Abstract
Neuroimaging studies of subjects who are gene-expanded for Huntington Disease, but not yet diagnosed (termed prodromal HD), report that the cortex is “spared,” despite the decrement in striatal and cerebral white-matter volume. Measurement of whole-cortex volume can mask more subtle, but potentially clinically relevant regional changes in volume, thinning, or surface area. The current study addressed this limitation by evaluating cortical morphology of 523 prodromal HD subjects. Participants included 693 individuals enrolled in the PREDICT-HD protocol. Of these participants, 523 carried the HD gene mutation (prodromal HD group); the remaining 170 were non gene-expanded and served as the comparison group. Based on age and CAG repeat length, gene-expanded subjects were categorized as “Far from onset,” “Midway to onset,” “Near onset,” and “already diagnosed.” MRI scans were processed using FreeSurfer. Cortical volume, thickness, and surface area were not significantly different between the Far from onset group and controls. However, beginning in the Midway to onset group, the cortex showed significant volume decrement, affecting most the posterior and superior cerebral regions. This pattern progressed when evaluating the groups further into the disease process. Areas that remained mostly unaffected included ventral and medial regions of the frontal and temporal cortex. Morphologic changes were mostly in thinning as surface area did not substantially change in most regions. Early in the course of HD, the cortex shows changes that are manifest as cortical thinning and are most robust in the posterior and superior regions of the cerebrum.
Keywords: Huntington disease, MRI, cerebral cortex, cortical thickness, surface area
Introduction
Huntington Disease (HD) is a neurodegenerative disorder that manifests in a triad of symptoms including cognitive, motor, and behavioral abnormalities. The discovery of the gene in 1993 (Huntington’s Disease Collaborative Research Group, 1993) provided an opportunity to study individuals who carry the mutant gene, but have not yet manifested significant disease signs and are therefore referred to as prodromal HD.
HD is part of a family of poly-glutamine diseases in which the mutant gene (huntingtin in the case of HD) contains a triplet CAG repeat coding for an expanding polyglutamine repeat and resulting in an abnormal or toxic protein. Another property of polygluatamine diseases is that despite the fact that the mutant proteins are expressed ubiquitously, the disease manifestation is specific to brain tissue and also to specific regions of the brain. For HD, the area of the brain most affected is that of the striatum. Therefore, most of the studies of HD, using either neuropathologic methods or neuroimaging methods have focused on measuring the effects of the disease on this brain region. However, more recently, there have been a number of studies evaluating “extra-striatal” brain structure such as the morphology of the cerebral white matter and cortex in subjects with early HD or prodromal HD. As a whole, these studies support an interesting pattern in which the cerebral white matter volume is substantially lower than normal, while in contrast, the cerebral cortex is relatively “spared” (Aylward et al., 1998; Beglinger et al., 2005; Ciarmiello et al., 2006; Douaud et al., 2006; Fennema-Notestine et al., 2004; Jernigan et al., 1991; Muhlau et al., 2007; Rosas et al., 2003).
PREDICT-HD is a long-term observational study of a large population of prodromal HD subjects (Paulsen et al., 2006). In a recent cross-sectional analysis of 657 participants, the volume of the cerebral cortex was indeed found to be significantly reduced in volume in the prodromal HD subjects. However, the effect size for the comparison of volume decrement in prodromal HD subjects to controls in the cerebral cortex (0.699) was substantially smaller than the effect size of the comparison of volumes across groups for the cerebral white matter (1.445) or striatum (2.456) (Paulsen et al., 2010). One important caveat for this and other analyses that use the global measure of cerebral cortex is that this measure is a gross one and may very well miss regional variation of structure within the cortex, as the cortex is divided up into many small structurally and functionally distinct regions.
Few studies have evaluated regional morphology of the cerebral cortex in HD. Rosas, in a study of HD subjects post-diagnosis, showed that cortical thinning was indeed gradual in onset and regionally specific (Rosas et al., 2002). In more recent studies, cortical thickness maps of both small (Rosas et al., 2005) and large (Tabrizi et al., 2009) samples of prodromal HD subjects show an interesting pattern in which cortical thinning occurs in an unexpected pattern that is heavily superior and posterior, with what appears to be frontal lobe sparing. This is surprising given the fact that, although the striatum receives input from all over the cortex, the output of the striatum (via basal ganglia and thalamic connections) is predominantly to the frontal cortex.
In regard to cerebral cortex morphology, studies have typically focused on measures of volume or cortical thickness. However, volume is a function of both thickness and surface area. Moreover, recent studies have shown the importance of distinguishing the measures of cortical thickness and cortical surface area. For instance, two studies have shown that the genetic influences of cortical thickness appear to be independent of those driving cortical surface area (Panizzon et al., 2009; Winkler et al., 2009). In addition, one of these studies (Winkler et al., 2009) showed that in a non-diseased population, measures of cortical volume were more highly correlated to surface area than to cortical thickness. These studies highlight the importance of looking at both thickness and surface area when evaluating corticsal morphology.
The current study is designed to extensively evaluate the morphology of the cerebral cortex in a large sample of prodromal HD subjects. This is the first study to comprehensively examine cortical morphology in prodromal HD subjects using volume, surface area, and thickness measures. Unlike previous studies that have reported only cortical thickness maps in prodromal HD, this study also measures and reports volumes of cortical regions.
Methods
Participants
Participants were recruited from the PREDICT-HD study, an ongoing longitudinal study conducted at 32 sites in the United States, Canada, Australia, Germany, Spain, and the United Kingdom (Paulsen et al., 2006). The study was approved by the Institutional Review Board at each participating institution. Study participants signed informed consents to participate and allow their de-identified research data to be analyzed by collaborative institutions. All participants underwent detailed motor, cognitive, psychiatric, and functional evaluations annually as previously described (Paulsen et al., 2006; Paulsen et al., 2008).
We use the term “prodromal HD” to describe the phase prior to the manifestation of the movement disorder clinically diagnosable as HD. Participants were classified as prodromal HD according to the diagnostic confidence level of the Unified Huntington Disease Rating Scale UHDRS (Huntington Study Group, 1996). Diagnostic confidence level is a scale ranging from 0 (normal) to 4 (defined as the unequivocal presence of an otherwise unexplained movement disorder in a participant at risk for HD). Participants with ratings less than 4 were classified as prodromal HD. All subjects were prodromal at the time of study enrollment, but some subjects converted to a diagnosis of HD at a follow-up assessment. Participants who received a rating of 4 were classified as converted and are referred to as “diagnosed.” Study participants consisted of 693 individuals. Of these participants, 523 carried the HD gene mutation, and 170 carried only normal alleles; the latter were used as the comparison group.
Proximity to Diagnosis
Estimated years to diagnosis and probability of receiving a diagnosis within the ensuing five years were calculated using a CAG-and-age based predictive model developed from a worldwide sample of 2,913 and validated with nearly 100 prospectively diagnosed patients from the PREDICT-HD study (Langbehn et al., 2004; Langbehn et al., 2009). Participants were considered ‘Far’ from diagnosis if estimated proximity to clinical diagnosis was greater than 15 years, ‘Mid’ to diagnosis if their estimated proximity was 9–15 years, and ‘Near’ to diagnosis if estimated proximity was less than 9 years. One group of participants was diagnosed as having HD. Table 1 displays the demographic and CAG repeat-lengths of the five groups. Gender was similar across groups (from 59% to 67% female), although participants in the FAR group were significantly younger than participants in the other groups.
