Abstract
Background
Recent genome-wide association studies have associated polymorphisms in the gene CACNA1C, which codes for Cav1.2, with a bipolar disorder and depression diagnosis.
Methods
The behaviors of wild type and Cacna1c heterozygous mice of both sexes were evaluated in a number of tests. Based upon sex differences in our mouse data, we assessed a gene x sex interaction for diagnosis of mood disorders in human subjects. Data from the NIMH-BP Consortium and the GenRED Consortium were examined utilizing a combined dataset that included 2,021 mood disorder cases (1,223 females) and 1,840 controls (837 females).
Results
In both male and female mice, Cacna1c haploinsufficiency is associated with lower exploratory behavior, decreased response to amphetamine, and antidepressant-like behavior in the forced swim and tail suspension tests. Female, but not male, heterozygous mice displayed decreased risk-taking behavior or increased anxiety in multiple tests, greater attenuation of amphetamine-induced hyperlocomotion, decreased development of learned helplessness, and a decreased acoustic startle response indicating a sex-specific role of Cacna1c. In humans, sex-specific genetic association was seen for two intronic single nucleotide polymorphisms (SNPs), rs2370419 and rs2470411, in CACNA1C, with effects in females (OR=1.64, 1.32), but not in males (OR=0.82, 0.86). The interactions by sex were significant after correction for testing 190 SNPs (P=1.4 x 10−4, 2.1 x 10−4; Pcorrected=0.03, 0.04), and were consistent across two large data sets.
Conclusions
Our preclinical results support a role for CACNA1C in mood disorder pathophysiology, and the combination of human genetic and preclinical data support an interaction between sex and genotype.
Keywords: CACNA1C, bipolar disorder, major depression, Cav1.2, animal model, gender, sex differences
Introduction
Bipolar disorder (BP) and major depressive disorder (MDD) are severe psychiatric disorders affecting about 1–3% and 15% of the world’s population, respectively, over a lifetime. The two disorders are closely related as depressive episodes are a core feature of BP and the illnesses often run together in families (1). Despite being common and often severe illnesses, there is limited knowledge regarding their underlying pathophysiology. It is becoming increasingly clear that gender differences are a critical consideration. For MDD, prevalence rates are approximately twice as high for women as for men, and this difference only arises after puberty and the associated increase in estrogen (2). In BP, some studies suggest the course of illness is different between genders, with women tending to have more depressive episodes, an increased risk for the rapid cycling form of the illness, and a later age of onset (3,4). While studies have suggested sex differences in genetic risk factors for depression (5) and BP (6), the underlying causes of these sex differences are unclear.
The heritability of BP and depression, ~70–90% and 40–50%, respectively, indicates that genetic variation in particular genes predisposes to the development of these disorders and that understanding the biological significance of this variation will provide insight into pathophysiology (7,8). The results of genome-wide association studies (GWAS) have converged to implicate a small number of genes in BP etiology. One candidate gene that has emerged from some (9,10), though not all (11), BP GWAS is CACNA1C. Additional association studies have correlated polymorphisms in CACNA1C with depression and schizophrenia (12–16). A recent GWAS meta-analysis of BP and MDD patients reported single nucleotide polymorphisms (SNPs) in CACNA1C as the most significant findings, with p-values that surpassed genome-wide significance, the strongest being p = 3.1 x 10−8 (17). In addition, reports have associated a SNP in CACNA1C with differences in mean gray matter volume (18), verbal fluency (19), and limbic activity (20) in healthy human subjects.
The L-type voltage-gated calcium channel family consists of four distinct isoforms referred to as Cav1.1–Cav1.4 (21). CACNA1C codes for the pore forming alpha-1C subunit of the Cav1.2 isoform. In the mouse brain Cav1.2 accounts for ~85% of the L-type channels with Cav1.3 accounting for most of the remainder (22). While it is currently unknown how the associated SNPs in CACNA1C affect function or levels of the protein, it is critical to understand how changes in protein levels may consequently affect behavior. We investigated the effects of Cacna1c haploinsufficiency on mouse behavior in both sexes in tests with relevance to human mood disorders. Based upon sex differences we observed in our mouse behavioral data, we assessed a gene x sex interaction of SNPs of CACNA1C with mood disorders in human subjects across 2,021 cases and 1,840 controls.
Materials & Methods
Animals
Founder mice were obtained from Jackson Laboratories (stock number 005783, Bar Harbor, ME) and had been backcrossed to C57BL/6J for at least seven generations. See online Supplement for additional details.
Behavioral Tests
Mice were tested in the open field, homecage activity, holeboard, elevated plus maze, light-dark box, novelty induced hypophagia, stress-induced hyperthermia, sensitization to d-amphetamine, acoustic startle, and forced swim tests using established methods. Minor alterations to the tail suspension tests were required to prevent the tendency of C57BL/6J mice to climb their tails (23). A clear plastic cylinder (4cm length, 1.5cm diameter) was placed around their tails. The learned helplessness procedure was performed in a Coulbourn Mouse Shuttle Cage (Coulbourn Instruments; Whitehall, PA) and consisted of 3 stages: Stage 1: 120 inescapable shocks, each 15 seconds at 0.3 mA with a 15 second intertrial interval. Stage 2: 30 trials, 15 second shocks, 0.3 mA, average intertrial interval of 20 seconds. In trials p1–p5, gate opened when the shock began. In trials 1–25, gate opened 3 seconds following shock. Stage 3: retest. See online Supplement for additional details for all behavioral tests.
Human genetics cohorts
Data were collected by the NIMH Genetics Initiative Bipolar Disorder Consortium (NIMH-BP) (24) and the Genetics of Recurrent Early-Onset Major Depression Consortium (GenRED) (25). The NIMH-BP SNP genotyping was performed on 1,001 BP cases and 1,034 unrelated controls as part of the Genetic Association Information Network Bipolar Initiative and Bipolar Genome Study (11). In a separate effort, genome-wide SNP genotyping was performed on 1,020 MDD cases from the GenRED sample and 1,636 unrelated controls (15). Genotyping in both samples was performed using the Affymetrix Genome-wide Human SNP Array 6.0 and we used the cleaned genotype data from each sample. Quality control measures and analytic methods are described in the online Supplement. We combined the data from the NIMH-BP and GenRED samples at the genotype level in a mega-analysis using PLINK (26), keeping only those SNPs common to both datasets. In addition, 830 controls were common to both NIMH-BP and GenRED and were included in the dataset only once. For the analysis of rs2370411 and rs2370419 by sub-study, unique controls were apportioned to each sub-study to avoid overlap. We extracted 190 SNPs located in the gene CACNA1C or within 10 kb of the gene boundaries for analysis. All alleles are presented in the forward strand orientation.
Statistical Analysis
Statistical analysis of the mouse behavioral data was performed using Graphpad Prism Version 5. Statistics used were 2-tailed t-test or repeated measure two-way ANOVA, either paired or unpaired dependent upon the experimental design and Bonferroni posthoc test was performed when indicated. Evaluation of equal variances was performed using Bartlett’s test. Detailed statistical results for mouse behavioral data are included in the online Supplement (Table S1). Data are reported as mean ± S.E.M. and P<0.05. Male and female mice were assessed on separate days and therefore (because of likely effects of day) direct statistical comparison between sexes was not made.
