Abstract
Conditioning procedures are used in many placebo studies because evidence suggests that conditioning-related placebo responses are usually more robust than those induced by verbal suggestions alone. However, it has not been shown whether there is a causal relation between the number of conditioning trials and the resistance to extinction of placebo and nocebo responses. Here we test the effects of either one or four sessions of conditioning on the modulation of both non-painful and painful stimuli delivered to the dorsum of the foot. Placebo and nocebo manipulations were obtained by pairing green or red light to a series of stimuli that were made lower or higher with respect to a yellow light associated with a series of control stimuli. Subjects were told that the lights would indicate a treatment that would reduce or increase non-painful and painful stimuli to the foot. They were randomly assigned to either Group 1 or 2. Group 1 underwent one session of conditioning and Group 2 received four sessions of conditioning. We found that one session of conditioning (Group 1) induced nocebo responses, but not placebo responses in no pain condition. After one session of conditioning, we observed both nocebo and placebo responses to painful stimulation. However, these effects extinguished over time. Conversely, four sessions of conditioning (Group 2) induced robust placebo and nocebo responses to both non-painful and painful stimuli that persisted over the entire experiment. These findings suggest that the strength of learning may be clinically important for producing long-lasting placebo effects.
Keywords: Expectations, Conditioning, Pain, Somatosensorial perception, Placebo, Nocebo
1. Introduction
The placebo effect has been related to several cognitive and biological factors determining the degree of the placebo response. Lately, there has been an increased emphasis on conditioning and learning as modulators of placebo effects [33]. The accumulated evidence increasingly suggests that the association of specific cues with the experience of the treatment effect induced by pharmacological or biological manipulations [1,5,16,34,41,48] powerfully changes behavior and clinical outcomes. Such associations have, generally speaking, produced the largest placebo effects, and the most convincing demonstrations of placebo effects on peripheral biological processes [40].
Early studies on the capacity of the human brain to learn and to mimic pharmacological effects found drug-like effects when placebos are given after consecutive and repetitive administrations of verum drugs [3,22,26,27]. Later research provided more evidence that painkillers [2,7] or analgesic simulations [10–13,24,29,30,35,43–47] are capable of evoking placebo analgesia. Wickramasekera (1985) interpreted this sort of healing responses in terms of associative learning by assuming that a conditioned placebo response (conditioned response, CR) may be acquired through repetitive pairings of a previous neutral stimulus (conditioned stimulus, CS, e.g. contextual elements) with an unconditioned stimulus (US, e.g. the treatment’s effectiveness) [48]. Although different kinds of learning modulate placebo analgesia [13], interesting questions related to associative learning and placebo analgesia remain unanswered, including the fundamental question of whether and how placebo analgesia can be maximized by employing conditioning procedures. In the broadest sense, the learning process consists of two distinct phases: acquisition, the mastery of learning, and outcome retention, the time course over which behavioral (and biological) modifications are retained. A number of parameters influence the learning and consolidation of outcomes, important among them the number of CS-US pairings and the nature of the US [36].
By using a CS reinforced on 100% of trials with different numbers of CS-US pairings, we probed the effects of training on both the magnitude of placebo and nocebo effects and their resistance to subsequent extinction. On the basis of our previous studies [9,11], we hypothesized that several exposures to positive and negative cues would enhance placebo and nocebo responses and their retention over time.
2. Materials and Methods
2.1. Subjects
A total of 46 healthy volunteers (30 females, 16 males; age 22.8 ± 3.4 years) provided written informed consent, as approved by local ethics committee of the University of Turin, and participated in the study. They were informed that the purpose of research was to investigate the modulation of both non-painful and painful perception. For the purpose of experimental control, subjects were not informed accurately about the research procedures. They were told that different- colored lights would indicate three different conditions. More specifically, the subjects were informed that the activation of electrodes attached to the ankle (actually, two sham electrodes), would reduce their perception of non-painful and painful stimuli, when a green light was displayed on the computer screen, whilst it would increase their non-painful or painful perception when a red light was displayed. Additionally, they were told that a yellow light would indicate the deactivation of the ankle electrodes and thus that no treatment would be given. At the end of the study, all the subjects were debriefed regarding the real experimental procedures and informed about the research results.
Before starting the experimental session, subjects underwent a brief clinical examination in order to rule out any major medical condition. None of them had any medical disorder or was on any medication. They were tested with the State-Trait Anxiety Inventory (STAI, 42) and Beck Depression Inventory (BDI, 4) to monitor respectively trait and state anxiety, and depression. The Interpersonal Reactivity Scale (IRI, 14) was also used to assess empathy trait.
Subjects were randomly subdivided into two experimental groups, whose characteristics are shown in Table 1. The two groups did not differ for age, sex, STAI-I, STAI-II, BDI, IRI scores, and baseline ratings of non-painful and painful stimuli. Group 1 underwent one session of conditioning and Group 2 received 4 sessions of conditioning for both non-painful and painful conditions (see below the details of the experimental procedure).
Table 1.
Characteristics of each experimental group.
| Group | learning paradigm | n | sex (F/M) | age (years) | STAI-I | STAI-II | BDI | IRI |
|---|---|---|---|---|---|---|---|---|
| 1 | one session | 23 | 15/8 | 23.3 ± 4 | 33 ± 6 | 39 ± 7 | 4.6 ± 4.5 | 80.2 ± 6.5 |
| 2 | four sessions | 23 | 15/8 | 22.3 ± 3 | 36 ± 7 | 39 ± 5.8 | 5 ± 4.6 | 81 ± 8 |
STAI, State-Trait Anxiety Inventory; BDI, Beck Depression Inventory; IRI, Interpersonal Reactivity Index
2.2. Non-painful, painful and visual stimuli
The non-painful and painful stimuli (USs) consisted of electric stimulation delivered to the dorsum of the left or right foot through two silver chloride electrodes (2.5 × 1 cm) connected to a constant current unit, thus avoiding the variability of skin-electrode impedance, according to a previously used procedure [9–11]. In each subject, only one foot was stimulated and the side of stimulation was randomized across subjects. Two sham electrodes were also attached to the left and right ankle, but they were not attached to any current source, and no electrical stimulation was actually delivered.
