Abstract
We hypothesized that in epileptic brains citric acid cycle intermediate levels may be deficient leading to hyperexcitability. Anaplerosis is the metabolic refilling of deficient metabolites. Our goal was to determine the anticonvulsant effects of feeding triheptanoin, the triglyceride of anaplerotic heptanoate. CF1 mice were fed 0-35% calories from triheptanoin. Body weights and dietary intake were similar in mice fed triheptanoin vs. standard diet. Triheptanoin feeding increased blood propionyl-carnitine levels, signifying its metabolism. 35%, but not 20%, triheptanoin delayed development of corneal kindled seizures. After pilocarpine-induced status epilepticus (SE), triheptanoin feeding increased the pentylenetetrazole tonic seizure threshold during the chronically epileptic stage. Mice in the chronically epileptic stage showed various changes in brain metabolite levels, including a reduction in malate. Triheptanoin feeding largely restored a reduction in propionyl-CoA levels and increased methylmalonyl-CoA levels in SE mice. In summary, triheptanoin was anticonvulsant in two chronic mouse models and increased levels of anaplerotic precursor metabolites in epileptic mouse brains. The mechanisms of triheptanoin's effects and its efficacy in humans suffering from epilepsy remain to be determined.
Keywords: anaplerosis, propionyl-CoA, corneal kindling, pilocarpine, seizure, epilepsy, pentylenetetrazole, β-hydroxybutyrate
Introduction
Up to 30% of epileptic patients, especially children, are drug-resistant and suffer from uncontrolled seizures. Evidence is increasing that epileptic disorders are linked to dysfunction of metabolic processes. For example, several studies show that manipulation of metabolic pathways and subsequently energy metabolism can be anticonvulsant. One of the few alternative and efficacious therapies for drug-resistant epilepsy is the ketogenic diet (Neal et al. 2008), a strict high fat diet that is very difficult to adhere to. The exact mechanism of seizure control exerted by the ketogenic diet is still unclear, although changes in energy metabolism appear to play a role (Bough et al. 2006, Hartman et al. 2007). Mild hypoglycemia and the replacement of glucose by “ketones” in energy metabolism may be important for the anticonvulsant effects. Also, it was recently found that fructose-1,6-bisphosphate (Lian et al. 2007) and 2-deoxy-D-glucose (Stafstrom et al. 2009, Garriga-Canut et al. 2006), which both also alter energy metabolism by reducing glycolysis, are effective in several rodent seizure models. These are all promising new therapies that are based on altering metabolism to treat epilepsy.
We hypothesized that in epileptic brains the levels of citric acid cycle (CAC) intermediates may be deficient leading to hyperexcitability. The oxidation of acetyl-CoA by the CAC and subsequent oxidative phosphorylation by the electron transport chain produces the most ATP in aerobic metabolism. The CAC intermediates α-ketoglutarate and oxaloacetate are precursors for the neurotransmitters glutamate, GABA and aspartate. Increased neurotransmission, such as during seizures, could therefore reduce the levels of CAC intermediates and subsequently acetyl-CoA oxidation and energy production. Anaplerosis is the refilling of reduced metabolites, including deficient catalytic intermediates of the CAC (Hassel 2000, Brunengraber & Roe 2006). It could therefore increase ATP production needed to keep neuronal membrane potentials stable and potentially prevent epileptic bursts.
Anaplerotic molecules include certain amino acids and odd chain fatty acids. Triheptanoin is the triglyceride of the anaplerotic C7 fatty acid heptanoate. Triheptanoin provides three anaplerotic propionyl-CoA molecules without overloading the system with nitrogen, sodium or acid. Triheptanoin has been successfully used as a dietary treatment for hereditary metabolic disorders in patients (Roe & Mochel 2006, Roe et al. 2002, Mochel et al. 2005). Heptanoate itself can enter the brain (Wang et al. 2007). Moreover, the liver metabolizes heptanoate to the “C5 ketone” bodies β-hydroxypentanoate & β-ketopentanoate. These are taken up by the brain, most likely through monocarboxylate transporters (Mochel et al. 2005). Each C5 ketone molecule is metabolized to one acetyl-CoA and one anaplerotic propionyl-CoA molecule. Alternatively, propionyl-CoA can be produced by β-oxidation of heptanoyl-CoA. Propionyl-CoA can replenish oxaloacetate via succinyl-CoA in rats (Kinman et al. 2006). Thus, it can increase acetyl-CoA oxidation and ATP production.
The purpose of this study was to establish a diet containing triheptanoin in mice and to evaluate its anticonvulsant effects in chronic seizure models. Our second aim was to determine to which extent the levels of brain metabolites are changed in epileptic mice and whether triheptanoin feeding would lead to alterations indicative of anaplerosis.
Materials and methods
Ethics
All experiments were approved by the Institutional Animal Care and Use Committee of Texas Tech University Health Sciences Center and conducted in accordance with its guidelines. Every effort was made to reduce animal suffering.
Diets and mice
All mice were housed under a 12 h light dark cycle with free access to food and water. Adult male CF1 mice (20-43 g, Charles River) were fed either a standard diet (TD.06316, as used by Samala et al. 2008), standard diet without sucrose, or diet containing either 20% or 35% of calories from triheptanoin (Sasol, Germany, Table 1). Triheptanoin replaced sucrose and some of the complex carbohydrates in the standard diet. The amounts of vitamins, minerals, antioxidants and protein match the newest nutritional standards and were equal among all diets relative to their caloric densities. Diets were mixed fresh every 3-4 days in the laboratory, dried and then supplied to mice.
Table 1.