Results
Baseline behaviors, activity, and exploration
As homozygous deletion of Cacna1c results in embryonic lethality in mice (27) we conducted studies with heterozygous (Cacna1c+/−, HET) Cacna1c knockout mice and compared these animals to their wild type (Cacna1c+/+, WT) littermates. Western blot analysis confirmed that heterozygous knockout of Cacna1c results in a significant decrease in levels of Cav1.2 without a change in Cav1.3, which is the most likely protein to compensate for such changes (Supplement: Figure S1). As expected, whole-cell voltage-clamp recordings made from CA1 pyramidal cells in ex vivo hippocampal slices revealed that the fraction of the current sensitive to nimodipine, a dihydropyridine L-type calcium channel blocker, was significantly larger in cells from WT mice than in cells from HET mice (P<0.05) (Supplement: Figure S1). Due to the widespread expression of Cacna1c in the mouse brain, and thus the potential for non-specific effects on behavior, baseline sensory motor function was assessed in male and female mice. We found no significant differences between genotypes in motor coordination and motor learning as assessed on the repeated accelerating rotarod, olfaction as assessed in the hidden cookie test, pain sensitivity in the hot plate test, or muscle strength in the hanging wire test (Supplement: Figure S2).
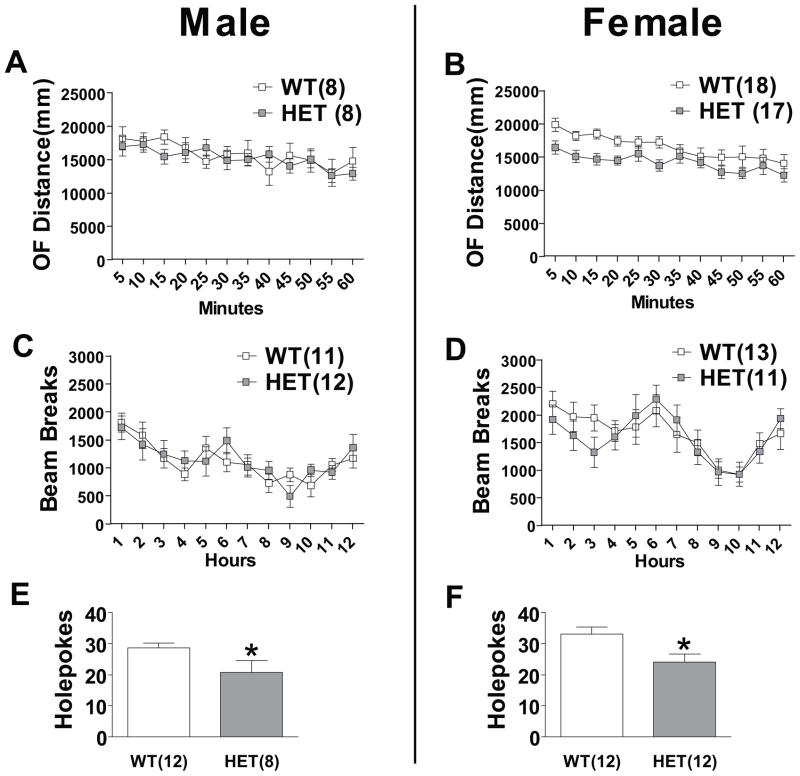
We assessed general locomotor activity in a 50 x 50 cm open field in both male and female mice (Figure 1a and b). Repeated measure two-way ANOVA indicated no significant difference in open field locomotion between male WT and HET mice. However, female HET mice displayed a subtle, yet statistically significant, decrease in locomotor activity in the open field (P<0.05). Bartlett’s test of equal variances revealed no significant differences in the variance between male and female mice of both genotypes suggesting estrous cycle was not influencing behavior (Supplement: Table S1).
Figure 1. Effect of Cacna1c HET knockout on activity and exploration.
(a) Male HET mice did not differ significantly from WT mice in open field locomotion. (b) In females, genotype had a significant effect on locomotion in the open field (P<0.05). Neither Male (c) nor Female (d) HET mice were significantly different than WT mice in homecage activity. (e) Male and (f) female HET mice had lower exploratory activity on the holeboard test. Data are expressed as mean ± SEM. *, P≤0.05.
To assess baseline activity in a non-novel environment we tracked home cage activity in both male and female mice. Mice were single housed for at least one week before homecage activity was monitored by an infrared tracking system during the 12 hours corresponding to the dark cycle. Neither male nor female HET mice displayed significant differences in home cage locomotion compared to WT mice (Figure 1c and d). The holeboard test measures exploratory behavior and previous work has reported that lithium, one of the most effective mood stabilizing medications, decreases holepoke behavior in mice (28). We found both male (P<0.05) and female (P<0.05) HET mice displayed a significantly lower number of holepokes during a five minute test (Figure 1e and f).
Anxiety/risk-taking behavior
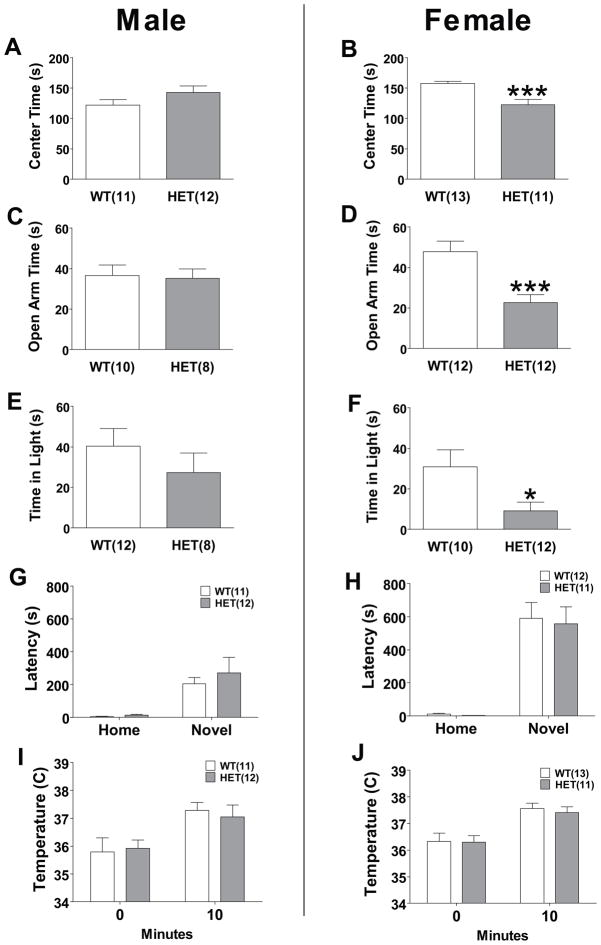
We explored possible differences between WT and HET mice in multiple tests of anxiety and risk-taking behavior. We tested WT and HET mice of both sexes in a 100x100cm open field, which is more sensitive to thigmotaxis than a smaller arena, over a 10 minute period (Figure 2a and b). Female HET mice only showed significantly decreased center time (P<0.001). This increased thigmotaxis observed in female, but not male, HET mice suggest an increased anxiety or a decreased risk-taking phenotype. The elevated plus maze explores the anxiety and risk-taking associated with entering an exposed, raised platform. Over five minutes, we measured time spent on the open arms in WT and HET mice (Figure 2c and d). In females only, HET mice spent significantly less time in the open arm (P<0.001) indicating lower risk-taking or greater anxiety; however, female HET mice also had a significant decrease in number of total arm entries (P<0.001) indicating lower overall activity. We also tested WT and HET mice in the light-dark box (Figure 2e and f). Increased time spent in the aversive light compartment is used as an inverse measure of anxiety, and is related to risk-taking. In females only, HET mice spent significantly less time in the light area compared to WT mice (P<0.05), but there was no significant difference in number of crosses between the two areas. Additionally, in both males and females, genotype had no significant effect on time for first emergence to the light area or total number of crosses.
Figure 2. Effect of Cacna1c HET knockout on risk-taking and anxiety-like behavior.
