Abstract
Anxiety disorders are frequently long-lasting and debilitating for more than 40 million American adults. Although stressor exposure plays an important role in the etiology of some anxiety disorders, the mechanisms by which exposure to stressful stimuli alters central circuits that mediate anxiety-like emotional behavior are still unknown. Substantial evidence has implicated regions of the central extended amygdala, including the bed nucleus of the stria terminalis (BNST) and the central nucleus of the amygdala (CeA) as critical structures mediating fear- and anxiety-like behavior in both humans and animals. These areas organize coordinated fear- and anxiety-like behavioral responses as well as peripheral stress responding to threats via direct and indirect projections to the paraventricular nucleus of the hypothalamus (PVN) and brainstem regions (Walker et al., 2003; Ulrich-Lai and Herman, 2009; Walker et al., 2009). In particular, the BNST has been argued to mediate these central and peripheral responses when the perceived threat is of long duration (Waddell et al., 2006) and/or when the anxiety-like response is sustained (Walker and Davis, 2008); hence, the BNST may mediate pathological anxiety-like states that result from exposure to chronic stress. Indeed, chronic stress paradigms result in enhanced BNST neuroplasticity that has been associated with pathological anxiety-like states (Vyas et al., 2003; Pego et al., 2008). Here we review evidence that suggests that pituitary adenylate cyclase activating polypeptide (PACAP) and corticotropin-releasing hormone (CRH) work together to modulate BNST function and increase anxiety-like behavior. Moreover, we have shown that BNST PACAP as well as its cognate PAC1 receptor are substantially upregulated following chronic stress, particularly in the BNST oval nucleus where PACAP-containing neurons closely interact with CRH-containing neurons (Kozicz et al., 1997; Hammack et al., 2009). We describe how interactions between PACAP and CRH in the BNST may mediate stress-associated behaviors, including anorexia and anxiety-like behavior. These studies have the potential to define specific mechanisms underlying anxiety disorders, and may provide important therapeutic strategies for stress- and anxiety-management.
Keywords: pituitary adenylate cyclase activating polypeptide (PACAP), corticotropin releasing hormone (CRH), bed nucleus of the stria terminalis (BNST), amygdala, chronic stress
Stress
The term "stress" has different meanings in the scientific literature, but is generally used to delineate any threat to an organism’s homeostasis. Hence, even manipulations that are associated with positive outcomes (such as exercise) can be considered stressors because they alter the resting state of the body. Homeostatic threats produce peripheral catabolic endocrine and autonomic stress responses through activation of corticotropin-releasing hormone (CRH)-containing neurons in the paraventricular nucleus (PVN) of the hypothalamus, which may help provide the organism with the energy required to cope with homeostatic challenges and/or may serve to regulate stress responses after they have been initiated (Sapolsky et al., 2000; Ulrich-Lai and Herman, 2009). Specific stressor types initiate the activation of the PVN via different brain regions. "Processive" or "exteroceptive" stressors, threats that require limbic structures, can activate peripheral stress response systems via projections to the PVN, and also stimulate central (behavioral) stress responding via extrahypothalamic projections to brain nuclei associated with fear- and anxiety-like behaviors (Herman et al., 1996; Ulrich-Lai and Herman, 2009; Figure 1). Notably, two CRH-rich areas that mediate responding to processive/exteroceptive stressors include the bed nucleus of the stria terminalis (BNST) and the central nucleus of the amygdala (CeA; Figure 2). Activation of either the BNST or CeA has been argued to coordinate a constellation of appropriate behavioral responses to cope with the perceived threat, while at the same time engaging peripheral catabolic systems that support these behavioral changes. Hence, both the CeA and BNST have been implicated in mediating the stress response as well as being critical for affective (fear- and anxiety-like) behavior. Unlike the CeA, which has been implicated to respond to short-duration (phasic) and perhaps predictable threats, the BNST has been argued to mediate long-term (tonic) anxiety-like responding to long-duration, diffuse and/or unpredictable negatively valenced threats (Walker et al., 2003; Waddell et al., 2006; Walker and Davis, 2008; Walker et al., 2009). Chronic exposure to negatively valenced processive stressors alters BNST function and leads to changes in anxiety-like behavior (Pego et al., 2008). Because chronic exposures to negatively valenced stressors are a critical component in the etiology of many anxiety disorders, understanding the mechanisms by which stress alters the BNST is critical in determining the relationships between stressor exposure and emotion.
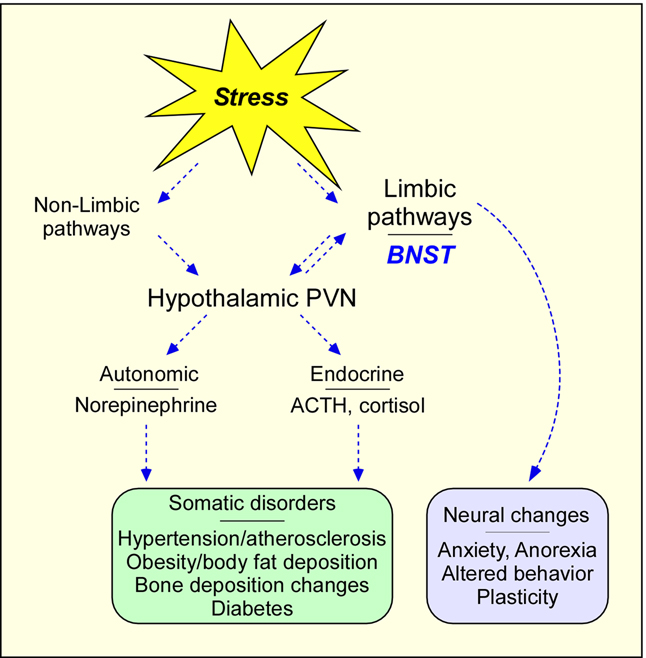
Figure 1. Stress pathway schematic.
Stress activates both limbic and nonlimbic pathways, which can converge on the paraventricular nucleus (PVN) of the hypothalamus for endocrine and autonomic responses. Stress-mediated activation limbic BNST either directly, or indirectly via the PVN, contribute to anxiety behavior. Chronic stress insults can result in long term neuroplasticity changes leading to anxiety and somatic disorders
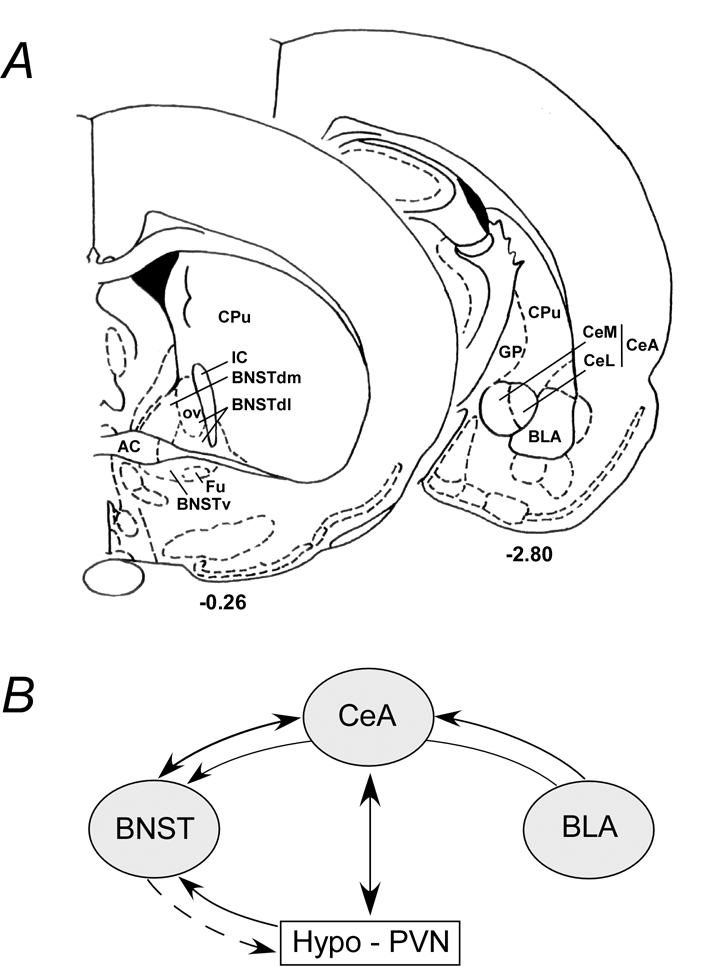
Figure 2. Schematic of extended amygdala areas and interactive pathways.