Composition of diets.
| Standard (TD06316) | 35% Triheptanoin diet | |
|---|---|---|
| g/kg | g/kg | |
| Casein | 200.0 | 215.0 |
| Corn Starch | 389.1 | 350.2 |
| Maltodextrin | 100.0 | 89.8 |
| Sucrose | 150.0 | |
| Cellulose | 50.0 | 102.6 |
| Mineral Mix, Ca-P Deficient (79055) | 13.4 | 14.3 |
| Vitamin Mix, Teklad 40060 | 10.0 | 10.7 |
| Calcium Phosphate Dibasic | 7.5 | 8.0 |
| Calcium Carbonate | 6.85 | 7.40 |
| DL-Methionine | 3.00 | 3.24 |
| Magnesium Oxide | 0.20 | 0.22 |
| TBHQ (Antioxidant) | 0.07 | 0.08 |
| Choline Bitartrate | 2.50 | |
| Pantothenic acid | 1.93 | |
| Vegetable Oil, Hydrogenated (Crisco) | 50.0 | |
| Coconut Oil, Hydrogenated | 20.0 | |
| Triheptanoin | 170.5 | |
| Corn oil | 23.5 | |
| total (g) | 1000 | 1000 |
| Kcal/g | 3.77 | 4.01 |
| %kcal/kcal diet: | ||
| Protein | 18.9 | 18.9 |
| Carbohydrates | 63.7 | 40.2 |
| Natural fat | 17.4 | 5.9 |
| Triheptanoin | 35 | |
Note that all diets contain the same levels of protein, calcium, magnesium, phosphate, TBHQ, vitamin mix and mineral mix relative to caloric content.
Caloric intake and metabolism studies
To compare caloric intake in mice fed standard vs. 35% triheptanoin diet, twelve 28-34 g mice were placed individually in metabolic chambers and fed respective diets for eight days. After 4 days habituation, food intake and body weight were determined daily and then averaged per day for each mouse. 24h urine samples were taken and organic acids isolated by liquid partition chromatography and trimethylsilyl derivatives quantified by gas chromatography mass spectrometry (Sweetman 1991). In a separate study mice were fed repective diets for 3 weeks and were decapitated after isoflurane anesthesia between 1-4pm. Trunk blood was collected on absorbent filter paper for measurement of acylcarnitine levels in dried blood spots using tandem mass spectrometry modified from the method of Rashed et al. (Rashed et al. 1997).
Corneal kindling
The corneal kindling model was slightly modified from the original protocol (Matagne & Klitgaard 1998) with kindling twice a day with at least 4 h intervals. A topical anesthetic (0.5% tetracaine hydrochloride ophthalmic solution) was applied to the corneas 10-15 min before stimulation. Electrodes were wet with 0.9% NaCl immediately before application of 3 sec stimuli of 9 mA 0.4 ms duration pulses at 50 Hz using a constant-current device (ECT Unit 57800, Ugo Basile). Mice were manually held during stimulation and then released for behavioral observation. Seizures were scored according to a modified Racine scale (Racine 1972) as described by Matagne and Klitgaard (1998) with 0 = no reaction or immobility; 1 = jaw clonus; 2 = myoclonic twitches in the forelimbs, sometimes with head nodding; 3 = clonic convulsions in the forelimbs; 4 = clonic convulsions in the forelimbs with rearing and falling; 5 = loss of balance.
In the first experiments, triheptanoin feeding was initiated three weeks prior to the first stimulation and was continued throughout the kindling process. To investigate if triheptanoin is anticonvulsant in the fully kindled stage, mice on standard diet were kindled for two weeks until at least four stage 5 seizures were obtained. Mice were then randomized and placed on 35% triheptanoin vs. standard diet. Both groups had the same average seizure scores for the full kindling period and the last three days of kindling. After one week on the respective diets and during the five following weeks, mice were rekindled every week twice a day on two consecutive days. The medians of the scores were calculated for each week.
Pilocarpine model with second hit seizure models
The pilocarpine CF1 mouse model used in this study was slightly modified from our previous description (Borges et al. 2003, Borges et al. 2008, Borges et al. 2006, Borges et al. 2004). 26-43 g CF1 mice were injected with methylatropine (2 mg/kg i.p. in 0.9% NaCl) to minimize peripheral side effects. 15-30 min later pilocarpine was administered (270-360 mg/kg, s.c.). About fifty percent of injected mice experienced behavioral status epilepticus (SE) lasting about four hours as defined by continuous seizure activity consisting mainly of whole body continuous clonic seizures. 4.5 h after pilocarpine injection, all mice were injected with pentobarbital (22.5 mg/kg, i.p.) followed by 1 ml 5% dextrose in lactate Ringer's solution (s.c.). After SE mice were monitored daily. They were hand-fed moistened cookies and injected with 5% dextrose in lactate Ringer's solution twice a day for about three days and thereafter when needed. Spontaneous and handling-induced seizures, including stage 3-5 seizures, jumping and wild running, were noted when observed in the animal house. No systematic observations were done to detect spontaneous seizures in all mice. When video-monitored all mice with SE developed spontaneous recurrent seizures (Borges et al. 2003), while those that do not develop SE (no SE mice) have never been observed to have handling-induced or spontaneous seizures or neuronal damage in our laboratory. “Sham control” mice received methylatropine and pentobarbital only. Out of a total of 205 mice injected with pilocarpine, we obtained 44% SE, 41% no SE mice, while 14% of mice died.
As a measure for seizure susceptibility of SE, no SE and control mice in the chronic stage of the pilocarpine model, we assessed the seizure thresholds for the first generalized clonic and tonic seizures induced by pentylenetetrazole (PTZ, i.v.). As previously described (Samala et al. 2008, Willis et al. 2009), 10 mg/ml PTZ dissolved in saline was infused into the tail vein at 150 μl/min. The latencies to the first generalized clonic seizure and tonic extension were determined and converted to seizure thresholds expressed as mg/kg body weight. Mice were euthanized by cervical dislocation immediately after the tonic extension seizure.
Brain and blood metabolite analysis
The brain metabolite profile of SE mice and “non-epileptic” no SE mice was compared during the chronic stage of the pilocarpine model. Both groups of mice received standard diet during the phase of epileptogenesis, the first two weeks after SE. They were then divided into two groups of equal average weight and received either standard or 35% triheptanoin diet for three weeks. To avoid metabolite changes induced by anesthesia, mice were killed by cervical dislocation and then decapitated. Within 22-32 seconds brains without cerebellum were frozen in liquid nitrogen. Trunk blood was collected in heparinized tubes for analysis of β-hydroxybutyrate and glucose using kits from Pointe Scientific and Raichem, respectively. Brains were stored at -80 C and shipped to the Metabolic Mouse Phenotyping Center at Case Western University for chromatography mass spectrometry (GC-MS/LC-MS) analysis and characterization of intermediates and products of the CAC and acyl-CoAs. Our brain collection method seems to be valid, because the levels of the most metabolites match those of the literature (see below).