Electrical stimuli were square pulses delivered by a somatosensory stimulator (Neurotravel Stim, Ates Medica Device srl, Verona, Italy), with a duration of 100 μs. The stimuli were delivered during the presentation of visual stimuli (red, green or yellow light), repetitively and pseudorandomly administered by a sequence randomizer (Stim2, Neuroscan, Compumedics, Charlotte, USA). The visual stimuli (CSs) were a red, green or yellow light, projected on a computer screen placed centrally in front of the participant at a distance of approximately 1 m, 15° over eye-level.
The intensity of stimulation was set accordingly to the individual non-painful and painful thresholds, the condition (non-painful or painful), the experimental manipulation (control, placebo or nocebo) and phase (conditioning or testing phase), as described below.
2.3. Design and procedures
The experiment started with the assessment of both non-painful tactile (t) and pain threshold (T) according to the following procedure. For each subject, the intensity of stimulation was set to elicit both non-painful and painful paresthesia in the area of stimulation. An ascending series of stimuli (steps of 0.5 mA) was delivered starting at a sub-tactile threshold, until non-painful tactile and painful sensations were induced.
After determination of t and T, the intensity of stimulation was approximately set in each subject at 1.5T for yellow stimuli, at 2.5T for red stimuli and, at T-2 mA for green stimuli. Similar proportions were used, in the non-painful condition with yellow stimuli at 1.5t, red stimuli at 2.5t and, green stimuli at t-2 mA.
The yellow light was associated to a medium intensity of pain (stimuli at 21.5±10 mA in Group 1; 19±9 mA in Group 2; with no statistical differences: p=0.378), the red light was associated to a high level of pain (stimuli at 34.7±19 mA, Group 1; 28±12 mA, Group 2, p=0.160) and, a low intensity stimulation (stimulus intensity at 14.2±5 mA, Group 1; 12.8±5 mA, Group 2; p=0.348) were associated to the green light. Similarly, in the non-painful condition the yellow light was associated to medium level of non-painful perception (stimuli at 8.2±2.8 mA, Group 1; 8.9±3.8 mA Group 2; comparison p=0.481), the red light was associated to high level of non-painful perception (stimuli at 12.6±3.4 mA, Group 1; 12.6±5 mA, Group 2; p=1) and, low non-painful stimuli (at 5±1.3 mA, Group 1; 4.7±1.9 mA, Group 2; p=0.913) were associated to green light. In order to control that stimuli were adequately set for obtaining respectively low, medium, and high perception both for non-painful and painful, subjects were also asked to rate each level during the phase of assessment. The low level coincided with the intensity which subjects rated as less than or equal to 3 on a 0 to 10 scale for assessing non-painful and painful stimuli (see below). The medium level coincided with the intensity that subjects rated between 4 and 6, and the high level corresponded at the intensity that subjects rated as greater than or equal to 7.
Based on this procedure, we expect that for the non-painful threshold, the intensity of stimulation was above the firing threshold of Aβ fibers (which convey non-painful tactile information) but below the threshold of nociceptive Aδ and C fibers. For painful intensity, the stimulus was expected to be above the firing threshold for nociceptive Aδ and C fibers [8].
As shown in Fig. 1A,B, depending on the experimental phases, the stimulus paired with the green light had either a reduced intensity with respect to the stimulus intensity following the control yellow light (conditioning phase) or the same intensity as the stimulus following the yellow light (testing phase). Conversely, the stimulus paired with the red light had either an increased intensity with respect to the stimulus intensity following the control yellow light (conditioning phase) or the same power as the stimulus following the yellow light (testing phase). Thus, in the phase of testing, placebo and nocebo responses were tested after the real direct experience of effectiveness (reduction or rise of their non-painful and painful perception) with respect to the baseline (no treatment condition).
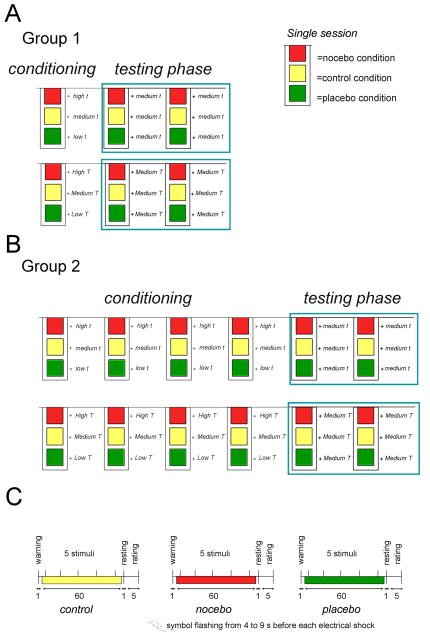
Figure 1. Experimental design.
Subjects were pseudorandomly assigned to either Group 1 (A) or Group 2 (B). Note that subjects of Group 1 underwent one session of conditioning, whereas subjects of Group 2 received four sessions of conditioning. In both the groups, there were two sessions of testing. As shown in legend (bottom right) each session consisted of a nocebo, control and placebo condition. Each condition was repeated twice for each session. A colored cue informed subjects about the condition under investigation throughout the experiment: they were told that the green cue would signal the procedure (a sub-threshold electric shock at the ankle) that in turn, induced a reduction of either non-painful or painful perception, the red cue would signal the procedure for increasing either non-painful or painful perception, whereas the yellow cue would indicate the control intensity of stimulation in which the sub-threshold electric shock at the ankle was turned off and no modulation of non-painful and painful perception was expected. The intensity of stimulation was respectively lower in green trials and increased in the red trials during the conditioning sessions, so that subjects experienced respectively real decrease and increase of their non-painful and painful perception. The characteristics of each trial of learning are detailed (C). At the beginning of each trial, the subjects received a visual warning about what condition was tested, either non-painful or painful. Then a visual cue, lasting 60s was presented on the monitor in front of the subject and a total of five stimuli were delivered at the same intensity but with a different inter-stimulus duration. A chain-light appeared from 4 to 9s before each electrical shock. Before the end of the trial, there was 1s for resting and 5s for rating. The inter-trial interval (ITI) was 35s. Behavioral ratings were assessed in each trial by means of two Visual Analogue Scales (VASs) differently anchored for non-painful and painful stimuli.
The impact of the number of learning trials in placebo and nocebo conditions was tested by training on either a single (Group 1) or four (Group 2) sessions of conditioning, as shown in Fig. 1. Each session consisted of the three different conditions (control, placebo and nocebo). Each condition was repeated twice in each session for both non-painful and painful stimuli. The order of condition presentations (US-CS) was pseudo-randomized. By contrast, the colored CSs were constantly paired with the specific US without any randomization because the significance of color treatment for placebo and nocebo responses is known: red color is commonly associated with avoidance motivation and green with safety [28].