A) Simplified rat coronal sections illustrating areas within the bed nucleus of stria terminalis (BNST, bregma −0.26) and the amygdala (bregma −2.80). The BNST oval nucleus (OV) in the dorsolateral BNST (BNSTdl) is illustrated; the anterolateral BNST encompasses the BNSTdl and may include the dorsomedial BNST (BNSTdm). Similarly, the two largest components of the central nucleus of the amygdala (CeA) are the medial (CeM) and lateral (CeL) divisions. CPu, caudate putamen; GP, globus pallidus; IC, internal capsule; BNSTv, ventral BNST; Fu, fusiform nucleus; BLA, basolateral amygdala. B) The BNST and CeA have reciprocal projections. The BLA projects not only to the CeA but have en passant fibers that can reach the BNST. The reciprocal hypothalamic (PVN) and CeA projections are best described although direct PVN fibers can be identified in the BNST. The BNST can influence hypothalamic function via direct or indirect neural pathways (dashed arrow).
The bed nucleus of the stria terminalis (BNST)
The BNST is a complex brain region and has historically been divided into anterior and posterior regions by the fibers of the stria terminalis, and further divided into medial and lateral subdivisions (Ju and Swanson, 1989; Ju et al., 1989). Swanson and colleagues have defined as many as 30 subdivisions based on cytoarchitecture, chemoarchitecture and connectivity (Dong et al., 2001). However, one anterolateral BNST region of particular note is the oval nucleus (Figure 2); the oval nucleus is activated by stressors, demonstrates high levels of PACAP and CRH expression, and is associated with affective behavior (Kozicz et al., 1997; Day et al., 1999; Walker et al., 2003; Funk et al., 2006).
The BNST and peripheral stress responding
As discussed above, threats to homeostasis activate many CNS-coordinated stress responses (Figure 1). The neuroendocrine stress response is initiated by increased hypothalamic PVN CRH biosynthesis and secretion into the pituitary portal system, which in turn elicits the release of anterior pituitary gland adrenocorticotropic hormone (ACTH) and downstream release of glucocorticoids from the adrenal cortex. Hypothalamic CRH neurons also regulate the autonomic stress response via fiber projections to brainstem and lower spinal nuclei (Arborelius et al., 1999; Ulrich-Lai and Herman, 2009); the activation of catecholaminergic sympathetic fibers results in the peripheral release of these catabolic neurotransmitters. The measurement of plasma glucocorticoid and catecholamine levels as end-points has long been used to confirm the stressful nature of experimental manipulations. Critically, CRH signaling from the PVN appears to be a central regulator of both the endocrine and autonomic pathways which provide the organism with coping strategies in the face of homeostatic threats (Herman and Cullinan, 1997; Ulrich-Lai and Herman, 2009).
Depending on the nature of the stressor, the hypothalamic PVN, in turn, can be driven by different upstream neural pathways (Figure 1). At least two pathways have been described by Herman and colleagues (Herman et al., 1996). The limbic-insensitive pathway regulates PVN responses to "systemic or interoceptive" stressors that include immediate and threatening visceral changes, such as respiratory and cardiovascular distress. These stimuli activate a direct pathway to the PVN and do not require higher-level cognitive processing associated with limbic brain regions. The limbic-sensitive pathway, by contrast, mediates PVN responses to "processive or exteroceptive" stressors and often requires not only higher-level central integration of sensory modalities, but also comparisons with prior experiences to determine the salience/valence of the perceived threat. The limbic structures that project to and stimulate the PVN include the hippocampus, medial prefrontal cortex, amygdala and BNST (Herman et al., 2005) (Figure 2). Among these regions, the BNST has intimate and direct connections with the PVN, and the anterolateral BNST stimulates PVN and stress responding (Dunn, 1987; Gray et al., 1993; Herman et al., 1994; Crane et al., 2003). Stimulation of the anterolateral BNST increases plasma corticosterone (Dunn, 1987), anterior BNST lesions reduce PVN CRH levels (Herman et al., 1994), and anterolateral BNST lesions attenuate prolactin, ACTH and corticosterone levels following a conditioned stress paradigm (Gray et al., 1993). While the BNST oval nucleus has few direct projections to the PVN (Dong and Swanson, 2001) this region likely increases PVN activity indirectly via projections to other BNST subregions. The posterior and medial regions of the BNST can elicit opposite effects (Dunn, 1987; Herman et al., 1994), reducing PVN activity, suggesting that neuronal projections from different BNST subregions can elicit distinct stress responses. These results demonstrate the importance of the BNST in the integration and modulation of PVN activity. Perhaps more interestingly, as reviewed below, the role of the BNST in stress is even broader to encompass anxiety-like behavior to negatively-valenced, diffuse and unpredictable threatening stimuli (Figure 1, right side) (Walker et al., 2003).
The BNST and anxiety-like behavior
In response to perceived threat, mammals display an array of defense behaviors that are adaptive for survival and the maintenance of homeostasis. The defensive responses are species- and stimulus-specific, centrally coordinated, and are often associated with changes in affect/motivation (Blanchard et al., 2003). The behavioral indices of emotion have been well studied; the defensive behaviors in rodents, such as freezing, avoidance of brightly lit areas, defensive withdrawal, and shock-probe burying, and the startle response are routinely measured and have been associated with changes in fear and anxiety-like responding (Davis, 1986; Fanselow and Helmstetter, 1988; Kliethermes, 2005; Legradi et al., 2007). Consistent with this interpretation, experimental manipulations such as the use of m-chlorophenylpiperazine (mCPP) and benzodiazepines to increase and decrease, respectively, the experience of fear and anxiety in humans also alter the expression of these defensive behaviors in rodents. Hence, the behavioral response to homeostatic challenge likely reflects changes in emotional state, particularly in fear and anxiety. Some of the underlying neurocircuits mediating stressor-responding coincide with the neurocircuitry mediating defensive responses, and these structures (such as the BNST) appear to be critical nodes for mediating stress-induced fear- and anxiety-like behavior. Hence, chronic exposure to stressors may produce maladaptive changes within these structures, and these changes likely underlie anxiety disorders in humans.