Statistics
All data points are presented as averages ± standard error of the mean (s.e.m.). Unpaired two-tailed t-tests were used to compare caloric intake and blood metabolite levels in two groups. Repeated measure ANOVAs with a subsequent post-test were employed to compare body weights in mice on different diets. To compare kindling scores over time, we calculated the areas under the curve for each mouse and compared them with unpaired two-tailed student's t-test for two diet groups. One way ANOVAs followed by the Newman-Keuls test were used to compare the areas under the curves between different diet groups and seizure thresholds in the PTZ test. To compare levels of individual metabolites after SE and with the triheptanoin diet, we used one way ANOVAs with a Bonferroni test with selected comparisons. GraphPad Prism version 5 was used for all statistical tests. Significance was set to p< 0.05. * depicts p<0.05, ** p<0.01, and *** p<0.001.
Results
Diets, body weights and triheptanoin metabolism
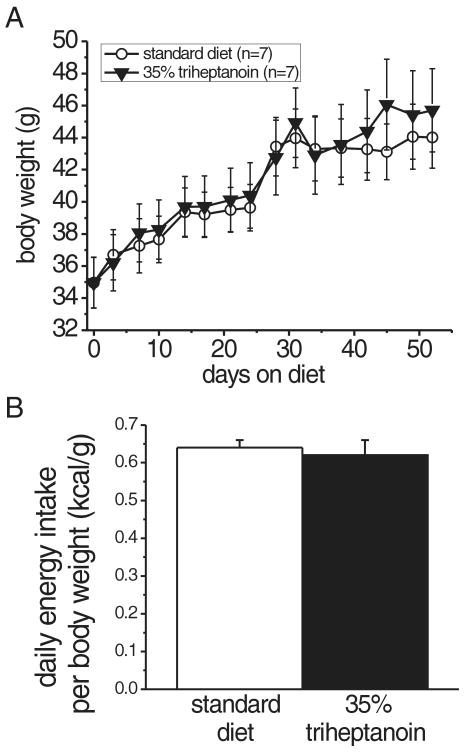
CF1 mice were fed 35% triheptanoin diet for up to 7.5 weeks. Their visual appearance was indistinguishable from mice fed standard chow and body weights were statistically indistinguishable (Fig. 1A, repeated measures ANOVA). Caloric intake measured in metabolic cages was also similar among triheptanoin and standard diet-fed mice, indicating that triheptanoin is well tolerated and metabolized in mice (Fig. 1B). We then investigated if triheptanoin is metabolized by fatty acid oxidation by measuring acylcarnitine levels in blood. Intracellular acylcarnitines are in equilibrium with fatty acid acyl-Coenyme A intermediates in mitochondrial fatty acid beta oxidation. The acylcarnitines can enter the blood where their levels reflect the intracellular levels of fatty acid acyl-Coenzyme As and can be used as an indicator of fatty acid metabolism. In triheptanoin-fed mice, heptanoyl-, pentanoyl- and propionyl-carnitines were elevated significantly two - 3.4-fold (p=0.002-0.012, n=5, Table 2). There were no differences in even chain fatty acid carnitine metabolites, indicating that triheptanoin is metabolized to C7-, C5- and C3-fatty acid metabolites. In this experiment, blood for acylcarnitine analysis collected in the afternoon. However, acylcarnitines are higher shortly after feeding, which occurs primarily at night.
Fig. 1.
The 35% triheptanoin diet is calorically equivalent to the standard diet.
(A) No significant difference in the growth curves of CF1 mice on standard and triheptanoin diets for 52 days (A, n=7; mean +/- SEM). (B) Similar daily energy intake per body weight (kcal/g/day) of standard and triheptanoin diets, showing the caloric equivalence of the two diets. The food intake was averaged for 4 days (n=6 mice per diet group).
Table 2. Blood acylcarnitines levels indicate that triheptanoin is metabolised.
| Acylcarnitine | Standard Diet | 35% Triheptanoin | fold increase | p |
|---|---|---|---|---|
| C3-Carnitine | 0.504 ± 0.065 | 1.622 ± 0.293 | 3.22 | 0.003 |
| C5-Carnitine | 0.078 ± 0.012 | 0.144 ± 0.012 | 1.85 | 0.002 |
| C7-Carnitine | 0.01 ± 0 | 0.022 ± 0.004 | 2.20 | 0.012 |
| C2-Carnitine | 20.80 ± 1.92 | 25.734 ± 3.53 | 1.24 | 0.21 |
| C4-Carnitine | 0.252 ± 0.035 | 0.278 ± 0.052 | 1.10 | 0.66 |
| Free Carnitine | 18.39 ± 1.29 | 20.77 ± 2.74 | 1.13 | 0.41 |
Mice fed either standard or triheptanoin diet for 3 weeks were sacrificed between 1-4 pm and acylcarnitines measured in blood by mass spectroscopy (μmoles/L, n=5 mice per diet group). Increases in C7-, C5- and C3 carnitines were significant at the indicated levels (unpaired t-tests) and indicate that triheptanoin is metabolized by mice.
Analysis of organic acids in urine showed some increases in markers for propionic acidemia, such as methylmalonate and methylcitrate. These levels were well below those found in patients suffering from propionic academia. This indicates that triheptanoin feeding does not lead to pathological propionic acid overload.