(a) Male HET mice had no significant difference in center time in a large open field. (b) Female HET mice spent less time in the center of a large open field. (c) There was no significant difference in time spent on the open arms of the elevated plus maze in male HET mice. (d) Female HET mice spent less time on the open arms of the elevated plus maze. (e) There was no significant difference in the time male HET mice spent in the light compartment of the light-dark box. (f) Female HET mice spent less time in the light compartment of the light-dark box. There was no significant effect of genotype in either (g) male or (h) female to modify latency to drink in the novel cage when compared to WT mice. There was no significant effect of genotype in either (i) male or (j) female subjects to modify hyperthermia in response to stress. Data are expressed as mean ± SEM. *, P≤0.05; ***, P<0.001.
We assessed the behavior of mice in two additional tests, novelty-induced hypophagia and stress-induced hyperthermia, that do not rely upon the animals entering an exposed area from a safer enclosed area. The novelty-induced hypophagia test assesses anxiety by comparing changes in latency to consume a familiar, palatable liquid between subjects’ home cage and a novel cage (29). In both males and females, a repeated measure two-way ANOVA revealed a significant increase in latency to consume the liquid in the novel cage (P<0.001), but no significant effect of genotype and no significant interaction (Figure 2g and h).
The physiological response to a stressful or anxiety-provoking event is an increase in body temperature and this increase is attenuated by anxiolytics (30). A repeated measure two-way ANOVA revealed both male and female WT and HET mice had a significant increase in temperature after stress (P<0.001), but there was no significant effect of genotype and no significant interaction (Figure 2i and j).
Response to d-amphetamine and acoustic startle
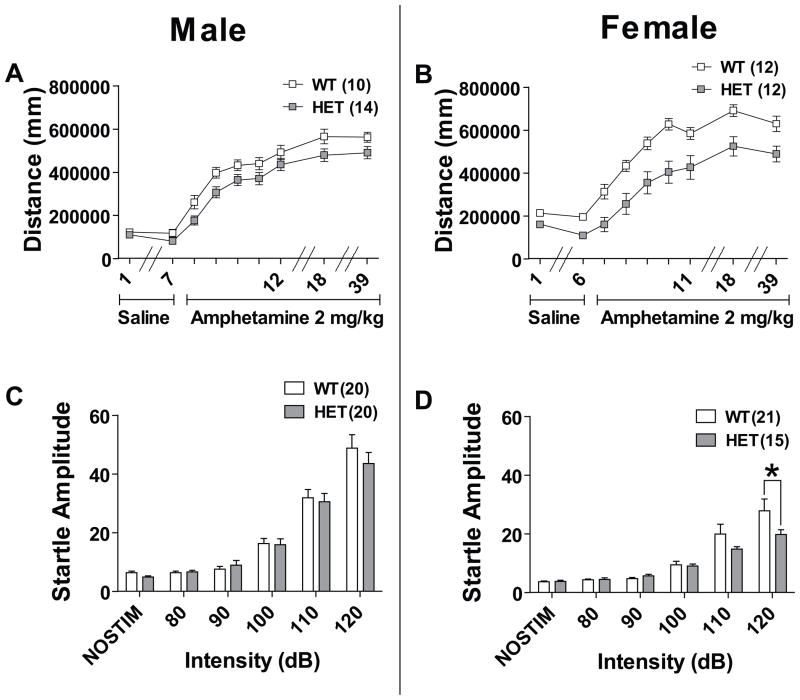
Amphetamine-induced hyperlocomotion is a model of the hyperactivity associated with bipolar mania; this hyperactivity is attenuated by lithium in both humans and mice (31–34). We conducted amphetamine sensitization in three stages: habituation, sensitization to d-amphetamine, and long-term sensitization or challenge (35). In males, both WT and HET mice habituated to the same level of locomotion with saline injections between the first and last days (Figure 3a). During d-amphetamine sensitization repeated measure two-way ANOVA revealed a significant effect of day (P<0.001) and a significant effect of genotype on distance traveled (P<0.05). Between days 18 and 39, a repeated measure two-way ANOVA revealed a trend for effect of genotype (P=0.063) indicating a long-lasting attenuation in response to d-amphetamine in male HET mice. In females, HET mice started at and habituated to a significantly lower level of locomotion compared to WT mice with a significant effect of day (P<0.001) and genotype (P<0.001) on distance traveled and a trend for an interaction between genotype and day (P=0.056) (Figure 3b). In females there was a significant effect of genotype on locomotion response to d-amphetamine during both sensitization days and challenge days 18 and 39 (P<0.01).
Figure 3. Effect of Cacna1c HET knockout on response to d-amphetamine and acoustic startle response.
(a) Male WT and HET mice habituated at the same level of locomotion during saline injection days 1–7. There was a significant effect of genotype to modify sensitization to d-amphetamine over days 8–12 in male mice (P<0.05). In males there was a trend for an effect of genotype to modify long-term sensitization to d-amphetamine on day 39 (P=0.063) but not day 18. (b) Female HET mice had lower locomotion during the saline habituation phase days 1–6 (P<0.001). In female HET mice there was a significant effect of genotype on sensitization to d-amphetamine (P<0.001). There remained a significant effect of genotype when females were re-challenged with d-amphetamine sensitization days 18 and 39 (P<0.001). (c) Male HET mice had no significant difference in response to multiple acoustic startle intensities. (d) Female HET mice had significantly lower acoustic startle response at 120dB. Data are expressed as mean ± SEM. *, P≤0.05 Bonferroni posttest.
Changes in acoustic startle response (ASR) can be induced by pharmacological (36) or genetic manipulations of the dopaminergic system (37). Changes in ASR habituation have also been reported in humans during the manic phase of BP (38). Mice were tested on their response to startle pulse intensities of 80, 90, 100, 110, and 120dB. In male mice, we found no significant difference between genotypes at all startle intensities (Figure 3c). In female mice, a repeated measure two-way ANOVA revealed a significant interaction between genotype and startle intensity (P<0.05) and a Bonferroni posthoc test indicated that HET mice startled significantly less at the 120dB pulse (Figure 3d; P<0.05). The normal startle response of female HET mice at intensities between 80–110dB suggest that the attenuated startle at 120dB is not due to a non-specific effect of Cacna1c haploinsufficiency on hearing.
Tests of antidepressant efficacy
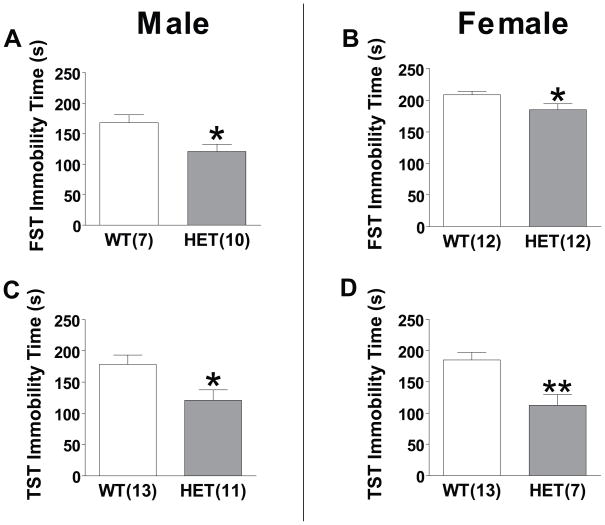
Previous studies indicate that administration of L-type calcium channel antagonists to rodents results in antidepressant-like effects in the forced swim test (FST) and tail suspension test (TST) (39). Similarly, lithium has been reported to decrease immobility time in these tests (28,31,40). In both male and female mice, HET mice spent significantly less time immobile than WT mice in the FST (P<0.05) (Figure 5a and b). Similarly, in the TST, both male and female HET mice had significantly less immobility time (P<0.05) (Figure 4).