While many CNS regions may participate in behavioral responses observed after stressor exposure, some forebrain nuclei have been argued to coordinate defensive responding in a manner similar to that described above. Of these forebrain areas, the central extended amygdala has garnered significant attention in mediating these behaviors in a variety of paradigms (Davis and Shi, 1999). The central extended amygdala includes the CeA and the anatomically-related BNST (Figure 2). As noted above, the BNST has been implicated in certain fear and anxiety-like behaviors that are not mediated by the CeA. Davis and colleagues (1997) initially found that lesions of BNST cell bodies blocked the enhanced startle responding (a behavioral measure of fear/anxiety) observed after central administration of CRH, whereas CeA lesions did not (Lee and Davis, 1997). Other fear-like behaviors that have subsequently been shown to be BNST-mediated include startle responding enhanced by bright light (Walker and Davis, 2002a, b), freezing behavior induced by predator odor (Fendt et al., 2003), fear responding to long-duration conditioned stimuli (Waddell et al., 2006), contextual fear conditioning (Sullivan et al., 2004), and the anxiogenic behavioral changes observed after uncontrollable stress (Hammack et al., 2004). Importantly, the BNST and CeA both receive substantial afferent information from the basolateral amygdala (BLA, Figure 2), and both project to many of the same regions involved in mediating individual fear responses (Walker et al., 2003). Based on these data, Davis and colleagues (2003, 2009, 2010) have suggested that the BNST mediates a "slower or sluggish" tonic fear response system that mediates behavioral responding to diffuse stimuli that persists long after the stimulus has terminated. By contrast, the CeA appears to mediate a rapid phasic response system to specific threat that dissipates when the threat is removed. Davis and colleagues have likened the former to an "anxiety" response system and have suggested that the maladaptive responding of the BNST may underlie some forms of anxiety disorders in humans, including post traumatic stress disorder (PTSD). Electrical stimulation of the anterolateral BNST region produces many of the endocrine, cardiovascular and respiratory responses that are elicited by anxiogenic stimuli (Casada and Dafny, 1991). Moreover, anxiogenic pharmacological agents, such as yohimbine and m-chlorophenylpiperazine (mCPP), increase BNST expression of activation markers such as c-fos (Singewald et al., 2003). The data implicating the BNST in the response to stressor exposure are not limited to rodents. Kalin et al. (2005) showed that increased BNST activity in rhesus monkeys was most positively correlated with individual differences in freezing behavior in response to eye contact with an intruder, which in this species represents a threatening stimulus (Kalin et al., 2005). The BNST has also been linked to fear behavior in humans; Straube et al. (2007) found that BNST activation was increased in spider phobic humans when they anticipated the presentation of an artificial spider (Straube et al., 2007). Notably, substantial evidence suggests that BNST activity mediates anxiety-like behavior, and altered function within the BNST likely mediates enhanced anxiety in humans. Our data suggest that chronic stress selectively increases multiple neurotrophic peptides and growth factors within the BNST, and this is consistent with previously published reports suggesting that chronic stress selectively activates the BNST (see below). Given the substantial data implicating the BNST in mediating anxiety-like behavior, we argue that the BNST is a critical site of confluence between stress responding and pathological anxiety.
Chronic stress and BNST plasticity
As noted above, stressor exposure can activate the BNST, and chronic exposure to stressors and/or pharmacological treatments (e.g., mCPP, corticosterone) have been shown to alter BNST plasticity. Several studies have found that the expression of BNST CRH was increased following prolonged social subjugation (chronic social stress), chronic "mild" stress, and chronic treatment with corticosterone (Makino et al., 1994; Watts and Sanchez-Watts, 1995; Schulkin et al., 1998). In addition, these treatments have been shown to increase signs of neuroplasticity within the BNST; exposure to a chronic unpredictable stress paradigm was associated with increased BNST volume and dendritic length (Pego et al., 2008), chronic immobilization increased the number of branch points observed in the dendritic arborization of BNST neurons (Vyas et al., 2002; Vyas et al., 2003), and a one-week variate stress paradigm increased BNST dendritic length (May, Braas and Hammack, unpublished data). Physiological correlates to BNST neuroplasticity have also been observed, so that chronic exposure to drugs of abuse has been shown to increase excitatory postsynaptic currents in ventral-tegmental area (VTA)-projecting BNST neurons (Dumont et al., 2008) as well as increase the expression of norepinephrine transporter within the BNST (Macey et al., 2003). Elevations in BNST CRH and neuroplasticity have been associated with increases in anxiety-like behavior and anhedonia (a symptom of depression, (Stout et al., 2000)). Hence, chronic exposure to stressors facilitates BNST function by changing neurochemistry, morphology and physiology within this structure, and these changes are associated with increases in fear- and anxiety-like behavior. From these data, increased neuroplasticity within the BNST has been argued to mediate anxiety disorders in humans whose etiology is associated with chronic stressor exposure.
Neuropeptides and the BNST
As discussed, the anterolateral BNST and CeA have high levels of stress-related peptides, including CRH. Extrahypothalamic CRH has been heavily implicated in mediating many of the behavioral responses to stressful stimuli, including fear and anxiety (Koob and Heinrichs, 1999), and CRH1 receptor knockout mice exhibit an anxiolytic behavioral profile (Timpl et al., 1998). CRH antagonists within the BNST reduce the anxiogenic response to intracerebroventricular (ICV) CRH injection (Lee and Davis, 1997). Other peptides associated with anxiety and stress in the extended amygdala include CRH-related urocortin peptides, neuropeptide Y (NPY), galanin, vasopressin and oxytocin (Walter et al., 1991; Veinante and Freund-Mercier, 1997; Koob and Heinrichs, 1999). As we discuss below, pituitary adenylate cyclase activating polypeptides (PACAP) are well studied pleiotropic factors and have diverse functions in development, physiology and injury responses. From tissue distribution studies, high levels of PACAP and its cognate G protein-coupled PAC1 receptor have been identified in the amygdala and BNST (Hashimoto et al., 1996; Hannibal, 2002), particularly in CRH-rich regions. Although several studies have implicated the PACAP family of peptides in behavior and extended amygdala function, few studies have investigated their function in the BNST. Recently, our studies have shown that BNST PACAP and PAC1 receptor mRNA levels are selectively induced in the extended amygdala after chronic stress (Hammack et al., 2009). Our data demonstrating the anxiogenic properties of PACAP are consistent with behavior studies in PACAP or PAC1 receptor knockout studies (Jamen et al., 2000; Hashimoto et al., 2001; Otto et al., 2001; Colwell et al., 2004; Girard et al., 2006); further, from its trophic properties, chronic PACAP signaling may be one mechanism underlying BNST neuronal cytoarchitectural remodeling and adaptation during stress (see below).
