Abstract
Rationale
Pyridine nucleotides regulate the cardiac Na+ current (INa) through generation of reactive oxygen species (ROS).
Objective
We investigated the source of ROS induced by elevated NADH.
Methods and Results
In HEK cells stably expressing the cardiac Na+ channel, the decrease of INa (52±9%; P<0.01) induced by cytosolic NADH application (100 μmol/L) was reversed by mitoTEMPO, rotenone, malonate, DIDS, PK11195 and 4′-chlorodiazepam, a specific scavenger of mitochondrial superoxide and inhibitors of the mitochondrial complex I, complex II, voltage-dependent anion channels, and benzodiazepine receptor, respectively. Antimycin A (20 μmol/L), a complex III inhibitor known to generate ROS, decreased INa (51±4%, P<0.01). This effect was blocked by NAD+, forskolin, or rotenone. Inhibitors of complex IV, nitric oxide synthase, the NADPH oxidases, xanthine oxidases, the mitochondrial permeability transition pore, and the mitochondrial ATP-sensitive K+ channel did not change the NADH effect on INa. Analogous results were observed in cardiomyocytes. Rotenone, mitoTEMPO, and 4′-chlorodiazepam also blocked the mutant A280V glycerol-3-phosphate dehydrogenase 1-like effect on reducing INa, indicating a role for mitochondria in the Brugada Syndrome caused by this mutation. Fluorescent microscopy confirmed mitochondrial ROS generation with elevated NADH and ROS inhibition by NAD+.
Conclusions
Altering the oxidized to reduced NAD(H) balance can activate mitochondrial ROS production, leading to reduced INa. This signaling cascade may help explain the link between altered metabolism, conduction block, and arrhythmic risk.
Keywords: metabolism, pyridine nucleotides, arrhythmia, sudden death
Introduction
Recently, we reported that mutations in glycerol-3-phosphate dehydrogenase 1-like (GPD1-L) protein, a gene associated with Brugada Syndrome and Sudden Infant Death Syndromes,1, 2 cause reduced cardiac sodium channel (Nav1.5) function by modulating pyridine nucleotides.3 Elevated intracellular NADH results in a rapid decrease in cardiac Na+ current (INa) in cardiomyocytes that is large enough to be clinically significant4 and of a magnitude seen in Brugada Syndrome.5 The effect is identical on heterologously expressed sodium channel in human embryonic kidney (HEK) cells. The immediacy of the NADH effect on reducing INa and the lack of change in mRNA abundance under various experimental conditions suggests that the effect of NADH is post-transcriptional.
NADH modulated Nav1.5 through PKC activation and increased oxidative stress.3 The finding that the balance of oxidized and reduced NAD(H) regulates INa suggests that the metabolic state of myocytes may influence Nav1.5. NADH is known to oscillate with myocardial ischemia, and mitochondrial injury is associated with increased NADH and ROS levels.6, 7 These changes in NADH could contribute to reduced INa, conduction block, and arrhythmic risk known to exist with ischemia. Moreover, heart failure is associated with increased oxidative stress, reduced NAD+, and increased NADH.8–10 The increased NADH level may contribute to the increased oxidative stress and diminished INa in heart failure.11, 12
Several metabolic pathways are known to produce ROS, including uncoupled nitric oxide synthase (NOS), the NAD(P)H oxidase, xanthine oxidase, and the mitochondrial electron transport chain (ETC). Cardiac oxidation leads to NOS uncoupling and diastolic dysfunction.13 NAD(P)H oxidases are an important source of superoxide in human atherosclerosis.14 Xanthine oxidase plays an important role in various forms of ischemic injury and in chronic heart failure.15 In ischemia/reperfusion injury, the ETC serves as the source of ROS.16 In chronic heart failure, ROS levels increase17, 18 and myocardial antioxidant reserve decreases.19, 20 In turn, ROS increases cell death by apoptosis, reduces cellular respiration, induces structural damage to proteins including ion channels, and impairs contractility.8 Here, we show that mitochondria are the main source of NADH-dependent ROS downregulating the cardiac Nav1.5.
Methods
Full descriptions of the methods are available in the supplemental material.
Cell Culture
We maintained a human embryonic kidney (HEK) cell line stably expressing the human cardiac Nav1.5 channel (SCN5A cells). Expression of Nav1.5 was linked to green fluorescent protein (GFP) expression by an internal ribosomal entry site (SCN5A-IRES-GFP). SCN5A cells were cultured in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum, 0.2 mg/mL geneticin (for antibiotic selection) and 1% penicillin/streptomycin in a 95% O2/5% CO2 incubator at 37°C. Rat neonatal ventricular myocytes (NVM) were isolated from neonatal rat hearts by collagenase treatment (Worthington Biochemical Corporation, Lakewook, NJ).
Nearly undetectable levels of GPD1-L protein are expressed in HEK cells.1 Therefore, for whole-cell patch clamping experiments to study GPD1-L effects on Nav1.5, SCN5A cells were transiently transfected with WT or A280V GPD1-L (a generous gift from Dr. Barry London, University of Pittsburgh, PA) and an expression vector containing red fluorescent protein (RFP) as described previously.2 In these experiments, cells expressing both GFP and RFP were studied.
Electrophysiology
Na+ currents were measured using the whole-cell patch clamp technique in voltage-clamp mode, while NVM action potentials (APs) were measured in current-clamp mode at room temperature. To measure Na+ currents, pipettes (1–2 M) were filled with a pipette solution containing (in mmol/L): CsCl 80, cesium aspartate 80, EGTA 11, MgCl2 1, CaCl2 1, HEPES 10, and Na2ATP 5 (adjusted to pH 7.4 with CsOH). The bath solution consisted of (in mmol/L): NaCl 130, CsCl 5, CaCl2 2, MgCl2 1.2, HEPES 10 and glucose 5 (adjusted to pH 7.4 with CsOH). A stepped voltage protocol from −100 to +60 mV with a holding potential of −100 mV was applied to establish the presence of Nav1.5. Peak currents obtained during steps to −20 or −30 mV were used for comparison in determining the relative reduction of INa. To minimize time-dependent driftin gating parameters, all protocols were initiated 2–5 minafter whole-cell configuration was obtained. The currents were normalized for cell capacitance prior to deriving ratios.