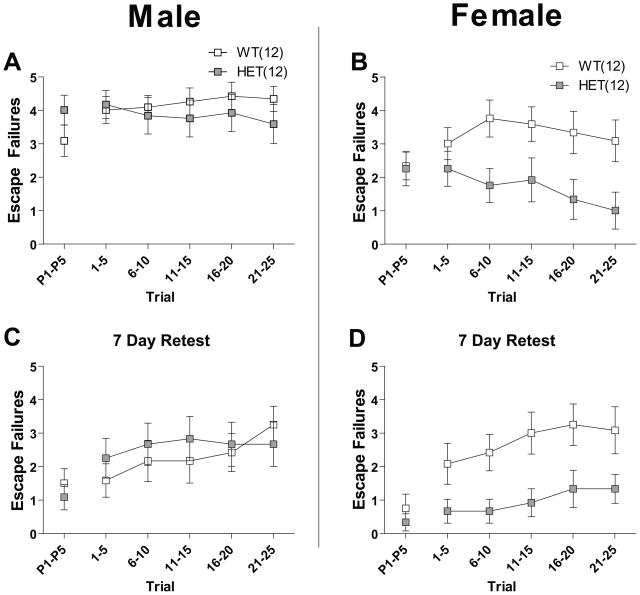
Figure 5. Effect of Cacna1c HET knockout on depression-like behavior in the learned helplessness model.
Mice were tested one and seven days after induction of learned helplessness with 0.3 mA inescapable shocks. In trials P1–P5, shock start and gate opening was simultaneous. In trials 1–25, a delay of three seconds was imposed between shock start and gate opening. In male mice on days 1 (a) and 7 (c), there was no significant effect of genotype in trials P1–P5, and in trials 1–25. In female mice on days 1 (b) and 7 (d), there were no significant differences in performance in trials P1–P5, but there was a significant effect of genotype in trials 1–25 (P<0.05). Data are expressed as mean ± SEM.
Figure 4. Effect of Cacna1c HET knockout in tests of antidepressant efficacy.
(a) Male HET mice spent significantly less time immobile on the forced swim test (FST) (b) Female HET mice spent significantly less time immobile on the FST. (c) Male HET mice spent significantly less time immobile in the tail suspension test (TST) (d) Female HET mice spent significantly less time immobile in the TST. Data are expressed as mean ± SEM. *, P≤0.05 **, P<0.01.
Learned helplessness model of depression
The learned helplessness test is a validated model of depression-like behavior (41). Twenty four hours following exposure to inescapable/uncontrollable shock we tested WT and HET mice in a shuttle box to measure their motivation to escape shock. We measured number of escape failures, defined as failure to escape and thus terminate shock, both 24 hours after induction of the learned helplessness effect and then seven days after first testing. In trials P1–P5, shock onset and gate opening occurred simultaneously. In subsequent trials, 1–25, gate opening occurred three seconds after shock onset. In females, there was a significant effect of genotype (P<0.05) on number of escape failures in trials 1–25 on both days (Figure 5b and d). Female HET mice showed fewer escape failures. Male HET mice did not significantly differ from WT mice on either day (Figure 5a and c). There was no significant difference between genotype in trials P1–P5 in either sex for either day. Female, but not male, HET mice also displayed significantly lower mean escape latencies (P<0.05) for trials 1–25 on both days (Supplement: Figure S3). In addition, there was no significant difference in escape latencies between naïve WT and HET mice not exposed to inescapable shock 24 hours prior to testing (Supplement: Figure S3). Together these data suggest that in female mice, Cacna1c haploinsufficiency attenuates the development of depressive-like behavior due to uncontrollable shock.
Analysis of human genetic CACNA1C data for female-specific association
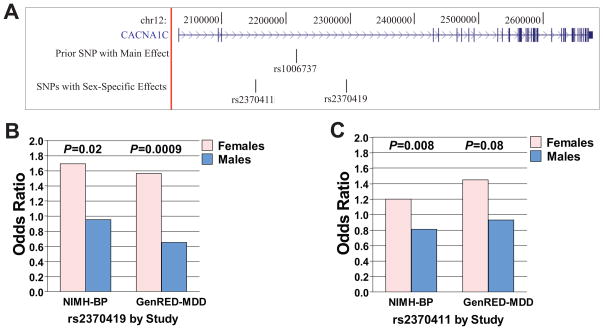
Given the sex-specific findings in some of our mouse behavioral studies, we hypothesized that in human mood disorders data sex-specific genetic association would be observed for SNPs in CACNA1C. We examined our data from the GWAS of the NIMH-BP (11) and GenRED samples (15). We utilized a combined dataset that included 2,021 mood disorder cases (1,223 females and 798 males) and 1,840 controls (837 females and 1,003 males) in a mega-analysis and focused our examination on 190 SNPs genotyped in CACNA1C and within 10 kb of the gene (Figure 6a). The 15 CACNA1C SNPs showing nominally significant interactions with sex are listed in Table 1 (full results are shown in Supplement: Table S2). Of these, rs2370419 showed an effect in females (OR=1.64; P=4.1 x 10−4), but not in males (OR=0.82; P=0.16), with an interaction that remained significant after correction for testing 190 SNPs (P=1.4 x 10−4; Pcorrected=0.03) (Figure 6b). A second SNP, rs2370411, also showed an interaction with sex that remained significant after correction (P=2.1 x10−4, Pcorrected=0.04). This SNP also showed an association with mood disorder in females (OR=1.32, P=1.9 x10−4), but not in males (OR=0.86, P=0.07) (Figure 6c). These effects were consistent across the two data sets (NIMH-BP and GenRED), with the minor alleles associated with increased risk of illness in females, but not males, in each set. These two SNPs are both intronic, are located ~142 kb from each other, and are in modest linkage disequilibrium (LD) with an r2=0.22 (D′=0.85). Their minor allele frequencies, by sex, are shown in Supplement: Table S2. Of note, this table also shows results for the overall male+female analyses, in which the strongest result yielded a p-value of 0.0051, an order of magnitude less strong than the best female-specific p-value.
Figure 6. Two human intronic CACNA1C single nucleotide polymorphisms (SNPs) show sex-specific association with mood disorders.
(a) CACNA1C is a 644.7 kb gene located on chromosome 12 at 2,032,677 bp to 2,677,376 bp (hg18). rs2340419 and rs2370411 are located within intron 3, which is 329 kb long. They are 142 kb from each other. rs1006737, the most significant result in a prior BP GWAS meta-analysis (10), is also in intron 3, located between rs2370411 and rs2370419. (b) Results by study for rs2370419. NIMH-BP: female odds ratio 1.69, male odds ratio, 0.95. GenRED-MDD: female odds ratio 1.56, male odds ratio 0.65. Both studies showed nominally significant p-values for the interaction of sex and diagnosis. Controls were non-overlapping. (c) Results by study for rs2370411. NIMH-BP: female odds ratio 1.20, male odds ratio, 0.81. GenRED-MDD: female odds ratio 1.45, male odds ratio 0.93. Both studies showed nominally significant p-values for the interaction of sex and diagnosis. Controls were non-overlapping.
Table 1.