PACAP peptides and receptors: a primer
There are many excellent recent reviews on the PACAP family of related peptides (Sherwood et al., 2000; Vaudry et al., 2009) and hence, only some of the fundamental details will be highlighted here to facilitate future discussions. PACAP, the archetypical member of vasoactive intestinal peptide (VIP)-secretin-glucagon family of bioactive peptides, was isolated from hypothalami based its ability to stimulate anterior pituitary gland adenylyl cyclase activity (Miyata et al., 1990; Ogi et al., 1990; Arimura, 1998; Vaudry et al., 2009). Two α-amidated forms of PACAP arise from alternative posttranslational processing of the precursor molecule; PACAP38 has 38 amino acid residues [pro-PACAP(131–68)] while PACAP27 corresponds to the amino terminus of PACAP38 [proPACAP(131–157)]. PACAP27 exhibits 68% amino acid identity with VIP (Kimura et al., 1990; Miyata et al., 1990; Ogi et al., 1990). The relative levels of the two forms of PACAP are tissue-specific, although PACAP38 predominates in most tissues (Arimura et al., 1991; Arimura, 1998). For example, the ratio of PACAP38 to PACAP27 can range from 500:1 in testes to 15:1 in the hypothalamus. The 28-amino acid VIP peptide is also α-amidated but, unlike PACAP, is not alternatively processed. From gene duplication, VIP and PACAP form one branch of the cladistic tree and are highly conserved among species implicating their physiological importance in evolution. For example, there is only one amino acid difference in PACAP27 between tunicates and mammals. The gene exon-intron organization, transcript untranslated regions and alternative splicing, and molecular conformation of PACAP and VIP have been reported (see Vaudry et al., 2009). Yet despite the similarities, PACAP and VIP can be differentially expressed and regulated (Pavelock et al., 2007; Vaudry et al., 2009) and the targeted deletion of one gene does not result in compensatory expression responses by the other (Girard et al., 2006). The cloning of cDNAs encoding three distinct G-protein-coupled receptors for PACAP and VIP has provided a molecular basis for understanding the complexity of PACAP and VIP signaling (Ishihara et al., 1992; Hashimoto et al., 1993; Hosoya et al., 1993; Lutz et al., 1993; Spengler et al., 1993; Inagaki et al., 1994; Laburthe et al., 2002). Only PACAP peptides exhibit high affinity for the PAC1 receptor, whereas VIP and PACAP have similar high affinities for VPAC1 and VPAC2 receptors. While VPAC receptors appear to be coupled principally to adenylyl cyclase, PAC1 receptor isoforms display unique patterns of adenylyl cyclase and PLC activation, which differ for PACAP27 and PACAP38 (Spengler et al., 1993; Vaudry et al., 2009). Isoforms are produced by alternative splicing of the PAC1 receptor transcript regions encoding the amino-terminal extracellular domain and third cytoplasmic loop. Variants resulting from the presence or absence of a 21-residue insert into the amino-terminal extracellular domain can affect PACAP38 and PACAP27 potency (Pantaloni et al., 1996). Other variants, from the alternative splicing of two 84 bp HIP and HOP exons in the region encoding the third cytoplasmic loop, exhibit differential patterns of adenylyl cyclase and PLC activation by PACAP (Spengler et al., 1993). Stimulation of the PAC1 null receptor (neither HIP nor HOP), for example, activates adenylyl cyclase; the presence of the HIP cassette (PAC1HIP receptor) can dampen the potency and efficacy of PAC1 receptor-mediated cyclic AMP production. The PAC1HOP receptor variant can be promiscuous in Gs/Gq coupling and allow both adenylyl cyclase and phospholipase C activation for diverse downstream intracellular signaling events. These potential multifactorial PAC1 receptor signaling in adenylyl cyclase, phospholipase C, MAPK and Akt pathways can be key in understanding neurotrophic signaling during development, plasticity and regeneration (Barrie et al., 1997; Villalba et al., 1997; May et al., 2010). The limited number of pharmacological tools have hampered studies on the roles of the different receptor subtypes in central and peripheral tissues. While the PACAP(6–38) antagonist acts at both PAC1 and VPAC2 receptors, M65 (also called max.d.4), a 19-amino acid deletion mutant of the sand fly vasodilatory maxadilan peptide, is a potent and selective PAC1 receptor antagonist (Moro and Lerner, 1997; Moro et al., 1999). Interestingly, several acyl hydrazides have now been identified as small non-peptide PAC1 receptor antagonists, but their relative selectivity at VPAC1 and VPAC2 receptors has not been described (Beebe et al., 2008). PG97-269 and PG99-465 have been developed as VPAC1 and VPAC2 receptor selective antagonists, respectively (Vanneste et al., 2004). The chimeric, substituted peptide Lys15Arg16Leu27-VIP(1–7)/GRF(8–27) is a high affinity VPAC1 agonist (Gourlet et al., 1997); Ro25-1553 and Hexa-VIP(1–28) are VPAC2-selective agonists (O'Donnell et al., 1994; Juarranz et al., 1999).
PACAP peptides in stress- and anxiety-related pathways
As described previously, PACAP peptides are widely distributed in the central/peripheral nervous systems (CNS/PNS) and in peripheral organs (Vaudry et al., 2009). But among CNS structures, both PACAP and PAC1 receptor mRNA and immunoreactivity are highly expressed in limbic areas including the hypothalamus, hippocampus, olfactory nuclei, and discrete regions of the amygdala (Hashimoto et al., 1996; Jaworski and Proctor, 2000; Hannibal, 2002), which are well known to mediate the diverse behavioral, physiological and endocrine responses observed following stressor exposure. Similar to CRH, some of the highest levels of PACAP mRNA expression and peptide content in the CNS are found in hypothamalmic nuclei, including the PVN where PACAP can be colocalized with a subpopulation of CRH neurons (Hannibal et al., 1995). Some of the highest densities of PAC1 receptor mRNA have also been identified in the many different regions of the hypothalamus emphasizing the importance of PACAPergic systems in neuroendocrine functions and homeostasis. Interestingly, PACAP and CRH have also been identified in specific regions of the central extended amygdala, often in complementary patterns (see below), to suggest additional functional parallels. Some of the highest extrahypothalamic CRH levels have been described in the BNST and CeA (Koob and Heinrichs, 1999); similarly, dense PACAP-immunoreactive fibers have been found in the dorsolateral BNST and in the capsular and central parts of the lateral CeA subdivision (Piggins et al., 1996; Hannibal, 2002). In all these studies, PACAP-immunoreactive fibers and terminals are far more prevalent than PACAP-immunopositive soma suggesting that the bulk of the PACAP fibers represent extrinsic long distance axonal projections to the CeA and BNST. The endogenous sources of the PACAP-containing axons in the PVN and BNST are still being evaluated but studies using retrograde tracers suggest that the PACAP fibers to the PVN may originate from diverse CNS regions such as local hypothalamic nuclei and more distant brainstem nuclei (Legradi et al., 1998), while those to the BNST stem significantly in part from PACAP neurons in the PVN (Kozicz et al., 1998). But in both PVN and BNST oval nucleus regions, the PACAP-immunoreactive fibers have been described to synapse on CRH neurons, implicating the two peptidergic systems in a common neural pathway neurons involved in stress responses. As specific regions of the BNST also project to the PVN, these results may also imply that the hypothalamus and BNST may have reciprocal regulatory functions. One potential caution in these observations rests in the use of colchicine in some of these studies. The microtubule polymerization inhibitor is frequently employed in many immunocytochemical peptide distribution studies to disrupt vesicle transport and secretion and enhance peptide detection in neuronal soma and fibers. As colchicine is a cellular, physiological and behavioral stressor, and can enhance cellular mRNA and expression, its effects are clearly not solely related to vesicular transport but more complex and transcriptional. While these treatments may not necessarily distort immunocytochemical staining patterns, they may bias staining intensities from levels that are more typically observed in naive animals. Nevertheless, the sparse number of BNST PACAP-immunoreactive soma mirror the scattering of PACAP mRNA expressing neurons in the BNST (Hannibal, 2002); hence even though the bulk of PACAP-positive fibers appear to arise from extrinsic projections, the BNST is capable of endogenous PACAP production for local and/or more long distant regulatory functions. As the cytoarchitecture of the BNST has been described to contain an inner "core" of projection neurons surrounded by an outer "shell" of interneurons (Larriva-Sahd, 2006), the localization of PACAP in the BNST may have functional significance. While PACAP is more prevalent in the capsular region of the CeA, CRH-immunoreactivity patterns appear complementary and restricted to the adjacent lateral and medial CeA regions. Interestingly, even though the BLA is also a central mediator of behavioral responses, neither PACAP nor CRH fibers and soma appear prevalent in the BLA. The distribution of PACAP-immunoreactive fibers correlates well with PAC1 receptor mRNA patterns in the hypothalamus, hippocampus and extended amygdala regions such as the BNST and CeA (Hashimoto et al., 1996). In aggregate these results implicate PACAP signaling, via CRH-dependent and/or independent mechanisms, as one of the regulators of neuroendocrine, autonomic, and behavioral responses to stressor exposure.
PACAP peptides in stress-related behavior - hints from knockout studies
Despite much evidence from neurochemical anatomy, the studies examining PACAP effects on behavior have been limited. The results from early studies evaluating the roles of PACAP in stress were modest; for example, whereas acute restraint, osmotic and ether stress have no apparent effects, other challenges including colchicine-, interleukin-1β- and kainic acid-induced stress, can increase PACAP mRNA expression in the hypothalamus (Hannibal et al., 1995). PACAP infusions into cerebral ventricles have been shown to augment plasma corticosterone levels, stimulate c-fos and phosphorylated CREB immunoreactivity in the hypothalamus, and increase PVN CRH mRNA levels, very much similar to the physiologic responses to stress, and yet the behavioral stress responses in these animals to PACAP have only been examined with respect to grooming behavior and motor activity (Agarwal et al., 2005; Norrholm et al., 2005). Further, ICV infusion studies, while instructive, cannot typically specify the central sites of PACAP activity. Some direct nuclei microinjections/infusions have been performed; for example, PACAP infusions into the CeA result in changes shock/probe aversion responses that are consistent with the manifestation of fear (Legradi et al., 2007).