For APs measurement, pipettes (2–4 MΩ) were filled with (in mmol/L): NaCl, 10, potassium glutamate 130, EGTA 1.0, MgCl2 0.5, KCl 9, HEPES 10, glucose 10, and MgATP 5 (adjusted to pH 7.4 with KOH). The bath solution consisted of (in mmol/L): NaCl 140, KCl 5, CaCl2 2, MgCl2 1.0, HEPES 10, glucose 10 (adjusted to pH 7.4 with NaOH). APs were evoked by 4-ms current injections applied at 0.8–1 Hz. The upstroke velocity of the action potential was taken as the maximum values of dV/dt.
Inhibitors or activators were applied directly to the pipette solution except for apocynin, forskolin, NAD+, and malonate, which were applied to bath solution. Concentrations were determined in our laboratory or by using values similar to those in the literatures.
Intracellular NADH Level
Intracellular NADH levels ([NADH]i) were detected by using the EnzyChromTM NAD+/NADH Assay Kit (BioAssay Systems, Hayward, CA) in SCN5A cells with or without treatment of 1 and 10 mmol/L pyruvate/lactate (PL) buffer for 10 min at room temperature. The intensity difference of the reduced product color, measured at 565 nm at time zero and 15 min later was proportional to the change in [NADH]i.
Confocal Microscopy
To measure mitochondrial ROS, the fluorecent probe MitoSOX™ Red was used according to the manufacturer’s protocol. Briefly, three groups of SCN5A cells or rat NVM were studied: untreated cells, the PL group (cells treated with the PL buffer for 10 min to increase intracellular NADH levels21–23, see “Results”), and the NAD-PL group (cells incubated with NAD+ for ~6 hours at 37 °C and then treated with the PL buffer buffer for 10 min). Cells were first stained with Hoechst 33342 (0.4 μg/ml) and then incubated with 2.5 μmol/L MitoSOX™ Red for 10 min at 37 °C, followed by washing three times with warm Hank’s balanced salt solution. Images were taken on a Zeiss LSM510 META confocal microscope (Carl Zeiss GmbH, Oberkochen, Germany) using an argon laser excitation (514 nm) with emisson collection through a 560 nm longpass filter. The cell area was calculated, and the whole cell fluorescence of MitoSOX™ Red was measured with ImageJ software. The number of pixels of the cell fluorescence divided by the cell area was used to determine the mitochondrial ROS generation.
To measure the effect of elevated intracellular NADH level on the mitochondrial membrane potential (ΔΨm), we applied the fluorescent probe tetramethylrhodamine methyl ester (TMRM), which is readily sequestered by mitochondria. SCN5A cells or rat NVM were loaded with TMRM (100 nmol/L)24 for 30 min at 37 °C. Cells were washed three times with the bath solution used in the voltage-clamp experiments before being placed in a 35°C holder on the stage of the Zeiss confocal microscope. TMRM was excited with a helium neon laser at (543 nm), and the emission was collected through a 560 nm longpass filter. Images were collected in time series. Then cells were exposed to the mitochondrial uncoupler carbonyl cyanide 3-chlorophenylhydrazone (CCCP, 10 μmol/L) for 1 min at 35 °C with cells, which is sufficient to completely depolarize ΔΨm.25 Images were collected in time series.
Statistical Evaluations
Data are shown as the mean ± SEM. Determinations of statistical significance were performed with ANOVA with the Bonferroni correction for comparisons of multiple means. A value of P<0.05 was considered statistically significant.
Results
Sources of ROS Induced by NADH
Since SOD is able to block the effect of NADH,3 ROS are implicated in the signaling cascade whereby NADH reduces INa. Sources of ROS within a cell include uncoupled NOS, the NAD(P)H oxidases, xanthine oxidase, and mitochondria. By using specific inhibitors, we tested which of these was the source of ROS modulating INa in response to increased cytosolic NADH.
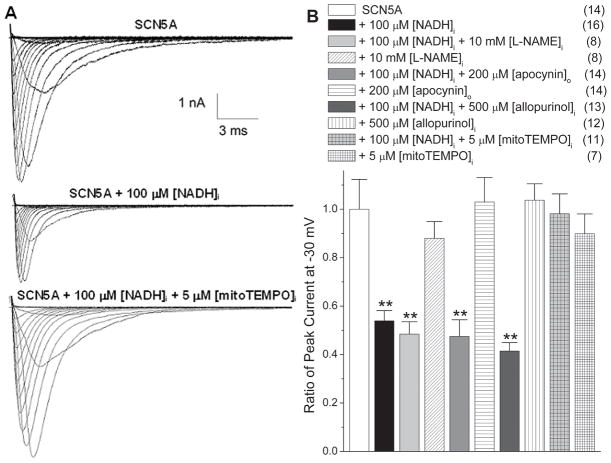
Figure 1 shows that apocynin, Nω-nitro-L-arginine methyl ester (L-NAME), and allopurinol did not affect INa, when they were applied alone in SCN5A cells. When applied with 100 μmol/L NADH, none of these blockers were able to inhibit the NADH effect on reducing cardiac INa. Steady state activation (SSA) was minimally affected by these compounds, and there were physiologically nonsignificant trends for hyperpolarizing shifts in steady state inactivation (SSI) with apocynin and allopurinol in the presence of NADH (Online Table I). These experiments indicate that the NAD(P)H oxidases, uncoupled NOS, and xanthine oxidases are not the source of ROS induced by NADH.
Figure 1.