Single nucleotide polymorphisms in CACNA1C with nominally significant sex-specific and interaction results in a combined bipolar disorder and major depression dataset.
| Female (N=2,060) | Male (N=1,801) | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|
| SNP | BP locationa | Minor Allele | ORb | Pc | ORb | Pc | ORb | Pc |
| rs2370419 | 2294118 | A | 1.64 | 0.00041 | 0.82 | 0.16 | 0.47 | 0.00014 |
| rs2370411 | 2152186 | C | 1.32 | 0.00019 | 0.86 | 0.071 | 0.67 | 0.00021 |
| rs7295089 | 2310725 | C | 1.25 | 0.015 | 0.87 | 0.13 | 0.7 | 0.0058 |
| rs17801265 | 2468876 | A | 1.19 | 0.052 | 0.86 | 0.11 | 0.56 | 0.0062 |
| rs4765966 | 2615463 | A | 1.24 | 0.13 | 0.74 | 0.035 | 0.7 | 0.0065 |
| rs11062241 | 2456847 | T | 1.53 | 0.046 | 0.79 | 0.25 | 0.77 | 0.018 |
| rs2239016 | 2153653 | G | 0.89 | 0.1 | 1.13 | 0.11 | 0.47 | 0.026 |
| rs104629 | 2625616 | A | 1.13 | 0.35 | 0.72 | 0.024 | 0.49 | 0.027 |
| rs1006737 | 2215556 | A | 1.17 | 0.025 | 0.94 | 0.36 | 0.8 | 0.029 |
| rs4765905 | 2219845 | C | 1.16 | 0.031 | 0.93 | 0.34 | 0.64 | 0.03 |
| rs2239015 | 2153456 | T | 0.9 | 0.11 | 1.12 | 0.13 | 0.82 | 0.031 |
| rs758170 | 2231721 | T | 1.15 | 0.041 | 0.93 | 0.34 | 1.24 | 0.039 |
| rs4765663 | 2049021 | C | 1.06 | 0.53 | 0.8 | 0.019 | 0.83 | 0.039 |
| rs2238043 | 2145924 | A | 1.18 | 0.015 | 0.96 | 0.59 | 1.25 | 0.04 |
| rs882194 | 2220713 | G | 1.06 | 0.37 | 0.88 | 0.071 | 0.83 | 0.048 |
BP=base pair. All bp locations are on chromosome 12 from NCBI Build 36/hg18. All SNPs listed here are intronic.
OR=odds ratio. Calculated under an additive model for the minor allele.
Uncorrected p-value
Discussion
Our data indicate that in male and female mice, Cacna1c haploinsufficiency is associated with decreased exploratory behavior, decreased hyperlocomotion in response to amphetamine, and antidepressant-like behavior in the FST and TST. There are also sex differences in the effects of Cacna1c haploinsufficiency: females exhibit more robust attenuation of amphetamine-induced hyperlocomotion than males and, unlike males, display an attenuated acoustic startle response and reduced development of learned helplessness, along with phenotypes of increased anxiety or decreased risk taking. Existing genetic animal models of mania-related behaviors display hyperactivity, increased risk-taking or decreased anxiety, increased responsiveness to amphetamine, and increased acoustic startle response (37,42–48). On the other hand, lithium treatment and a model of lithium action, the GSK-3β heterozygous knockout mouse, results in decreased immobility time in the FST and TST, decreased exploratory behavior in the holeboard test, and decreased response to amphetamine (28,31,40,49,50). Taken together with this evidence, our results suggest that Cacna1c haploinsufficiency in mice results in a resilient, or “mood stabilized”, phenotype that is modified by sex for some but not all behaviors.
It remains unclear why some behaviors are modified by sex, while others are not. In particular, both male and female HET mice showed antidepressant-like behavior in the TST and FST, but only female HET mice are resistant to depression-like behavior in the learned helplessness test. This may be related to the fact that the TST and FST we utilized are models of antidepressant efficacy, while the learned helplessness model has better validity as a measure of depression. However, overall, there is an imprecise relationship between mouse behavioral tests and human psychiatric conditions.
The behavior of female HET mice in the elevated plus maze, light-dark box, and open field could be interpreted as either increased anxiety or decreased risk taking. Indeed, these are well-validated tests of anxiety-like behavior, which also involve a significant risk component. This distinction is important, as increased risk taking, but not decreased anxiety, is associated with the manic phase of BP (43,51). Several lines of evidence oppose the conclusion that female HET mice are more anxious. First, we did not observe increased anxiety-like behavior in HET mice in the novelty-induced hypophagia and stress-induced hyperthermia tests. While the elevated plus maze, light-dark box, and open field thigmotaxis all assess exploration into a naturally dangerous, open area as a measure of anxiety, the novelty-induced hypophagia and stress-induced hyperthermia tests do not rely on entry into potentially dangerous areas, and thus may be less related to taking risks. Secondly, increased acoustic startle is associated with other measures of anxiety, and increased startle responses can be decreased by administration of anxiolytic drugs (52,53), and we observed decreased acoustic startle in female HET mice.
Female HET mice also displayed decreased locomotion in the open field, which may confound some of our results. There was no significant difference in homecage locomotion in either male or female HET mice when compared to their WT littermates. Similarly, the lack of difference in the accelerating rotarod test of coordination or the hanging wire test, as well as the increase in activity observed in the FST and TST suggests that there are no innate motor deficits in these mice. However, due to baseline differences in open field locomotion, the effects of genotype on amphetamine-induced hyperlocomotion in females should be interpreted with caution.
Because we observed sex differences in behavioral alterations in Cacna1c haploinsufficient mice, we hypothesized that CACNA1C genotype may interact with sex to influence a mood disorder diagnosis in humans. We found evidence for a female-specific association of two intronic SNPs, with the minor alleles being more prevalent in mood-disordered women than in control women. While SNPs in CACNA1C associated with a mood disorder diagnosis currently have no known function, rs2370411 is within ~25–50 bp of a region with high regulatory potential (54) and rs2370419 is within ~800 bp of such a region, while numerous variants in additional high regulatory potential regions are in LD with both SNPs. We also note that the minor allele frequencies in control women were lower than in control men. This could be the result of random fluctuation, though it also could be related to our screened control sample. Because we excluded 35% of controls due to evidence of mood disorders, the remainder might be depleted for mood disorder susceptibility alleles, a phenomenon which would be sex-specific in the case of alleles conferring sex-specific disease vulnerability. Consistent with earlier work (10), we did not observe a robust sex-specific effect for rs1006737, the SNP previously most strongly implicated in BP, although we did see a nominally significant one, as shown in Table 1. rs1006737 is located roughly midway between the two SNPs that we have highlighted, and is in partial LD with them (with rs2370419: r2=0.096, D′=0.81; with rs2370411: r2=0.033, D′=0.26).
Previous GWAS reports in mood disorders have also shown strong associations with rs10848632 (9), rs10774037 (10), rs1024582 (10) and rs11614275 (15). These SNPs are all located within 97 kb of each other in the same intron of CACNA1C, again located between the two SNPs we have highlighted, rs2370419 and rs2370411. Given that all of these SNPs are located within a large block of linkage disequilibrium encompassing ~150 kb, it is possible that the varied association signals in different samples all reflect a single regulatory element in the region, which has both sex-independent and sex-dependent variants within it. Alternatively, there could be multiple regulatory elements within this block. Meta-analysis of data on SNPs across this region and dissection of potential sex-specific effects using a very large combined mood disorders set will soon be possible due to the efforts of the Psychiatric GWAS Consortium (55).
