Abstract
Excessive cervical facet capsular ligament stretch has been implicated as a cause of whiplash-associated disorders following rear-end impacts, but the pathophysiological mechanisms that produce chronic pain in these cases remain unclear. Using a rat model of C6/C7 cervical facet joint capsule stretch that produces sustained mechanical hyperalgesia, the presence of neuronal hyperexcitability was characterized 7 days after joint loading. Extracellular recordings of spinal dorsal horn neuronal activity between C6 and C8 (117 neurons) were obtained from anesthetized rats, with both painful and non-painful behavioral outcomes established by the magnitude of capsule stretch. The frequency of neuronal firing during noxious pinch (p<0.0182) and von Frey filaments applications (4–26 g) to the forepaw was increased (p<0.0156) in the painful group compared to the non-painful and sham groups. In addition, the incidence and frequency of spontaneous and afterdischarge firing were greater in the painful group (p<0.0307) relative to sham. The proportion of cells in the deep laminae that responded as wide dynamic range neurons also was increased in the painful group relative to non-painful or sham groups (p<0.0348). These findings suggest that excessive facet capsule stretch, while not producing visible tearing, can produce functional plasticity of dorsal horn neuronal activity. The increase in neuronal firing across a range of stimulus magnitudes observed at day 7 post-injury provides the first direct evidence of neuronal modulation in the spinal cord following facet joint loading, and suggests that facet-mediated chronic pain following whiplash injury is driven, at least in part, by central sensitization.
Introduction
The annual incidence of neck pain in the general population is estimated at nearly 20% [11], and for many individuals, the symptoms can become chronic and debilitating [23]. The cervical facet joint is a frequent source of neck pain in 54–60% of the chronic neck pain cases originating from injury and idiopathic causes [3,34,37]. Cadaveric studies of facet joint kinematics during whiplash implicate excessive stretch of the cervical facet capsular ligament as a cause of neck pain following whiplash [14,28,40–41,50,55–56]. However, despite clinical and biomechanical studies linking facet capsule stretch to pain, the pathophysiological mechanisms by which facet capsular ligament loading can produce sustained pain have yet to be fully elucidated.
The cervical facet capsule is innervated by proprioceptive and nociceptive primary afferents that encode the magnitude of load transmitted through the structure [25, 38, 53]. Nerve fibers in the facet capsule reactive for neuropeptides demonstrate the potential for nociceptive signaling [4,16,25,27,54]. Application of substance P to facet joints produces an excitatory effect on the mechanical stimulation of proprioceptive and nociceptive afferents in that tissue [54]. In the rat, increased substance P expression in the dorsal root ganglia is sustained at 7 days after facet joint stretch that also produces mechanical hyperalgesia and allodynia [33]. In a caprine model, both nociceptor firing during cervical facet joint loading and sustained afferent discharges after loading were produced in the absence of rupture of the joint’s capsule [5,8,35–36]. These models demonstrate that certain degrees of facet capsule stretch can induce nociceptive firing, alter neurotransmitter expression in the peripheral nervous system, and produce persistent behavioral hypersensitivity. However, the mechanisms governing the maintenance of pain after injury-related facet joint loading scenarios remain unclear.
Patients with chronic pain after whiplash injury report mechanical hyperalgesia and allodynia along the neck and upper extremities [1,12,17,47], which suggests the development and maintenance of the increased sensitivity of spinal neurons. Central sensitization has been implicated as a driving mechanism responsible for many chronic pain states [26,45,52]. Wide dynamic range neurons in the dorsal horn may be capable of modulating central sensitization in many chronic pain states [9–10,18,21–22,44,46]. However, the role of such spinal neurons in facet-mediated pain remains unclear owing to a lack of investigations probing neuronal plasticity in the spinal cord.
The goal of this study was to investigate the development of neuronal hyperexcitability in the spinal cord of the rat after loading of the C6/C7 facet joints. Facet capsule stretch was applied using separate magnitudes that do and do not produce behavioral hypersensitivity in the neck and forepaw at day 7 [31,33] to assess whether painful facet capsule stretch is associated with neuronal hyperexcitability in the spinal cord. We hypothesized that spinal dorsal horn neurons are more excitable in response to forepaw stimulation 7 days after facet joint loading that induces mechanical hyperalgesia. Extracellular voltage recordings were made in the deep laminae of the dorsal horn at day 7, and the frequency of baseline, evoked, and afterdischarge firing was assessed to characterize the neuronal response to mechanical stimuli.
Methods
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania and followed the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain [57]. Male Holtzman rats (356–460 g; Harlan Sprague-Dawley, Indianapolis, IN) were housed under USDA- and AAALAC-compliant conditions with food and water available ad libitum.
Facet capsule stretch injury
Rats were anesthetized with isoflurane (4% for induction, 2.5% for maintenance). A controlled bilateral displacement was applied across the cervical C6/C7 facet joints using a custom loading device, as previously described [31]. Rats were placed in a prone position and the paraspinal musculature was carefully separated from the spinous processes between C4 and T2. The laminae and facet joints at the C6 and C7 levels were exposed and musculature was cleared from the dorsal surface of the facet capsule. Microforceps were attached to the spinous processes of both C6 and C7. Vertebral displacements were imposed by separating the microforceps to apply tensile deformation across the facet joint’s capsule; the C6 microforceps translated rostrally while C7 remained stationary. Each rat underwent a single prescribed vertebral displacement at one of three magnitudes (n=6 per group) to induce known behavioral outcomes: 0.7 mm to induce sustained behavioral sensitivity (painful), 0.2 mm to stretch the capsule but not induce behavioral sensitivity (non-painful), or 0 mm (sham) [33,43]. Previous work with this facet model has demonstrated no detectable difference in the pain or inflammatory responses between naïve rats and those having undergone a sham surgery [15, 31–33]. The magnitude of joint displacement that was actually applied was measured by tracking markers placed on each lamina at C6 and C7. The maximum relative distance measured between the centroids of those markers during applied vertebral displacements was taken as the magnitude of each facet capsule stretch. Following surgery, the incisions were closed using 3-0 polyester suture and surgical staples; rats were monitored as they recovered in room air.