Effect of the triheptanoin diet on seizure susceptibility
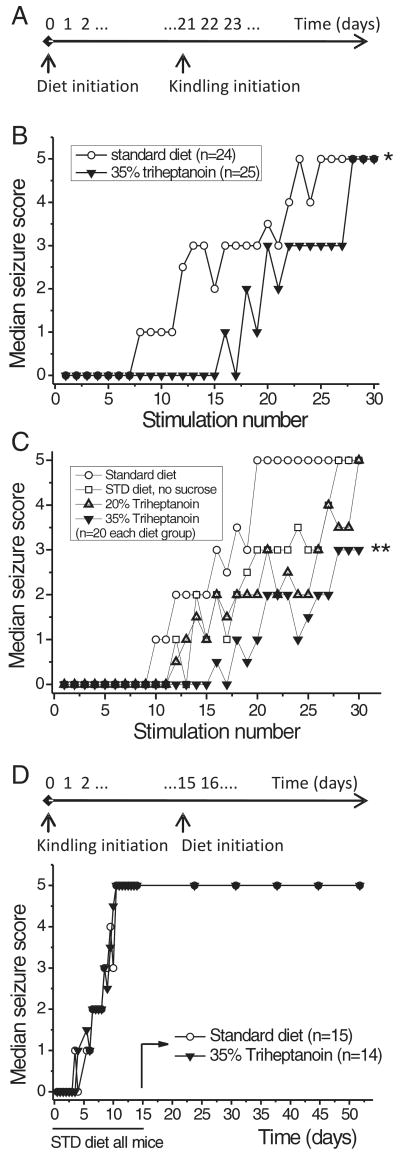
The effect of 35% triheptanoin feeding on corneal kindling development was investigated in two independent experiments. Corneal kindling was chosen over amygdala kindling because it does not require surgery. In both experiments triheptanoin feeding was initiated three weeks prior to the first stimulation and was continued throughout the kindling process. This feeding regimen delayed the development of kindled seizures (Fig. 2A, B p<0.05, t-test; C p<0.01 one way ANOVA and subsequent Newman-Keuls test). In the second experiment, we also evaluated a) if the omission of sucrose in the triheptanoin diet accounts for the anticonvulsant effect and b) if 20% triheptanoin is sufficient to affect kindling. The kindling development was not statistically different with omission of sucrose or 20% triheptanoin relative to the standard diet (Fig. 2C), indicating that more than 20% of triheptanoin is required. We then investigated if 35% triheptanoin feeding affects seizure severity by initiating the triheptanoin diet in fully kindled mice. There was no change in the severity of elicited seizures in mice fed 35% triheptanoin compared to standard diet (Fig. 2D).
Fig. 2.
The development of corneal kindling-induced seizures is delayed by 35% triheptanoin feeding.
(A) Timeline of experiments B and C indicating that the experimental diets were initiated three weeks before the first corneal stimulation. (B, C) The median seizure thresholds of each diet group are plotted against the stimulation number, showing significant differences in seizure development with 35% triheptanoin feeding during the kindling process (comparisons of areas under the curve B p<0.05 student's t-test *, C P=0.006 one way ANOVA; P<0.01 35% triheptanoin vs. standard diet, Newman-Keuls test **). (D) Triheptanoin and standard diets were initiated in fully kindled mice. No significant differences in the severity of behavioral seizures were found between diets in fully kindled mice.
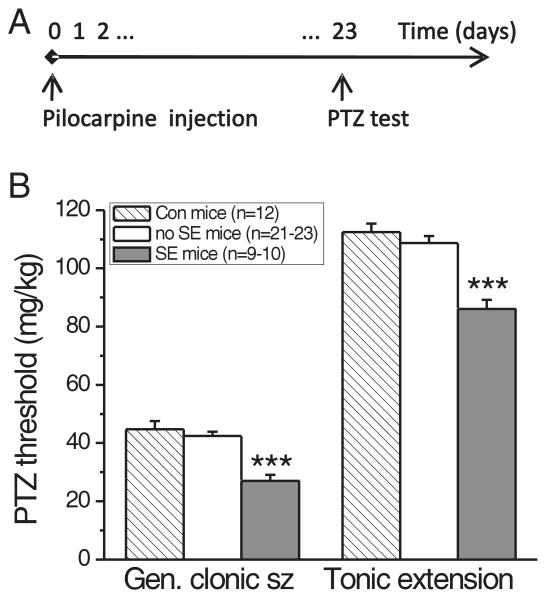
To confirm the anticonvulsant effect found in the corneal kindling model, we developed another chronic epilepsy model. Video-EEG analysis of spontaneous seizures in chronic epilepsy models is a complicated and time-consuming procedure. We therefore used a second hit model, in which mice in the chronic stage of the pilocarpine model show increased susceptibility to induced seizures. Fourteen to 24 days after pilocarpine-induced SE, mice with SE were significantly more sensitive to PTZ-induced seizures than “sham control” and no SE mice (Fig. 3, p<0.001, n=4 experiments). The latter two groups showed no statistically significant difference in their thresholds to the PTZ-induced first generalized clonic and tonic extension seizures (p>0.05 Newman-Keuls post-test after ANOVA), justifying the use of no SE mice as control mice (Fig. 3).
Fig. 3.
Lowered PTZ seizure thresholds in the chronic stage of the pilocarpine model.
(A) Timeline of the experiment. (B) Twenty-three days after pilocarpine injection, SE mice were more sensitive than no SE and “sham control” mice to PTZ-induced clonic generalized and tonic seizures (p<0.001, Newman-Keuls post-test *** after one-way ANOVA with p<0.0001). There were no statistically significant differences between thresholds in no SE and sham control mice (p>0.05, Newman-Keuls post-test).
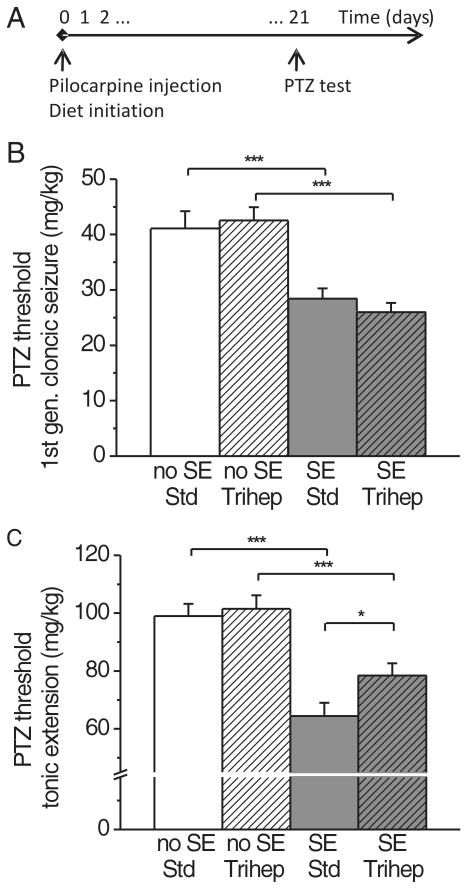
Two experiments were performed to determine the effect of triheptanoin after the epileptogenic insult of SE. In the first experiment, triheptanoin or standard diets were initiated immediately after pilocarpine or sham injection to all mice for three weeks until the PTZ threshold test (Fig. 4). The PTZ seizure thresholds to generalized clonic seizures and tonic extension were 30-35% lower in SE mice compared to the combined no SE and control mouse group (P<0.0001 ANOVA, p<0.001 post-hoc Newman-Keuls test, n=10-16 mice). Triheptanoin reversed this increased susceptibility to the tonic extension by about 40% in the SE mice (p<0.05 Newman-Keuls test), indicating that triheptanoin is anticonvulsant in mice with chronic epilepsy. Triheptanoin had no effect on PTZ clonic seizure thresholds in SE or no SE mice. Spontaneous and handling-induced seizures were observed in SE mice after 2-25 days on either diet to a similar extent, i.e. six out of 16 SE mice on standard diet and eight out of 17 SE mice on triheptanoin diet (p=0.73, Fisher's exact test), showing that triheptanoin does not prevent epileptogenesis after pilocarpine-induced SE. There was no difference in PTZ seizure thresholds between mice that were observed to have spontaneous or handling-induced seizures vs. those that did not.