Table 1.
Demographic Information
| Controls | Far from Onset (>15 years) | Midway to Onset (9–15 years) | Near to Onset (<9 years) | Diagnosed HD | |
|---|---|---|---|---|---|
| # Females | 108 (63.5) | 113 (66.5) | 109 (66.9) | 80 (59.3) | 37 (67.3) |
| # Males | 62 (36.5) | 57 (33.5) | 54 (33.1) | 55 (40.7) | 18 (32.7) |
| Total # | 170 | 170 | 163 | 135 | 55 |
| Age mean (s.d.) | 44.9 (12.1) | 38.1 (8.4) | 44.1 (10.4) | 47.1 (10.1) | 47.3 (9.5) |
| CAG length mean (s.d.) | 20.1 (3.3) | 41.0 (1.5) | 42.3 (2.2) | 43.6 (2.4) | 43.7 (3.5) |
MRI Procedures
All scans were obtained using a standard multi-modal protocol that included an axial 3D volumetric spoiled-gradient echo series (~1×1×1.5 mm voxels) and a dual echo proton density/T2 (~1×1×3 mm voxels) series. Thirty sites used General Electric 1.5 Tesla scanners, and two sites used Siemens 1.5 Tesla scanners.
Each multi-modal scan series was processed through a standardized morphometric processing pipeline that corrected for common multi-site data differences (Magnotta et al., 2002). Briefly, outputs of the morphometric processing pipeline included a brain mask used for computing the Intracranial Volume (ICV) and a T1 weighted image supplied to FreeSurfer for cortical thickness processing.
The brain mask was derived from all three image intensity modes to derive robust estimates of ICV, which include tissue and surface CSF that extends to the border of dura mater. The T1 weighted image was created with isotropic (1.0 mm3) voxels. In addition, the T1 images were normalized so that the tissue intensities across the spatial domain of a single image and scans from different sites were placed in a consistent intensity range. Spatial intensity inhomogeneities were removed by applying a parametric correction (Styner et al., 2000) that used estimates of the tissue intensities based on tissue classes from the muliti-modal tissue classification (Harris et al., 1999). Each scan’s intensity range was placed on a consistent scale by linearly scaling to maximize the dynamic range inside the brain region. A reoriented, inhomogeneity and intensity corrected T1 scan for each subject was then clipped to the brain mask to be used as input for cortical parcellation.
Cortical reconstruction was performed with the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/). Briefly, processing includes removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), tessellation of the gray-matter white-matter boundary, and automated topology correction (Fischl et al., 2001; Segonne et al., 2007). In addition, surface deformation, following intensity gradients, is done to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale and Sereno, 1993; Dale et al., 1999; Fischl and Dale, 2000). Once the cortical models are complete, a number of deformable procedures can perform further data processing and analysis including surface inflation (Fischl et al., 1999a), registration to a spherical atlas, which utilizes individual cortical folding patterns to match cortical geometry across subjects (Fischl et al., 1999b), parcellation of the cerebral cortex into units based on gyral and sulcal structure (Desikan et al., 2006; Fischl et al., 2004), and creation of a variety of surface-based data including maps of curvature and sulcal depth. This method uses both intensity and continuity information from the entire three-dimensional MR volume in segmentation and deformation procedures to produce representations of cortical thickness, calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface (Fischl and Dale, 2000). The maps are created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. The maps produced are not restricted to the voxel resolution of the original data and thus, are capable of detecting subvoxel differences between groups. Procedures for the measurement of cortical thickness have been validated against histological analysis (Rosas et al., 2002) and manual measurements (Kuperberg et al., 2003; Salat et al., 2004). FreeSurfer morphometric procedures show good test-retest reliability across scanner manufacturers and field strengths (Han et al., 2006).
Processing was completed without manual intervention. Since FreeSurfer uses random initiation of certain processes in the pipeline, each scan was processed five times, and a similarity index was calculated for each run in comparison to the other runs for that scan. The run that was most similar to the other runs for a given scan was chosen to represent the best result for that scan, eliminating spurious outliers due to the random initialization. Visual inspection was then completed to review the final surfaces and parcellation.
Statistical analysis
The thickness at each vertex (brain map analysis), the total volume of each region of interest, and the surface area of each region of interest were the outcomes in a general linear-model tests of prognostic group (Control, Far, Mid, Near, or Diagnosed), which was the predictor variable of primary interest. Gender and age were covariates. For the region of interest analysis, all volumes were adjusted for ICV. To determine if potential group differences depended on the age or gender of individuals, the two-way interactions (age*gender, age*group, and gender*group) were tested and removed from the model if not statistically significant, as our objective was to identify the most parsimonious model of disease progression. All statistical tests were corrected for multiple comparisons using a false discovery rate (FDR) of 0.05 (Genovese et al., 2002).
Results
Regional Cortical Volume Analysis of Prognostic Groups Compared to Controls
Table 2 displays results of the ANOVAs comparing mean cortical volumes of 33 regions among all prodromal HD subjects combined and the control group. Volumes in this table are ordered by group effect sizes. After correction for multiple comparisons, 20 of the 33 regions showed significant group differences. In all of these 20 regions, volumes were reduced in the HD group compared to controls, with the exception of the rostral anterior cingulate region wherein volume was increased. Most of the regions that are significantly affected are in the occipital and parietal lobes as well as lateral temporal regions. The areas affected most in the frontal lobes are superior frontal, paracentral (medial surface), precentral, and two small lateral regions, the rostral middle frontal and pars opercularis (Broca’s area). No regions on the ventral frontal lobe are structurally different in the prodromal HD groups compared to controls. Also spared are the temporal pole and the ventromedial areas of the entorhinal and parahippocampal cortex.
Table 2.