Behavioral testing
Mechanical hyperalgesia was assessed prior to facet capsule stretch injury and on the day of electrophysiological testing to verify that behavioral hypersensitivity in each group in the current study were consistent with previous reports using the same vertebral displacement magnitudes [33]. Hyperalgesia was measured in the forepaw using a modified Chaplan’s up/down method to quantify the threshold for tactile sensitivity to a von Frey stimulus [7,24,31,33]. For both the left and right forepaw, the threshold to elicit a withdrawal response was determined in three rounds of testing. For each round, a series of filaments with logarithmically-increasing strengths (0.4, 0.6, 1.4, 2, 4, 6, 8, 15, 26 g) (Stoelting Co., Wood Dale, IL) was applied to the forepaw. Each filament was applied five times before using a stronger filament, and if two consecutive filament strengths elicited a response, the lower of the two filament strengths was recorded as the threshold. Any rat failing to respond to any filaments was assigned a threshold of 26 g for that round. The average threshold from the three rounds was calculated for each forepaw of each rat on both the baseline and electrophysiological testing days.
Electrophysiology
To determine the effects of facet capsule stretch on neuronal excitability in the dorsal horn of the C6–C8 spinal cord, electrophysiological recordings were acquired on day 7 after facet joint injury for both the painful and non-painful groups. Electrophysiological recordings were taken on day 6 for the sham group to provide a more liberal estimate of the physiological effects of surgery. For surgical procedures, anesthesia was induced with isoflurane (4% in O2, then 2.5% in O2 for maintenance), and the left lateral tail vein was cannulated to administer fluids over the course of the experiment (1:1 mixture of lactated Ringer’s and 6% hetastarch solutions; 4 ml/kg/h i.v.). The mid-cervical trachea was exposed ventrally and cannulated to allow mechanical ventilation at 60–70 cycles/min with a 2.5–3.0 ml tidal volume (Harvard Small Animal Ventilator Model 683; Harvard Apparatus; Holliston, MA), and the end tidal concentration of CO2 was monitored continuously (Capnogard; Novametrix Medical Systems; Wallingford, CT). The right femoral artery was also exposed and cannulated to monitor arterial blood pressure (Model P122; Grass Telefactor; West Warrick, RI). Following surgical instrumentation, the rat was immobilized in a stereotaxic frame using ear bars and a vertebral clamp at T2. Core temperature was maintained between 36–37°C using a heating plate with temperature controller and isolated rectal probe (model TCAT-2DF; Physitemp Instruments, Inc.; Clifton, NJ). A thoracotomy was performed with a lateral intercostal approach to minimize respiratory-related spinal cord movement during extracellular recordings. The C6–C8 spinal cord was then exposed via bilateral dorsal laminectomy, and the overlying dura was resected. Rats were then converted to urethane anesthesia (1.2 g/kg i.v.) as isoflurane was slowly discontinued, and a 1:1 mixture of O2 and N2 (FIO2 0.50) was delivered via mechanical ventilation for the remainder of the experiment. After conversion to urethane anesthesia, 1.5 hours elapsed before neuronal recordings began. Anesthetic depth was continuously monitored and was maintained by urethane injection (0.12 g/kg i.v.) following any withdrawal response or mean arterial blood pressure increase in response to a hind paw pinch.
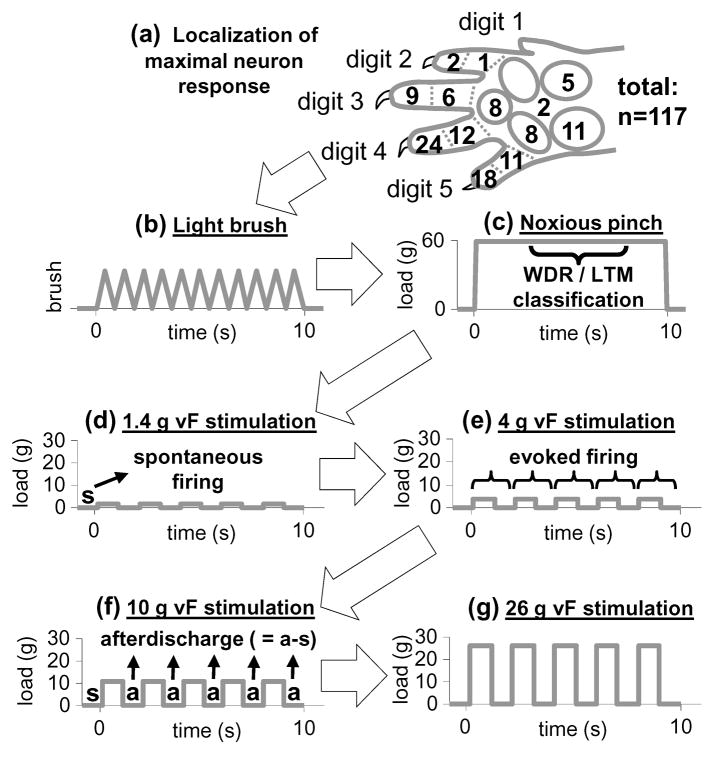
Extracellular voltage potentials were continuously recorded using a 5–8 μm diameter carbon fiber electrode (Carbostar-3; Kation Scientific, Inc.; Minneapolis, MN) and were amplified with a gain of 3000 (ExAmp-20KB; Kation Scientific, Inc.; Minneapolis, MN). The amplified signal was processed with a 60 Hz noise eliminator (Hum Bug; Quest Scientific; North Vancouver, BC), and then digitized and stored at 25 kHz (MK1401/Spike 2; CED; Cambridge, UK). Sensory neurons within the C7 dorsal horn (400–1000 μm below the pial surface) were identified using a light brush stroke applied to the plantar surface of the forepaw with a cotton swab. Search times were limited to 2.5 hours for each side of the spinal cord, and only the forepaw ipsilateral to the electrode was used to identify sensory neuronal activity. Once an evoked potential was identified, the forepaw location that evoked the maximum response was marked, and a stimulation protocol was performed that included brushing, noxious pinch, and a series of non-noxious and noxious von Frey filaments (Fig. 1). Extracellular recordings were stored for the entire duration of this stimulation protocol. Specifically, 10 consecutive brush strokes were applied to the targeted location on the forepaw with a cotton swab, and the location was then pinched for 10 seconds using a vascular clip calibrated to apply a 60 g force (World Precision Instruments, Inc.; Sarasota, FL). This vascular clip was selected because it did not produce any tissue damage or leave any permanent redness to the application area. Von Frey filaments were mounted to a load cell (5 N capacity; SMT S-Type Model; Interface, Inc., Scottsdale, AZ) and the load cell position was adjusted to apply the filaments to the identified location on the forepaw. Load cell voltages were recorded by the Spike 2 acquisition system to synchronize the mechanical stimulus application with the extracellular recordings (Fig. 2). Four logarithmically-spaced filament strengths (1.4, 4, 10, 26 g) spanning the range of filaments used for the behavioral assessment were applied to the targeted forepaw site; five stimulations spaced approximately 1–2 seconds apart were applied with each of the four filament strengths (Fig. 1 & 2). Approximately 60 seconds elapsed between the brush and pinch stimuli, and the use of the different von Frey filament magnitudes.