The source of ROS induced by NADH is the mitochondria. (A) Representative traces of INa demonstrate the decrease in current in the presence of [NADH]i (100 μmol/L) was blocked by mitoTEMPO (5 μmol/L). (B) The downregulation of peak INa by [NADH]i at 100 μmol/L (**P<0.01 versus SCN5A group) is not reversed by L-NAME, apocynin, or allopurinol (P>0.05 versus NADH group), but is reversed by mitoTEMPO at 5 μmol/L (P>0.05 versus SCN5A group, P<0.01 versus NADH group). All these compounds have no effect on INa when applied alone (P>0.05 versus SCN5A group). Numbers in parentheses indicate the number of experiments.
MitoTEMPO is a highly positively charged TEMPO derivative that is concentrated in the mitochondria matrix and acts there as a superoxide scavenger.26, 27 MitoTEMPO at 5 μM blocked the NADH effect on reducing INa but had no effect on INa when applied alone (Fig. 1). The SSA and SSI were not affected by mitoTEMPO with or without the presence of NADH (Online Table I). This implied that the mitochondria were a likely source of ROS induced by increased NADH.
Mitochondrial ROS Generation Induced by Elevated NADH
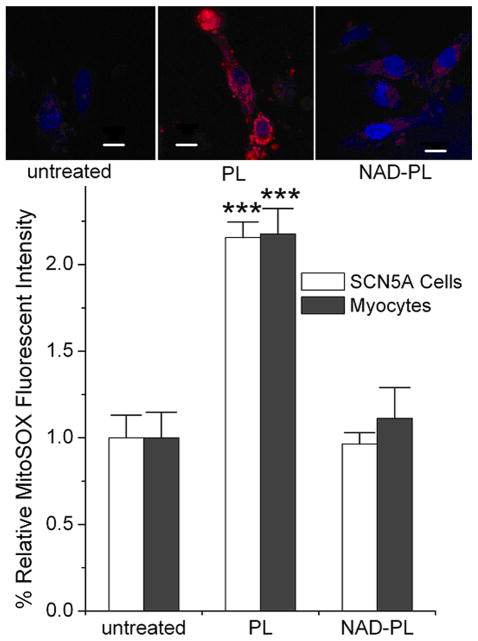
Mitochondrial ROS generation was monitored with MitoSOX™ Red in SCN5A cells and rat NVM, respectively. MitoSOX™ Red is a membrane permeant, fluorogenic dye for selective detection of superoxide in the mitochondria. Once in the mitochondria, the dye is oxidized by superoxide and exhibits red fluorescence. Application of MitoSOX™ Red in untreated cells revealed a low level of red fluorescence, indicating low levels of mitochondrial ROS (Fig. 2). SCN5A cells and rat NVM were treated with 1 and 10 mmol/L PL buffer (PL group in Fig. 2). This PL buffer increased intracellular NADH level by 1.7±0.1-fold and decreased INa to 0.54±0.04 of control (P<0.01).3 Treatments showed 2.06 ± 0.09-fold and 2.18 ± 0.15-fold increases in mitochondrial ROS levels for SCN5A cells and rat NVM as compared to untreated cells, respectively. This increase in ROS was blocked by NAD+ pre-incubation (NAD-PL group in Fig. 2, 0.96 ± 0.06 and 1.11 ± 0.18-fold of untreated cells, respectively). These observations are in agreement with the electrophysiological studies and confirm that mitochondria are the source of ROS overproduction induced by elevated NADH.
Figure 2.
Mitochondrial ROS production in response to [NADH]i was monitored by MitoSOX™ Red with SCN5A cells and myocytes. The control groups were untreated, the PL groups were treated with 1 and 10 mmol/L pyruvate/lactate for 10 min, and the NAD-PL groups were incubated with 500 μmol/L NAD+ for ~6 hours and then treated with pyruvate/lactate buffer for 10 min. The pictures in the upper panel are representative images of myocytes of three groups. The scale bar indicates 10 μm. The lower panel shows the relative MitoSOX™ Red fluorescent intensity, ***P<0.001 versus the untreated cells or NAD-PL groups. For each group, 9–16 samples were averaged.
The ETC as a Source of NADH-Induced ROS
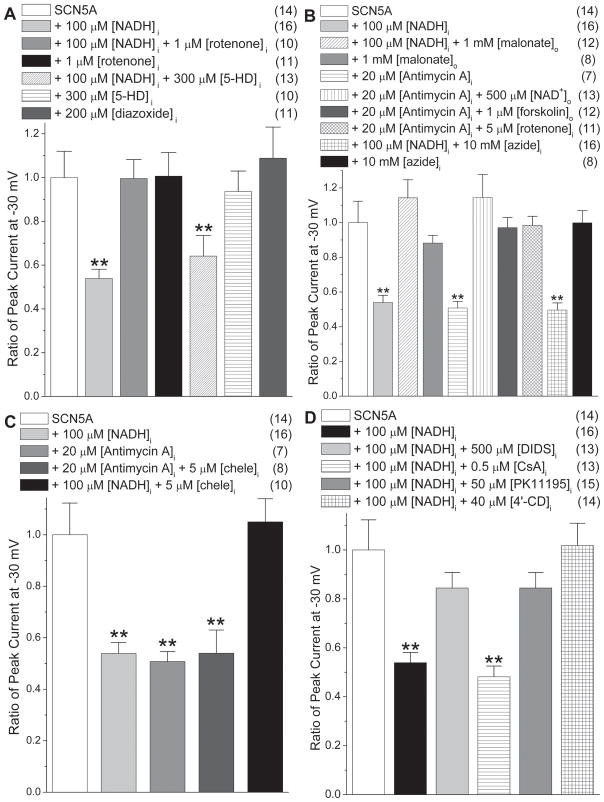
Our previous work has shown that PKC activation is required for ROS production in response to NADH.3 The ETC and mitochondrial ATP-sensitive K+ channel (mitoKATP) are targets of PKC activation,28 and both have been shown to be involved in ROS generation and release from mitochondria.6, 16, 29, 30 An inhibitor and an opener of the mitoKATP channel, 5-hydroxydecanoate (5-HD)31 and diazoxide32 respectively, were applied to study whether they would have any effect on INa. As shown in Fig. 3A, 5-HD neither blocked the NADH effect on reducing INa nor showed any effect on INa when applied alone. Diazoxide did not affect INa, either. For 5-HD, there were minor shifts of V1/2 values of the SSA and SSI relationships that were not enough to affect the evaluation of the peak currents (Online Table I). These experiments indicate that the mitoKATP channel is not involved in NADH modulation of Nav1.5.