Analysis of Regional Volumes Comparing all prodromal HD subjects to Controls
| Region | Lobe | F value | P (FDR corrected) | Direction |
|---|---|---|---|---|
| Lateral occipital | Occipital | 16.47 | <0.0001 | Control > prHD |
| Superior temporal | Temporal | 11.75 | <0.0001 | Control > prHD |
| Pre-cuneus | Parietal | 10.90 | <0.0001 | Control > prHD |
| Inferior parietal | Parietal | 10.76 | <0.0001 | Control > prHD |
| Cuneus | Occipital | 8.47 | <0.0001 | Control > prHD |
| Post-central | Parietal | 8.01 | <0.0001 | Control > prHD |
| Lingual | Occipital | 7.14 | 0.0001 | Control > prHD |
| Bank Superior Temporal Sulcus | Temporal | 6.70 | 0.0001 | Control > prHD |
| Pars opercularis | Frontal | 6.69 | 0.0001 | Control > prHD |
| Fusiform | Temporal | 6.49 | 0.0001 | Control > prHD |
| Rostral middle frontal | Frontal | 5.91 | 0.0003 | Control > prHD |
| Superior frontal | Frontal | 5.27 | 0.0010 | Control > prHD |
| Transverse temporal | Temporal | 5.18 | 0.0011 | Control > prHD |
| Supra-marginal | Parietal | 4.86 | 0.0018 | Control > prHD |
| Middle temporal | Temporal | 4.68 | 0.0022 | Control > prHD |
| Pre-central | Frontal | 4.48 | 0.0030 | Control > prHD |
| Superior parietal | Parietal | 4.44 | 0.0030 | Control > prHD |
| Para-central | Parietal | 4.16 | 0.0047 | Control > prHD |
| Rostral anterior cingulate | Frontal | 4.04 | 0.0055 | prHD > Control |
| Inferior temporal | Temporal | 3.54 | 0.0123 | Control > prHD |
| Entorhinal | Temporal | 2.54 | 0.0624 | n/a |
| Peri-calcarine | Occipital | 2.31 | 0.0871 | n/a |
| Caudal middle frontal | Frontal | 2.06 | 0.1244 | n/a |
| Lateral orbitofrontal | Frontal | 2.04 | 0.1244 | n/a |
| Pars orbitalis | Frontal | 1.74 | 0.1833 | n/a |
| Pars triangularis | Frontal | 1.74 | 0.1833 | n/a |
| Caudal anterior cingulate | Frontal | 1.22 | 0.3787 | n/a |
| Frontal pole | Frontal | 1.14 | 0.4101 | n/a |
| Posterior cingulate | Frontal | 1.11 | 0.4129 | n/a |
| Isthmus cingulate | Frontal | 0.80 | 0.5933 | n/a |
| Medial orbitofrontal | Frontal | 0.61 | 0.7176 | n/a |
| Para-hippocampal | Temporal | 0.52 | 0.7645 | n/a |
| Temporal pole | Temporal | 0.40 | 0.8336 | n/a |
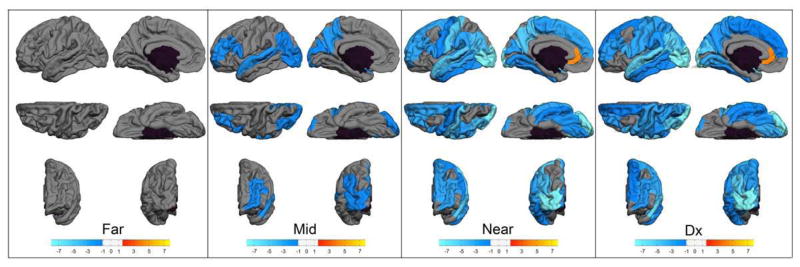
Figure 1 displays volumes of cortical regions that significantly differed between the control group and each prodromal HD prognostic group. No differences between the Far and control groups were found in cortical volume. However, in the Mid, Near, and Diagnosed groups, patterns of volume loss occur, starting in a few areas in the mid to onset group (lateral and medial occipital and parietal, superior temporal, pars triangularis), then extending through the most of the cortex in the diagnosed groups. Consistent with the combined prodromal HD group analysis (Table 2), the areas with the most severe volume loss (the lightest shades of blue) include lateral and medial occipital regions along with the superior temporal gyrus while the regions not different from the controls include a contiguous area of ventral and medial frontal and temporal cortex.
Fig. 1.
Cortical Regions Volume. Starting from the left, each group of six maps represent the comparison of volume of separate cortical region between each prodromal HD group and the controls (Dx = diagnosed). Left hemisphere only is shown. The top row shows (from left to right) lateral and medial views, the second row shows dorsal and ventral views, the last row shows anterior and posterior views, respectively.
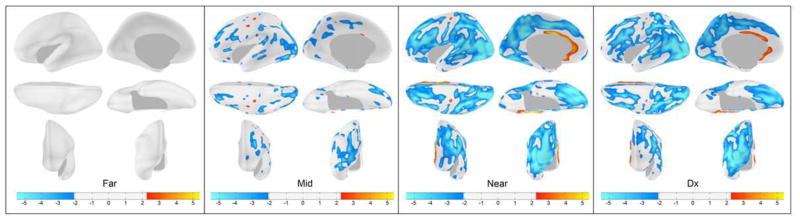
Cortical Thickness Maps of Prognostic Groups Compared to Controls
Figure 2 shows the cortical thickness maps of each prognostic group (Far, Mid, Near, Diagnosed) derived from comparisons with the controls. Each map is corrected for multiple comparisons so that only statistically significant differences are displayed. The pattern is similar to that of the cortical volume analysis. In the Far group, there were no significant differences in cortical thickness compared to controls. Going from the Mid to the Near to the Diagnosed groups, there emerges a pattern of progressively thinner regions of the cortex. Initially, this is present predominantly in the lateral parietal and occipital regions, but by the time of HD diagnosis, thinning is observed throughout most of the cerebral cortex. The most affected regions (lightest shade of blue), however, remain posterior. Ventral and medial areas of the frontal and temporal lobe are largely spared as are both the pre and post central gyri. The exception to the widespread thinning is in the area of the rostral anterior cingulate where there appears to be significant thickening in the HD groups compared to controls, in parallel with the volumetric analysis.
Fig. 2.
Cortical Thickness Maps. Starting from the left, each group of six maps represent the comparison of cortical thickness between each prodromal HD group and the controls (Dx = diagnosed). Left hemisphere only is shown. The top row shows (from left to right) lateral and medial views, the second row shows dorsal and ventral views, the last row shows anterior and posterior views, respectively.
One interesting exception to the similar patterns in the cortical volume and cortical thickness maps are the precentral and postcentral gyri, which showed volume loss in the Near and Diagnosed groups, but relative sparing of cortical thickness, even in the diagnosed group.
Regional Surface Area Analysis of Prognostic Groups Compared to Controls
Differences between the prodromal HD group and controls in surface area were significant for only five regions: transverse temporal, postcentral, precentral, cuneus, and superior temporal (see Table 3). For all of these regions, the prodromal HD group had lower surface area compared to controls. The findings of the pre- and post-central areas having significant smaller surface area helps to clarify the apparent inconsistent finding between the volume and thickness maps of these regions. That is, although the volume is significantly lower, cortical thickness was not abnormal, yet surface area was.
Table 3.