Fig. 4.
Triheptanoin feeding increases the lowered PTZ tonic extension threshold in the chronic stage of the pilocarpine SE model.
(A) Timeline of the experiment showing that experimental diets were given immediately after pilocarpine injection. (B, C) Three weeks after pilocarpine injection, SE mice were more sensitive than no SE and non-injected control mice to PTZ-induced clonic generalized and tonic seizures (p<0.001, Newman-Keuls post-tests *** after ANOVA with p<0.0001). Triheptanoin partially reversed the increased susceptibility to the tonic extension in SE mice (C, p<0.05, Newman-Keuls post-test *).
In the second experiment, three week triheptanoin feeding was initiated in the chronic stage of the model two weeks after pilocarpine injection (the same time course as the metabolite analysis, see below). Again, triheptanoin counteracted a 30% increased susceptibility to tonic extension seizure in SE mice by about 50% (P<0.0001 ANOVA, p<0.05 post hoc Newman-Keuls test, n= 14-16 mice). This finding indicates that triheptanoin may also be anticonvulsant when administered after epilepsy has developed.
Blood and brain metabolites in the chronic stage of the pilocarpine model
To investigate to what extent triheptanoin feeding induced changes in brain metabolism that could account for the anticonvulsant effects found, we compared brain metabolite levels in the chronic stage of the pilocarpine model in SE relative to “non-epileptic” no SE mice (Fig. 5, Table 3). Two weeks after pilocarpine injection, mice were placed on triheptanoin vs. standard diet for three weeks. All SE mice, except for one mouse on the standard diet, were observed with either spontaneous or handling-induced behavioral seizures between 3 and 26 days after SE.
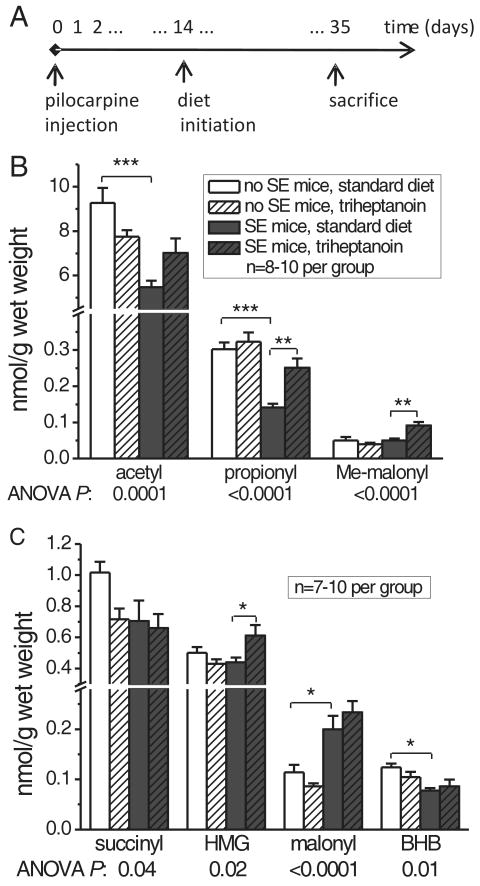
Fig. 5.
Quantification of CoA metabolites in brain shows that triheptanoin feeding increases the levels of anaplerotic molecules in SE mice.
(A) Timeline of the experiment. After pilocarpine injection, SE mice and no SE mice were first fed standard diet for two weeks, then divided into groups of equal average weight and received either standard or 35% triheptanoin-containing diet for following three weeks until metabolite quantification. (B, C) The brain levels of CoA-coupled metabolites are plotted for the different mouse groups in nmol/g wet brain weight, white bars - no SE mice, black bars -SE mice, clear bars – standard diet, striped bars – 35% triheptanoin diet. BHB - β-hydroxybutyrate, HMG – 3-hydroxy-3-methylglutaryl, Me-malonyl – methylmalonyl.
Table 3. Few changes in brain citric acid cycle intermediates and metabolites in SE and no SE mice fed triheptanoin.
| Metabolites | No SE mice Standard Diet | No SE mice Triheptanoin | SE mice Standard Diet | SE mice Triheptanoin | ANOVA P |
|---|---|---|---|---|---|
| CAC INTERMEDIATE | |||||
| citrate | 0.20 ± 0.01 | 0.21 ± 0.01 | 0.20 ± 0.02 | 0.2 ± 0.01 | 0.72 |
| succinate | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.74 |
| fumarate (%) | 100 ± 6.9 | 82.8 ± 3.8* | 86.1 ± 4.7 | 81.4 ± 2.9 | 0.028 |
| malate (%) | 100.0 ± 4.2 | 125.5 ± 7.7** | 77.1± 4.3 *** | 80.1 ± 3.9 | <0.0001 |
| CAC PRODUCTS | |||||
| glutamate | 23.64 ± 1.13 | 21.59 ± 0.92 | 21.01 ± 0.80 | 22.01 ± 1.65 | 0.52 |
| glutamine | 19.17 ± 5.23 | 12.91 ± 0.49 | 12.89 ± 0.40 | 13.15 ± 0.48 | 0.21 |
| GABA | 2.55 ± 0.11 | 2.36 ± 0.13 | 2.17± 0.07* | 2.16 ± 0.05 | 0.011 |
| aspartate (%) | 100.0 ± 13.9 | 104.0 ± 7.0 | 71.4 ± 6.3 | 76.6 ± 4.1 | 0.006 |
| OTHERS | |||||
| BHB | 55.6 ± 4.5 | 108.9 ± 9.6*** | 63.9 ± 3.3 | 127.4 ± 15.7*** | <0.0001 |
Two weeks after pilocarpine injection, no SE and SE mice were fed standard or 35% triheptanoin for three weeks. Brain homogenate levels are given in μmol/g wet weight or % of control. One-Way ANOVAs with Tukey post test for each metabolite. With the exception of malate, which was significantly lower in SE mice relatively to no SE mice on standard diet, steady-state levels of levels of CAC intermediates and products were unaffected by SE or diet.