Figure 1. Forepaw stimulation protocol.
(a) Once a neuron was identified, the forepaw location that evoked maximal firing was defined. This location was most frequently found at the distal end of digits 4 and 5. (b) Light brush, (c) noxious pinch, and (d–g) von Frey (vF) filament stimulation were applied to that forepaw location. Neurons were classified as wide dynamic range (WDR) or low threshold mechanoreceptive (LTM) based on their response measured between 3–8 seconds during noxious pinch, as in (c). Spontaneous firing was characterized during the 1-second prior to von Frey stimulation in (d–g). Evoked firing from each von Frey application in (d–g) was defined by the response during each stimulation and immediately after. Afterdischarge following each von Frey application in (d–g) was defined as the difference between firing immediately after stimulation (a) and spontaneous firing (s).
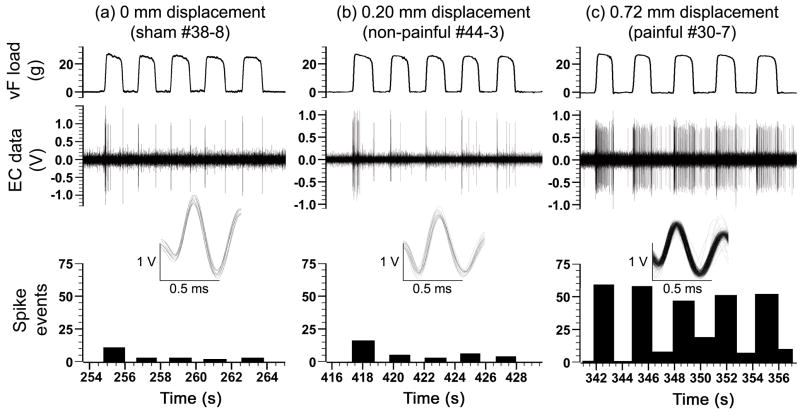
Figure 2. Representative extracellular recordings during the application of a 26 g von Frey filament to the forepaw 7 days after facet joint loading.
For each neuron, a series of 5 stimulations was applied with each filament magnitude. Extracellular (EC) data were spike-sorted to isolate the response of single units. Superimposed traces of all single unit activity counted in the histograms are provided. (a) Firing was evoked in sham rats during the initial application of the filament to the forepaw and upon its removal. (b) Rats that underwent a targeted 0.2 mm vertebral displacement also responded to forepaw stimulation predominantly during the initial application of the filament and upon its removal. (c) Neuronal firing was more frequent throughout the entire application of the filament and after removal for rats that underwent a vertebral displacement capable of producing a painful behavioral response.
Data analysis
Recordings during the stimulation protocol of each neuron were spike-sorted using Spike 2 software (CED; Cambridge, UK) to ensure that only the firing of a single unit was measured from each recording. The total number of spikes during the 10 light brush strokes and number of spikes during the 10-second noxious pinch were counted for each neuron. Neurons were classified as either a low threshold mechanoreceptive (LTM) or a wide dynamic range (WDR) neuron based on their response to the noxious pinch. Neurons were classified as WDR if firing exceeded one action potential during the period between the application and removal of the clip (3–8 seconds into pinch application). A nociceptive-specific neuron classification was not considered in this study because neurons were identified based on an evoked response to light brushing and this cell type is typically not found in the deep laminae of the dorsal horn (400–1000 μm) in rats [18].
The number of spikes from the initial application of a von Frey filament to 1-second after the removal of the filament was also counted as evoked firing for each neuron (Fig. 1). Baseline firing prior to stimulation with each von Frey filament was assessed by counting the number of spikes during the 1-second immediately before the first of the five applications of a filament (Fig. 1). Each neuron was classified as spontaneously firing or not based on whether baseline firing had occurred during the 1-second prior to the first application of any of the four von Frey filaments. Afterdischarge following each von Frey application was computed as the difference between the firing rate recorded during the 1-second after the stimulus and the baseline firing rate recorded during the 1-second prior to the first von Frey application with that filament (Fig. 1).
Statistical analyses were performed using JMP 8 (SAS Institute Inc.; Cary, NC). Differences in the amount of vertebral displacement between the painful and non-painful groups were measured using an unpaired Student’s t-test. Changes in behavioral sensitivity from baseline to the day of electrophysiological testing were assessed for each group using a paired t-test. Electrophysiological data were log-transformed due to a positive skew, and a normal distribution was verified after the transformation by plotting the residuals from the statistical models. To test for differences in the firing responses to light brush and noxious pinch between the painful, non-painful, and sham groups, mixed-effect ANOVAs were used with neurons nested within rats, and rats nested within groups. Post-hoc Tukey HSD tests evaluated differences between the three individual groups. A mixed-effect ANOVA with the same levels of nesting was used to analyze differences between groups, von Frey stimulation magnitudes, the order of stimulus application, and their interactions. This mixed-effect ANOVA structure was also used to evaluate afterdischarge following von Frey stimuli. The number of neurons that were spontaneously firing during any of the baseline recordings was compared between groups through Pearson’s chi-square tests to evaluate whether spontaneous firing differed among injury groups. Differences in the proportion of WDR and LTM neurons between injury groups were also assessed though Pearson’s chi-square tests. All statistical tests were performed with α=0.05, and all values are expressed as means ± S.E.
Results
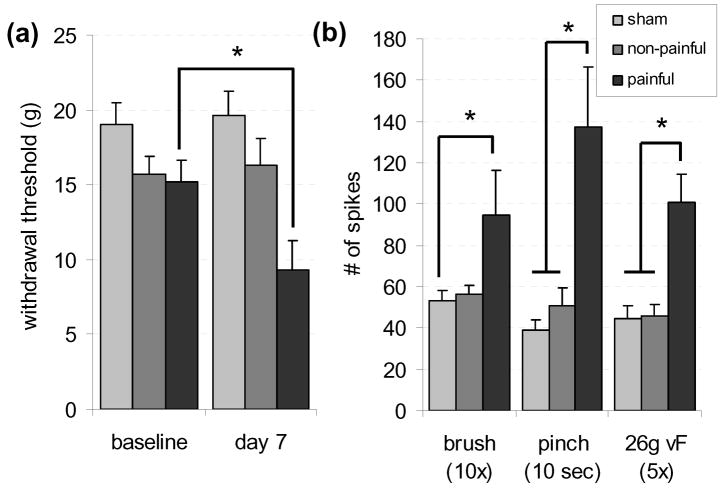
The mean vertebral displacement imposed in the painful group was 0.684±0.009 mm, and was significantly greater (p<0.0001) than the mean displacement applied in the non-painful group (0.229±0.006 mm). The threshold for paw withdrawal in the mechanical hyperalgesia testing was not significantly different between the left and right side for any of the groups, so the withdrawal threshold was computed as the average of both sides for each rat. The withdrawal threshold did not change significantly in the sham group between baseline values (19.75±1.46 g) and day 6 (19.58±1.69 g) or in the non-painful group between baseline (15.72±1.150 g) and day 7 (16.33±1.77 g) (Fig. 3). However, there was a significant decrease (p=0.004) in the withdrawal threshold measured at day 7 (9.3±4.7g) in the painful injury group compared to its baseline values (16.19±1.45 g) (Fig. 3).