Analysis of Regional Cortical Surface Area Comparing all prodromal HD subjects to Controls
| Region | Lobe | F value | P (FDR corrected) | Direction |
|---|---|---|---|---|
| Transverse Temporal | Temporal | 16.52 | <0.0001 | Control > prHD |
| Post-central | Parietal | 9.51 | <0.0001 | Control > prHD |
| Cuneus | Occipital | 8.39 | <0.0003 | Control > prHD |
| Pre-central | Frontal | 5.04 | <0.0003 | Control > prHD |
| Superior Temporal | Temporal | 5.00 | <0.0196 | Control > prHD |
| Lateral occipital | Occipital | 3.96 | 0.0957 | n/a |
| Lingual | Occipital | 2.94 | 0.1118 | n/a |
| Entorhinal | Temporal | 2.77 | 0.1219 | n/a |
| Inferior Temporal | Temporal | 2.65 | 0.1381 | n/a |
| Middle Temporal | Temporal | 2.51 | 0.3024 | n/a |
| Pre-cuneus | Parietal | 1.91 | 0.3024 | n/a |
| Peri-calcarine | Occipital | 1.91 | 0.3071 | n/a |
| Supra-marginal | Parietal | 1.85 | 0.3622 | n/a |
| Para-central | Frontal | 1.68 | 0.3622 | n/a |
| Superior Frontal | Frontal | 1.65 | 0.3739 | n/a |
| Pars Opercularis | Frontal | 1.58 | 0.3850 | n/a |
| Fusiform | Temporal | 1.52 | 0.3999 | n/a |
| Medial orbitofrontal | Frontal | 1.44 | 0.3999 | n/a |
| Para-hippocampal | Temporal | 1.42 | 0.3999 | n/a |
| Inferior Parietal | Parietal | 1.39 | 0.4513 | n/a |
| Rostral anterior cingulate | Frontal | 1.27 | 0.4513 | n/a |
| Temporal pole | Temporal | 1.21 | 0.4513 | n/a |
| Pars triangularis | Frontal | 1.20 | 0.4513 | n/a |
| Pars orbitalis | Frontal | 1.15 | 0.4513 | n/a |
| Caudal middle Frontal | Frontal | 0.21 | 0.5147 | n/a |
| Lateral orbitofrontal | Frontal | 0.98 | 0.5147 | n/a |
| Isthmus cingulate | Frontal | 0.96 | 0.5147 | n/a |
| Frontal pole | Frontal | 0.93 | 0.5147 | n/a |
| Posterior cingulate | Frontal | 0.91 | 0.5147 | n/a |
| Rostral middle Frontal | Frontal | 0.87 | 0.5275 | n/a |
| Superior Parietal | Parietal | 0.81 | 0.5353 | n/a |
| Bank Superior Temporal Sulcus | Temporal | 0.80 | 0.5353 | n/a |
| Caudal anterior cingulate | Frontal | 0.71 | 0.5791 | n/a |
With the exception of the pre- and post-central regions, the cortical thickness maps follow a pattern almost identical to the cortical volume maps. Moreoever, despite relatively widespread cortical volume decrements, Table 3 is impressively nonsignificant with only five of the 33 regions abnormal. This is in comparison to Table 2 (volume) where 20 of the 33 regions are abnormal, and both the Figures (volumes and thickness mapped according to prognostic group) show widespread, significant volume and thickness abnormalities in the prodromal HD subjects compared to controls. Thus, volume differences between the groups appear to be mostly driven by changes in cortical thickness, rather than by decrements in surface area.
Discussion
This study is the first comprehensive analysis of cortical morphology in a large sample of prodromal HD subjects compared to healthy controls. Beginning in the midway to onset group (9–15 years from diagnosis), the cortex shows significant decrement in volume in a pattern that is initially specific to superior and posterior regions of the cortex, but by onset of diagnosis represents a pattern that is quite extensive and includes most of the entire cerebrum. Nevertheless, the superior and posterior regions of the cerebrum remain the most significantly abnormal while regions of the ventral and medial frontal and temporal lobe remain mostly unaffected. Unique to this study in particular is the finding that the volume loss appears to be related mostly to cortical thinning rather than decrements in surface area.
The current study showed that individuals far from onset had no significant changes in cortical thickness or volume compared to controls. In a previous preliminary study on prodromal HD subjects evaluating cortex morphology, we found a pattern in which the cortex was enlarged in the area of gyri, but substantially thinner in the sulci (Nopoulos et al., 2007). The current study is partially supportive in that the thinning found in the Mid and Near groups were primarily located in the sulci, as identified in the previous study. In addition, the current study also found areas of gyral thickening in the Far from onset group, similar to the previous study; however none of these areas of thickening survived the more strict analysis of correction for multiple comparison. How far back the changes in the cortex are occurring is uncertain. For instance, cortical thickening could be present even prior to the Far from onset group as a manifestation of abnormal development. A recent study of a knock-in mouse model of HD showed substantial abnormalities in brain development supporting the notion that changes in the brain may be present decades prior to onset of disease (Molero et al., 2009).
The current findings in which substantial thinning is not present until mid and near onset are consistent with the theme of relative gray matter sparing early in the course of the disease seen in our previous volumetric analysis of a larger group of prodromal HD subjects enrolled in PREDICT-HD. In that study, the total cerebral-cortex volume was reduced in the prodromal HD group relative to controls. However, the effect size of this finding was substantially smaller than the effect size for the decrement in white-matter volume or for the effect size of the decrement in volume of the striatum (Paulsen et al., 2010).
The observations of relative gray matter sparing in early prodromal HD (Far group) are interesting, given that widespread cortical involvement is evident in later stages (Heinsen et al., 1994; Selemon et al., 2004; Wagster et al., 1994). Yet lack of cortical findings in other neuroimaging studies of subjects who are prodromal HD or newly diagnosed suggest that cortical degeneration is a relatively late phenomenon in the course of the illness, occurring in a slow and patchy process. In addition, longitudinal studies of prodromal HD subjects (Kipps et al., 2005) show very little or only regionally specific gray matter loss in prodromal HD and/or early HD subjects, while the remaining cortex is unaffected.
Recent studies of cortical thinning in prodromal HD and diagnosed subjects have shown a similar pattern to the current study, with substantial thinning first in the posterior cerebrum (occipital lobe), then superior parietal, and superior frontal regions (Rosas et al., 2005; Tabrizi et al., 2009). Even as the disease progresses through diagnosis and areas of the temporal lobe and lateral frontal lobe become thin, there remains remarkable sparing of the medial and ventral aspects of the frontal and temporal lobe. These findings are consistent with one pathologic study in which cortical atrophy (cell count using planimetry) was found to be greatest in the occipital lobe and least in the frontal lobe (Lange, 1981). Given the circuitry of the basal ganglia, the cortical regions that appear spared (ventral and medial frontal and temporal lobe) are mostly among those that receive output projections from the basal ganglia to the cortex (Middleton and Strick, 1996a, 1996b, 2000). However, given the somewhat patchy nature of change in the cortex (some parts of the frontal lobe significantly affected, other parts spared), there appears to be a relative separation between striatal atrophy and cerebral cortical changes early in the disease process.
Also similar to the two other studies evaluating the thickness of the cortex in prodromal HD subjects (Rosas et al., 2005; Tabrizi et al., 2009), the current study found an increase in the volume and thickness of the rostral anterior cingulate in the Near and Diagnosed groups. As similar methods were used, one interpretation could be that the increased volume is related to a methodologic bias. However, in individuals far from onset of motor diagnosis, functional blood flow (fMRI) studies have found both decreased blood flow (Reading et al., 2004) and increased blood flow (Paulsen et al., 2004) in the anterior cingulate. Though these different patterns may be related to the cognitive context during neuroimaging, dysfunction in this brain area is seen early in the course of the disease. Of interest is that two recent studies have shown the anterior cingulate to behave different than any other region of the cortex in the context of aging (Abe et al., 2008; Salat et al., 2004). That is, whereas most cortical regions decrease in volume with age, the cingulate is either unchanged or increased in size with age. Since the prodromal HD subjects in the current study were compared against healthy controls, it suggests that this phenomenon of increased volume in the prodromal HD subjects as disease onset approached was above and beyond what is seen in the context of aging within the normal healthy group. The mechanisms by which this brain region, in contrast to other regions, would thicken substantially as the disease progresses are unclear.