Fig. 1C shows the timeline of each trial. Red, green or yellow light was presented for a time of 60s in which a total of five stimuli at the same intensity were delivered. Thus, during the conditioning phase, subjects of Group 1 received a total number of 10 light-pairing stimuli in each condition, whereas subjects of Group 2 received a total of 40 light-pairings in each condition. A flash of light was displayed before each stimulus alerting subjects that the shock would be given. The inter-stimuli interval (ISI) varied randomly from 9 to 12s. At the end of the colored light stimuli, subjects rated their perception (1s of resting and 5s for rating) by means of two Visual Analogue Scale (VAS) differently anchored (see the description below). Each trial lasted 67s and the inter-trial interval was 35s. The total duration of the experiment (explanation of experimental procedure, assessing of t and T, conditioning and testing, and filling out the psychological tests) was about 2 hours for Group 1 and 4 hours for Group 2. In Group 1 and 2, each session was separated by 3 min. In Group 2, there was also a time lag of 15 min following the first three sessions.
2.4. Psychophysical scale and inventories
In both the experimental groups, subjects rated non-painful and painful perception at the end of each trial. They were trained to use a VAS ranging from 0=no perception (the lower extreme) to 10=maximum perception (the upper extreme) for rating non-painful stimuli and, a VAS raging from 0=no pain (the lower extreme) to 10=maximum imaginable pain (the upper extreme) for painful stimuli. Subjects were informed that the upper extreme of non-painful VAS coincided with a clear perception that in some case might be disturbing but never painful and, that “maximum imaginable pain” referred to a bearable pain with respect to the specific experimental context. They were asked to use the scales consistently with the condition (non-painful or painful) under investigation (Fig. 1C), although the perception assessment might result as a continuum from no perception to maximum pain.
Subjects were also required to complete STAI-I and BDI at the beginning of the experiment, and a repeated administration of the STAI (STAI-II) and IRI were filled at the end of conditioning and testing experimental phases. The significance of correlations between these psychological tests and placebo ad nocebo ratings are sum up in Table 2.
Table 2.
Correlations between VAS and psychological scores
| Group | Condition | Stimulation | STAI-I | STAI-II | BDI | IRI |
|---|---|---|---|---|---|---|
| 1 | Nocebo | non-painful | r=−0.424;p<0.044 | r=−0.430;p<0.04 | n.s. | n.s. |
| painful | r=0.509;p<0.01 | r=0.547;p<0.007 | n.s. | n.s. | ||
| Placebo | non-painful | n.s. | n.s. | n.s. | r=0.384;p<0.07 | |
| painful | n.s. | n.s. | n.s. | r=0.418;p<0.05 | ||
| 2 | Nocebo | non-painful | n.s. | n.s. | n.s. | n.s. |
| painful | n.s. | n.s. | n.s. | n.s. | ||
| Placebo | non-painful | n.s. | n.s. | n.s. | n.s. | |
| painful | n.s. | n.s. | n.s. | n.s. | ||
STAI, State-Trait Anxiety Inventory; BDI, Beck Depression Inventory; IRI, Interpersonal Reactivity Index
2.5. Statistical analysis
The normality assumption underlying standard inferential tests was checked with the Kolmogorov-Smirnov test. In no case was a significant deviation from normality found. Statistical comparisons were performed using repeated measures ANOVA with Groups as between-factors and Treatment (yellow-, red- and green-stimuli) and Time (trials) as within-subjects factors. Thus, separate ANOVAs were performed for Group 1 and 2, non-painful tactile and painful condition, including the following within-subjects factors: treatment (yellow-, red- and green-stimuli) and time (trials). F-tests were followed by the Bonferroni post-hoc tests for multiple comparisons. Sphericity condition was also assessed and when it was not verified, the Greenhouse-Geisser correction was applied. In addition, a series of single-sample paired t-tests were performed on ratings in placebo (green) vs. neutral (yellow) conditions, and on nocebo (red) vs. neutral conditions. In order to compare the effects of the different number of learning trials on the magnitude of placebo and nocebo responsiveness, we expressed the placebo and nocebo responses as the difference between green-associated and red-associated VAS scores with respect to yellow-associated reports and we performed a univariate analysis with Group as between-subject factor. Cohen’s d, a standardized measure of effect sizes for two independent groups, was also calculated to contrast the magnitude of placebo and nocebo effects in Group 1 and 2. d was computed as the difference between the means, |MGroup1| − |MGroup 2|, divided by the standard deviation, б, of either group. Negative values indicate higher effects in Group 2 compared to Group 1 (and vice versa). Linear regressions were calculated to test the correlation between nocebo and placebo responses and psychological scores. All the analyses were carried out using the SPSS software package (SSPS Inc, Chicago, Illinois, USA, version 17). The level of significance was set at P<0.05.
3. Results
Before testing for placebo and nocebo effects on non-painful and painful condition, we verified the effectiveness of training phases. We verified that during the conditioning phase the red-and green-associated stimuli were rated respectively higher and lower than those paired to yellow light (control condition). In both the experimental groups, the conditioning phase (Fig. 2A,C and 3A,C) successfully set the three distinct levels of non-painful and painful perception. Thus, we focused on the testing phase, and we first probed the interaction between the factor Treatment (yellow-, red- and green-stimuli) and Group (short- vs. long- conditioning) by calculating repeated measures ANOVA of the VAS scores. As there was a significant Treatment × Group interaction in both non-painful (F(2,88)=4.120, p<0.019) and painful (F(2,88)=8.680, p<0.0001) condition, we performed a series of separate ANOVAs in each Group and Condition.