When on standard diet, levels of certain metabolites were statistically significantly different in SE compared to no SE mice, including a 1.8-fold increase in malonyl-CoA concentrations and decreases in the levels of propionyl- (50% loss), acetyl- and β-hydroxybutyryl-CoA (both 40%, all ANOVAs p ≤; 0.01 and post-hoc Bonferroni tests with selected comparisons P<0.05; Fig. 5). Also, in SE mice the levels of aspartate (29%), and γ-aminobutyric acid (GABA, 15%) were decreased relative to no SE mice, with statistical significance of <0.05 (post-hoc Bonferroni tests with selected comparisons; both ANOVAs p ≤; 0.011, Table 3). These data support our hypothesis that epileptic tissue shows changes in metabolism, potentially including CAC activity.
Triheptanoin feeding to SE mice restored only the brain propionyl-CoA levels (Fig. 5, ANOVA p<0.001, Bonferroni test with selected comparisons p<0.001), but not acetyl-CoA levels. Methylmalonyl- (1.4-fold) and HMG-CoA levels (1.8-fold) were largely increased by triheptanoin feeding in chronically epileptic SE mice, but not in no SE mice. Yet, triheptanoin feeding to no SE mice increased malate (26%) and decreased fumarate (17%) levels (p<0.05, both Bonferroni post-hoc tests with selected comparisons), suggesting a potential shift in the malate/fumarate equilibrium.
The 35% triheptanoin diet doubled the brain β-hydroxybutyrate levels in both SE and no SE brains (Table 3) which may be explained by the high dietary fat content in the absence of sucrose. Yet, there were no significant changes in plasma levels of β-hydroxybutyrate (0.17-0.28 mM) or glucose (174-228 mg/dl). No significant changes by SE or diet were found in the steady state brain levels of the CAC intermediates succinyl-CoA, succinate and citrate and its metabolites glutamate and glutamine (Table 3). In summary, these measurements of steady state metabolite levels do not show any apparent evidence of anaplerosis by triheptanoin in the “non-epileptic” or “epileptic” brain.
4. Discussion
Our principal findings on the effects of triheptanoin in chronic mouse epilepsy models are: 1) Our 35% triheptanoin diet is calorically equivalent to a rodent standard diet and is well-tolerated by outbred CF1 mice over up to 7.5 weeks of feeding. 2) 35% triheptanoin was repeatedly anticonvulsant in two chronic mouse epilepsy models, during the development of corneal kindling and the PTZ threshold test in mice in the chronic epileptic stage of the pilocarpine model. The omission of sucrose in the diet does not account for this effect and 20% triheptanoin was not sufficient to delay corneal kindling. 3) Mice in the chronic stage of the pilocarpine model showed changes in steady state brain metabolite levels, some which were restored by triheptanoin feeding. Taken together these findings indicate that triheptanoin is anticonvulsant, but its effect on brain metabolism and its anticonvulsant mechanism is still unclear.
Anticonvulsant profile of triheptanoin
Our metabolic analyses showed that CF1 mice metabolized triheptanoin and did not suffer from propionic acidemia, similarly to the findings when anaplerotic C5-ketones were given to dogs (Leclerc et al. 1995). To our knowledge this is the first time that triheptanoin feeding was successful in mice. Triheptanoin feeding was anticonvulsant at 35% of the caloric intake in two chronic seizure models, the second hit PTZ model in pilocarpine-SE mice and corneal kindling. Efficacy in certain seizure models has been correlated to efficacy in different human seizure types, (e.g. White 2003, Smith et al. 2007). Triheptanoin's anticonvulsant profile is unusual and its activity in the two chronic mouse models employed is promising, but also difficult to interpret. To our knowledge, only a few anticonvulsant drugs have been previously tested during corneal kindling development, namely brivaracetam and leviteracetam (Matagne et al. 2008). Triheptanoin delays kindling in a similar fashion as low concentrations of those two drugs. Also, the delay of kindling by triheptanoin in the corneal kindling model mirrors the effect of valproate, phenobarbital and lacosamide during amygdala kindling in the rat (Silver et al. 1991, Brandt et al. 2006). To our knowledge the combination of the chronic pilocarpine mouse model with a second hit seizure susceptibility test has not been described before, although second hit models have been used before (e.g. Vezzani et al. 1994; Blanco et al. 2009). Blanco and colleagues treated Wistar rats with pilocarpine and one month later SE and no SE rats were subjected to the subcutaneous PTZ test. The PTZ-induced seizures were sensitive to valproate, phenobarbital and phenytoin in no SE rats, but not in SE rats. These data suggest that the PTZ model in animals with SE may be a useful tool to find treatments with efficacy in pharmacoresistant epilepsy. Rats and mice show similarities in many seizure models, including their response to different anticonvulsant drugs in the PTZ model (Loscher et al. 1991) and the pathophysiological changes after pilocarpine-induced SE (e.g. Borges et al. 2003, Turski et al. 1983, Turski et al. 1984, Curia et al. 2008). Therefore, similar to the second hit PTZ rat model it is likely that our mouse model is pharmacoresistant. In addition, short-term, but not long-term feeding of a ketogenic diet containing triheptanoin inhibited cortical spreading depression in young rats (de Almeida Rabello Oliveira et al. 2008), indicating that triheptanoin may be useful in the context of a ketogenic diet. At this time it is too difficult to compare the anticonvulsant mechanisms of the triheptanoin vs. the ketogenic diet, because the ketogenic diet has been tested in other seizure models. In summary, triheptanoin's efficacy in chronic epilepsy models suggests that it may be a powerful anticonvulsant in human patients.
Metabolite changes in brain
The metabolites levels measured are within range of published levels in rodents (e.g. Goldberg et al. 1966, Bough et al. 2006, Nordstrom et al. 1978, Puchowicz et al. 2008). The levels vary to some degree, which can be explained by different brain extraction methods (Goldberg et al. 1966).