Our study is the first to comprehensively examine cortical morphology in prodromal HD using volume, surface area, and thickness measures. Since volume is the product of surface area and thickness, these measures can have unique and independent contributions. Consistent with this notion are studies reporting cortical thickness and cortical surface area measures to be genetically independent (Panizzon et al., 2009; Winkler et al., 2009). In the current study, most of the brain regions that show significant volume decrement compared to controls seem to be lower due to a thinning of the cortex rather than a reduction in surface area. This is in contrast to the Winkler et al. study (2009), who measured cortical thickness and surface area in a large sample of randomly ascertained extended pedigrees of families and found that cortical volume was more closely related to surface area than cortical thickness (Winkler et al., 2009). The difference between our study and the Winkler study may be that the current study was an assessment of a disease process whereas the Winkler study was, in general, a sample of related subjects, not representative of a disease, but more representative of a healthy control sample. Manifest HD pathological studies (Hedreen et al., 1991; Selemon et al., 2004; Sotrel et al., 1991) have indicated relatively selective loss of neurons in deep cortical layers (especially layers V and VI, and some loss in layer III as well). These changes would be consistent with the thinning that we see. However cortical thinning could also represent morphologic changes in cell size, dendritic number and shape, and volume of glial components (DiProspero et al., 2004). Thus early changes might represent cellular abnormalities with or without substantial cell death. These early changes would be more likely to be reversible with therapeutics. Thus therapeutics begun during the prodromal HD period might be able to forestall further irreversible pathologic changes in cortex. Though these changes could in fact manifest in cognitive changes and other symptoms, they would more likely represent the earliest stages of dysfunction in a process that eventually leads to cell death.
Another important aspect to investigate further is the relationship between clinical symptoms and regions of cortex abnormality. In a recent pathologic study, cell loss in the primary motor strip was associated with movement abnormalities, while cell loss in the anterior cingulated was associated with significant mood symptoms in subjects with manifest disease (Thu et al, 2010). These are findings that, at first glance, may seem inconsistent with the current findings since the motor strip and the anterior cingulated appear ‘spared’ in some aspects compared to other regions of the brain that are affected. However, although the primary motor strip was normal in thickness, it had substantial reduction in surface area (and therefore volume), which could be correlated with cell loss. Moreover, although the anterior cingulate is enlarged, there is still possibility that this could represent morphology such as cell loss with accompanying gliosis. Finally, the Thu study was done on patients with manifest disease and therefore represent morphology that is most likely years advanced beyond the current study. Future work in which neuropathologic changes and neuroimaging changes can be assessed on patients in comparable time frames of disease would help shed light on whether MRI findings truly mirror that of histopathologic findings.
Cerebral white matter changes may potentially be considered as “secondary” to either striatal or cortical abnormality (functional deterioration or frank degeneration). Yet some have suggested that the white matter abnormalities reported in HD may be the result of a primary pathology in white matter rather than a secondary one (Bartzokis et al., 2007; Bartzokis et al., 2006). Findings from the current study may support the theory of a primary problem with white matter pathology. If the white matter changes in prodromal HD are seen as secondary cortical change, there should be a clear association between the regions of cortical change and the white matter associated with those regions. However in the current study, there is a lack of connection between the regions of the cortex that are abnormal and the regions of white matter that are abnormal. From the PREDICT-HD sample, a longitudinal analysis of brain morphology in prodromal HD subjects shows that white-matter volumes significantly decrease far from onset (greater than 15 years from diagnosis), and although the changes are throughout the cerebrum, they are most prominent in the frontal lobes (Aylward et al., forthcoming). This is in contrast to the current analysis that shows cortical thinning mostly in the posterior regions with no appreciable change in relatively large parts of the frontal lobes (ventral and medial). This further supports the notion that the changes in cerebral white matter may be a primary process rather than secondary to structural changes in the cortex or striatum (Bartzokis et al., 2007; Bartzokis et al., 2006).
In summary, the current study evaluates extensively the cortical morphology of prodromal HD subjects. Changes in the cortex of prodromal HD subjects appear to occur much later in the disease process (within a decade or nearer to onset) compared to changes in the striatum or white matter (seen as far out as greater than 15 years from onset). When changes are apparent, they appear as cortical thinning (rather than decrement in surface area) in mostly superior and posterior regions. Ventral and medial aspects of the frontal and temporal lobes remain largely unchanged even throughout the progression to formal clinical diagnosis of the cortical changes early in the course of the disease may be amenable to therapeutic intervention and seem to be unrelated to changes in white matter.
Research Highlights.
Changes of cerebral cortex are detected in subjects up to a decade prior to onset.
Structural changes are mostly in thinning rather than in reduction of surface area.
Affected areas include superior and posterior cerebrum while ventral frontal is spared.
Acknowledgments
This research is supported by the National Institutes for Health, National Institute of Neurological Disorders and Stroke (NS40068) and CHDI Foundation, Inc. We thank the PREDICT-HD sites, the study participants, and the National Research Roster for Huntington Disease Patients and Families.