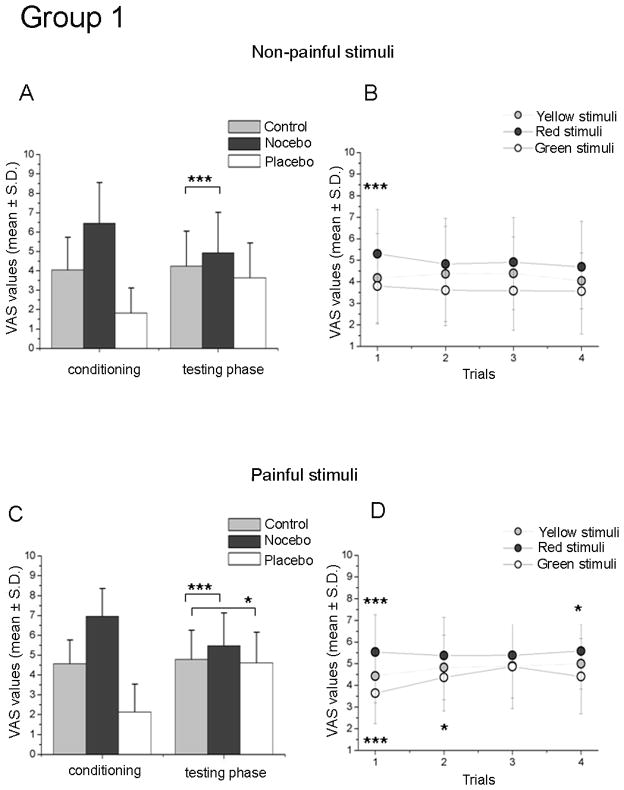
Figure 2. Results following a short-lasting module of learning (Group 1).
The histograms show the conditioning and testing phase for the non-painful (A) and painful stimuli (C) following a short-lasting training. Note that in the testing phase only nocebo responses were found for the non-painful stimuli and both placebo and nocebo responses for the painful intensity. The graphics on the right present the time-course of VAS reports respectively for non-painful (C) and painful (D) trials. We can observe that a short-training of learning induced nocebo responses which were inconstant over the entire experimental session and placebo responses which extinguished very early. The asterisks show the level of significance related to the comparison between red- vs. yellow- stimuli and green- vs. yellow stimuli (*** p<0.001; **p<0.01; * p<0.05).
Figure 3. Results following a long-lasting module of learning (Group 2).
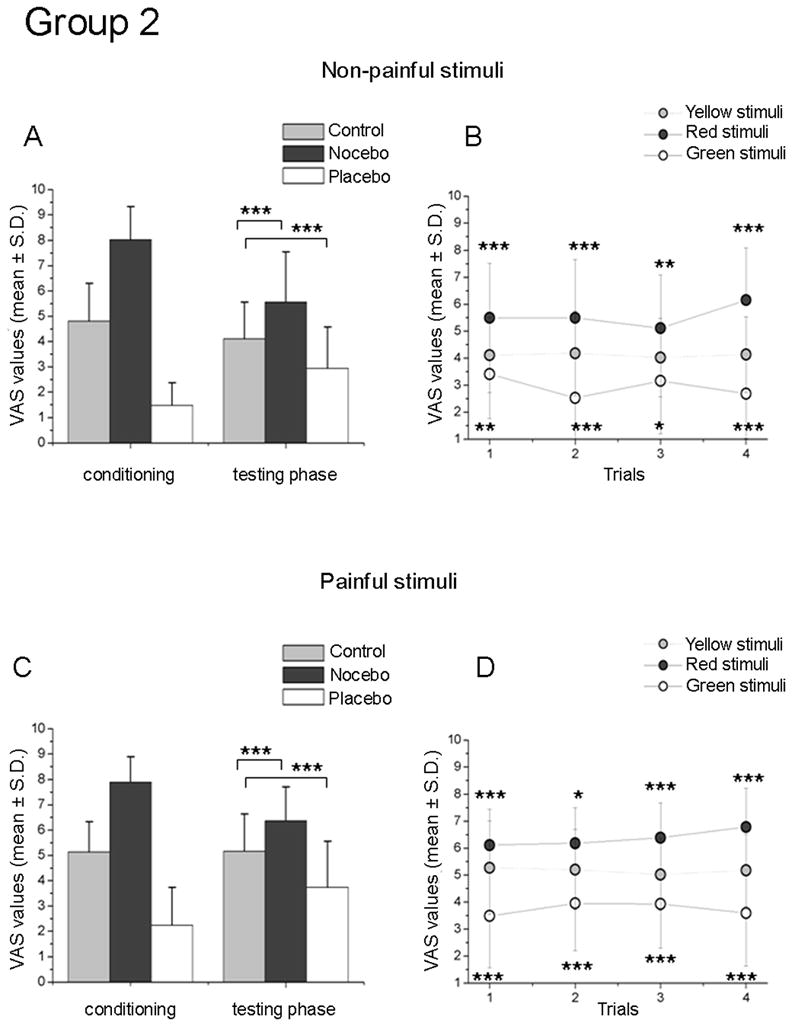
The histograms present the conditioning and testing phase for the non-painful (A) and painful condition (C) following a long-term module of learning. Note that robust nocebo and placebo responses were constantly found in the testing phase for both the conditions. The graphics on the right show the time-course of non-painful (B) and painful (D) VAS values. Enhancing the number of learning trials induced responses which lasted over the entire experiments. The asterisks show the level of significance related to the comparison between red- vs. yellow- stimuli and green- vs. yellow stimuli (*** p<0.001; **p<0.01; * p<0.05).
3.1.1. Group 1. Short-lasting training. Non-painful stimuli
Repeated measures ANOVA of the VAS scores in the testing phase revealed a main effect for Treatment (F(2,42)=9.498, p<0.0001), indicating that a difference was present between yellow-, red- and green-associated subjective reports. The post-hoc Bonferroni test for multiple comparisons showed that the VAS reports of red-associated stimuli were different with respect to those given to yellow (p<0.002) and green stimuli (p<0.009), indicating a nocebo effect on pain ratings. In contrast, no difference in the testing phase was found for the green vs. yellow condition (p=0.155), indicating a relative absence of placebo effects. These results are shown in Fig. 2A. An additional series of single-sample paired t-tests was performed to determine whether each red-associated stimulus was rated as higher than the control. We found that nocebo reports reached significance only at the first evaluation, exhibiting extinction of the effect during the rest of trials (p values are presented in Fig. 2B).
3.1.2. Group 1. Short-lasting training. Painful stimuli
Repeated measures ANOVA of the VAS scores indicated a main effect for Treatment (F(2,42)=15.749, p<0.0001) with a trend to significance for the factor Time (F(3,63)=2.559, p=0.063) and for interaction between the two factors (F(6,126)=2.125, p<0.055). The post-hoc Bonferroni test for multiple comparisons indicated that red pairings were rated differently with respect to yellow (p<0.009) and green stimuli (p<0.0001), indicating the presence of nocebo effects. Similarly VAS reports of green-associated stimuli were different with respect to yellow (p<0.021), indicating the presence of placebo effects (see the histograms in Fig. 2C, testing phase). An additional series of single-sample paired t-tests was performed to analyze the time-course of placebo and nocebo effects. Fig. 2D shows the trials that reached significance, indicating that placebo responses extinguished very early and nocebo responses occurred at different times after a short training in Group 1.