Although these results are indicative and served as a prelude for additional studies, the ensuing behavior data from several PACAP or PAC1 receptor null mouse strains developed by several independent laboratories have also provided complementary and revealing results (Jamen et al., 2000; Hashimoto et al., 2001; Otto et al., 2001; Colwell et al., 2004; Girard et al., 2006). In general, the PAC1 and PACAP knockout animals share some defects such as hyperactive psychomotor behavior, poor fertility, circadian rhythm irregularities, and abnormalities in glucose/lipid homeostasis. Furthermore, the PACAP null mice appear to have high early postnatal mortality, which may be related to acute thermosensitivity, apnea or other metabolic dysfunctions (Gray et al., 2001; Gray et al., 2002; Cummings et al., 2004b; Cummings et al., 2004a; Cummings et al., 2008). Yet despite these hurdles, some of the best evidence for PACAP/PAC1 receptor signaling in behavior has been presented in studies using these knockout animals. PAC1 receptor knockouts demonstrate abnormalities in social and sexual behavior associated with pheromone processing (Nicot et al., 2004) and appear to present deficits in contextual fear conditioning, a hippocampus-dependent associative learning paradigm (Sauvage et al., 2000; Otto et al., 2001). However, some of the most consistent and striking phenotypes in the PACAP or PAC1 receptor knockout mice are the changes in psychomotor behavior (Hashimoto et al., 2001; Otto et al., 2001; Girard et al., 2006). In open field tests, PACAP null mice demonstrate reduced anxiety-like behavior, exaggerated jumping behavior and dramatically increased locomotor activity with little evidence for habituation to the environment. PACAP knockout animals move quicker, travel longer distances per unit time, spend less time resting and access the center of the open field more frequently than wildtype mice, which travel preferentially along the test chamber perimeter (Hashimoto et al., 2001; Girard et al., 2006). PACAP knockouts show greater tendencies to enter and spend more time in the open arms on the elevated-plus maze test, also consistent with a phenotype of reduced anxiety. In emergence tests, in which mice within a safe open cylinder are placed in a novel open-field environment, PACAP knockout mice demonstrate less latency emerging from the cylinder and a near 2-fold increase in exploratory times (Hashimoto et al., 2001). Other related test paradigms have also shown the peptide knockouts are less anxious and spent more time exploring novel objects. The same psychomotor behaviors have been observed in PAC1 receptor knockout mice. Interestingly, unlike mice with a global PAC1 receptor deficiency, the PAC1CaMCRE2 mice with targeted disruption of cortical and hippocampal PAC1 receptors, did not present psychomotor behavioral defects (Otto et al., 2001), suggesting that PACAP signaling outside of these areas, such as the extended amygdala, may control the anxiety responses. The hyperactive behavior the PACAP null animals can be ameliorated with the dopamine antagonist haloperidol and the explosive jumping behavior can be attenuated with serotonin reuptake inhibitors. While the monoaminergic system does not appear to be perturbed in embryonic knockout brains, cortical and striatal 5-HIAA (a serotonin metabolite) may be diminished slightly in adult PACAP-deficient animals, which implicates a late developmental role for PACAP in serotoninergic system function. Other stress-related responses are also attenuated in PACAP knockout animals. Intraperitoneal Injections with trimethyltin to instill neuronal damage and systemic stress failed to induce corticosterone levels in knockout animals compared to wildtype (Morita et al., 2006). More recently, the increase in hypothalamic CRH mRNA and serum corticosterone observed in wildtype mice following prolonged restraint stress has been shown to be blunted in PACAP-deficient mice (Stroth and Eiden, 2009). As a caution, analogous behavioral studies have not been performed using conditional knockout animals. Clearly, maternal gestational influences and even postnatal handling can impact development, and these issues should be considered as additional animal resources become available. But, despite the many related observations suggesting that PACAP modulates anxiety-like behavior and stress-responding, the neuroanatomical substrates, characteristics and mechanisms of PACAP action have not been identified. Our recent studies suggest that one primary locus for PACAP signaling in anxiety-like behavioral responding to stressor exposure resides in the BNST and that the ensuing long term changes in PACAP expression may underlie the long lasting neurological and physiological defects that may have permanence in allosteric load.
Chronic variate stress increases PACAP, PAC1 receptor and neurotrophin-related transcripts expression in the BNST
Chronic stress elicits diverse neurotransmitter and hormonal responses through activation of multiple signaling pathways, including SAPK/JNK (Shen et al., 2004), one critical regulator of neuronal PACAP expression following metabolic stress. To evaluate whether chronic stress regulates PACAP expression in brain regions associated with stress-responding and/or anxiety-like behavior, Sprague-Dawley rats were exposed to a chronic variate stress paradigm in which rats received a different stressor in each of 7 days to reduce habituation of the stress response (Hammack et al., 2009). Twenty-four hours after the last stressor, several different brain regions associated with stress and fear behavior were microdissected and frozen on dry ice before RNA processing for quantitative RT-PCR measurement. As reported in Hammack et al., 2009, among the 12 neural regions examined, chronic variate stress selectively increases PACAP transcript levels more than 10-fold only in the dorsolateral BNST (dorsal aspect of anterolateral BNST); a smaller but significant 2-fold increase in PACAP mRNA is also apparent in the hypothalamic PVN. PACAP levels in the remaining brain regions, including the central and basolateral amygdala, and ventral aspect of the BNST, are not different from non-stressed control animal tissues. The stress-induced increase in dorsolateral BNST PACAP expression is associated with a 2-fold increase in PAC1 receptor mRNA expression; like all CNS tissues examined to date, the PAC1null receptor isoform predominates in the BNST. The increase in dorsolateral BNST PACAP and PAC1 receptor appears unique as VIP, VPAC1 and VPAC2 receptor transcript levels in the same samples are not changed (Hammack et al., 2009).
Within the same tissue sets, the chronic variate stress paradigm also augmented other stress-related transcripts in the PVN and amygdala, some of which may have been consequences of PACAP signaling. For example, chronic variate stress also increased brain-derived neurotrophic factor (BDNF) and TrkB mRNA levels nearly 3-fold and 2-fold, respectively, in the dorsolateral BNST when compared with non-stress control tissues. PACAP can stimulate neuronal BDNF and/or TrkB expression and function in a variety of paradigms (Yaka et al., 2003; Braas et al., 2007) and CNS BDNF expression appears diminished in PAC1 receptor knockout animal (Zink et al., 2004). Since chronic stress results in long term changes in dorsolateral BNST cellular plasticity, and BDNF function has been associated with anxiety and behavior disorder, PACAP-stimulated BDNF expression may be one mechanism underlying that plasticity. To that effect, PACAP treatment of dorsolateral BNST explants in serum-free cultures can increase BDNF transcript expression (unpublished observations). BDNF transcripts in a few select CNS regions are also increased following chronic variate stress, including the PVN (approximately 1.4-fold) and dorsal raphe nucleus (3-fold). As these CNS areas are targets of BNST projections, the observed changes in BDNF and TrkB transcript expression in these regions may also reflect stress-mediated PACAP production and signaling from the BNST. Importantly, these data suggest that the dorsolateral BNST is a critical CNS target of stress-induced plasticity and such plasticity within the BNST may represent a likely mechanism underlying anxiety disorders in humans. It is notable that neither PACAP nor BDNF was increased in the BLA, which like the BNST can exhibit enhanced neuroplasticity after some chronic stress paradigms (Vyas et al., 2002). These data might suggest that BLA enhancements in neuroplasticity are not PACAP dependent; however, it is currently unclear whether the chronic variate stress paradigm enhances BLA neuroplasticity, as Vyas and colleagues (2002) only found enhanced neuroplasticity in the BLA following chronic immobilization stress and not after a chronic unpredictable stress paradigm.