A limitation of our study is that we did not investigate the molecular mechanism through which Cacna1c haploinsufficiency exerts its effects on behavior. While we identified significant reductions in Cav1.2 protein levels and dihydropyridine sensitive calcium current in male mice (Supplement: Figure S1), it is possible that such measures are modified by sex. Cav1.2 is important in modifying the effects of synaptic activity on cell survival, synaptic plasticity, and gene expression (56). Previous work has linked Cav1.2 to MAPK pathway activation (57), increased immediate early gene expression (58), and a proteolytically cleaved C-terminal fragment of the channel acts as a transcription factor (59). Intracellular calcium levels regulated specifically by Cav1.2 influence a number of intracellular signaling cascades that modify expression of calcium-dependant genes including Bcl-2, BDNF, and c-fos (60). Other studies have focused on Cav1.2′s role in learning and memory using such conditional knockout mice (61,62). While a conditional knockout approach may better isolate Cav1.2 function in adult neurons, the heterozygous knockout model used in our study likely more closely mimics a possible clinical situation, where a nearly complete absence of Cav1.2 function is unlikely. In addition, Cav1.2 mRNA levels increase in the VTA following repeated amphetamine administration, which may be related to the attenuated response to amphetamine observed in Cacna1c HET mice (63). We further note that significantly increased CACNA1C mRNA levels were seen in postmortem brains from BP patients in the array collection of the Stanley Medical Research Institute (www.stanleygenomics.org). Relevant to the sex-differences we observed, physiological levels of estrogen potentiate L-type calcium channels in vitro (64) and some of the neuroprotective effects of estrogen on glutamate-induced cell death have been linked to L-type calcium channels (65).
Overall, our behavioral results provide evidence that decreases in Cav1.2 function may play a protective role in the development of mood disorders, and our human genetic and preclinical data support an interaction between CACNA1C and sex. Further research is needed to investigate the mechanisms by which Cav1.2 produces its sex-dependent spectra of behavioral effects.
Supplementary Material
Table 2.
Minor allele frequencies, by sex, in cases and controls, for two intronic CACNA1C SNPs.
| rs2370419 | rs2370411 | |||
|---|---|---|---|---|
| Case | Control | Case | Control | |
| Female | ||||
| Combineda (N=2,060) | 0.075 | 0.049 | 0.276 | 0.225 |
| NIMH-BP (N=929) | 0.077 | 0.048 | 0.275 | 0.237 |
| GenRED (N=1,131) | 0.073 | 0.049 | 0.276 | 0.213 |
| Male | ||||
| Combineda (N=1,801) | 0.062 | 0.075 | 0.236 | 0.259 |
| NIMH-BP (N=930) | 0.068 | 0.072 | 0.236 | 0.277 |
| GenRED (N=871) | 0.051 | 0.077 | 0.237 | 0.245 |
NIMH-BP and GenRED samples combined
Acknowledgments
Bipolar Genome Study Co-Authors: John R. Kelsoe, Tiffany A. Greenwood, Paul Shilling, Caroline Nievergelt, Nicholas, Schork, Erin N. Smith, Cinnamon Bloss, John Nurnberger, Howard J. Edenberg, Tatiana Foroud, Elliot Gershon, Chunyu Liu, Judith A. Badner, William Sheftner, William B. Lawson, Evaristus A. Nwulia, Maria Hipolito William, John Rice, William Byerley, Francis McMahon, Thomas G. Schulze, Thomas Barrett, Wade Berrettini, Melvin G. McInnis, Sebastian Zöllner, David Craig, Szabolics Szelinger. This work was supported by National Institutes of Health grants MH078151 (J.R.K.), MH059542 (W.C.), MH059552 (J.B.P.), MH061686 and K24MH64197 (D.F.L.), MH059541 (W.A.S.) and MH060912 (M.M.W.).
The GenRED cell and data collections used in this study included contributions from Dr. George S. Zubenko and Dr. Wendy N. Zubenko, Department of Psychiatry, University of Pittsburgh School of Medicine, that were supported by R01 grant MH60866 from the National Institute of Mental Health (GSZ, PI). The NIMH Cell Repository at Rutgers University and the NIMH Center for Collaborative Genetic Studies on Mental Disorders made essential contributions to this project. Data and biomaterials were collected in six projects that participated in the National Institute of Mental Health (NIMH) Genetics of Recurrent Early-Onset Depression (GenRED) project. From 1999–2003, the Principal Investigators and Co-Investigators were: New York State Psychiatric Institute, New York, NY, R01 MH060912, Myrna M. Weissman, Ph.D. and James K. Knowles, M.D., Ph.D.; University of Pittsburgh, Pittsburgh, PA, R01 MH060866, George S. Zubenko, M.D., Ph.D. and Wendy N. Zubenko, Ed.D., R.N., C.S.; Johns Hopkins University, Baltimore, R01 MH059552, J. Raymond DePaulo, M.D., Melvin G. McInnis, M.D. and Dean MacKinnon, M.D.; University of Pennsylvania, Philadelphia, PA, RO1 MH61686, Douglas F. Levinson, M.D. (GenRED coordinator), Madeleine M. Gladis, Ph.D., Kathleen Murphy-Eberenz, Ph.D. and Peter Holmans, Ph.D. (University of Wales College of Medicine); University of Iowa, Iowa City, IW, R01 MH059542, Raymond R. Crowe, M.D. and William H. Coryell, M.D.; Rush University Medical Center, Chicago, IL, R01 MH059541-05, William A. Scheftner, M.D. Rush-Presbyterian. Data and biomaterials for the NIMH samples were collected as part of 10 projects that participated in the NIMH Bipolar Disorder Genetics Initiative. From 1991 to 1998, the Principal Investigators and Co-Investigators were: Indiana University, Indianapolis, IN, U01 MH46282 John Nurnberger, MD, PhD, Marvin Miller, MD and Elizabeth Bowman, MD; Washington University, St Louis, MO, U01 MH46280 Theodore Reich, MD, Allison Goate, PhD and John Rice, PhD; Johns Hopkins University, Baltimore, MD U01 MH46274 J Raymond DePaulo Jr, MD Sylvia Simpson, MD, MPH and Colin Stine, PhD; NIMH Intramural Research Program, Clinical Neurogenetics Branch, Bethesda, MD Elliot Gershon, MD, Diane Kazuba, BA and Elizabeth Maxwell, MSW. From 1999 to 2003, the Principal Investigators and Co-Investigators were: Indiana University, Indianapolis, IN, R01 MH59545 John Nurnberger, MD, PhD, Marvin J Miller, MD, Elizabeth S Bowman, MD, N Leela Rau, MD, P Ryan Moe, MD, Nalini Samavedy, MD, Rif El-Mallakh, MD (at University of Louisville), Husseini Manji, MD (at Wayne State University), Debra A Glitz, MD (at Wayne State University), Eric T Meyer, MS, Carrie Smiley, RN, Tatiana Foroud, PhD, Leah Flury, MS, Danielle M Dick, PhD and Howard Edenberg, PhD; Washington University, St Louis, MO, R01 MH059534, John Rice, PhD, Theodore Reich, MD, Allison Goate, PhD and Laura Bierut, MD; Johns Hopkins University, Baltimore, MD, R01 MH59533 Melvin McInnis, MD, J Raymond DePaulo Jr, MD, Dean F MacKinnon, MD, Francis M Mondimore, MD, James B Potash, MD, Peter P Zandi, PhD, Dimitrios Avramopoulos and Jennifer Payne; University of Pennsylvania, PA, R01 MH59553 Wade Berrettini, MD, PhD; University of California at Irvine, CA, R01 MH60068 William Byerley, MD and Mark Vawter, MD; University of Iowa, IA, R01 MH059548 William Coryell, MD and Raymond Crowe, MD; University of Chicago, Chicago, IL, R01 MH59535 Elliot Gershon, MD, Judith Badner, PhD, Francis McMahon, MD, Chunyu Liu, PhD, Alan Sanders, MD, Maria Caserta, Steven Dinwiddie, MD, Tu Nguyen, Donna Harakal; University of California at San Diego, CA, R01 MH59567 John Kelsoe, MD, Rebecca McKinney, BA; Rush University, IL, R01 MH059556 William Scheftner, MD, Howard M Kravitz, DO, MPH, Diana Marta, BA, Annette Vaughn-Brown, MSN, RN and Laurie Bederow, MA; NIMH Intramural Research Program, Bethesda, MD, 1Z01MH002810-01, Francis J McMahon, MD, Layla Kassem, PsyD, Sevilla Detera-Wadleigh, PhD, Lisa Austin, PhD, Dennis L Murphy, MD. Control