Figure 3. Neuronal hyperexcitability in the dorsal horn are produced at day 7 following facet joint loading that produces mechanical hyperalgesia.
(a) The withdrawal threshold was significantly lower (*p=0.004) at day 7 in the painful group compared to the corresponding baseline values. (b) Neuronal excitability was significantly greater (*p<0.038) in the painful group compared to sham for brush, pinch, and 26 g von Frey stimuli; the painful group was also significantly greater (p<0.0182) than the non-painful group for pinch and von Frey stimulation.
A total of 117 neurons were identified at an average depth of 638±14.56 μm. For 34 neurons, light brushing of one of the pads on the forepaw produced the most robust firing response, while firing from the remaining 83 neurons was most robust in response to stimulation of one of the digits of the forepaw (Fig. 1). Light brushing produced significantly more firing (p=0.038) in the painful group (94±22 spikes/10 strokes) relative to sham (53±5 spikes/10 strokes), but the non-painful group (56±5 spikes/10 strokes) was not significantly different from either the painful or sham group (Fig. 3). Noxious pinch to the forepaw evoked significantly more firing (p<0.0182) in the painful group (137±29 spikes/10 s) than compared to either the non-painful (51±8 spikes/10 s) or sham (39±5spikes/10 s) groups (Fig. 3). In the painful group, 69% of neurons were classified as wide dynamic range neurons, which was significantly higher than that found in non-painful (44% WDR; p=0.0348) or sham (43% WDR; p=0.0251) rats.
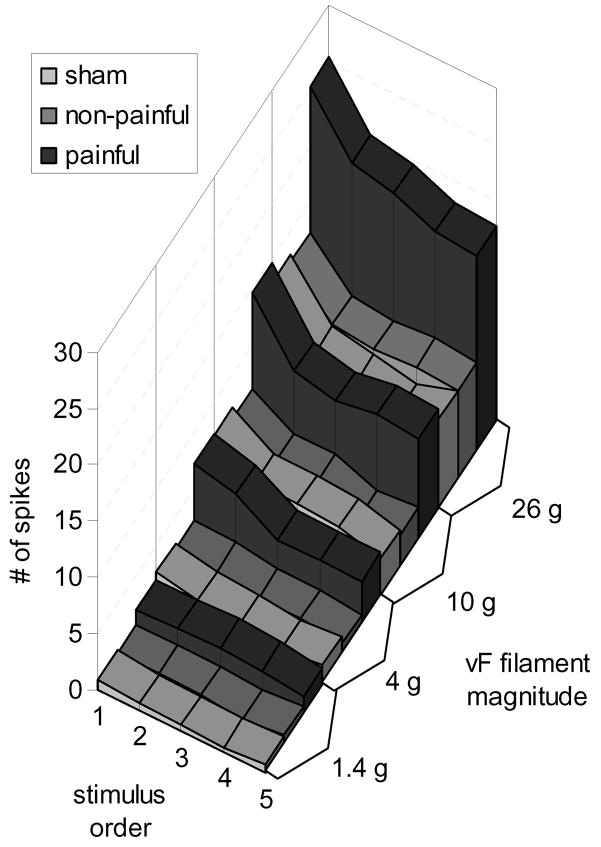
Average evoked firing was significantly different between each of the von Frey stimulus magnitudes (p<0.0001) (Fig. 4). Using each von Frey filament magnitude, firing among the five applications was also significantly different (p<0.0001), with the first application producing significantly more firing than the subsequent four applications (p<0.0001), and the second application producing significantly more firing than the fourth and fifth applications (Fig. 5). A significant interaction was also found between the magnitude of the von Frey stimulus applied to the forepaw and the order of application (p<0.0001). Firing during the first application of the 26 g von Frey filament was significantly greater than all other combinations of stimulus magnitude and order of application (p<0.0001) (Fig. 5).
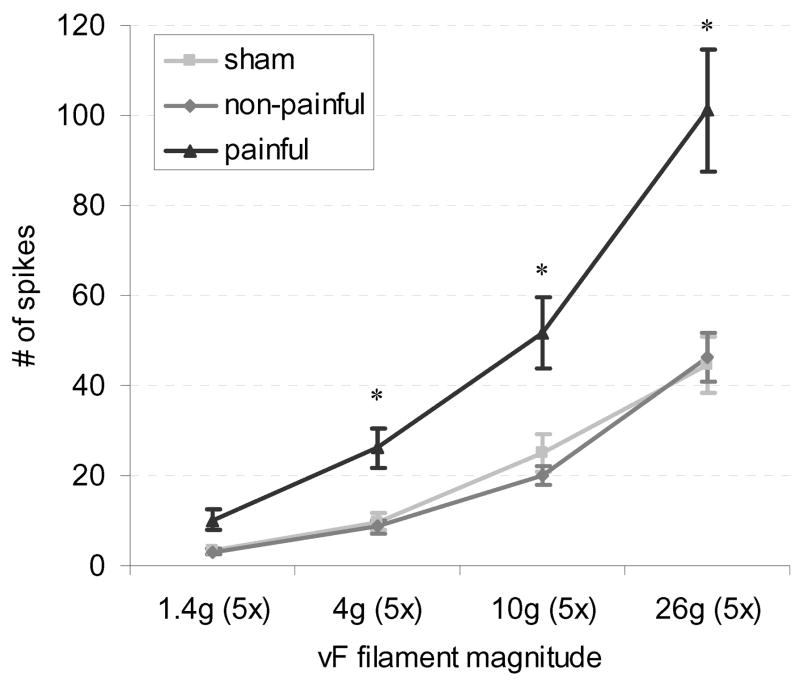
Figure 4. Evoked neuronal firing increases with increasing von Frey stimulus magnitude.
The number of evoked spikes in the painful group was significantly greater than the non-painful and sham groups at 4, 10, and 26 g (*p<0.0156).