Figure 3.
PKC, the electron transport chain and the IMAC are involved in downregulation of INa by [NADH]i. (A) Downregulation of INa by [NADH]i (**P<0.01 versus SCN5A) is reversed by rotenone (1 μmol/L), but not by 5-HD. Diazoxide does not affect INa (P>0.05 versus SCN5A). (B) Malonate (1 mmol/L) blocks the NADH effect on reducing INa, and antimycin A (20 μmol/L) reproduces the [NADH]i effect (**P<0.01 versus SCN5A group). The antimycin A-induced reduction in INa is prevented by [NAD+]o, forskolin, or rotenone. Azide failed to block the NADH effect. (C) Chelerythrine failed to block the antimycin A effect on reducing INa, confirming that PKC activation is required for ROS generation. (D) Downregulation of INa by [NADH]i is reversed by DIDS, PK11195 and 4′-CD, but not by CsA (**P<0.01 versus SCN5A groups). Numbers in parentheses indicate the number of experiments.
Complexes I and III are the main sources of ROS production of ETC.6, 16, 33 Rotenone, which decreases ROS generation by inhibiting complex I (i.e. NADH dehydrogenase),6, 33 blocked entirely the NADH effect on INa (Fig. 3A), indicating that the ETC was the source of ROS overproduction induced by NADH. Malonate, an inhibitor complex II,33 also blocked the NADH effect and reversed the decrease in INa (Fig. 3B). Fig. 3B also shows that azide, which inhibits complex IV,33 failed to block NADH effect on reducing INa. Antimycin A blocks the electron transfer from the Qi to Qo sites of complex III and increases ROS generation in the intermembrane space of mitochondria.6, 33 We found that antimycin A gave rise to an equivalent decrease of INa as did NADH. Comparably to NADH, the antimycin A effect was blocked by NAD+, forskolin, or rotenone as shown in Fig. 3B.3 A PKC inhibitor, chelerythrine, failed to block the antimycin A effect to reduce INa, as shown in Fig. 3C. This confirmed that PKC activation was necessary for ROS generation from complex III.3 Shifts of V1/2 values of SSI were observed with rotenone and azide in the presence of NADH, and with antimycin A alone. These were minor and unlikely to be sufficient to affect Na+ channel availability significantly at the holding potential used (Online Table I).
NADH-Induced ROS Release from Mitochondria was through the Mitochondrial Inner Membrane Anion Channel (IMAC)
Mitochondrial respiration is ordinarily accompanied by low-level ROS generation. In the event of significant cellular ROS, mitochondria respond by increasing their own ROS production, a phenomenon termed ROS-induced ROS release (RIRR).34, 35 Two modes of RIRR have been reported: the mitochondrial inner membrane anion channel (IMAC)-dependent and the mitochondrial permeability transition pore (MPTP)-dependent mechanisms. These two anions channels along with the voltage-dependent anion channel (VDAC) are thought to be the predominant paths for cytosolic release of superoxide generated by the ETC. Cycloporine A (CsA) and 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) are inhibitors of MPTP and IMAC/VDAC, respectively. Fig. 3D shows that DIDS blocked the NADH effect on reducing INa, but CsA did not. Measurements of the mitochondrial ΔΨm with TMRM showed that elevated NADH levels did not affect the ΔΨm (data not shown). This indicated that the IMAC or VDAC but not MPTP are involved in ROS release in response to NADH.
IMAC is regulated by the mitochondrial benzodiazepine receptor (mBzR). It has been reported that ROS generation and oscillations are prevented by inhibiting IMAC with mBzR ligands such as 4′-chlorodiazepam (4′-CD) and PK11195.6 Inhibition of mitochondria ROS release by 4′-CD is thought to prevent reperfusion arrhythmias.24 As shown in Fig. 3D, both 4′-CD and PK11195 were capable of blocking the NADH effect on INa. Since the mBzR modifies ROS release through the IMAC, these data strenthen the idea that IMAC is involved in mitochondrial ROS release in response to NADH. FGIN-1-27 (500 μmol/L), an activator of mBzR6, 24, showed that simply opening the mBzR was not enough to decrease INa (1.01 ± 0.14 of SCN5A group, P>0.05). When FGIN-1-27 and NADH were applied together, FGIN-1-27 showned no influence on the reduction in INa mediated by NADH. NADH (100 μmol/L) alone reduced INa to 0.54 ± 0.04 of SCN5A group (P<0.01)3, while in the presence of FGIN-1-27 (500 μmol/L), the reduction of INa by NADH was 0.51 ± 0.04 (P<0.01). This implies that the mBzR is fully activated in the presence of NADH.