Appendix
PREDICT-HD Investigators, Coordinators, Motor Raters, Cognitive Raters
January 5, 2010
Peg Nopoulos, MD, Robert Rodnitzky, MD, Ergun Uc, MD, BA, Leigh J. Beglinger, PhD, Vincent A. Magnotta, PhD, Stephen Cross, BA, Nicholas Doucette, BA, Andrew Juhl, BS, Jessica Schumacher, BA, Mycah Kimble, BA, Pat Ryan, MS, MA, Jessica Wood, MD, PhD, Eric Epping, MD, PhD, Thomas Wassink, MD, and Teri Thomsen, MD (University of Iowa Hospitals and Clinics, Iowa City, Iowa, USA);
David Ames, MD, Edmond Chiu, MD, Phyllis Chua, MD, Olga Yastrubetskaya, PhD, Joy Preston, Anita Goh, D. Psych, and Angela Komiti, BS, MA (The University of Melbourne, Kew, Victoria, Australia);
Lynn Raymond, MD, PhD, Rachelle Dar Santos, BSc, Joji Decolongon, MSC, and David Weir, BSc (University of British Columbia, Vancouver, British Columbia, Canada);
Adam Rosenblatt, MD, Christopher A. Ross, MD, PhD, Barnett Shpritz, BS, MA, OD, and Claire Welsh (Johns Hopkins University, Baltimore, Maryland, USA);
William M. Mallonee, MD and Greg Suter, BA (Hereditary Neurological Disease Centre, Wichita, Kansas, USA);
Ali Samii, MD, Hillary Lipe, ARNP, and Kurt Weaver, PhD (University of Washington and VA Puget Sound Health Care System, Seattle, Washington, USA);
Randi Jones, PhD, Cathy Wood-Siverio, MS, Stewart A. Factor, DO, and Claudia Testa, MD, PhD (Emory University School of Medicine, Atlanta, Georgia, USA);
Roger A. Barker, BA, MBBS, MRCP, Sarah Mason, BSC, Anna Goodman, PhD, and Anna DiPietro (Cambridge Centre for Brain Repair, Cambridge, UK);
Elizabeth McCusker, MD, Jane Griffith, RN, and Kylie Richardson, PhD (Westmead Hospital, Sydney, Australia);
Bernhard G. Landwehrmeyer, MD, Daniel Ecker, MD, Patrick Weydt, MD, Michael Orth MD, PhD, Sigurd Süβmuth, MD, RN, Katrin Barth, RN, and Sonja Trautmann, RN (University of Ulm, Ulm, Germany);
Kimberly Quaid, PhD, Melissa Wesson, MS, and Joanne Wojcieszek, MD (Indiana University School of Medicine, Indianapolis, IN);
Mark Guttman, MD, Alanna Sheinberg, BA, Adam Singer, and Janice Stober, BA, BSW (Centre for Addiction and Mental Health, University of Toronto, Markham, Ontario, Canada);
Susan Perlman, MD and Arik Johnson, PsyD (University of California, Los Angeles Medical Center, Los Angeles, California, USA);
Michael D. Geschwind, MD, PhD and Jon Gooblar, BA (University of California San Francisco, California, USA);
Tom Warner, MD, PhD, Stefan Kloppel, MD, Maggie Burrows, RN, BA, Marianne Novak, MD, Thomasin Andrews, MD, BSC, MRCP, Elisabeth Rosser, MBBS, FRCP, and Sarah Tabrizi, BSC, PhD (National Hospital for Neurology and Neurosurgery, London, UK);
Anne Rosser, MD, PhD, MRCP and Kathy Price, RN (Cardiff University, Cardiff, Wales, UK);
Amy Chesire, LCSW-R, MSG, Frederick Marshall, MD, and Mary Wodarski, BA (University of Rochester, Rochester, New York, USA);
Oksana Suchowersky, MD, FRCPC, Sarah Furtado, MD, PhD, FRCPC, and Mary Lou Klimek, RN, BN, MA (University of Calgary, Calgary, Alberta, Canada);
Peter Panegyres, MB, BS, PhD, Carmela Connor, BP, MP, DP, and Elizabeth Vuletich, BSC (Neurosciences Unit, Graylands, Selby-Lemnos & Special Care Health Services, Perth, Australia);
Joel Perlmutter, MD and Stacey Barton, MSW, LCSW (Washington University, St. Louis, Missouri, USA);
Sheila A. Simpson, MD and Daniela Rae, RN (Clinical Genetics Centre, Aberdeen, Scotland, UK);
David Craufurd, MD, Ruth Fullam, BSC, and Elizabeth Howard, MD (University of Manchester, Manchester, UK)
Pietro Mazzoni, MD, PhD, Karen Marder, MD, MPH, Carol Moskowitz, MS, and Paula Wasserman, MA (Columbia University Medical Center, New York, New York, USA);
Diane Erickson, RN, Dawn Miracle, BS, MS, and Rajeev Kumar, MD (Colorado Neurological Institute, Englewood, Colorado, USA);
Vicki Wheelock, MD, Terry Tempkin, RNC, MSN, Nicole Mans, BA, MS, and Kathleen Baynes, PhD (University of California Davis, Sacramento, California, USA);
Joseph Jankovic, MD, Christine Hunter, RN, CCRC, and William Ondo, MD (Baylor College of Medicine, Houston, Texas, USA);
Justo Garcia de Yebenes, MD, Monica Bascunana Garde, Marta Fatas, BA, and Jose Luis Lópenz Sendon, MD (Hospital Ramón y Cajal, Madrid, Spain);
Martha Nance, MD, Dawn Radtke, RN, and David Tupper, PhD (Hennepin County Medical Center, Minneapolis, Minnesota, USA);
Wayne Martin, MD, Pamela King, BScN, RN, and Satwinder Sran, BSC (University of Alberta, Edmonton, Alberta, Canada);
Anwar Ahmed, PhD, Stephen Rao, PhD, Christine Reece, BS, Janice Zimbelman, PhD, PT, Alexandra Bea, BA, and Emily Newman, BA (Cleveland Clinic Foundation, Cleveland, Ohio, USA);
Steering Committee
Jane Paulsen, PhD, Principal Investigator, Eric Epping, MD, PhD, Douglas Langbehn, MD, PhD, Hans Johnson, PhD, Megan Smith, PhD, Janet Williams, PhD, RN, FAAN (University of Iowa Hospitals and Clinics, Iowa City, IA); Elizabeth Aylward, PhD (Seattle Children’s Research Institute, WA); Kevin Biglan, MD (University of Rochester, Rochester, NY); Blair Leavitt, MD (University of British Columbia, Vancouver, BC, Canada); Marcy MacDonald, PhD (Massachusetts General Hospital); Martha Nance, MD (Hennepin County Medical Center, Minneapolis, MN); Jean Paul Vonsattel, PhD (Columbia University Medical Center, New York, NY).
Scientific Sections
Bio Markers: Blair Leavitt, MDCM, FRCPC (Chair) and Michael Hayden, PhD (University of British Columbia); Stefano DiDonato, MD (Neurological Insitute “C. Besta,” Italy); Ken Evans, PhD (Ontario Cancer Biomarker Network); Wayne Matson, PhD (VA Medical Center, Bedford, MA); Asa Peterson, MD, PhD (Lund University, Sweden), Sarah Tabrizi, PhD (National Hospital for Neurology and Neurology and Neurosurgery, London).
Cognitive: Deborah Harrington, PhD (Chair, University of California, San Diego), Tamara Hershey, PhD and Desiree White, PhD (Washington University Cognitive Science Battery Development); Holly Westervelt, PhD, Jennifer Davis, PhD, Pete Snyder, PhD, and Geoff Tremont, PhD, MS (Chair, Quality Control and Training, Brown University); Megan Smith, PhD (Chair, Administration), David J. Moser, PhD, Leigh J. Beglinger, PhD (University of Iowa); Lucette Cysique, PhD (St. Vincent’s/University of Melbourne, Australia); Carissa Gehl, PhD (VA Medical Center, Iowa City, IA); Robert K. Heaton, PhD, David Moore, PhD, Joanne Hamilton, PhD, and David Salmon, PhD (University of California, San Diego); Kirsty Matheson (University of Aberdeen); Paula Shear, PhD (University of Cincinnati); Karen Siedlecki, PhD (Fordham University); Glenn Smith, PhD (Mayo Clinic); and Marleen Van Walsem (EHDN).
Functional Assessment: Janet Williams, PhD (Co-Chair), Leigh J. Beglinger, PhD, Anne Leserman, MSW, LISW, Justin O’Rourke, MA, Bradley Brossman, MA, Eunyoe Ro, MA (University of Iowa); Rebecca Ready, PhD (University of Massachusetts); Anthony Vaccarino, PhD (Ontario Cancer Biomarker Network); Sarah Farias, PhD (University of California, Davis); Noelle Carlozzi, PhD (Kessler Medical Rehabilitation Research & Education Center); and Carissa Gehl, PhD (VA Medical Center, Iowa City, IA).