Figure 5. The average number of spikes evoked during von Frey filament stimulation of the forepaw increases with respect to the filament strength and depends on the order of application.
A significant interaction effect was found between the von Frey magnitude and the ordinal rank of the stimulus application (p<0.0001); at greater filament magnitudes, firing was greater in response to the first application of the filament relative to the subsequent applications at that magnitude.
Overall, neuronal firing in response to von Frey stimuli was significantly higher (p<0.001) in the painful group than either the non-painful or sham group (Fig. 3–5). The 26 g filament evoked an average of 101±13 spikes over the five applications in the painful group, and this was significantly greater (p<0.004) than the number of spikes produced during the five applications of the filament in either the non-painful (46±5 spikes) or sham (45±6 spikes) groups (Fig. 2 & 3). Firing was also significantly higher in the painful group compared to non-painful and sham groups for the 10 g (p<0.0156) and 4 g (p<0.005) filaments (Fig. 4), but no significant differences in firing were found between groups for stimulation with the 1.4 g filament.
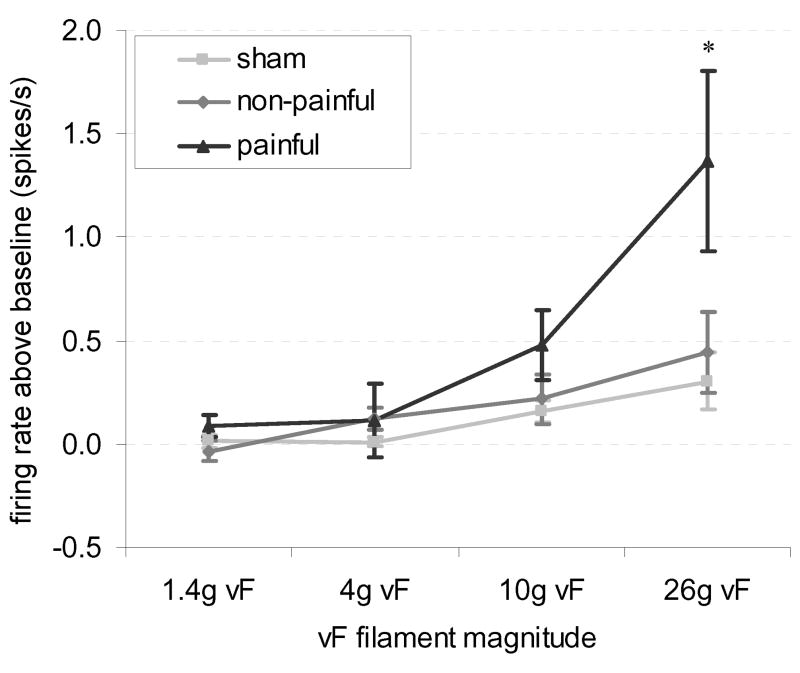
The overall average spontaneous firing rate prior to von Frey filament stimulation was 0.068±0.3132 spikes/s, and firing only occurred in 21 of the 117 neurons. The number of neurons spontaneously firing in the painful group (11 of 32) was significantly greater (p=0.0042) than expected when compared to the sham group (3 of 40), but not the non-painful group (7 of 45). Afterdischarge rates, measured by the difference in firing immediately following forepaw stimulation relative to the spontaneous rate, were significantly greater (p=0.0307) overall in the painful group compared to sham; yet, this was not significantly greater than that in the non-painful group. The average afterdischarge rate following a 26 g stimulus (1.37±0.44 spikes/s increase over spontaneous discharge rates) was significantly greater (p<0.0002) than the non-painful (0.44±0.20 spikes/s increase) or sham (0.31±0.14 spikes/s increase) groups (Fig. 6). Significant group-magnitude (p=0.0013) and group-magnitude-application order (p=0.0290) interactions were found for afterdischarge firing. These interactions were attributable to the significantly higher afterdischarge rates in the painful group after a 26 g stimulus compared to all other combinations of groups and magnitudes (p>0.0006) (Fig. 6). Furthermore, afterdischarge in the painful group after a 26 g stimulus was significantly lower for the first application compared to the second and third (p>0.0029).
Figure 6. Afterdischarge rates increase after painful facet injury in response to von Frey filament stimulation of the forepaw.
Afterdischarge was measured as the increase in firing rate above spontaneous baseline firing rates. The afterdischarge rate was significantly higher after 26 g filament stimulation in the painful group compared to non-painful and sham groups (*p<0.0006).
Discussion
This is the first study to identify changes in neuron firing in the central nervous system at any time (immediately or at a later time point) after mechanically-induced facet joint injury. Seven days after a subfailure C6/C7 facet joint capsule stretch, mechanical stimulation of the forepaw evoked an exaggerated firing response across a range of mechanical stimuli compared to responses in rats with a less-severe capsule stretch magnitude (Fig. 2–4). Additionally, neurons 7 days after painful joint loading were more likely to spontaneously fire than neurons in rats having undergone sham procedures. Noxious von Frey stimulation (26 g) produced a significantly higher afterdischarge firing rate in the painful group relative to non-painful or sham groups (Fig. 6). These findings suggest that facet capsule stretch of a sufficient magnitude to induce mechanical hypersensitivity will also sensitize dorsal horn neurons and cause increases in firing to non-noxious and noxious forepaw stimulation (Fig. 3 & 4), which may drive the mechanical allodynia [15,32] and hyperalgesia [31,33] (Fig. 3) observed in this model and also reported in patients with whiplash-associated disorders [1,12,49].
This study provides the first direct evidence of the modulation of secondary somatosensory neurons following a facet capsule stretch. Previous work in a caprine model demonstrated that primary afferent firing may be altered during, and immediately after, certain magnitudes of capsule stretch [5,8,36]. The nociceptor firing and mechanoreceptor afterdischarge in response to the initial mechanical injury in that study may provide evidence of a sufficient input to initiate the changes in the firing response of spinal neurons that were measured seven days after injury in the current study. Increased levels of substance P mRNA [33] and increased expression of metabotropic glutamate receptor 5 [15] in the spinal cord at day 7 following similar degrees of facet capsule stretch also provide evidence of sustained, facet-mediated cellular changes in the spinal cord. Although those findings strongly suggest neuronal plasticity they were not able to localize those spinal changes to the dorsal horn nor were they able to identify the cellular source of those modifications. The characterization of neuronal hyperexcitability in the current study in response to graded vertebral displacements suggests that dorsal horn neuron plasticity may be related to the previous evidence of transcriptional changes in the spinal cord.