Neonatal Ventricular Myocytes Show Similar Results
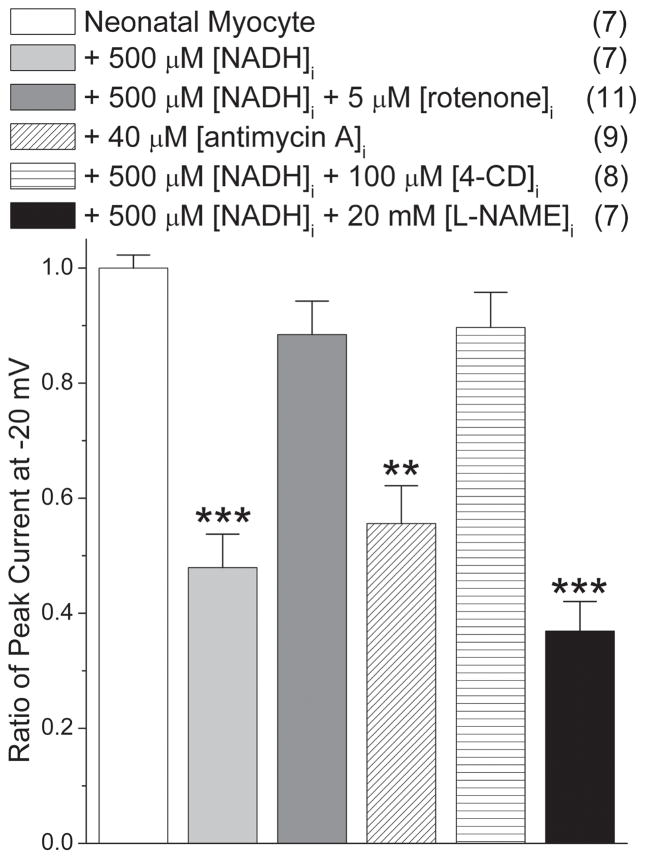
Analogous experiments were repeated using rat NVM to confirm the effects of rotenone, antimycin A, 4′-CD, and L-NAME on NADH regulation of Nav1.5. As shown in Fig. 4, rotenone and 4′-CD blocked the NADH effect on INa, while L-NAME did not. Antimycin A reduced INa to 55 ± 7% in myocytes. These results were in agreement with the findings obtained with SCN5A cells, confirming the mitochondrial role on NADH regulation of Nav1.5 in myocytes.
Figure 4.
Neonatal ventricular myocytes show analogous downregulation of INa by [NADH]i. Downregulation can be blocked by rotenone and 4′-CD, but not L-NAME. Antimycin A decreases INa similarly to that of [NADH]i (**P<0.01 and ***P<0.001 versus control myocytes). Numbers in parentheses indicate the number of experiments.
NADH treatment did not affect the maximum diastolic membrane potential. The value for untreated NVM was −66.9 ± 1.4 mV and was −64.3 ± 1.8 mV for myocytes treated with 500 μmol/L NADH (p=NS). On the other hand, treatment with NADH decreased the maximum upstroke velocity of the action potential to 0.68 ± 0.12 of untreated NVM (P<0.05).
A280V GPD1-L and NADH Affect INa Correspondingly
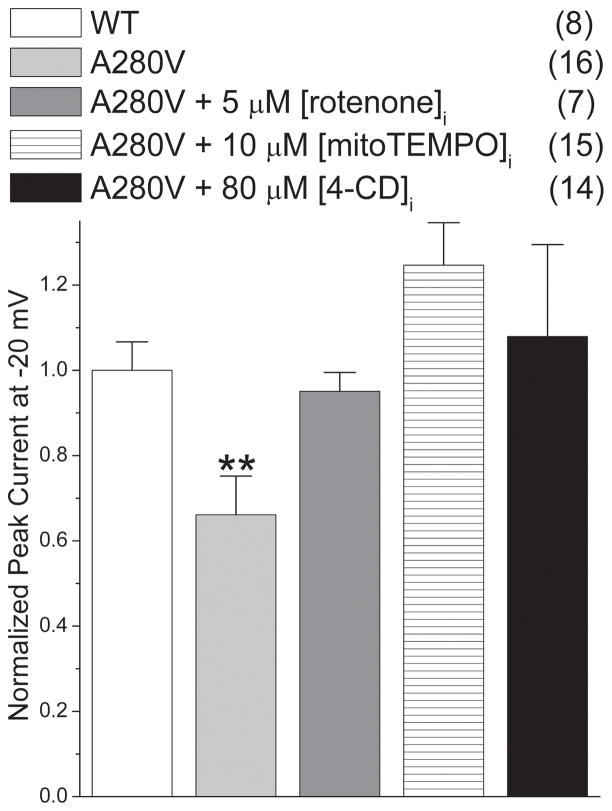
Previously, we have found that the mutant A280V GPD1-L reduces INa by increasing intracellular NADH.3 Similarly to the NADH-mediated INa reduction, mitoTEMPO, rotenone, and 4′-CD all reversed the INa decrease caused by A280V GPD1-L (Fig. 5). When these compounds were applied to cells expressing WT GPD1-L, the INa was unvaried (data not shown). These results imply that increased NADH mediates the effect of A280V GPD1-L to downregulate Nav1.5 and that mitochondrial ETC and IMAC are involved in the pro-arrhythmic effect of this mutation.
Figure 5.
Downregulation of INa by A280V GPD1-L is reversed by mitoTEMPO, rotenone, and 4′-CD (**P<0.01 versus all other groups). ). Peak currents at −20 mV were normalized to cell capacitance and divided by the current obtained with SCN5A cells transfected with WT GPD1-L. Numbers in parentheses indicate the number of experiments.
Discussion
Many signaling pathways involved in cardiomyopathy and cardioprotection converged on the mitochondria. Mitochondria comprise ~30–40% of the myocyte volume and generate >90% of the ATP.36, 37 Also, they are a major site of physiological ROS production in the cardiomyocyte, with 1–3% of the electrons flowing through the ETC leaking to produce ROS.38, 39 ROS generation within the mitochondrial matrix depends critically on the proton motive force, the NADH/NAD+ ratio, the CoQH2/CoQ ratio, and the local O2 concentration. Under conditions of a high NADH/NAD+ ratio, complex I and perhaps other enzymes linked to the NADH pool may contribute to ROS production.40
In the present study, we found that the oxidative stress induced by NADH is derived from mitochondria. Experiments with different inhibitors for the uncoupled NOS, NAD(P)H oxidases, xanthine oxidases, mitoKATP, and the ETC revealed that the mitochondrial ETC plays a critical role in NADH regulation of Nav1.5. Blockade of the NADH effect to reduce INa was observed with rotenone and malonate, complex I and II blockers, respectively. Because malonate inhibited the NADH-induced ROS but cannot prevent ROS release from complex I, it seemed likely that complex III was the source of ROS in our study. Another possibility is reverse electron transfer from complex II to complex I can also lead to ROS production.41 This is also blocked by malonate and rotenone. Antimycin A inhibits complex III at the Qi center and increases superoxide generation from the Qo center.42 In the present study, antimycin A caused a significantly reduced INa, supporting the idea that complex III is the source of ROS induced by NADH. At the same time, the antimycin A effect could be blocked by NAD+, forskolin, and rotenone. These results are comparable to the inhibition of the NADH effect on INa reported in this and previous work.3 Taken together, the data suggest that complex III is the main source of NADH-induced ROS generation and that blockade of electron flow upstream of complex III minimizes ROS production induced by NADH.