subjects from the National Institute of Mental Health Schizophrenia Genetics Initiative (NIMH-GI), data and biomaterials were collected by the “Molecular Genetics of Schizophrenia II” (MGS-2) collaboration. The investigators and coinvestigators are: ENH/Northwestern University, Evanston, IL, MH059571, Pablo V. Gejman, M.D. (Collaboration Coordinator; PI), Alan R. Sanders, M.D.; Emory University School of Medicine, Atlanta, GA,MH59587, Farooq Amin, M.D. (PI); Louisiana State University Health Sciences Center; New Orleans, Louisiana, MH067257, Nancy Buccola APRN, BC, MSN (PI); University of California-Irvine, Irvine, CA,MH60870, William Byerley, M.D. (PI); Washington University, St. Louis, MO, U01, MH060879, C. Robert Cloninger, M.D. (PI); University of Iowa, Iowa, IA,MH59566, Raymond Crowe, M.D. (PI), Donald Black, M.D.; University of Colorado, Denver, CO, MH059565, Robert Freedman, M.D. (PI); University of Pennsylvania, Philadelphia, PA, MH061675, Douglas Levinson M.D. (PI); University of Queensland, Queensland, Australia, MH059588, Bryan Mowry, M.D. (PI); Mt. Sinai School of Medicine, New York, NY,MH59586, Jeremy Silverman, Ph.D. (PI). Genome-wide SNP genotyping of the NIMH samples was performed through the Genetic Association Information Network under the direction of the Bipolar Genetics Studies (BiGS) Collaboration. The BiGS Principal Investigators and Co-Investigators were: University of California San Diego, La Jolla, CA, John R. Kelsoe, M.D. (PI), Tiffany A. Greenwood, Ph.D., Caroline Nievergelt, Ph.D.; Scripps Research Institute, La Jolla, CA: Nicholas Schork, Ph.D. (PI), Erin N. Smith, Ph.D., Cinnamon Bloss, Ph.D.; Indiana University, Bloomington, IN, John Nurnberger, M.D. (PI), Howard J. Edenberg, Ph.D., Tatiana Foroud, Ph.D.; University of Chicago, Chicago, IL, Elliot Gershon, M.D. (PI), Chunyu Liu, Ph.D., Judith A. Badner, Ph.D.; Rush University Medical Center, Chicago, IL, William A. Scheftner, M.D.; Howard University, Washington, DC, William B. Lawson, M.D. (PI), Evaristus A. Nwulia, M.D., Maria Hipolito, M.D.; University of Iowa, Iowa City, IA, William Coryell, M.D. (PI); Washington University, St. Louis, MO, John Rice, Ph.D. (PI); University of California San Francisco, San Francisco, CA, William Byerley, M.D. (PI); National Institute of Mental Health, Bethesda, MD, Francis McMahon, M.D. (PI), Thomas G. Schulze, M.D.; University of Pennsylvania, Philadelphia, PA, Wade Berrettini, M.D., Ph.D. (PI); Johns Hopkins University, Baltimore, MD, James B. Potash, M.D. (PI), Peter P. Zandi, Ph.D., Pamela Belmonte Mahon, PhD; University of Michigan, Ann Arbor, MI, Melvin G. McInnis, M.D. (PI), Sebastian Zöllner, Ph.D.; Translation Genomic Research Institute, Phoenix, AZ, David Craig, Ph.D. (PI), Szabolics Szelinger. Genotyping services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, contract number N01-HG-65403. The authors express their profound appreciation to the families who participated in this project, and to the many clinicians who facilitated the referral of participants to the study.
Footnotes
Financial Disclosures
JAK is on the Scientific Advisory Committee for Next Generation Sequencing of Life Technologies, Inc. and is a technical advisor to SoftGenetics, Inc. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gershon ES, Hamovit J, Guroff JJ, Dibble E, Leckman JF, Sceery W, et al. A family study of schizoaffective, bipolar I, bipolar II, unipolar, and normal control probands. Archives of general psychiatry. 1982;39:1157–1167. doi: 10.1001/archpsyc.1982.04290100031006. [DOI] [PubMed] [Google Scholar]
- 2.Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr Scand. 2003;108:163–174. doi: 10.1034/j.1600-0447.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- 3.Leibenluft E. Women with bipolar illness: clinical and research issues. Am J Psychiatry. 1996;153:163–173. doi: 10.1176/ajp.153.2.163. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy N, Boydell J, Kalidindi S, Fearon P, Jones PB, van Os J, et al. Gender differences in incidence and age at onset of mania and bipolar disorder over a 35-year period in Camberwell, England. Am J Psychiatry. 2005;162:257–262. doi: 10.1176/appi.ajp.162.2.257. [DOI] [PubMed] [Google Scholar]
- 5.Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- 6.McMahon FJ, Stine OC, Meyers DA, Simpson SG, DePaulo JR. Patterns of maternal transmission in bipolar affective disorder. Am J Hum Genet. 1995;56:1277–1286. [PMC free article] [PubMed] [Google Scholar]
- 7.McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Archives of general psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- 8.McGuffin P, Katz R, Watkins S, Rutherford J. A hospital-based twin register of the heritability of DSM-IV unipolar depression. Archives of general psychiatry. 1996;53:129–136. doi: 10.1001/archpsyc.1996.01830020047006. [DOI] [PubMed] [Google Scholar]
- 9.Sklar P, Smoller JW, Fan J, Ferreira MAR, Perlis RH, Chambert K, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;13:558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira MAR, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith EN, Bloss CS, Badner JA, Barrett T, Belmonte PL, Berrettini W, et al. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry. 2009;14:755–763. doi: 10.1038/mp.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan PF, de Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T, et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry. 2009;14:359–375. doi: 10.1038/mp.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moskvina V, Craddock N, Holmans P, Nikolov I, Pahwa JS, Green E, et al. Gene-wide analyses of genome-wide association data sets: evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk. Mol Psychiatry. 2009;14:252–260. doi: 10.1038/mp.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi J, Potash JB, Knowles JA, Weissman MM, Coryell W, Scheftner WA, et al. Genome-wide assocation study of recurrent early-onset major depressive disorder. Mol Psych. 2010 Feb 2; doi: 10.1038/mp.2009.124. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyegaard M, Demontis D, Foldager L, Hedemand A, Flint TJ, Sorensen KM, et al. CACNA1C (rs1006737) is associated with schizophrenia. Mol Psychiatry. 2010;15:119–121. doi: 10.1038/mp.2009.69. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Blackwood DH, Caesar S, de Geus EJ, Farmer A, Ferreira MA, et al. Meta-analysis of genome-wide association data of bipolar disorder and major depressive disorder. Mol Psychiatry. 2010 Mar 30; doi: 10.1038/mp.2009.107. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kempton MJ, Ruberto G, Vassos E, Tatarelli R, Girardi P, Collier D, et al. Effects of the CACNA1C risk allele for bipolar disorder on cerebral gray matter volume in healthy individuals. Am J Psychiatry. 2009;166:1413–1414. doi: 10.1176/appi.ajp.2009.09050680. [DOI] [PubMed] [Google Scholar]
- 19.Krug A, Nieratschker V, Markov V, Krach S, Jansen A, Zerres K, et al. Effect of CACNA1C rs1006737 on neural correlates of verbal fluency in healthy individuals. Neuroimage. 2010;49:1831–1836. doi: 10.1016/j.neuroimage.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Wessa M, Linke J, Witt SH, Nieratschker V, Esslinger C, Kirsch P, et al. The CACNA1C risk variant for bipolar disorder influences limbic activity. Mol Psychiatry. 2010 Mar 30; doi: 10.1038/mp.2009.103. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 22.Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, et al. Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol. 2009;75:407–414. doi: 10.1124/mol.108.049981. [DOI] [PubMed] [Google Scholar]