The underlying mechanism that drives chronic pain in whiplash is poorly understood due to the frequent absence of any evidence of injury to spinal tissues or other structures [42]. Given that anesthetic facet joint blocks can provide short-term relief of chronic neck pain for about 50% of patients [2–3,34], it is likely that peripheral neuron firing is a requisite for pain in these cases. The current study suggests that when facet joint loading is sufficient to induce persistent hypersensitivity, the wide dynamic range neurons in the dorsal horn respond to 4g von Frey stimulation of the forepaw as though it were a more noxious 10 g filament stimulation (Fig. 4). Future work is needed to establish whether non-noxious proprioceptive information from the facet joints and other spinal structures may be misinterpreted as nociceptive under certain neck motions. Although neck musculature and other connective tissues were disrupted during the surgical approaches used in all groups in this study, recent advances in electrophysiological techniques using minimally invasive surgery may enable direct measurements of the effect of proprioceptive afferent signaling in the neck and facet joints in this model [30, 51]. Furthermore, a less-invasive surgical technique is needed for future work to better characterize the acute and long-term effects of the surgical procedures used in this model of facet joint loading.
The significant increase in the number of wide dynamic range neurons classified in the painful group (69% of neurons; p>0.0348) in this study suggests that a phenotypic shift in the response of the neuronal population in the deep laminae of the dorsal horn may play a key role in modulating chronic pain after facet joint injury. The classification of neurons following sham procedures in this study (43% WDR and 57% LTM) is similar to the proportion of WDR cells reported in electrophysiological studies of neuron properties in dorsal horn of rat and sheep [13,18,22] and supports the classification methodology used in our approach. The increase in the number of WDR neurons identified in the deep laminae following facet capsule stretch in the painful group in our study is similar to the phenotypic shift identified in the dorsal horn following spinal cord hemisection [18]. An increased responsiveness of dorsal horn neurons to noxious stimuli has also been reported in models of joint inflammation [29], peripheral neuropathy [39], peripheral burn injury [6], and spinal cord injury [18–20]. The shift in the dorsal horn neuron population towards a wide dynamic range response, and the hyperexcitability of these neurons across a range of mechanical stimuli (Fig. 3 & 4) suggests that facet capsule stretch that produces persistent pain symptoms may result from central sensitization brought on by sustained changes to peripheral neurons and/or wide dynamic range neuron plasticity in the spinal cord.
Hypersensitivity is frequently observed along the back of the shoulder and neck in whiplash patients [1,12,48] and has also been reported in this rat model of facet capsule stretch [31,33]. Although the design of the current study provides a direct comparison to mechanical hyperalgesia assessments using similar forepaw stimulation protocols, its scope did not encompass all regions along the C6–C8 dermatomes that may be sensitized following whiplash-like facet joint injury conditions. Additionally, this study did not characterize differences in the size of the receptor field of neurons. However, it should be noted that unlike other studies of neuronal hypersensitivity, the site of injury in this study (facet joints) was not directly related to the stimulation region, and by identifying neuronal hyperexcitability in the forepaw, this study suggests widespread secondary hyperalgesia. The order of the brush, pinch, and filament stimuli used in this study was based on previous electrophysiological studies of dorsal horn hypersensitivity [6, 18–20] and does not account for an effect noxious pinch may have on the firing evoked by von Frey filament stimulation. However, immediately after noxious pinch, the five applications of the 1.4 g von Frey filament did not exhibit any dependence on the order of application (Fig. 5), suggesting that the 60 g pinch magnitude did not produce a significant effect on spinal neuron firing. Dorsal horn neurons were identified based on an evoked response to light brush, but spontaneous discharges were also identified in 18% of these mechanosensitive neurons. However, the average spontaneous discharge rate (0.068±0.3132 spikes/s) was substantially lower than the firing frequencies observed during evoked responses (Fig. 2–4). This study characterized the neuronal response to forepaw stimulation at a single time point (7 days) after a capsule stretch that produced hypersensitivity. The development of neuronal plasticity and the long-term effects remain unknown; however, behavioral hypersensitivity in our model of facet capsule stretch has been shown to persist for up to 42 days after initial injury [43].
These electrophysiological findings support the hypothesis that chronic pain following whiplash may be driven in part by central sensitization. Certainly, additional studies are needed to elucidate the biochemical or anatomical changes that produce neuronal hyperexcitability and a change in the phenotypic response of neurons to noxious stimuli in this study of the deep laminae of the dorsal horn. Nonetheless, this study provides the first direct evidence of spinal neuron plasticity in the lower cervical spinal cord at a non-acute time point after facet capsule stretch. This work provides a foundation to continue to understand the neuronal mechanisms driving the maintenance of chronic pain and the relationship between mechanical tissue loading and pain in the neck for whiplash injury.
Acknowledgments
The project described was supported by Grant Number AR056288 from NIAMS (BAW), a University Research Foundation award from the University of Pennsylvania (BAW), and a Craig H. Neilsen Foundation Research Grant (FJG). There are no conflicts of interests for any authors with any aspect of this study. The authors would like to thank Jeffrey V. Kras and Sharon Martinez for their technical assistance and advice during these experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banic B, Petersen-Felix S, Andersen OK, Radanov BP, Villiger PM, Arendt-Nielsen L, Curatolo M. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain. 2004;107(1–2):7–15. doi: 10.1016/j.pain.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Barnsley L, Lord S, Bogduk N. Comparative local anaesthetic blocks in the diagnosis of cervical zygapophysial joint pain. Pain. 1993;55(1):99–106. doi: 10.1016/0304-3959(93)90189-V. [DOI] [PubMed] [Google Scholar]