ROS produced by leakage of electrons from the ETC can trigger the opening of the mitochondrial IMAC and subsequent release of O2•− to the cytoplasm.6, 34 IMAC-dependent ROS release is regulated by the mBzR. Localized mitochondrial ROS release can propagate throughout cardiac cells in the form of oscillations or waves.6, 34 Mitochondrial depolarization associated with increase ROS and activation of the MPTP has been correlated with opening of the mitoKATP channel and conduction block, referred to as a metabolic sink.43 We show a second possible mechanism for conduction impairment involving mitochondrial ROS, ROS induced decreased INa, which is dependent on the mBzR and IMAC but not the MPTP. CsA failed to block the NADH effect on reducing INa while PK11195 and 4′-CD inhibited the NADH effect. This suggests that Na+ channel-mediated changes in conduction may precede those of mitoKATP, since the mitoKATP effect requires mitochondrial MPTP activation and mitochondrial depolarization whereas the NADH effect requires less extreme mitochondrial ROS production.
Studies of metabolic stress in isolated cardiac cells reveal that energy-sensitive K+ channels in the sarcolemmal membrane can be activated spontaneously in an oscillatory manner.44 These K+ current oscillations are closely associated with whole cell metabolic oscillations. Modulation of the cellular action potential by these metabolic oscillations could result in arrhythmias in the heart after ischemia-reperfusion. Mitochondria have been identified as the source of the oscillations. K+ channel opening compounds like diazoxide and nicorandil have been found to protect heart cells from ischemic or oxidative stress through a mechanism that involves the opening of mitoKATP channel.32 In our work, the blocker for mitoKATP, 5-HD, was unable to protect against the NADH-mediated reduction in INa, and an opener of mitoKATP, diazoxide, did not affect INa, either. These results indicate that the NADH effect is unique and independent of mitoKATP.
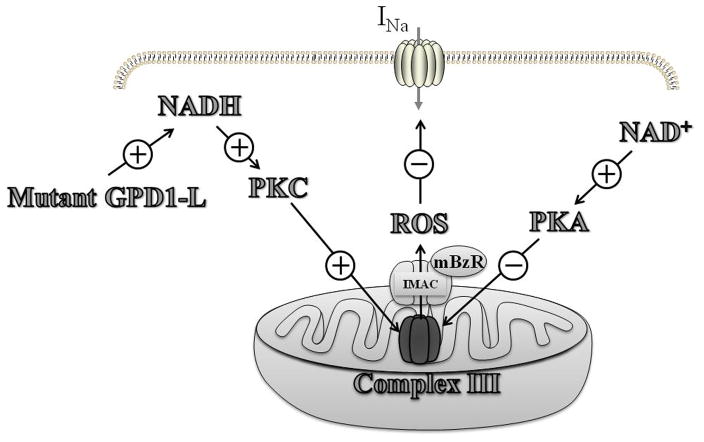
In summary, elevated intracellular NADH leads to mitochondrial ROS overproduction that results in downregulation of the cardiac Na+ channel. Mitochondrial ROS overproduction is mainly derived from complex III of the electron transport chain, and ROS is probably released into the cytoplasm through the IMAC, which is regulated by the mBzR (Fig. 6). A similar mechanism likely explains the arrhythmia syndromes induced by mutant GPD1-L protein,1, 3 since the mutant GPD1-L A280V leads to an increase of intracellular NADH level and mitoTEMPO, rotenone, and 4′-CD block the A280V GPD1-L effect to reduce INa. Valdivia et al.45 presented a somewhat different possible signaling pathway to explain the reduction in INa with mutations of GPD1-L. Nevertheless, the two proposals share many elements, including elevated NADH and PKC activation being involved in the signaling cascade. In experiments not shown, the lack of effect on INa of raising intracellular dihydroxyacetone phosphate, which should increase glycerol-3-phosphate production by glycerol-3-phosphate dehydrogenase catalysis without raising NADH levels, suggests that NADH and not glycerol-3-phosphate is mediating the reduction in current.
Figure 6.
A proposed signaling pathway by which the mutant GPD1-L and NADH downregulate cardiac Na+ channel by causing PKC activation and ROS overproduction from the complex III of mitochondrial electron transport chain. Reactive oxygen species (ROS) are released from the mitochondria by the IMAC that is modulated by the mBzR. NAD+ upregulates the cardiac Na+ channel through PKA activation and inhibition of ROS overproduction.
Our experiments do not unequivocally establish a mechanism by which mitochondrial ROS reduce INa. ROS could be having a direct effect on the channel, cause the channel to be excluded from the membrane, or alter channel post-translational modifications known to decrease INa. Preliminary experiments suggest that the disulfide reducing agent, dithiothreitol, does not prevent the NADH effect. Moreover, preliminary total internal reflection fluoroscopy experiments with labeled sodium channels do not show any channel internalization in response to NADH. It seems reasonable that PKC acts directly on the channel, as proposed by Valdivia et al45. Changes in the SSA and SSI relationships support this assertion. It is interesting to note, however, that the effect of only one of two GPD1-L mutations known to cause sudden death is fully reversed by eliminating a Na+ channel PKC phosphorylation site, suggesting the possibility of multiple mechanisms or sites being involved in the current reduction. Our results represent a heretofore unknown regulation of the cardiac Na+ channel by NADH through mitochondria ROS production that may help explain the link between altered metabolism and arrhythmic risk.
Novelty and Significance.