As in other stress paradigms, the same chronic variate stress treatment also increases CRH transcripts in the PVN. Though BNST CRH transcript level appears unchanged in this paradigm, expression of the different BNST CRH receptor subtypes is enhanced following chronic stress. Interestingly, the greatest increase in CRH mRNA is noted in the lateral and medial CeA which may be related to fear-like responses. Again, as PACAP-immunoreactive fibers can impinge and regulate CRH neurons, and as stress-induced expression of hypothalamic CRH is diminished in PACAP knockout animals, these result further implicate an integration of PACAP and CRH pathways.
Chronic variate stress increases PACAP immunoreactivity and transcript levels in the oval nucleus of the BNST
The methods used to dissect BNST subregions for quantitative RT-PCR measurements have some limitations in distinguishing PACAP expression in neuronal vs non-neuronal compartments, identifying the distinct BNST subnuclei affected, and demonstrating associated changes in PACAP peptide levels. Hence, the RT-PCR data have been reexamined using immunocytochemistry and in situ hybridization techniques and from these results, PACAP immunoreactivity and transcript expression is increased by chronic stress selectively in the BNST oval nucleus (Hammack et al., 2009; May et al., 2009; Roman et al., 2009). Notably, unlike previous work, colchicine was not used in these studies to allow expression change detection between control (unstressed) and chronically stressed animals. As for many neuropeptides including CRH, dark punctate PACAP immunoreactivity was localized predominantly to neuronal fibers, varicosities and terminals in the oval nucleus of the dorsolateral BNST, identified by high levels CRH staining in the same region on adjacent tissue sections. The oval nucleus PACAP staining area and density is vastly more extensive after chronic stress (Figure 3); by pixel analyses, chronic stress increased BNST PACAP immunoreactivity more than 4-fold. Interestingly, even with high sensitivity methods, PACAP- and CRH-immunoreactivities are not colocalized in the BNST, again illustrating how the two peptidergic systems maybe separate yet integrated. As mature amidated peptides are typically transported along axons, PACAP staining in fiber structures is more robust than the soma. However, parallel in situ hybridization approaches to label soma have also demonstrated increased PACAP neuronal number in the BNST after chronic stress, in good agreement with quantitative PCR measurements suggesting that the BNST oval nucleus neurons can express PACAP. Hence the increase in BNST oval nucleus PACAP fiber staining may reflect augmented peptide production from both extrinsic and intrinsic sources. As noted above, PACAP transcripts are increased in the PVN after chronic stress and PVN PACAP projections to the BNST may represent one extrinsic source. Depending on the location of the intrinsic PACAP BNST neurons (shell vs core), BNST PACAP may be important for local BNST neurocircuits or long distance target projections. Some preliminary analyses suggest the latter possibility. PACAP-immunoreactive fibers in the medial and subcommissural BNST regions appear diffuse and unchanged between the two groups. PACAP staining levels in the capsular region of the CeA is also unchanged after chronic variate stress. In contrast to the BNST, CRH immunoreactivity in the CeA appears enhanced after chronic stress. Hence the changes in PACAP and CRH immunoreactive staining patterns in the BNST and CeA appear to be reciprocal.
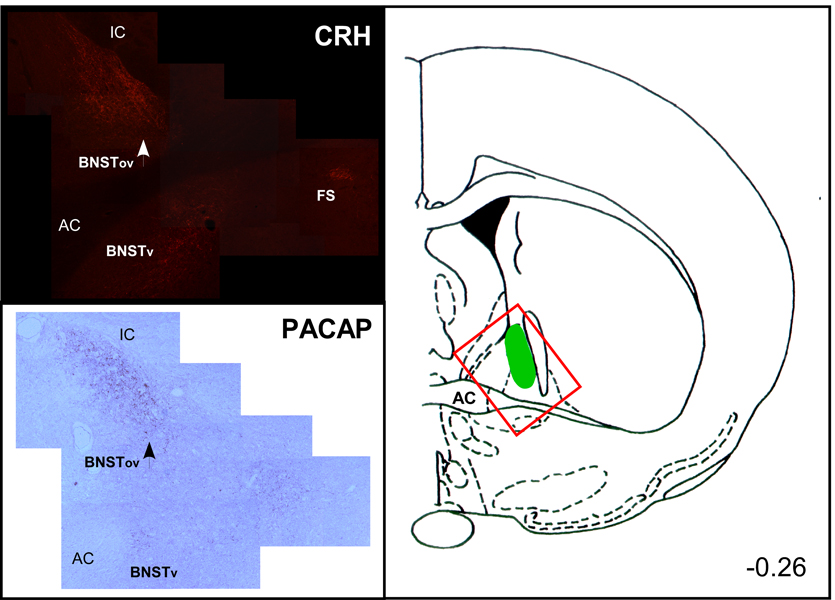
Figure 3. BNST CRH and PACAP immunoreactivity after chronic stress.
(A) Cryosections from chronically stressed rats were processed and CRH immunoreactivity was detected using Cy3-conjugated secondary antibody fluorescence. In the photomontage, CRH immunoreactivity n the oval nucleus of the BNST (BNSTov) is dense compared to staining levels in the ventral zone (BNSTv); from the plane of section CRH staining is compact in the fundus striatum (FS). (B) Adjacent cryosection from the same chronically stressed brain was processed for PACAP immunoreactivity using tyramide amplification and diaminobenzidine as substrate. Though the distribution of PACAP staining in the BNST can be broad, the highest density of PACAP is found in the BNSTov. Even in chronically stressed animals, PACAP staining in the BNSTv appears relatively diffuse compared to PACAP immunoreactivity in the oval nucleus.
Acute PACAP injection into the BNST induces stress-related behavioral consequences
The ability of chronic variate stress to selectively increase BNST PACAP and PAC1 receptors transcripts has suggested that this system plays an important role in chronic stress-mediated anxiety-like behavior. These observations, coupled with other PACAP-related stress studies and the anxiolytic behavioral changes observed in the PACAP and PAC1 receptor knockout mice, in aggregate implicate BNST PACAP signaling as a potential mechanism for anxiogenic responses and the long-term neuroplasticity changes that accompany anxiety-like behavior. To evaluate that possibility, adult male rats were implanted bilaterally with cannula aimed at the anterior BNST and subsequently infused with one of several PACAP38 doses (0.1 – 1 µg) or vehicle at a flow rate of 0.25 µl/min. Consistent with hypotheses, PACAP38 was anxiogenic in a concentration-dependent manner as measured by increased baseline startle behavior (Hammack et al., 2009). Perhaps more interestingly, BNST treated rats continue to exhibit an elevation in baseline startle amplitude even one week after the single PACAP38 injection. As PACAP can elicit sustained neurotrophic effects, these results suggest that PACAP-induce BNST neuroplasticity may be one mechanism underlying the long-term elevation in anxiety-like behavior.