- 3.Barnsley L, Lord SM, Wallis BJ, Bogduk N. The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine. 1995;20(1):20–25. doi: 10.1097/00007632-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Beaman DN, Graziano GP, Glover RA, Wojtys EM, Chang V. Substance P innervation of lumbar spine facet joints. Spine. 1993;18(8):1044–1049. doi: 10.1097/00007632-199306150-00014. [DOI] [PubMed] [Google Scholar]
- 5.Cavanaugh JM, Lu Y, Chen C, Kallakuri S. Pain generation in lumbar and cervical facet joints. J Bone Joint Surg Am. 2006;88 (Suppl 2):63–67. doi: 10.2106/JBJS.E.01411. [DOI] [PubMed] [Google Scholar]
- 6.Chang YW, Tan A, Saab C, Waxman S. Unilateral focal burn injury is followed by long-lasting bilateral allodynia and neuronal hyperexcitability in spinal cord dorsal horn. J Pain. 2009;11(2):119–130. doi: 10.1016/j.jpain.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Lu Y, Cavanaugh JM, Kallakuri S, Patwardhan A. Recording of neural activity from goat cervical facet joint capsule using custom-designed miniature electrodes. Spine. 2005;30(12):1367–1372. doi: 10.1097/01.brs.0000166193.39389.21. [DOI] [PubMed] [Google Scholar]
- 9.Christensen MD, Hulsebosch CE. Chronic central pain after spinal cord injury. J Neurotrauma. 1997;14(8):517–537. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- 10.Coghill RC, Mayer DJ, Price DD. Wide dynamic range but not nociceptive-specific neurons encode multidimensional features of prolonged repetitive heat pain. J Neurophysiol. 1993;69(3):703–716. doi: 10.1152/jn.1993.69.3.703. [DOI] [PubMed] [Google Scholar]
- 11.Croft PR, Lewis M, Papageorgiou AC, Thomas E, Jayson MI, Macfarlane GJ, Silman AJ. Risk factors for neck pain: a longitudinal study in the general population. Pain. 2001;93(3):317–325. doi: 10.1016/S0304-3959(01)00334-7. [DOI] [PubMed] [Google Scholar]
- 12.Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Giani C, Zbinden AM, Radanov BP. Central hypersensitivity in chronic pain after whiplash injury. Clin J Pain. 2001;17(4):306–315. doi: 10.1097/00002508-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Dado RJ, Katter JT, Giesler GJ., Jr Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. II. Responses to innocuous and noxious mechanical and thermal stimuli. J Neurophysiol. 1994;71(3):981–1002. doi: 10.1152/jn.1994.71.3.981. [DOI] [PubMed] [Google Scholar]
- 14.Deng B, Begeman PC, Yang KH, Tashman S, King AI. Kinematics of human cadaver cervical spine during low speed rear-end impacts. Stapp Car Crash J. 2000;44:171–188. doi: 10.4271/2000-01-SC13. [DOI] [PubMed] [Google Scholar]
- 15.Dong L, Winkelstein BA. Simulated whiplash modulates expression of the glutamatergic system in the spinal cord suggesting spinal plasticity is associated with painful dynamic cervical facet loading. J Neurotrauma. 2010;27(1):163–174. doi: 10.1089/neu.2009.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.el-Bohy A, Cavanaugh JM, Getchell ML, Bulas T, Getchell TV, King AI. Localization of substance P and neurofilament immunoreactive fibers in the lumbar facet joint capsule and supraspinous ligament of the rabbit. Brain Res. 1988;460(2):379–382. doi: 10.1016/0006-8993(88)90386-1. [DOI] [PubMed] [Google Scholar]
- 17.Greening J, Dilley A, Lynn B. In vivo study of nerve movement and mechanosensitivity of the median nerve in whiplash and non-specific arm pain patients. Pain. 2005;115(3):248–253. doi: 10.1016/j.pain.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Hains BC, Johnson KM, Eaton MJ, Willis WD, Hulsebosch CE. Serotonergic neural precursor cell grafts attenuate bilateral hyperexcitability of dorsal horn neurons after spinal hemisection in rat. Neuroscience. 2003;116(4):1097–1110. doi: 10.1016/s0306-4522(02)00729-7. [DOI] [PubMed] [Google Scholar]
- 19.Hains BC, Willis WD, Hulsebosch CE. Differential electrophysiological effects of brain-derived neurotrophic factor on dorsal horn neurons following chronic spinal cord hemisection injury in the rat. Neurosci Lett. 2002;320(3):125–128. doi: 10.1016/s0304-3940(02)00026-5. [DOI] [PubMed] [Google Scholar]
- 20.Hains BC, Willis WD, Hulsebosch CE. Temporal plasticity of dorsal horn somatosensory neurons after acute and chronic spinal cord hemisection in rat. Brain Res. 2003;970(1–2):238–241. doi: 10.1016/s0006-8993(03)02347-3. [DOI] [PubMed] [Google Scholar]
- 21.Hao JX, Xu XJ, Yu YX, Seiger A, Wiesenfeld-Hallin Z. Transient spinal cord ischemia induces temporary hypersensitivity of dorsal horn wide dynamic range neurons to myelinated, but not unmyelinated, fiber input. J Neurophysiol. 1992;68(2):384–391. doi: 10.1152/jn.1992.68.2.384. [DOI] [PubMed] [Google Scholar]
- 22.Herrero JF, Headley PM. The dominant class of somatosensory neurone recorded in the spinal dorsal horn of awake sheep has wide dynamic range properties. Pain. 1995;61(1):133–138. doi: 10.1016/0304-3959(94)00152-5. [DOI] [PubMed] [Google Scholar]
- 23.Hogg-Johnson S, van der Velde G, Carroll LJ, Holm LW, Cassidy JD, Guzman J, Cote P, Haldeman S, Ammendolia C, Carragee E, Hurwitz E, Nordin M, Peloso P. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(4 Suppl):S39–51. doi: 10.1097/BRS.0b013e31816454c8. [DOI] [PubMed] [Google Scholar]
- 24.Hubbard RD, Winkelstein BA. Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and spinal glial activation: mechanical factors in painful neck injuries. Spine. 2005;30(17):1924–1932. doi: 10.1097/01.brs.0000176239.72928.00. [DOI] [PubMed] [Google Scholar]
- 25.Inami S, Shiga T, Tsujino A, Yabuki T, Okado N, Ochiai N. Immunohistochemical demonstration of nerve fibers in the synovial fold of the human cervical facet joint. J Orthop Res. 2001;19(4):593–596. doi: 10.1016/S0736-0266(00)00048-6. [DOI] [PubMed] [Google Scholar]
- 26.Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8(1):1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 27.Kallakuri S, Singh A, Chen C, Cavanaugh JM. Demonstration of substance P, calcitonin gene-related peptide, and protein gene product 9.5 containing nerve fibers in human cervical facet joint capsules. Spine. 2004;29(11):1182–1186. doi: 10.1097/00007632-200406010-00005. [DOI] [PubMed] [Google Scholar]