3.2.1. Group 2. Long-lasting training. Non-painful stimuli
After a long-lasting first-hand experience of both reduction and increase of non-painful perception, red-and green-associated stimuli were rated respectively as significantly higher and lower compared to yellow-associated stimuli. Repeated measures ANOVA on the VAS indicated a main effect for Treatment (F(2,42)=45.289, p<0.0001) but not for Time (F(3,63)=1.123, p=0.347). The difference across the three experimental conditions (control, nocebo and, placebo) was further investigated with the post-hoc Bonferroni test for multiple comparisons confirming that the VAS reports of red were higher than yellow (p<0.0001), indicating the presence of nocebo effects. Similarly, green-associated stimuli were different from yellow (p<0.0001), indicating the presence of placebo effect (see Fig. 3A). As expected, the placebo vs. nocebo comparison was also significant (p<0.0001), with nocebo-paired stimuli reported as more painful than placebo-paired stimuli. Additionally, a separate analysis for each trial by means of single-sample t-tests, indicated a stable condition for placebo and nocebo responses over the entire experimental session, without either habituation or sensitization (Fig. 3B).
3.2.2. Group 2. Long-lasting training. Painful stimuli
The repeated measures ANOVA of the VAS scores showed a main effect for Treatment (F(2,42)=54.087, p<0.0001) with no significance for the factor Time (F(3,63)=0.688, p=0.563), indicating that the long training significantly impacted nocebo and placebo responsiveness. The post-hoc Bonferroni test for multiple comparisons confirmed that red pairings were rated differently with respect to yellow (p<0.0001) and green stimuli (p<0.0001); similarly, VAS reports of green-associated stimuli were different with respect to yellow (p<0.0001) and red stimuli (p<0.0001) (see histograms of Fig. 3C). An additional series of single-sample paired t-tests was performed to further investigate the time-course of placebo and nocebo responses. The persistence of the effects was confirmed (Fig. 3D).
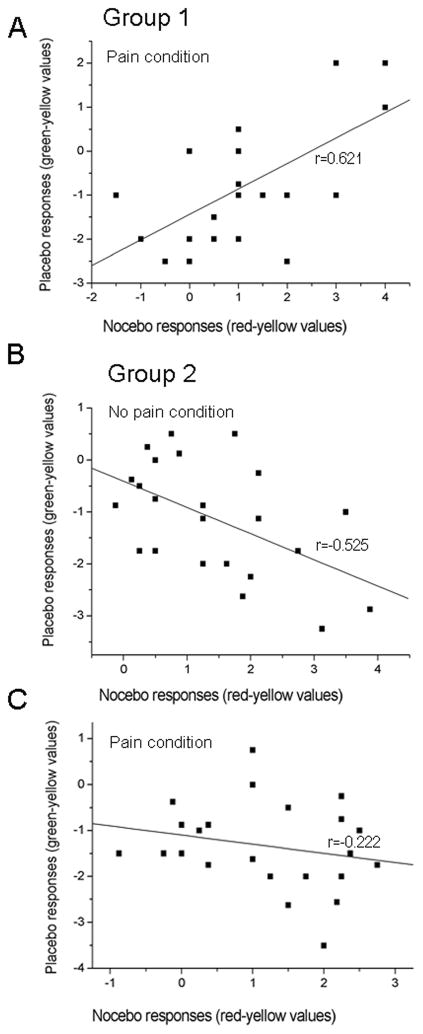
3.3. Correlation between nocebo and placebo responses
We correlated nocebo and placebo responses when both were significantly present in each experimental group (Group 1, painful condition; Group 2 non-painful and painful conditions). In Group 1, placebo and nocebo responses for painful intensity (first trial) were significantly correlated (r=0.621; p<0.001; Fig. 4A). In Group 2, the correlations between placebo and nocebo responses in each single trial were not statistically significant. However, as placebo and nocebo responses in Group 2 were persistent across the entire experimental session, we averaged the values from trial 1 to 4 by expressing the responses as mean difference (red-yellow and, green-yellow values). In this case, a significant negative correlation between placebo and nocebo responses was found for non-painful stimuli (r=−0.525; p<0.01; Fig. 4B) but not for pain (r=−0.222; p=0.308; Fig. 4C). Based on these findings, the relationship between nocebo and placebo responses in the same experimental subject seems to be sensitive to length of the training and to the intensity of stimulation, thus making difficult any definitive conclusions about the predictability of nocebo and placebo responses with respect to each other.
Figure 4. Placebo and nocebo correlations.
After a short-training in Group 1, placebo and nocebo responses were correlated for the painful condition (p<0.0001). A significant correlation was found between placebo and nocebo responses for the non-painful (p<0.01), but not for the painful condition (p=0.308) in Group 2.
3.4. Non-painful versus painful features of stimulation
We also verified whether placebo and nocebo responses depended on stimulus intensity by performing a within- comparison between non-painful and painful nocebo and placebo responses in Group 2 and where both positive and negative modulations were present constantly. After a long-training of conditioning, no significant changes between non-painful and painful conditions were found for the placebo condition (F(1,22)=1.304, p=0.226). As to the nocebo counterpart, the repetitive exposure to a high level of painful stimulation, induced a difference with respect to the intensity (F(1,22)=3.361, p<0.021), suggesting that the US-feature is one factor (but not the only) that has an effect on nocebo responses. In fact, after a short-term conditioning in Group 1, nocebo responses did not differ with respect to the non-painful versus painful condition (F(1,22)=.000, p=0.985).