Although BNST PACAP signaling may be anxiogenic, whether chronic stress-induced anxiety is mediated by central PACAP functioning is currently under study. In preliminary work, the rats were infused continuously into the lateral ventricles with vehicle or the PAC1/VPAC2 antagonist PACAP(6–38) via osmotic minipumps (1 µl/h, 12 µg/day) during the 7 day chronic stress paradigm and 24 hours after the last stressor, rats were tested for anxiety-like behavior on an elevated plus-maze. While the antagonist-treated animals appeared to venture into the open arms more frequently in these preliminary studies, the results on anxiety-like behavior were equivocal from variability within the small animal groups. However, in the same study, chronic variate stress, previously shown to produce anorexia in rodents, dramatically reduced weight gain more than 70%. Interestingly, chronic central PACAP(6–38) antagonist infusion dramatically reversed the stress-induced weight change such that the body weights of stressed/PACAP(6–38) treated animals were comparable to those of non-stressed controls. These antagonist responses were so dramatic that additional studies were performed to identify the sites and roles of PACAP in weight change. Recently in assessing the role of the BNST in stress-mediated weight change, excitotoxic N-methyl-D-aspartate (NMDA) BNST lesions were performed prior to chronic stress treatments. In parallel with the PACAP antagonist infusion studies, chronic stress-induced weight loss was reduced in the BNST-lesioned animals compared to sham or non-stress control animals, demonstrating that the BNST is an important site for stress-induced anorexia. To determine whether BNST PACAP alone was sufficient to produce anorexia in the absence of stress, PACAP38 (0.1 – 1 µg) was injected directly into the BNST and within 24-hour post-infusion, PACAP dose-dependently produced a dramatic reduction in weight and food/water intake. Hence, in addition to anxiety-like behavior, these results suggest that intra-BNST PACAP expression and signaling represent an important locus for weight/feeding regulation and the anorexic effects of stress.
Summary and overview
The many separate lines of work are beginning to coalesce and implicate PACAP expression and signaling in stress-related behaviors. To encapsulate our recent observations, our work suggests that PACAP/PAC1 receptor signaling has acute and sustained stress-associated behavioral effects when injected into the BNST. PACAP and PAC1 receptor expression is augmented selectively in the BNST after chronic stress, particularly in BNST subregions that express stress-related CRH. BNST CRH activation has been shown to be necessary and sufficient for the expression of anxiety-like behavior, and the distribution and functional similarities between the two peptidergic systems suggest that the PACAP and CRH pathways may be integrated to increase BNST activity and coordinate these behavioral changes. As noted above, the increase in BNST neuroplasticity has been argued to represent a key underlying mechanism to sustain behavioral changes, such as anxiety after chronic stress. In addition to its transmitter activities, PACAP has well established neurotrophic functions through G protein-coupled PAC1 receptor function or downstream induction of neurotrophins, especially BDNF. As chronic stress and PACAP can upregulate BDNF and TrkB expression, PACAP may be an important mediator of stress-induced increases in BNST neuroplasticity. Hence, the effects of PACAP may be multidimensional with other transmitters, peptides and growth factor systems to enhance not only acute but also long term stress-induced behavioral changes. These studies have the potential of defining specific mechanisms for the etiology of anxiety disorders with important implications for clinical therapeutic adaptations. PACAP systems have also been implicated in depression and schizophrenia-like disorders (Hashimoto et al., 2007; Hashimoto et al., 2009a; Hashimoto et al., 2009b). Although not directly associated, depression and anxiety are two of the most comorbid disorders known (Simon, 2009); the presence of anxiety in schizophrenia-like disorders is also highly prevalent (Achim et al., 2009). Future PACAP studies may identify other clinical correlations with chronic debilitating anxiety, PTSD (posttraumatic stress disorder), anorexia-like behavior and related feeding-related disorders.
Acknowledgements
This work was supported by grants HD27468 and NS37179 (VM and KMB), and MH072088 (SEH) from the National Institutes of Health. Portions of the work was also supported by National Alliance for Research on Schizophrenia and Depression (NARSAD). The use of the Molecular Biology Core Facility at the University of Vermont College of Medicine supported by National Institute of Health NCRR P20RR16435 is also gratefully acknowledged.
References
- Achim AM, Maziade M, Raymond E, et al. How prevalent are anxiety disorders in schizophrenia? A meta analysis and critical review on a significant association. Schizophr Bull. 2009 doi: 10.1093/schbul/sbp148. 2009 Dec 3 [Epub ahead of print] PMID: 19959704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res. 2005;138:45–57. doi: 10.1016/j.molbrainres.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuro-endocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;8:301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- Arimura A, Somogyvari-Vigh A, Miyata A, et al. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- Barrie AP, Clohessy AM, Buensuceso CS, et al. Pituitary adenylyl cyclase-activating peptide stimulates extracellular signal-regulated kinase 1 or 2 (ERK1/2) activity in a Ras-independent, mitogen-activated protein Kinase/ERK kinase 1 or 2-dependent manner in PC12 cells. J Biol Chem. 1997;272:19666–19671. doi: 10.1074/jbc.272.32.19666. [DOI] [PubMed] [Google Scholar]
- Beebe X, Darczak D, Davis-Taber RA, et al. Discovery and SAR of hydrazide antagonists of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor type 1 (PAC1-R) Bioorg Med Chem Lett. 2008;18:2162–2166. doi: 10.1016/j.bmcl.2008.01.052. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1177–1185. doi: 10.1016/j.pnpbp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Braas KM, Schutz KC, Bond JP, et al. Microarray analyses of pituitary adenylate cyclase activating polypeptide (PACAP)-regulated gene targets in sympathetic neurons. Peptides. 2007;28:1856–1870. doi: 10.1016/j.peptides.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Res Bull. 1991;27:207–212. doi: 10.1016/0361-9230(91)90069-v. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, et al. Selective deficits in the circadian light response in mice lacking PACAP. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1194–R1201. doi: 10.1152/ajpregu.00268.2004. [DOI] [PubMed] [Google Scholar]
- Crane JW, Buller KM, Day TA. Evidence that the bed nucleus of the stria terminalis contributes to the modulation of hypophysiotropic corticotropin-releasing factor cell responses to systemic interleukin-1beta. J Comp Neurol. 2003;467:232–242. doi: 10.1002/cne.10918. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Willie C, Wilson RJ. Pituitary adenylate cyclase-activating polypeptide maintains neonatal breathing but not metabolism during mild reductions in ambient temperature. Am J Physiol Regul Integr Comp Physiol. 2008;294:R956–R965. doi: 10.1152/ajpregu.00637.2007. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Pendlebury JD, Sherwood NM, et al. Sudden neonatal death in PACAP-deficient mice is associated with reduced respiratory chemoresponse and susceptibility to apnoea. J Physiol. 2004a;555:15–26. doi: 10.1113/jphysiol.2003.052514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Pendlebury JD, Jirik FR, et al. A SIDS-like phenotype is associated with reduced respiratory chemoresponses in PACAP deficient neonatal mice. Adv Exp Med Biol. 2004b;551:77–83. doi: 10.1007/0-387-27023-x_13. [DOI] [PubMed] [Google Scholar]
- Davis M. Pharmacological and anatomical analysis of fear conditioning using the fear-potentiated startle paradigm. Behav Neurosci. 1986;100:814–824. doi: 10.1037//0735-7044.100.6.814. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Day HE, Curran EJ, Watson SJJ, et al. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol. 1999;413(1):113–128. [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, et al. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in the adult rat brain. J Comp Neurol. 2001;536:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Rycroft BK, Maiz J, et al. Morphine produces circuit-specific neuroplasticity in the bed nucleus of the stria terminalis. Neuroscience. 2008;153:232–239. doi: 10.1016/j.neuroscience.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JD. Plasma corticosterone responses to electrical stimulation of the bed nucleus of the stria terminalis. Brain Res. 1987;407:327–331. doi: 10.1016/0006-8993(87)91111-5. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Helmstetter FJ. Conditional analgesia, defensive freezing, and benzodiazepines. Behav Neurosci. 1988;102:233–243. doi: 10.1037//0735-7044.102.2.233. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Girard BM, Lelievre V, Braas KM, et al. Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. J Neurochem. 2006;99:499–513. doi: 10.1111/j.1471-4159.2006.04112.x. [DOI] [PubMed] [Google Scholar]