- 28.Kaneoka K, Ono K, Inami S, Hayashi K. Motion analysis of cervical vertebrae during whiplash loading. Spine. 1999;24(8):763–769. doi: 10.1097/00007632-199904150-00006. [DOI] [PubMed] [Google Scholar]
- 29.Kitagawa J, Kanda K, Sugiura M, Tsuboi Y, Ogawa A, Shimizu K, Koyama N, Kamo H, Watanabe T, Ren K, Iwata K. Effect of chronic inflammation on dorsal horn nociceptive neurons in aged rats. J Neurophyiol. 2005;93(6):3594–3604. doi: 10.1152/jn.01075.2004. [DOI] [PubMed] [Google Scholar]
- 30.Lam DK, Sessle BJ, Hu JW. Surgical incision can alter capsaicin-induced central sensitization in rat brainstem nociceptive neurons. Neuroscience. 2008;156(3):737–747. doi: 10.1016/j.neuroscience.2008.07.058. [DOI] [PubMed] [Google Scholar]
- 31.Lee KE, Davis MB, Winkelstein BA. Capsular ligament involvement in the development of mechanical hyperalgesia after facet joint loading: behavioral and inflammatory outcomes in a rodent model of pain. J Neurotrauma. 2008;25(11):1383–1393. doi: 10.1089/neu.2008.0700. [DOI] [PubMed] [Google Scholar]
- 32.Lee KE, Thinnes JH, Gokhin DS, Winkelstein BA. A novel rodent neck pain model of facet-mediated behavioral hypersensitivity: implications for persistent pain and whiplash injury. J Neurosci Methods. 2004;137(2):151–159. doi: 10.1016/j.jneumeth.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Lee KE, Winkelstein BA. Joint distraction magnitude is associated with different behavioral outcomes and substance P levels for cervical facet joint loading in the rat. J Pain. 2009;10(4):436–445. doi: 10.1016/j.jpain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Lord SM, Barnsley L, Wallis BJ, Bogduk N. Chronic cervical zygapophysial joint pain after whiplash. A placebo-controlled prevalence study. [see comment] Spine. 1996;21(15):1737–1744. doi: 10.1097/00007632-199608010-00005. [DOI] [PubMed] [Google Scholar]
- 35.Lu Y, Chen C, Kallakuri S, Patwardhan A, Cavanaugh JM. Neural response of cervical facet joint capsule to stretch: a study of whiplash pain mechanism. Stapp Car Crash J. 2005;49:49–65. doi: 10.4271/2005-22-0003. [DOI] [PubMed] [Google Scholar]
- 36.Lu Y, Chen C, Kallakuri S, Patwardhan A, Cavanaugh JM. Neurophysiological and biomechanical characterization of goat cervical facet joint capsules. J Orthop Res. 2005;23(4):779–787. doi: 10.1016/j.orthres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Manchikanti L, Singh V, Rivera J, Pampati V. Prevalence of cervical facet joint pain in chronic neck pain. Pain Physician. 2002;5(3):243–249. [PubMed] [Google Scholar]
- 38.McLain RF. Mechanoreceptor endings in human cervical facet joints. Iowa Orthop J. 1993;13:149–154. [PMC free article] [PubMed] [Google Scholar]
- 39.Palecek J, Dougherty PM, Kim SH, Paleckova V, Lekan H, Chung JM, Carlton SM, Willis WD. Responses of spinothalamic tract neurons to mechanical and thermal stimuli in an experimental model of peripheral neuropathy in primates. J Neurophysiol. 1992;68(6):1951–1966. doi: 10.1152/jn.1992.68.6.1951. [DOI] [PubMed] [Google Scholar]
- 40.Panjabi MM, Cholewicki J, Nibu K, Grauer J, Vahldiek M. Capsular ligament stretches during in vitro whiplash simulations. J Spinal Disord. 1998;11(3):227–232. [PubMed] [Google Scholar]
- 41.Pearson AM, Ivancic PC, Ito S, Panjabi MM. Facet joint kinematics and injury mechanisms during simulated whiplash. Spine. 2004;29(4):390–397. doi: 10.1097/01.brs.0000090836.50508.f7. [DOI] [PubMed] [Google Scholar]
- 42.Riley LH, 3rd, Long D, Riley LH., Jr The science of whiplash. Medicine. 1995;74(5):298–299. doi: 10.1097/00005792-199509000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Rothman SM, Hubbard RD, Lee KE, Winkelstein BA. Detection, transmission, and perception of pain. In: Slipman CW, Simeone FA, Derby R, Mayer TG, editors. Interventional spine: an algorithmic approach. Philadelphia: Saunders; 2008. pp. 29–38. [Google Scholar]
- 44.Sandkuhler J. Learning and memory in pain pathways. Pain. 2000;88(2):113–118. doi: 10.1016/S0304-3959(00)00424-3. [DOI] [PubMed] [Google Scholar]
- 45.Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci. 2002;5 (Suppl):1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- 46.Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462(7273):651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheather-Reid RB, Cohen ML. Psychophysical evidence for a neuropathic component of chronic neck pain. Pain. 1998;75(2–3):341–347. doi: 10.1016/s0304-3959(98)00013-x. [DOI] [PubMed] [Google Scholar]
- 48.Sterling M, Jull G, Kenardy J. Physical and psychological factors maintain long-term predictive capacity post-whiplash injury. Pain. 2006;122(1–2):102–108. doi: 10.1016/j.pain.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 49.Sterling M, Jull G, Vicenzino B, Kenardy J. Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. Pain. 2003;104(3):509–517. doi: 10.1016/S0304-3959(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 50.Sundararajan S, Prasad P, Demetropoulos CK, Tashman S, Begeman PC, Yang KH, King AI. Effect of Head-Neck Position on Cervical Facet Stretch of Post Mortem Human Subjects during Low Speed Rear End Impacts. Stapp Car Crash J. 2004;48:331–372. doi: 10.4271/2004-22-0015. [DOI] [PubMed] [Google Scholar]
- 51.Vernon H, Sun K, Zhang Y, Yu X, Sessle BJ. Central sensitization induced in trigeminal and upper cervical dorsal horn neurons by noxious stimulation of deep cervical paraspinal tissues in rats with minimal surgical trauma. J Manip Physiol Ther. 2009;32(7):506–514. doi: 10.1016/j.jmpt.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 53.Yamashita T, Cavanaugh JM, el-Bohy AA, Getchell TV, King AI. Mechanosensitive afferent units in the lumbar facet joint. J Bone Joint Surg Am. 1990;72:865–870. [PubMed] [Google Scholar]
- 54.Yamashita T, Cavanaugh JM, Ozaktay AC, Avramov AI, Getchell TV, King AI. Effect of substance P on mechanosensitive units of tissues around and in the lumbar facet joint. J Orthop Res. 1993;11(2):205–214. doi: 10.1002/jor.1100110208. [DOI] [PubMed] [Google Scholar]
- 55.Yang KH, King AI. Neck kinematics in rear-end impacts. Pain Res Manag. 2003;8(2):79–85. doi: 10.1155/2003/839740. [DOI] [PubMed] [Google Scholar]
- 56.Yoganandan N, Pintar FA, Klienberger M. Cervical spine vertebral and facet joint kinematics under whiplash. J Biomech Eng. 1998;120(2):305–307. doi: 10.1115/1.2798318. [DOI] [PubMed] [Google Scholar]
- 57.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]