3.5. Comparison between short- and long-term learning training
The most important difference between Group 1 and 2 is the persistence of effects observed after a long-lasting conditioning. Furthermore, we compared the effect of number of learning trials (Group 1 versus 2) on magnitude of placebo and nocebo responses by averaging the differences between red- and yellow-associated VAS reports and green- and yellow-associated reports. For painful stimulation, a significant change between Group 1 and 2 was found in the placebo condition (F(1,44)=11.237, p<0.002), but not for nocebo responses (F(1,44)=2.810, p=0.101). These findings indicate that placebo responses were stronger in the extended training group (Group 2), whereas nocebo responses were relatively strong in both groups. For non-painful stimuli, we observed an opposite trend. The comparison between placebo responses in Group 1 and 2 was not significant (F(1,44)=1.795, p=0.187), whereas the nocebo responses in Group 1 and 2 were significantly differently (F(1,44)=6.129, p<0.017), indicating that salience of stimulation may impact learning effects (Fig. 5). The magnitude of nocebo and placebo responses in Group 1 and 2 was also contrasted by effect size calculation (nocebo no pain condition: d = −0.729; nocebo pain condition: d = −0.494; placebo no pain condition: d = −0.393; placebo pain condition: d = −0.988). Expressed in percentages, long- lasting training induced an increase on VAS reports of 51 ± 26 % in no pain nocebo condition, and of 42 ± 0.07 % in pain. Similarly, long-compared to short-lasting-training, produced a decrease of VAS reports of 46 ± 38 % for non-painful stimuli, and a decrease of 65 ± 14% for painful stimuli.
Figure 5. Average of the responses in each group and condition.
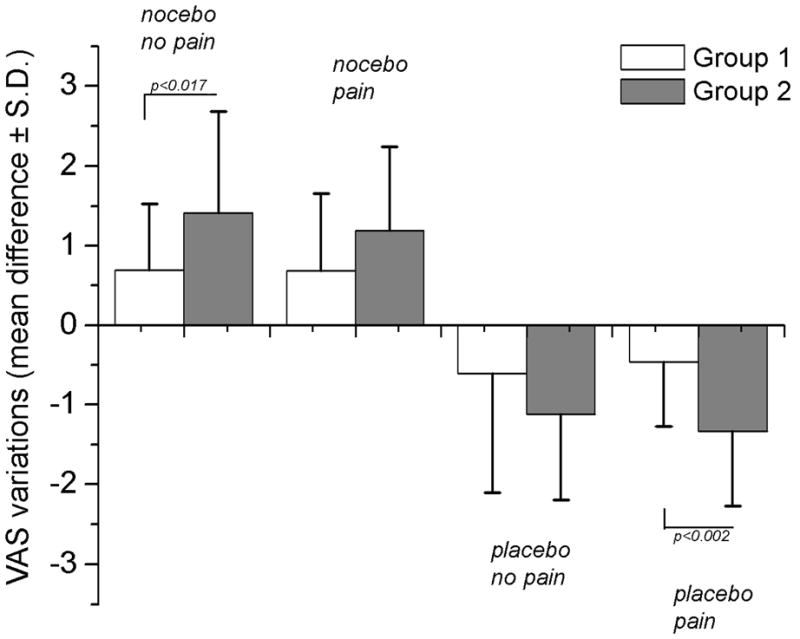
It can be noted that in pain condition, the mean nocebo responses were not different between Group 1 and 2. Conversely, the mean placebo responses in pain condition, were sensitive to the length of learning trials. In no pain condition, the magnitude of modulation varied between Group 1 and 2 for nocebo responses, but not for placebo responses.
3.6. Correlations between psychological tests and placebo and nocebo reports
After the short-lasting training, the nocebo responses in no pain condition were negatively correlated with STAI-I (r=−0.424; p<0.044) and STAI-II (r=−0.430; p<0.04) scores whereas, the placebo responses showed a weak trend to be positively correlated with total IRI scores (r=0.384; p<0.07). In pain condition, the short conditioning induced nocebo responses that were positively correlated with STAI-I (r=0.509; p<0.01) and STAI-II (r=0.547; p<0.007) scores, and placebo responses that were positively correlated with total IRI scores (r=0.418; p<0.05). These correlations were not found after the long-lasting conditioning (Table. 2), suggesting that a long conditioning may interfere with dispositional and situational attitudes.
Discussion
The present findings suggest that there is a causal relation between the number of conditioning trials and the resistance to extinction of the ensuing placebo and nocebo responses. Using a two-phase conditioning, we found that the persistence of placebo and nocebo responses was firmly connected to the number of exposures to effective treatments. In fact, a long-lasting positive or negative conditioning paradigm resulted in the formation of sustained nocebo and placebo responses. Conversely, a short-lasting training induced nocebo responses to both non-painful and painful condition and, placebo responses only for painful stimuli. The intensity of stimulation (non-painful versus painful) influenced the occurrence of nocebo and placebo responses, thus suggesting that learning effects vary in different contexts.
Several lines of research suggest that conditioning is a crucial factor for shaping the placebo and nocebo responses. Conditioned responses are present across different systems such as somatosensorial perception [5], motor system [6,15,32], hormone secretion [7], immune responses [20,21], and emotion [31], whereby drug-like effects have been observed when active treatments are replaced with inert treatments (e.g. saline solutions or sugar pills). Here we tested the relationship between the number of learning trials and the modulation of placebo (and nocebo) responsiveness. The longer the module of conditioning was, the more robust and persistent the placebo and nocebo responses were.
With respect to the role of the number of learning trials, it is worth mentioning some animals and humans studies [17,20,21]. For example, Garcia et al. (1955) demonstrated that conditioned aversion in rats exposed concurrently to gamma radiation and saccharin-flavored drinking water was dose-dependent in terms of the size of saccharin aversion and its persistence [17]. Human studies by Goebel et al. [20,21] have elegantly demonstrated that more than a single associative learning trial is required for producing human immune conditioned effect. Goebel and co-workers observed that the association between a distinctively flavored/colored solution (CS) with interferon-β injections (US) did not evoke an immune conditioned response [20]. However, after four pairings between the distinctive taste and immunosuppressive drug, exposure to the drink alone resulted in conditioned inhibition of ex vivo IL-2 and IFN-γ cytokine mRNA expression, and in a decreased proliferative responsiveness of peripheral blood lymphocytes [21]. Thus, these results indicate that a CS may mimic the properties of active treatment if repetitively paired with it in a conditioning procedure. Nevertheless, the studies by Goebel and co-workers investigated a link between associative learning and the immune system [20,21], which is likely to operate largely outside conscious control. Similarly to hormonal placebo effects [7], merely telling people that their immune blood levels are going to change might not produce any effects. Indeed, conditioned immune and hormonal placebo responses might be notably different from effects induced by reinforcement learning in systems consciously accessible such as pain.