Epileptic tissue showed changes in metabolite levels, some of which may contribute to seizures. It is conceivable that this may include the decreases found in malate and propionyl-CoA levels, which may impair function of the CAC. Also, the lower levels of aspartate found in SE mice are likely indicators of low oxaloacetate, which was not analyzed here. There are few studies that investigate brain metabolite levels in chronically epileptic rodent tissue, although energy metabolism and mitochondrial dysfunction have been clearly implicated in epilepsy (Kudin et al. 2009, Pan et al. 2008). Our data also indicate that metabolism of triheptanoin is different in epileptic tissue compared to normal brains, e.g. triheptanoin increased the levels of propionyl- and methyl-malonyl-CoA only in epileptic mice. Therefore, triheptanoin as well as other treatments may have more pronounced effects in “diseased” compared to normal brain tissue.
The increases in the levels of the anaplerotic molecules propionyl- and methylmalonyl-CoA are consistent with increased anaplerotic flux. The anaplerotic need of the CAC is expected to be increased in epileptic tissue, because CAC intermediates are the precursors of neurotransmitters, such as glutamate and GABA, which are excessively released during seizures. If this need is not fully met, energy shortage ensues, which could lead to depolarization of neuronal membranes and lower seizure threshold. We hypothesized that triheptanoin feeding would be anaplerotic in the epileptic brain, which could provide additional ATP and potentially GABA and may therefore protect against seizure generation. While we found that triheptanoin was anticonvulsant, it is yet unclear to which extent triheptanoin feeding was anaplerotic in the brain. Our analysis of steady state brain metabolite levels revealed that triheptanoin feeding increased levels of anaplerotic precursor molecules in chronically epileptic brains. However, there were no significant changes in the steady state levels of the CAC intermediates or metabolites quantified in either the SE or no SE mice that point to anaplerosis. On the other hand, the turnover of the CAC is fast and small undetectable changes in steady state metabolite levels may still largely increase the capacity to produce ATP. Future work using 13C tracers and mass isotopomer analysis is needed to determine the degree of anaplerotic fluxes and to understand the metabolic fates of propionyl-CoA. Taken together there is dire need for more research in brain metabolism and its role in seizure development.
Conclusion
We developed a new anticonvulsant diet that was well-tolerated diet by out mice. Our triheptanoin diet was repeatedly anticonvulsant in two chronic mouse epilepsy models. In the chronic epileptic stage in pilocarpine-SE mice, a significant reduction in brain propionyl-CoA levels was revealed, which was largely restored by triheptanoin feeding. Whether these metabolic changes underlie triheptanoin's anticonvulsant effect remains to be studied. This work provides the foundation to future studies aimed at optimizing this diet for the treatment of human epilepsy and the deciphering of its anticonvulsant mechanism.
Acknowledgments
We thank Drs. Bjoernar Hassel, Henri Brunengraber, Charles Roe and Terri Bottiglieri for helpful discussions throughout this work and David Lust for comments on the manuscript. Ramakrishna Samala and Naomi Wangler provided excellent help with some of the experiments. We are grateful to Sasol for donating triheptanoin and to the CASE Mouse Metabolic Phenotyping Center, U24 DK76169, and Dr. Michelle Puchowicz for quantifications of brain metabolite levels. This project was funded by the Citizens United for Research in Epilepsy (CURE) and the NIH (1R15NS060105-01A2). We have filed for a US provisional patent.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
G. Literature Cited
- Blanco MM, dos Santos JG, Jr, Perez-Mendes P, Kohek SR, Cavarsan CF, Hummel M, Albuquerque C, Mello LE. Assessment of seizure susceptibility in pilocarpine epileptic and nonepileptic Wistar rats and of seizure reinduction with pentylenetetrazole and electroshock models. Epilepsia. 2009;50:824–831. doi: 10.1111/j.1528-1167.2008.01797.x. [DOI] [PubMed] [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Borges K, Gearing M, Rittling SR, Sorensen ES, Kotloski R, Denhardt DT, Dingledine R. Characterization of osteopontin expression and function after status epilepticus. Epilepsia. 2008;49:1675–1685. doi: 10.1111/j.1528-1167.2008.01613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges K, McDermott D, Irier H, Smith Y, Dingledine R. Degeneration and proliferation of astrocytes in the mouse dentate gyrus after pilocarpine-induced status epilepticus. Exp Neurol. 2006;201:416–427. doi: 10.1016/j.expneurol.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges K, McDermott DL, Dingledine R. Reciprocal changes of CD44 and GAP-43 expression in the dentate gyrus inner molecular layer after status epilepticus in mice. Exp Neurol. 2004;188:1–10. doi: 10.1016/j.expneurol.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- Brandt C, Heile A, Potschka H, Stoehr T, Loscher W. Effects of the novel antiepileptic drug lacosamide on the development of amygdala kindling in rats. Epilepsia. 2006;47:1803–1809. doi: 10.1111/j.1528-1167.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- Brunengraber H, Roe CR. Anaplerotic molecules: Current and future. J Inherit Metab Dis. 2006;29:327–331. doi: 10.1007/s10545-006-0320-1. [DOI] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Rabello Oliveira M, da Rocha Ataide T, de Oliveira SL, de Melo Lucena AL, de Lira CE, Soares AA, de Almeida CB, Ximenes-da-Silva A. Effects of short-term and long-term treatment with medium- and long-chain triglycerides ketogenic diet on cortical spreading depression in young rats. Neurosci Lett. 2008;434:66–70. doi: 10.1016/j.neulet.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, Roopra A. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- Goldberg ND, Passonneau JV, Lowry OH. Effects of changes in brain metabolism on the levels of citric acid cycle intermediates. J Biol Chem. 1966;241:3997–4003. [PubMed] [Google Scholar]