Genetics: Marcy MacDonald, PhD (Co-Chair), Jim Gusella, PhD, and Rick Myers, PhD (Massachusetts General Hospital); Michael Hayden, PhD (University of British Columbia); Tom Wassink, MD (Co-Chair) and Eric Epping, MD, PhD (University of Iowa).
Imaging
Administrative: Ron Pierson, PhD (Chair), Kathy Jones, BS, Jacquie Marietta, BS, William McDowell, AA, Steve Dunn, BA, Greg Harris, BS, Eun Young Kim, MS, and Yong Qiang Zhao, PhD (University of Iowa); John Ashburner, PhD (Functional Imaging Lab, London); Vince Calhoun, PhD (University of New Mexico); Steve Potkin, MD (University of California, Irvine); Klaas Stephan, MD, PhD (University College of London); and Arthur Toga, PhD (University of California, Los Angeles).
Striatal: Elizabeth Aylward, PhD (Chair, Seattle Children’s Research Institute) and Kurt Weaver, PhD (University of Washington and VA Puget Sound Health Care System, Seattle, Washington).
Surface Analysis: Peg Nopoulos, MD (Chair), Eric Axelson, BSE, and Jeremy Bockholt, BS (University of Iowa).
Shape Analysis: Christopher A. Ross (Chair), MD, PhD, Michael Miller, PhD, and Sarah Reading, MD (Johns Hopkins University); Mirza Faisal Beg, PhD (Simon Fraser University).
DTI: Vincent A. Magnotta, PhD (Chair, University of Iowa); Karl Helmer, PhD (Massachusetts General Hospital); Kelvin Lim, MD (University of Ulm, Germany); Mark Lowe, PhD (Cleveland Clinic); Sasumu Mori, PhD (Johns Hopkins University); Allen Song, PhD (Duke University); and Jessica Turner, PhD (University of California, Irvine).
fMRI: Steve Rao, PhD (Chair), Erik Beall, PhD, Katherine Koenig, PhD, Mark Lowe, PhD, Michael Phillips, MD, Christine Reece, BS, and Jan Zimbelman, PhD, PT (Cleveland Clinic).
Motor: Kevin Biglan, MD (University of Rochester), Karen Marder, MD (Columbia University), and Jody Corey-Bloom, MD, PhD (University of California, San Diego) all Co-Chairs; Michael Geschwind, MD, PhD (University of California, San Francisco); and Ralf Reilmann, MD (Muenster, Germany).
Psychiatric: Eric Epping, MD, PhD (Chair), Nancy Downing, RN, MSN, Jess Fedorowicz, MD, Robert Robinson, MD, and Megan Smith, PhD (University of Iowa); Karen Anderson, MD (University of Maryland); David Craufurd, MD (Manchester University); Mark Groves, MD (Columbia University); Anthony Vaccarino, PhD and Ken Evans, PhD (Ontario Cancer Biomarker Network); Hugh Rickards, MD (Queen Elizabeth Psychiatric Hospital); and Eric van Duijn, MD (Leiden University Medical Center, Netherlands).
Core Sections
Statistics: Douglas Langbehn, MD, PhD (Chair) and James Mills, MEd, MS (University of Iowa); and David Oakes, PhD (University of Rochester).
Recruitment/Retention: Martha Nance, MD (Chair, University of Minnesota); Anne Leserman, MSW, LISW, Stacie Vik, BA, Christine Anderson, BA, Nick Doucette, BA, Kelly Herwig, BA, MS, Mycah Kimble, BA, Pat Ryan, MSW, LISW, MA, Jessica Schumacher, BA, Kelli Thumma, BA, and Elijah Waterman, BA (University of Iowa); and Norm Reynolds, MD (University of Wisconsin, Milwaukee).
Ethics: Cheryl Erwin, JD, PhD, (Chair, McGovern Center for Health, Humanities and the Human Spirit); Eric Epping, MD, PhD and Janet Williams, PhD (University of Iowa); and Martha Nance, MD (University of Minnesota).
IT/Management: Hans Johnson, PhD (Chair), R.J. Connell, BS, Paul Allen, AASC, Sudharshan Reddy Bommu, MS, Karen Pease, BS, Ben Rogers, BA, BSCS, Jim Smith, AS, Kent Williams, BSA, MCS, MS, Shuhua Wu, MCS, and Roland Zschiegner (University of Iowa).
Program Management
Administrative: Chris Werling-Witkoske (Chair), Karla Anderson, BS, Kristine Bjork, BA, Ann Dudler, Stacey Jones, BS, Jamy Schumacher, Sean Thompson, BA (University of Iowa).
Financial: Steve Blanchard, MSHA (Co-Chair), Machelle Henneberry, and Kelsey Montross, BA (University of Iowa).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Peggy C. Nopoulos, Email: peggy-nopoulos@uiowa.edu.
Elizabeth H. Aylward, Email: elizabeth.aylward@seattlechildrens.org.
Christopher A. Ross, Email: caross@jhu.edu.
Hans J. Johnson, Email: hans-johnson@uiowa.edu.
Vincent A. Magnotta, Email: vincent-magnotta@uiowa.edu.
Andrew R. Juhl, Email: andrew-juhl@uiowa.edu.
Ronald K. Pierson, Email: ronald-pierson@uiowa.edu.
James Mills, Email: jamills@healthcare.uiowa.edu.