In our experimental conditions, both conscious and sub-conscious components of learning may shape behavior. Although placebo and nocebo responses were strongly modulated by US exposure, the integration of other factors such as beliefs and information provided by the investigators who conducted the experiment may have contributed to creating the final outcome. Subjects received explicit instruction about the modulatory effect of the ankle (sham) electrode by a colored cue (CS-US contingency). However, they may not have been conscious of the changes of US-feature during the testing phase, when they received all the stimulations at the same intensity but different overt information. The experience which had been set in the training sessions was violated by the change in afferent inputs. It seems that what was learnt in the conditioning phase was strong enough to bias the perception.
Consistently with cognitive theories of learning [9,23,25,37–39], such conditioning would lead to inducing expectations that a given event will follow another event, and this would occur on the basis of the information that the conditioned stimulus provides about the unconditioned stimulus. Increasing the number of US exposures would enhance the predictability about the occurrence of an event, thus strengthening positive or negative expectations.
In line with the ideas above, we have previously demonstrated that a conditioning manipulation is graded according to prior experience [10]. The exposure to an effective conditioning procedure elicited long-lasting placebo responses which were present after both a few minutes and after a time lag of four to seven days. Conversely, when the same conditioning procedure was repeated after a totally ineffective analgesic procedure, the placebo responses were remarkably reduced in comparison to the first group. This shows that prior experience of pain treatment, both effective and ineffective, has sequential and lasting effects on how the subsequent treatments of the same conditions are perceived.
It is also interesting to compare the placebo effect with the nocebo effect. For painful stimuli, the placebo response was significantly stronger after the long conditioning compared with the short conditioning, whereas there was no difference on magnitude of the nocebo responses depending on the conditioning length (Fig 5). The sensitivity of placebo analgesia to the number of learning trials, is consistent with our previous studies showing that conditioning enhanced the magnitude of placebo analgesic responses, and that they were relatively small with verbal suggestions alone [11,12]. We also showed that non-painful and low painful stimuli were perceived as painful (nocebo responses) after both verbal suggestions of hyperalgesia and a conditioning procedure [11]. Moreover, in the present study, we observed a different trend for no pain condition where subjects were informed that the non-painful red-associated stimuli would become disturbing but never painful thus, indicating that variations in the information result in different effects of conditioning.
A prominent aspect of placebo research is to predict who will be a more efficient placebo responder. Here we ask whether the nocebo effect predicts the placebo effect (i.e. whether a subject defined as “nocebo responder” also is a “placebo responder” and, viceversa). In the non-painful intensity, a positive correlation between placebo and nocebo responses was found. We also observed that placebo and nocebo responses were correlated after a short-training, but not after a long-training for pain intensity. Possibly, learning of positive and negative events may be influenced by the salience of the US cue as well as the training length; accordingly, our ability to creating placebo and nocebo responses would be context-dependent.
Some studies have also noted that higher level of optimism and social desirability are associated with higher placebo responses [18,19,30]. Although this study was not aimed at identifying a placebo-and nocebo-prone psychological profile, we observed a significant relationship between the nocebo responses and state-trait-anxiety scores and, between the placebo responses and empathy scores in the short but not in longer training. Although speculative, this suggests that longer conditioning interferes with dispositional and situational attitudes by changing the initial state of beliefs or creating new expectations. Certainly, this issue is worthy of further investigation.
This study was designed to investigate the role of the number of US exposures in placebo and nocebo responsiveness among healthy volunteers and therefore has a limitation in its implications for clinical practices. Suffering patients may have expectations and beliefs that are different from healthy volunteers. Also, the relationship between physician and patient may involve a much stronger dependency as compared to the researcher-experimental subject relationship. However, in spite of this limitation, our results may point to a mechanism for harnessing placebo effect in clinical practice. Therapeutic conditioned placebo responses might be elicited by administering active treatments (USs) with some CSs and then, replacing the USs with inert substances accordingly to a dose-extender model.
Overall, this research suggests that learning via prior exposure to effective treatments may represent a promising strategy to harness placebo mechanisms in the clinical setting. At the same time, it is mandatory to avoid repetitive exposures to negative and ineffective treatments to prevent nocebo outcome consolidation.
Acknowledgments
This research was supported by grants from International Association for Study of Pain (IASP) Collaborative Research Grant, from Regione Piemonte, from Istituto San Paolo di Torino, and from the Volkswagen Foundation.
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ader R. The role of conditioning in pharmacotherapy. In: Harrington A, editor. The Placebo Effect: An Interdisciplinary Exploration. Cambrige, MA: Harvard UP; 1997. pp. 138–165. [Google Scholar]
- 2.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19(1):484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batterman RC, Lower WR. Placebo responsiveness--influence of previous therapy. Curr Ther Res Clin Exp. 1968;10(3):136–143. [PubMed] [Google Scholar]
- 4.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 5.Benedetti F. Mechanisms of placebo and placebo-related effects across diseases and treatments. Annu Rev Pharmacol Toxicol. 2008;48:33–60. doi: 10.1146/annurev.pharmtox.48.113006.094711. [DOI] [PubMed] [Google Scholar]