- Hartman AL, Gasior M, Vining EP, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatr Neurol. 2007;36:281–292. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel B. Carboxylation and anaplerosis in neurons and glia. Mol Neurobiol. 2000;22:21–40. doi: 10.1385/MN:22:1-3:021. [DOI] [PubMed] [Google Scholar]
- Kinman RP, Kasumov T, Jobbins KA, Thomas KR, Adams JE, Brunengraber LN, Kutz G, Brewer WU, Roe CR, Brunengraber H. Parenteral and enteral metabolism of anaplerotic triheptanoin in normal rats. Am J Physiol Endocrinol Metab. 2006;291:E860–866. doi: 10.1152/ajpendo.00366.2005. [DOI] [PubMed] [Google Scholar]
- Kudin AP, Zsurka G, Elger CE, Kunz WS. Mitochondrial involvement in temporal lobe epilepsy. Exp Neurol. 2009;218:326–332. doi: 10.1016/j.expneurol.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Leclerc J, Des Rosiers C, Montgomery JA, Brunet J, Ste-Marie L, Reider MW, Fernandez CA, Powers L, David F, Brunengraber H. Metabolism of R-beta-hydroxypentanoate and of beta-ketopentanoate in conscious dogs. Am J Physiol. 1995;268:E446–452. doi: 10.1152/ajpendo.1995.268.3.E446. [DOI] [PubMed] [Google Scholar]
- Lian XY, Khan FA, Stringer JL. Fructose-1,6-bisphosphate has anticonvulsant activity in models of acute seizures in adult rats. J Neurosci. 2007;27:12007–12011. doi: 10.1523/JNEUROSCI.3163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Honack D, Fassbender CP, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. III. Pentylenetetrazole seizure models. Epilepsy Res. 1991;8:171–189. doi: 10.1016/0920-1211(91)90062-k. [DOI] [PubMed] [Google Scholar]
- Matagne A, Klitgaard H. Validation of corneally kindled mice: a sensitive screening model for partial epilepsy in man. Epilepsy Res. 1998;31:59–71. doi: 10.1016/s0920-1211(98)00016-3. [DOI] [PubMed] [Google Scholar]
- Matagne A, Margineanu DG, Kenda B, Michel P, Klitgaard H. Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br J Pharmacol. 2008;154:1662–1671. doi: 10.1038/bjp.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochel F, DeLonlay P, Touati G, Brunengraber H, Kinman RP, Rabier D, Roe CR, Saudubray JM. Pyruvate carboxylase deficiency: clinical and biochemical response to anaplerotic diet therapy. Mol Genet Metab. 2005;84:305–312. doi: 10.1016/j.ymgme.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- Nordstrom CH, Rehncrona S, Siesjo BK. Effects of phenobarbital in cerebral ischemia. Part II: restitution of cerebral energy state, as well as of glycolytic metabolites, citric acid cycle intermediates and associated amino acids after pronounced incomplete ischemia. Stroke. 1978;9:335–343. doi: 10.1161/01.str.9.4.335. [DOI] [PubMed] [Google Scholar]
- Pan JW, Williamson A, Cavus I, Hetherington HP, Zaveri H, Petroff OA, Spencer DD. Neurometabolism in human epilepsy. Epilepsia. 2008;49 3:31–41. doi: 10.1111/j.1528-1167.2008.01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchowicz MA, Zechel JL, Valerio J, Emancipator DS, Xu K, Pundik S, LaManna JC, Lust WD. Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J Cereb Blood Flow Metab. 2008;28:1907–1916. doi: 10.1038/jcbfm.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rashed MS, Bucknall MP, Little D, Awad A, Jacob M, Alamoudi M, Alwattar M, Ozand PT. Screening blood spots for inborn errors of metabolism by electrospray tandem mass spectrometry with a microplate batch process and a computer algorithm for automated flagging of abnormal profiles. Clin Chem. 1997;43:1129–1141. [PubMed] [Google Scholar]
- Roe CR, Mochel F. Anaplerotic diet therapy in inherited metabolic disease: Therapeutic potential. J Inherit Metab Dis. 2006;29:332–340. doi: 10.1007/s10545-006-0290-3. [DOI] [PubMed] [Google Scholar]
- Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest. 2002;110:259–269. doi: 10.1172/JCI15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samala R, Willis S, Borges K. Anticonvulsant profile of a balanced ketogenic diet in acute mouse seizure models. Epilepsy Res. 2008;81:119–127. doi: 10.1016/j.eplepsyres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Silver JM, Shin C, McNamara JO. Antiepileptogenic effects of conventional anticonvulsants in the kindling model of epilespy. Ann Neurol. 1991;29:356–363. doi: 10.1002/ana.410290404. [DOI] [PubMed] [Google Scholar]
- Smith M, Wilcox KS, White HS. Discovery of antiepileptic drugs. Neurotherapeutics. 2007;4:12–17. doi: 10.1016/j.nurt.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Ockuly JC, Murphree L, Valley MT, Roopra A, Sutula TP. Anticonvulsant and antiepileptic actions of 2-deoxy-D-glucose in epilepsy models. Ann Neurol. 2009;65:435–447. doi: 10.1002/ana.21603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman L. Organic Acid Analysis Techniques in Diagnostic Human Biochemical Genetics: A Laboratory Manual. In: Hommes F, editor. Techniques in Diagnostic Human Biochemical Genetics: A Laboratory Manual. Wiley-Liss Inc; New York: 1991. pp. 143–176. [Google Scholar]
- Turski WA, Cavalheiro EA, Bortolotto ZA, Mello LM, Schwarz M, Turski L. Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res. 1984;321:237–253. doi: 10.1016/0006-8993(84)90177-x. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Civenni G, Rizzi M, Monno A, Messali S, Samanin R. Enhanced neuropeptide Y release in the hippocampus is associated with chronic seizure susceptibility in kainic acid treated rats. Brain Res. 1994;660:138–143. doi: 10.1016/0006-8993(94)90847-8. [DOI] [PubMed] [Google Scholar]
- Wang X, Allen FJ, Sayre C, Wan D, Minkler PE, Hoppel CL, Brunengraber H. Anaplerosis from heptanoate - a propionyl-CoA precursor - in mouse brain. FASEB. 2007;21:541.512. [Google Scholar]
- White HS. Preclinical development of antiepileptic drugs: past, present, and future directions. Epilepsia. 2003;44 7:2–8. doi: 10.1046/j.1528-1157.44.s7.10.x. [DOI] [PubMed] [Google Scholar]
- Willis S, Samala R, Rosenberger TA, Borges K. Eicosapentaenoic and docosahexaenoic acids are not anticonvulsant or neuroprotective in acute mouse seizure models. Epilepsia. 2009;50:138–142. doi: 10.1111/j.1528-1167.2008.01722.x. [DOI] [PubMed] [Google Scholar]