Douglas R. Langbehn, Email: douglas-langbehn@uiowa.edu.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, et al. Aging in the CNS: comparison of gray/white matter volume and diffusion tensor data. Neurobiol Aging. 2008;29(1):102–16. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Nopoulos P, Ross CA, Langbehn DR, Pierson R, Mills J, et al. Longitudinal change in regional brain volumes in preclinical Huntington disease. J Neurol Neurosurg Psychiatry. Forthcoming doi: 10.1136/jnnp.2010.208264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Anderson NB, Bylsma FW, Wagster MV, Barta PE, Sherr M, et al. Frontal lobe volume in patients with Huntington’s disease. Neurology. 1998;50(1):252–8. doi: 10.1212/wnl.50.1.252. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tishler TA, Fong SM, Oluwadara B, Finn JP, et al. Myelin breakdown and iron changes in Huntington’s disease: pathogenesis and treatment implications. Neurochem Res. 2007;32(10):1655–64. doi: 10.1007/s11064-007-9352-7. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tishler TA, Perlman S. In vivo assessment of iron in Huntington’s disease and other age-related degenerative brain diseases. In: Sigel A, Sigel H, Sigel RKO, editors. Metal Ions in life sciences. Chichester: Wiley; 2006. pp. 151–77. [Google Scholar]
- Beglinger LJ, Nopoulos PC, Jorge RE, Langbehn DR, Mikos AE, Moser DJ, et al. White matter volume and cognitive dysfunction in early Huntington’s disease. Cogn Behav Neurol. 2005;18(2):102–7. doi: 10.1097/01.wnn.0000152205.79033.73. [DOI] [PubMed] [Google Scholar]
- Ciarmiello A, Cannella M, Lastoria S, Simonelli M, Frati L, Rubinsztein DC, et al. Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington’s disease. J Nucl Med. 2006;47(2):215–22. [PubMed] [Google Scholar]
- Dale A, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5:162–76. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- DiProspero NA, Chen EY, Charles V, Plomann M, Kordower JH, Tagle DA. Early changes in Huntington’s disease patient brains involve alterations in cytoskeletal and synaptic elements. J Neurocytol. 2004;33(5):517–33. doi: 10.1007/s11068-004-0514-8. [DOI] [PubMed] [Google Scholar]
- Douaud G, Gaura V, Ribeiro MJ, Lethimonnier F, Maroy R, Verny C, et al. Distribution of grey matter atrophy in Huntington’s disease patients: a combined ROI-based and voxel-based morphometric study. Neuroimage. 2006;32(4):1562–75. doi: 10.1016/j.neuroimage.2006.05.057. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Archibald SL, Jacobson MW, Corey-Bloom J, Paulsen JS, Peavy GM, et al. In vivo evidence of cerebellar atrophy and cerebral white matter loss in Huntington disease. Neurology. 2004;63(6):989–95. doi: 10.1212/01.wnl.0000138434.68093.67. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8(4):272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180–94. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, et al. Improving tissue segmentation in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr. 1999;23:144–54. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- Hedreen JC, Peyser CE, Folstein SE, Ross CA. Neuronal loss in layers V and VI of cerebral cortex in Huntington’s disease. Neurosci Lett. 1991;133(2):257–61. doi: 10.1016/0304-3940(91)90583-f. [DOI] [PubMed] [Google Scholar]
- Heinsen H, Strik M, Bauer M, Luther K, Ulmar G, Gangnus D, et al. Cortical and striatal neurone number in Huntington’s disease. Acta Neuropathol (Berl) 1994;88(4):320–33. doi: 10.1007/BF00310376. [DOI] [PubMed] [Google Scholar]
- Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72(6):971–83. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Huntington Study Group. Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11(2):136–42. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Salmon DP, Butters N, Hesselink JR. Cerebral structure on MRI, Part II: Specific changes in Alzheimer’s and Huntington’s diseases. Biol Psychiatry. 1991;29(1):68–81. doi: 10.1016/0006-3223(91)90211-4. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Duggins AJ, Mahant N, Gomes L, Ashburner J, McCusker EA. Progression of structural neuropathology in preclinical Huntington’s disease: a tensor based morphometry study. J Neurol Neurosurg Psychiatry. 2005;76(5):650–5. doi: 10.1136/jnnp.2004.047993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60(9):878–88. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;65(4):267–77. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- Langbehn DR, Hayden MR, Paulsen JS. CAG-repeat length and the age of onset in Huntington disease (HD): A review and validation study of statistical approaches. Am J Med Genet Part B. 2009;153B:397–408. doi: 10.1002/ajmg.b.30992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange HW. Quantitative changes of telencephalon, diencephalon, and mesencephalon in Huntingon’s chorea, postecephalitic, and adiopathic parkinsonism. Verh Anat Ges. 1981;75:923–5. [Google Scholar]
- Magnotta V, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26(4):251–64. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar output influences non-motor function. Mol Psychiatry. 1996a;1(6):429–33. [PubMed] [Google Scholar]
- Middleton FA, Strick PL. The temporal lobe is a target of output from the basal ganglia. Proc Natl Acad Sci U S A. 1996b;93(16):8683–7. doi: 10.1073/pnas.93.16.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31(2–3):236–50. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Molero AE, Gokhan S, Gonzalez S, Feig JL, Alexandre LC, Mehler MF. Impairment of developmental stem cell-mediated striatal neurogenesis and pluripotency genes in a knock-in model of Huntington’s disease. Proc Natl Acad Sci U S A. 2010;106(51):21900–5. doi: 10.1073/pnas.0912171106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlau M, Weindl A, Wohlschlager AM, Gaser C, Stadtler M, Valet M, et al. Voxel-based morphometry indicates relative preservation of the limbic prefrontal cortex in early Huntington disease. J Neural Transm. 2007;114(3):367–72. doi: 10.1007/s00702-006-0571-x. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Magnotta VA, Mikos A, Paulson H, Andreasen NC, Paulsen JS. Morphology of the cerebral cortex in preclinical Huntington’s disease. Am J Psychiatry. 2007;164(9):1428–34. doi: 10.1176/appi.ajp.2007.06081266. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19(11):2728–35. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Hayden M, Stout JC, Langbehn DR, Aylward E, Ross CA, et al. Preparing for preventive clinical trials: the Predict-HD study. Arch Neurol. 2006;63(6):883–90. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, et al. Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79(8):874–80. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Nopoulos P, Aylward E, Ross CA, Johnson H, Magnotta VA, et al. Striatal and white matter predictors of estimated diagnosis for Huntington disease. Brain Res Bull. 2010;82:201–7. doi: 10.1016/j.brainresbull.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Zimbelman JL, Hinton SC, Langbehn DR, Leveroni CL, Benjamin ML, et al. An fMRI biomarker of early neuronal dysfunction in presymptomatic Huntington’s disease. AJNR Am J Neuroradiol. 2004;25(10):1715–21. [PMC free article] [PubMed] [Google Scholar]
- Reading SA, Dziorny AC, Peroutka LA, Schreiber M, Gourley LM, Yallapragada V, et al. Functional brain changes in presymptomatic Huntington’s disease. Ann Neurol. 2004;55(6):879–83. doi: 10.1002/ana.20121. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65(5):745–7. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Koroshetz WJ, Chen YI, Skeuse C, Vangel M, Cudkowicz ME, et al. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2003;60(10):1615–20. doi: 10.1212/01.wnl.0000065888.88988.6e. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58(5):695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26(4):518–29. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Evidence for progression in frontal cortical pathology in late-stage Huntington’s disease. J Comp Neurol. 2004;468(2):190–204. doi: 10.1002/cne.10938. [DOI] [PubMed] [Google Scholar]
- Sotrel A, Paskevich PA, Kiely DK, Bird ED, Williams RS, Myers RH. Morphometric analysis of the prefrontal cortex in Huntington’s disease. Neurology. 1991;41(7):1117–23. doi: 10.1212/wnl.41.7.1117. [DOI] [PubMed] [Google Scholar]
- Styner M, Brechbuhler C, Szekely G, Gerig G. Parametric estimate of intensity inhomogeneities applied to MRI. IEEE Trans Med Imaging. 2000;19(3):153–5. doi: 10.1109/42.845174. [DOI] [PubMed] [Google Scholar]
- Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, et al. Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8(9):791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu DC, Oorschot DE, Tippett LJ, Nana AL, Hogg VM, Synek BJ, et al. Cell loss in the motor and cingulate cortex correlates with symptomatology in Huntington’s disease. Brain. 2010;133(Pt 4):1094–110. doi: 10.1093/brain/awq047. [DOI] [PubMed] [Google Scholar]
- Wagster MV, Hedreen JC, Peyser CE, Folstein SE, Ross CA. Selective loss of [3H]kainic acid and [3H]AMPA binding in layer VI of frontal cortex in Huntington’s disease. Exp Neurol. 1994;127(1):70–5. doi: 10.1006/exnr.1994.1081. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]