. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 83.Li J, Sherman-Baust CA, Tsai-Turton M, Bristow RE, Roden RB, Morin PJ. Claudin-containing exosomes in the peripheral circulation of women with ovarian cancer. BMC Cancer. 2009;20:244. doi: 10.1186/1471-2407-9-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–3388. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 85.Van Ierssel SH, Van Craenenbroeck EM, Conraads VM, Van Tendeloo VF, Vrints CJ, Jorens PG, Hoymans VY. Flow cytometric detection of endothelial microparticles (EMP): Effects of centrifugation and storage alter with the phenotype studied. Thromb Res. 2010;125:332–339. doi: 10.1016/j.thromres.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 86.Ratajczak MZ. Microvesicles: from “dust to crown”. Blood. 2006;108:2885. [Google Scholar]
- 87.Pap E, Pállinger É, Pásztói M, Falus A. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1–8. doi: 10.1007/s00011-008-8210-7. [DOI] [PubMed] [Google Scholar]
- 88.Geiser T, Sturzenegger M, Genewein U, Haeberli A, Beer JH. Mechanisms of cerebrovascular events as assessed by procoagulant activity,cerebral microemboli, and platelet microparticles in patients with prosthetic heart valves. Stroke. 1998;29:1770–1777. doi: 10.1161/01.str.29.9.1770. [DOI] [PubMed] [Google Scholar]
- 89.Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, Tedgui A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2001;101:841–843. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- 90.Gawaz M, Neumann JF, Ott I, Schiessler A, Schömig A. Platelet function in acute myocardial infarction treated with direct angioplasty. Circulation. 1996;93:229–337. doi: 10.1161/01.cir.93.2.229. [DOI] [PubMed] [Google Scholar]
- 91.Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211–217. doi: 10.1161/01.hyp.0000049760.15764.2d. [DOI] [PubMed] [Google Scholar]
- 92.Leroyer AS, Tedgui A, Boulanger CM. Microparticles and type 2 diabetes. Diab Metab. 2008;34:27–31. doi: 10.1016/S1262-3636(08)70100-9. [DOI] [PubMed] [Google Scholar]
- 93.Goichot B, Grunebaum L, Desprez D, Vinzio S, Meyer L, Schlienger JL, Lessard M, Simon C. Circulating procoagulant microparticles in obesity. Diabetes Metab. 2006;32:82–85. doi: 10.1016/s1262-3636(07)70251-3. [DOI] [PubMed] [Google Scholar]
- 94.Koga H, Sugiyama S, Kugiyama K, Fukushima H, Watanabe K, Sakamoto T, Yoshimura M, Jinnouchi H, Ogawa H. Elevated levels of remnant lipoproteins are associated with plasma platelet microparticles in patients with type-2 diabetes mellitus without obstructive coronary artery disease. European Heart Journal. 2006;27:817–823. doi: 10.1093/eurheartj/ehi746. [DOI] [PubMed] [Google Scholar]
- 95.Nieuwland R, Berckmans RJ, McGregor S, Böing AN, Romijn F, Westendorp RGJ, Hack CE, Sturk A. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930–935. [PubMed] [Google Scholar]
- 96.Taylor DD, Doellgast GJ. Quantitation of eroxidise-antibody binding to membrane fragments using column chromatography. Anal Biochem. 1979;98:53–59. doi: 10.1016/0003-2697(79)90704-8. [DOI] [PubMed] [Google Scholar]
- 97.Taylor DD, Homesley HD, Doellgast GJ. Binding of specific peroxidiselabeled antibody to placental-type alkaline phospohatase on tumor-derived membrane fragments. Cancer Res. 1980;40:4964–4969. [PubMed] [Google Scholar]
- 98.Coumans FA, Doggen CJ, Attard G, de Bono JS, Terstappen LW. All circulating EpCAM+CK+CD45− objects predict overall survival in castration-resistant prostate cancer. Ann Oncol. 2010 Feb 10; doi: 10.1093/annonc/mdq030. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 99.Baran J, Baj-Krzyworzeka M, Weglarczyk K, Szatanek R, Zembala M, Barbasz J, Czupryna A, Szczepanik A, Zembala M. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Cancer Immunol Immunother. 2010;59:841–850. doi: 10.1007/s00262-009-0808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Redman CW, I, Sargent L. Placental debris, oxidative stress and preeclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 101.Amabile N, Heiss C, Real WM, Minasi P, McGlothlin D, Rame EJ, Grossman W, De Marco T, Yeghiazarians Y. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1268–1275. doi: 10.1164/rccm.200710-1458OC. [DOI] [PubMed] [Google Scholar]
- 102.Zernecke A, Bidzhekov K, Noels H. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;8:1–11. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 103.Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67:30–38. doi: 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 104.Al-Nedawia K, Meehana B, Kerbelb RS, Allison AC, Raka J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. PNAS. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]