What Is Known?
Cardiac arrhythmias are more prevalent when cardiac metabolism is abnormal.
A mutation in glycerol-3-phosphate dehydrogenase like-1 protein (GPDL-1) alters pyridine nucleotide levels and reduces cardiac sodium current (INa), potentially explaining how this mutation leads to the Brugada Syndrome, which increases the likelihood of sudden cardiac death.
What New Information Does This Article Contribute?
Elevation in NADH results in activation of protein kinase C (PKC) and a subsequent increase in mitochondrial complex III-derived reactive oxygen species (ROS) through ROS-induced ROS release involving the mitochondrial inner membrane anion channel (IMAC).
Mitochondrial superoxide release is responsible for the downregulation of INa.
Inhibition of mitochondrial ROS overproduction by several strategies prevents INa downregulation by NADH.
Altered cardiac metabolism is associated with increased risk of arrhythmias and sudden death. In part, this occurs because of reduced electrical conduction in the cardiomyocytes, but the mechanisms for this are not clear. We have shown previously that a mutation in GPD1-L protein, causing the sudden death condition Brugada syndrome, reduces INa by raising intracellular NADH levels and inducing ROS. Here, we investigated the source of ROS induced by elevated NADH. We found that elevated NADH induced ROS production from mitochondria and that ROS release from the mitochondria was mediated by the IMAC. NAD+, inhibition of mitochondrial electron transport, a mitochondrial targeted antioxidant, and an IMAC modulator could prevent the reduction in INa by reducing mitochondrial ROS production. These findings contribute to our understanding of the mechanisms of conduction block and arrhythmia when cardiac metabolism is dysfunctional. Also, the results suggest possible therapeutic strategies to reduce arrhythmic risk associated with cardiomyopathy.
Supplementary Material
Acknowledgments
We thank Dr. Sergey Dikalov (Emory University, Atlanta, GA) for the gift of mitoTEMPO.
Sources of funding
This work was supported by NIH R01 HL085558, R01 HL073753, and P01 HL058000 (SCD).
Non-standard Abbreviations and Acronyms
- 4′-CD
4′-chlorodiazepam
- 5-HD
5 –hydroxydecanoate
- AP
action potential
- CCCP
carbonyl cyanide 3-chlorophenylhydrazone
- CsA
Cycloporine A
- DIDS
4′-diisothiocyanatostilbene-2,2′-disulfonic acid
- ETC
electron transport chain
- FGIN-1-27
[N,N-dihexyl-2-(4-fluorophenyl)indole-3-acetamide
- GFP
green fluorescent protein
- GPD1-L
glycerol-3-phosphate dehydrogenase 1-like
- HEK
human embryonic kidney
- IMAC
the mitochondrial inner membrane anion channel
- L-NAME
Nω-nitro-L-arginine methyl ester
- mBzR
the mitochondrial benzodiazepine receptor
- mitoKATP
mitochondrial ATP-sensitive K+ channel
- MPTP
the mitochondrial permeability transition pore
- Nav1.5
cardiac sodium channel
- NOS
nitric oxide synthase
- NVM
neonatal ventricular myocyte
- PK
protein kinase
- PL
pyruvate/lactate
- RFP
red fluorescent protein
- RIRR
ROS-induced ROS release
- SCN5A
cardiac sodium channel
- SOD
superoxide dismutase
- SSA
steady state activation
- SSI
steady state inactivation
- TMRM
tetramethylrhodamine methyl ester
- VDAC
the voltage-dependent anion channel
Footnotes
Disclosures
Dr. Dudley has filed provisional patents related to this work: 1) Modulation of sodium current by nicotinamide adenine dinucleotide and 2) Modulating mitochondrial reactive oxygen species to increase cardiac sodium channel current and mitigate sudden death.
Reference List
- 1.London B, Michalec M, Mehdi H, Zhu X, Kerchner L, Sanyal S, Viswanathan PC, Pfahnl AE, Shang LL, Madhusudanan M, Baty CJ, Lagana S, Aleong R, Gutmann R, Ackerman MJ, McNamara DM, Weiss R, Dudley SC., Jr Mutation in glycerol-3-phosphate dehydrogenase 1-like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007;116:2260–8. doi: 10.1161/CIRCULATIONAHA.107.703330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Norstrand DW, Valdivia CR, Tester DJ, Ueda K, London B, Makielski JC, Ackerman MJ. Molecular and functional characterization of novel glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) mutations in sudden infant death syndrome. Circulation. 2007;116:2253–9. doi: 10.1161/CIRCULATIONAHA.107.704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu M, Sanyal S, Gao G, Gurung IS, Zhu X, Gaconnet G, Kerchner LJ, Shang LL, Huang CLH, Grace A, London B, Dudley SC., Jr Cardiac Na+ current regulation by pyridine nucleotides. Circ Res. 2009;105:737–45. doi: 10.1161/CIRCRESAHA.109.197277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue: roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997;81:727–41. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu W, Aiba T, Kamakura S. Mechanisms of disease: current understanding and future challenges in Brugada syndrome. Nat Clin Pract Cardiovasc Med. 2005;2:408–14. doi: 10.1038/ncpcardio0268. [DOI] [PubMed] [Google Scholar]
- 6.Aon MA, Cortassa S, Marban E, O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 7.Di LF, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–5. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 8.Choudhary G, Dudley SC., Jr Heart failure, oxidative stress, and ion channel modulation. Congest Heart Fail. 2002;8:148–55. doi: 10.1111/j.1527-5299.2002.00716.x. [DOI] [PubMed] [Google Scholar]
- 9.Pillai JB, Isbatan A, Imai Si, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2α deacetylase activity. J Biol Chem. 2005;280:43121–30. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- 10.Dzhanashiya PK, Vladytskaya OV, Salibegashvili NV. Efficiency and mechanisms of the antioxidant effect of standard therapy and refracterin in the treatment of chronic heart failure in elderly patients with postinfarction cardiosclerosis. Bull Exp Biol Med. 2004;138:412–4. doi: 10.1007/s10517-005-0056-1. [DOI] [PubMed] [Google Scholar]