- 23.Mayorga AJ, Lucki I. Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology (Berl) 2001;155:110–112. doi: 10.1007/s002130100687. [DOI] [PubMed] [Google Scholar]
- 24.Dick DM, Foroud T, Flury L, Bowman ES, Miller MJ, Rau NL, et al. Genomewide linkage analyses of bipolar disorder: a new sample of 250 pedigrees from the National Institute of Mental Health Genetics Initiative. American journal of human genetics. 2003;73:107–114. doi: 10.1086/376562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmans P, Zubenko GS, Crowe RR, DePaulo JR, Jr, Scheftner WA, Weissman MM, et al. Genomewide significant linkage to recurrent, early-onset major depressive disorder on chromosome 15q. American journal of human genetics. 2004;74:1154–1167. doi: 10.1086/421333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kuhbandner S, et al. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem. 2000;275:39193–39199. doi: 10.1074/jbc.M006467200. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, et al. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Olivier B, Zethof T, Pattij T, van Boogaert M, van Oorschot R, Leahy C, et al. Stress-induced hyperthermia and anxiety: pharmacological validation. Eur J Pharmacol. 2003;463:117–132. doi: 10.1016/s0014-2999(03)01326-8. [DOI] [PubMed] [Google Scholar]
- 31.O’Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: leads to develop novel therapeutics. Neurosci Biobehav Rev. 2007;31:932–962. doi: 10.1016/j.neubiorev.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Kammen DP, Murphy DL. Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia. 1975;44:215–224. doi: 10.1007/BF00428897. [DOI] [PubMed] [Google Scholar]
- 33.Cox C, Harrison-Read PE, Steinberg H, Tomkiewicz M. Lithium attenuates drug-induced hyperactivity in rats. Nature. 1971;232:336–338. doi: 10.1038/232336a0. [DOI] [PubMed] [Google Scholar]
- 34.Gould TD, O’Donnell KC, Picchini AM, Manji HK. Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology. 2007;32:1321–1333. doi: 10.1038/sj.npp.1301254. [DOI] [PubMed] [Google Scholar]
- 35.Gould TD, O’Donnell KC, Picchini AM, Dow ER, Chen G, Manji HK. Generation and behavioral characterization of beta-catenin forebrain-specific conditional knock-out mice. Behav Brain Res. 2008;189:117–125. doi: 10.1016/j.bbr.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halberstadt AL, Geyer MA. Habituation and sensitization of acoustic startle: opposite influences of dopamine D1 and D2-family receptors. Neurobiol Learn Mem. 2009;92:243–248. doi: 10.1016/j.nlm.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ralph-Williams RJ, Paulus MP, Zhuang X, Hen R, Geyer MA. Valproate attenuates hyperactive and perseverative behaviors in mutant mice with a dysregulated dopamine system. Biol Psychiatry. 2003;53:352–359. doi: 10.1016/s0006-3223(02)01489-0. [DOI] [PubMed] [Google Scholar]
- 38.Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol Psychiatry. 2001;50:418–424. doi: 10.1016/s0006-3223(01)01184-2. [DOI] [PubMed] [Google Scholar]
- 39.Cohen C, Perrault G, Sanger DJ. Assessment of the antidepressant-like effects of L-type voltage-dependent channel modulators. Behav Pharmacol. 1997;8:629–638. doi: 10.1097/00008877-199711000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Gould TD, O’Donnell KC, Dow ER, Du J, Chen G, Manji HK. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology. 2008;54:577–587. doi: 10.1016/j.neuropharm.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anisman H, Merali Z. Learned Helplessness in Mice. In: Gould TD, editor. Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests. New York: Humana Press; 2009. pp. 177–196. [Google Scholar]
- 42.Engel SR, Creson TK, Hao Y, Shen Y, Maeng S, Nekrasova T, et al. The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Mol Psychiatry. 2009;14:448–461. doi: 10.1038/sj.mp.4002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Einat H. Modelling facets of mania--new directions related to the notion of endophenotypes. J Psychopharmacol. 2006;20:714–722. doi: 10.1177/0269881106060241. [DOI] [PubMed] [Google Scholar]
- 44.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaltiel G, Maeng S, Malkesman O, Pearson B, Schloesser RJ, Tragon T, et al. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berl) 2009 doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen G, Henter ID, Manji HK. Translational research in bipolar disorder: emerging insights from genetically based models. Mol Psychiatry. doi: 10.1038/mp.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, et al. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 51.Chandler RA, Wakeley J, Goodwin GM, Rogers RD. Altered Risk-Aversion and Risk-Seeking Behavior in Bipolar Disorder. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.05.011. in press. [DOI] [PubMed] [Google Scholar]
- 52.Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biol Psychiatry. 1997;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- 53.Plappert CF, Pilz PK. Difference in anxiety and sensitization of the acoustic startle response between the two inbred mouse strains BALB/cAN and DBA/2N. Genes Brain Behav. 2002;1:178–186. doi: 10.1034/j.1601-183x.2002.10306.x. [DOI] [PubMed] [Google Scholar]
- 54.King DC, Taylor J, Elnitski L, Chiaromonte F, Miller W, Hardison RC. Evaluation of regulatory potential and conservation scores for detecting cis-regulatory modules in aligned mammalian genome sequences. Genome research. 2005;15:1051–1060. doi: 10.1101/gr.3642605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cichon S, Craddock N, Daly M, Faraone SV, Gejman PV, Kelsoe J, et al. Genomewide association studies: history, rationale, and prospects for psychiatric disorders. Am J Psychiatry. 2009;166:540–556. doi: 10.1176/appi.ajp.2008.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hardingham GE, Cruzalegui FH, Chawla S, Bading H. Mechanisms controlling gene expression by nuclear calcium signals. Cell Calcium. 1998;23:131–134. doi: 10.1016/s0143-4160(98)90111-7. [DOI] [PubMed] [Google Scholar]
- 57.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 58.Murphy TH, Worley PF, Baraban JM. L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron. 1991;7:625–635. doi: 10.1016/0896-6273(91)90375-a. [DOI] [PubMed] [Google Scholar]
- 59.Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, et al. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White JA, McKinney BC, John MC, Powers PA, Kamp TJ, Murphy GG. Conditional forebrain deletion of the L-type calcium channel Ca V 1.2 disrupts remote spatial memories in mice. Learn Mem. 2008;15:1–5. doi: 10.1101/lm.773208. [DOI] [PubMed] [Google Scholar]
- 62.Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Muller J, et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajadhyaksha A, Husson I, Satpute SS, Kuppenbender KD, Ren JQ, Guerriero RM, et al. L-type Ca2+ channels mediate adaptation of extracellular signal-regulated kinase 1/2 phosphorylation in the ventral tegmental area after chronic amphetamine treatment. J Neurosci. 2004;24:7464–7476. doi: 10.1523/JNEUROSCI.0612-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarkar SN, Huang RQ, Logan SM, Yi KD, Dillon GH, Simpkins JW. Estrogens directly potentiate neuronal L-type Ca2+ channels. Proc Natl Acad Sci U S A. 2008;105:15148–15153. doi: 10.1073/pnas.0802379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sribnick E, Del Re A, Ray S, Woodward J, Banik N. Estrogen attenuates glutamate-induced cell death by inhibiting Ca2+ influx through L-type voltage-gated Ca2+ channels. Brain Res. 2009;1276:11. doi: 10.1016/j.brainres.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.