- 6.Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, Bergamasco B, Lopiano L. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci. 2004;7(6):587–588. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 2003;23(10):4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess PR, Perl ER. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol. 1967;190(3):541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chater N. Rational and mechanistic perspectives on reinforcement learning. Cognition. 2009;113:350–364. doi: 10.1016/j.cognition.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Colloca L, Benedetti F. How prior experience shapes placebo analgesia. Pain. 2006;124(1–2):126–133. doi: 10.1016/j.pain.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Colloca L, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. Pain. 2008;136(1–2):211–218. doi: 10.1016/j.pain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Colloca L, Tinazzi M, Recchia S, Le Pera D, Fiaschi A, Benedetti F, Valeriani M. Learning potentiates neurophysiological and behavioral placebo analgesic responses. Pain. 2008;139(2):306–314. doi: 10.1016/j.pain.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. Pain. 2009;144(1–2):28–34. doi: 10.1016/j.pain.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Davis MA. A multidimensional approach to individual differences in empathy. JSAS Cat Selected Docs Psychol. 1980;10:85. [Google Scholar]
- 15.de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293(5532):1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- 16.Enck P, Benedetti F, Schedlowski M. New insights into the placebo and nocebo responses. Neuron. 2008;59(2):195–206. doi: 10.1016/j.neuron.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- 18.Geers AL, Helfer SG, Kosbab K, Weiland PE, Landry SJ. Reconsidering the role of personality in placebo effects: dispositional optimism, situational expectations, and the placebo response. J Psychosom Res. 2005;58(2):121–7. doi: 10.1016/j.jpsychores.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Gelfand DM, Gelfand S, Rardin MW. Some personality factors associated with placebo responsivity. Psychol Rep. 1965;17:555–62. doi: 10.2466/pr0.1965.17.2.555. [DOI] [PubMed] [Google Scholar]
- 20.Goebel MU, Hubell D, Kou W, Janssen OE, Katsarava Z, Limmroth V, Schedlowski M. Behavioral conditioning with interferon beta-1a in humans. Physiol Behav. 2005;84(5):807–814. doi: 10.1016/j.physbeh.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Goebel MU, Trebst AE, Steiner J, Xie YF, Exton MS, Frede S, Canbay AE, Michel MC, Heemann U, Schedlowski M. Behavioral conditioning of immunosuppression is possible in humans. FASEB J. 2002;16(14):1869–1873. doi: 10.1096/fj.02-0389com. [DOI] [PubMed] [Google Scholar]
- 22.Kantor TG, Sunshine A, Laska E, Meisner M, Hopper M. Oral analgesic studies: pentazocine hydrochloride, codeine, aspirin, and placebo and their influence on response to placebo. Clin Pharmacol Ther. 1966;7(4):447–454. doi: 10.1002/cpt196674447. [DOI] [PubMed] [Google Scholar]
- 23.Kirsch I. Conditioning, expectancy, and the placebo effect: comment on Stewart-Williams and Podd (2004) Psychol Bull. 2004;130(2):341–343. doi: 10.1037/0033-2909.130.2.341. discussion 344–345. [DOI] [PubMed] [Google Scholar]
- 24.Klinger R, Soost S, Flor H, Worm M. Classical conditioning and expectancy in placebo hypoalgesia: a randomized controlled study in patients with atopic dermatitis and persons with healthy skin. Pain. 2007;128(1–2):31–39. doi: 10.1016/j.pain.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Kirsch I, Lynn SJ, Vigorito M, Miller RR. The role of conditioning in classical and operant conditioning. J Clin Psychol. 2004b;60(4):369–392. doi: 10.1002/jclp.10251. [DOI] [PubMed] [Google Scholar]
- 26.Lasagna L, Mosteller F, Von Felsinger JM, Beecher HK. A study of the placebo response. Am J Med. 1954;16(6):770–779. doi: 10.1016/0002-9343(54)90441-6. [DOI] [PubMed] [Google Scholar]
- 27.Laska E, Sunshine A. Anticipation of analgesia. A placebo effect. Headache. 1973;13(1):1–11. doi: 10.1111/j.1526-4610.1973.hed1301001.x. [DOI] [PubMed] [Google Scholar]
- 28.Mehta R, Zhu RJ. Blue or red? Exploring the effect of color on cognitive task performances. Science. 2009;323(5918):1226–1229. doi: 10.1126/science.1169144. [DOI] [PubMed] [Google Scholar]
- 29.Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72(1–2):107–113. doi: 10.1016/s0304-3959(97)00016-x. [DOI] [PubMed] [Google Scholar]
- 30.Morton DL, Watson A, El-Dereby W, Jones AKP. Reproducibility of placebo analgesia: Effect of dispositional optimism. Pain. 2009;146:194–198. doi: 10.1016/j.pain.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing--induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46(6):957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Pollo A, Carlino E, Benedetti F. The top-down influence of ergogenic placebos on muscle work and fatigue. Eur J Neurosci. 2008;28(2):379–388. doi: 10.1111/j.1460-9568.2008.06344.x. [DOI] [PubMed] [Google Scholar]
- 33.Porro CA. Open your mind to placebo conditioning. Pain. 2009;145(1–2):2–3. doi: 10.1016/j.pain.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 35.Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83(2):147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 36.Rescorla R, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: current research and theory. New York: Apleton-Century-Crofts; 1972. [Google Scholar]
- 37.Reiss S. Pavlovian conditioning and human fear: an expectancy model. Behav Ther. 1980;11:380–396. [Google Scholar]
- 38.Rescorla RA. Pavlovian conditioning: it is not what you think it is. Am Psychologist. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- 39.Shanks DR. Learning: From association to Cognition. Annu Rev Psychol. 2010;61:17.1–17.29. doi: 10.1146/annurev.psych.093008.100519. [DOI] [PubMed] [Google Scholar]
- 40.Schedlowski M, Pacheco-Lopez G. The learned immune response: Pavlov and beyond. Brain Behav Immun. 2010;24(2):176–85. doi: 10.1016/j.bbi.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Siegel S. Explanatory mechanisms for placebo effects: Pavlovian conditioning. In: Guess HA, Kleinman A, Kusek JW, Engel LW, editors. The Science of the Placebo: Toward an Interdisciplinary Research Agenda. London: BMJ Books; 2002. pp. 133–157. [Google Scholar]
- 42.Spielberger CD, Gorsuch RL, Lushene RE. Forma X. Organizzazioni Speciali; Firenze: 1980. S.T.A.I. (State-Trait-Anxiety Inventory). Questionario di autovalutazione per l’ansia di stato e di tratto. [Google Scholar]
- 43.Voudouris NJ, Peck CL, Coleman G. Conditioned placebo responses. J Pers Soc Psychol. 1985;48(1):47–53. doi: 10.1037//0022-3514.48.1.47. [DOI] [PubMed] [Google Scholar]
- 44.Voudouris NJ, Peck CL, Coleman G. Conditioned response models of placebo phenomena: further support. Pain. 1989;38(1):109–116. doi: 10.1016/0304-3959(89)90080-8. [DOI] [PubMed] [Google Scholar]
- 45.Voudouris NJ, Peck CL, Coleman G. The role of conditioning and verbal expectancy in the placebo response. Pain. 1990;43(1):121–128. doi: 10.1016/0304-3959(90)90057-K. [DOI] [PubMed] [Google Scholar]
- 46.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 47.Watson A, El-Deredy W, Iannetti GD, Lloyd D, Tracey I, Vogt BA, Nadeau V, Jones AK. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain. 2009;145(1–2):24–30. doi: 10.1016/j.pain.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wickramasekera I. A conditioned response model of the placebo effect: predictions from the model. In: White L, Tursky B, Schwartz GE, editors. Placebo: theory, research, and mechanisms. New York: Guilford Press; 1985. pp. 255–287. [Google Scholar]