- 11.Makielski JC, Farley A. Na+ current in human ventricle: implications for sodium loading and homeostasis. J Cardiovasc Electrophysiol. 2006;17:S15–S20. doi: 10.1111/j.1540-8167.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 12.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–83. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Silberman GA, Fan T-H, Liu H, Jiao Z, Xiao HD, Lovelock JD, Boulden B, Widder J, Fredd S, Bernstein KE, Wolska B, Dikalov S, Harrison DG, Dudley SC., Jr Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–28. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–35. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 15.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrukhiv A, Costa ADT, West I, Garlid KD. Opening of mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol. 2006;291:H2067–H2074. doi: 10.1152/ajpheart.00272.2006. [DOI] [PubMed] [Google Scholar]
- 17.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura Ki, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–63. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 18.Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Elevated levels of 8-iso-prostaglandin F2α in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97:1536–9. doi: 10.1161/01.cir.97.16.1536. [DOI] [PubMed] [Google Scholar]
- 19.Hill MF, Singal PK. Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation. 1997;96:2414–20. doi: 10.1161/01.cir.96.7.2414. [DOI] [PubMed] [Google Scholar]
- 20.Dhalla AK, Singal PK. Antioxidant changes in hypertrophied and failing guinea pig hearts. Am J Physiol Heart Circ Physiol. 1994;266:H1280–H1285. doi: 10.1152/ajpheart.1994.266.4.H1280. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi K, Neely JR. Control of maximum rates of glycolysis in rat cardiac muscle. Circ Res. 1979;44:166–75. doi: 10.1161/01.res.44.2.166. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Hwang YC, Ananthakrishnan R, Oates PJ, Guberski D, Ramasamy R. Polyol pathway and modulation of ischemia-reperfusion injury in Type 2 diabetic BBZ rat hearts. Cardiovasc Diabetol. 2008;7:33–44. doi: 10.1186/1475-2840-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moir AM, Zammit VA. Insulin-independent and extremely rapid switch in the partitioning of hepatic fatty acids from oxidation to esterification in starved-refed diabetic rats. Biochem J. 1995;305:953–8. doi: 10.1042/bj3050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akar FG, Aon MA, Tomaselli GF, O’Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005;115:3527–35. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Raam B, Sluiter W, de Wit E, Roos D, Verhoeven A, Kuijpers T. Mitochondrial membrane potential in human neutropils is maintained by complex III activity in the absence of supercomplex organisation. PLoS ONE. 2008;3:e2013. doi: 10.1371/journal.pone.0002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang HL, Arsenault J, Mortensen J, Park F, Johnson CP, Nilakanta V. Partial attenuation of cytotoxicity and apoptosis by SOD1 in ischemic renal epithelial cells. Apoptosis. 2009;14:1176–89. doi: 10.1007/s10495-009-0393-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–16. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budas G, Mochly-Rosen D. Mitochondrial protein kinase Cε (PKCε): emerging role in cardiac protection from ischaemic damage. Biochem Soc Trans. 2007;35:1052–4. doi: 10.1042/BST0351052. [DOI] [PubMed] [Google Scholar]
- 29.Costa ADT, Pierre SV, Cohen MV, Downey JM, Garlid KD. cGMP signalling in pre- and postconditioning: the role of mitochondria. Cardiovasc Res. 2008;77:344–52. doi: 10.1093/cvr/cvm050. [DOI] [PubMed] [Google Scholar]
- 30.Ogbi M, Chew CS, Pohl J, Stuchlik O, Ogbi S, Johnson JA. Cytochrome c oxidase subunit IV as a marker of protein kinase Cε function in neonatal cardiac myocytes: implications for cytochrome c oxidase activity. Biochem J. 2004;382:923–32. doi: 10.1042/BJ20040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato T, O’Rourke B, Marban E. Modulation of mitochondrial ATP-dependent K+ channels by protein kinase C. Circ Res. 1998;83:110–4. doi: 10.1161/01.res.83.1.110. [DOI] [PubMed] [Google Scholar]
- 32.O’Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res. 2004;94:420–32. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Q, Vazquez E, Moghaddas S, Hoppel C, Lesnefsky E. Production of reactive oxygen species by mitochondria. J Biol Chem. 2003;278:36027–31. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 34.Brady N, Hamacher-Brady A, Westerhoff H, Gottlieb R. A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxid Redox Signal. 2006;8:1651–65. doi: 10.1089/ars.2006.8.1651. [DOI] [PubMed] [Google Scholar]
- 35.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–14. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu Rev Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 37.Barth E, Stammler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992;24:669–81. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 38.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128:617–31. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batandier C, Fontaine E, Keriel C, Leverve X. Determination of mitochondrial reactive oxygen species: methodological aspects. J Cell Mol Med. 2002;6:175–87. doi: 10.1111/j.1582-4934.2002.tb00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panov A, Schonfeld P, Dikalov S, Hemendinger R, Bonkovsky HL, Brooks BR. The Neuromediator glutamate, through specific substrate interactions, enhances mitochondrial ATP production and reactive oxygen species generation in monsynaptic brain mitochondria. J Biol Chem. 2009;284:14448–56. doi: 10.1074/jbc.M900985200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–63. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 43.Brown D, Aon MA, Akar FG, Liu T, Sorarrain N, O’Rourke B. Effects of 4′-chlorodiazepam on cellular excitation-constraction coupling and ischaemia-reperfusion injury in rabbit heart. Cardiovasc Res. 2008;79:141–9. doi: 10.1093/cvr/cvn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Rourke B, Ramza B, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994;265:962–6. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- 45.Valdivia CR, Ueda K, Ackerman MJ, Makielski JC. GPD1L links redox state to cardiac excitability by PKC-dependent phosphorylation of the sodium channel SCN5A. AJP - Heart and Circulatory Physiology. 2009;297:H1446–H1452. doi: 10.1152/ajpheart.00513.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.