- Gourlet P, Vandermeers A, Vertongen P, et al. Development of high affinity selective VIP1 receptor agonists. Peptides. 1997;18:1539–1545. doi: 10.1016/s0196-9781(97)00228-3. [DOI] [PubMed] [Google Scholar]
- Gray SL, Cummings KJ, Jirik FR, et al. Targeted disruption of the pituitary adenylate cyclase-activating polypeptide gene results in early postnatal death associated with dysfunction of lipid and carbohydrate metabolism. Mol Endocrinol. 2001;15:1739–1747. doi: 10.1210/mend.15.10.0705. [DOI] [PubMed] [Google Scholar]
- Gray SL, Yamaguchi N, Vencova P, et al. Temperature-sensitive phenotype in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology. 2002;143:3946–3954. doi: 10.1210/en.2002-220401. [DOI] [PubMed] [Google Scholar]
- Gray TS, Piechowski RA, Yracheta JM, et al. Ibotenic acid lesions in the bed nucleus of the stria terminalis attenuate conditioned stress-induced increases in prolactin, ACTH and corticosterone. Neuroendocrinology. 1993;57:517–524. doi: 10.1159/000126400. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, et al. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, et al. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J. Pituitary adenylate cyclase activating peptide in the rat central nervous system: an immunocytochemical and in situ hybridization study. J Comp Neurol. 2002;453:389–417. doi: 10.1002/cne.10418. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Mikkelsen JD, Fahrenkrug J, et al. Pituitary adenylate cyclase-activating peptide gene expression in corticotropin-releasing factor-containing parvicellular neurons of the rat hypothalamic paraventricular nucleus is induced by colchicine, but not by adrenalectomy, acute osmotic, ether, or restraint stress. Endocrinology. 1995;136:4116–4124. doi: 10.1210/endo.136.9.7649120. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Ishihara T, Shigemoto R, et al. Molecular cloning and tissue distribution of a receptor for pituitary adenylate cyclase-activating polypeptide. Neuron. 1993;11:333–342. doi: 10.1016/0896-6273(93)90188-w. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Nogi H, Mori K, et al. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J Comp Neurol. 1996;371:567–577. doi: 10.1002/(SICI)1096-9861(19960805)371:4<567::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Hashimoto R, Shintani N, et al. Depression-like behavior in the forced swimming test in PACAP-deficient mice: amelioration by the atypical antipsychotic risperidone. J Neurochem. 2009a;110:595–602. doi: 10.1111/j.1471-4159.2009.06168.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanaka K, et al. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP) Proc Natl Acad Sci U S A. 2001;98:13355–13360. doi: 10.1073/pnas.231094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Hashimoto H, Shintani N, et al. Possible association between the pituitary adenylate cyclase-activating polypeptide (PACAP) gene and major depressive disorder. Neurosci Lett. 2009b doi: 10.1016/j.neulet.2009.11.019. [Epub ahead of print] PMID: 19914336. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Hashimoto H, Shintani N, et al. Pituitary adenylate cyclase-activating polypeptide is associated with schizophrenia. Mol Psychiatry. 2007;12:1026–1032. doi: 10.1038/sj.mp.4001982. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Watson SJ. Involvement of the bed nucleus of the stria terminalis in tonic regulation of paraventricular hypothalamic CRH and AVP mRNA expression. J Neuroendocrinol. 1994;6:433–442. doi: 10.1111/j.1365-2826.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, et al. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hosoya M, Onda H, Ogi K, et al. Molecular cloning and functional expression of rat cDNAs encoding the receptor for pituitary adenylate cyclase activating polypeptide (PACAP) Biochem Biophys Res Commun. 1993;194:133–143. doi: 10.1006/bbrc.1993.1795. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Yoshida H, Mizuta M, et al. Cloning and functional characterization of a third pituitary adenylate cyclase-activating polypeptide receptor subtype expressed in insulin-secreting cells. Proc Natl Acad Sci USA. 1994;91:2679–2683. doi: 10.1073/pnas.91.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T, Shigemoto R, Mori K, et al. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal peptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Jamen F, Persson K, Bertrand G, et al. PAC1 receptor-deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J Clin Invest. 2000;105:1307–1315. doi: 10.1172/JCI9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski DM, Proctor MD. Developmental regulation of pituitary adenylate cyclase-activating polypeptide and PAC(1) receptor mRNA expression in the rat central nervous system. Brain Res Dev Brain Res. 2000;120:27–39. doi: 10.1016/s0165-3806(99)00192-3. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. Cytoarchitecture. J Comp Neurol. 1989;280:587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW, Simerly RB. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. Chemoarchitecture. J Comp Neurol. 1989;280:603–621. doi: 10.1002/cne.902800410. [DOI] [PubMed] [Google Scholar]
- Juarranz MG, Van Rampelbergh J, Gourlet P, et al. Different vasoactive intestinal polypeptide receptor domains are involved in the selective recognition of two VPAC(2)-selective ligands. Mol Pharmacol. 1999;56:1280–1287. doi: 10.1124/mol.56.6.1280. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, et al. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura C, Ohkubo S, Ogi K, et al. A novel peptide which stimulates adenylate cyclase: molecular cloning and characterization of the ovine and human cDNAs. Biochem Biophys Res Commun. 1990;166:81–89. doi: 10.1016/0006-291x(90)91914-e. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Vigh S, Arimura A. Axon terminals containing PACAP- and VIP-immunoreactivity form synapses with CRF-immunoreactive neurons in the dorsolateral division of the bed nucleus of the stria terminalis in the rat. Brain Res. 1997;767:109–119. doi: 10.1016/s0006-8993(97)00737-3. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Vigh S, Arimura A. The source of origin of PACAP- and VIP-immunoreactive fibers in the laterodorsal division of the bed nucleus of the stria terminalis in the rat. Brain Res. 1998;810:211–219. doi: 10.1016/s0006-8993(98)00692-1. [DOI] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A, Marie JC. VPAC receptors for VIP and PACAP. Receptors Channels. 2002;8:137–153. [PubMed] [Google Scholar]
- Larriva-Sahd J. Histological and cytological study of the bed nuclei of the stria terminalis in adult rat. II. Oval nucleus: extrinsic inputs, cell types, neuropil, and neuronal modules. J Comp Neurol. 2006;497:772–807. doi: 10.1002/cne.21011. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legradi G, Hannibal J, Lechan RM. Pituitary adenylate cyclase-activating polypeptide-nerve terminals densely innervate corticotropin-releasing hormone-neurons in the hypothalamic paraventricular nucleus of the rat. Neurosci Lett. 1998;246:145–148. doi: 10.1016/s0304-3940(98)00255-9. [DOI] [PubMed] [Google Scholar]
- Legradi G, Das M, Giunta B, et al. Microinfusion of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural Plast. 2007:79102. doi: 10.1155/2007/79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz EM, Sheward WJ, West KM, et al. The VIP2 receptor: molecular characterization of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Smith HR, Nader MA, et al. Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. J Neurosci. 2003;23:12–16. doi: 10.1523/JNEUROSCI.23-01-00012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Res. 1994;657:141–149. doi: 10.1016/0006-8993(94)90961-x. [DOI] [PubMed] [Google Scholar]
- May V, Roman CW, Schutz KC, et al. Chronic variate stress alters the expression of transcript for several stress-related peptides in the anterolateral bed nucleus of the stria terminalis (BNST). Program No 4695 Neuroscience 2009. Chicago, IL: Society for Neuroscience; 2009. Online. [Google Scholar]
- May V, Lutz E, Mackenzie C, et al. Pituitary adenylate cyclase activating polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through PI3Kgamma and vesicle endocytosis for neuronal survival. J Biol Chem. 2010 Jan 21; doi: 10.1074/jbc.M109.043117. [Epub ahead of print] PMID: 20093365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata A, Jiang L, Dahl RR, et al. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38) Biochem Biophys Res Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- Morita Y, Yanagida D, Shintani N, et al. Lack of trimethyltin (TMT)-induced elevation of plasma corticosterone in PACAP-deficient mice. Ann N Y Acad Sci. 2006;1070:450–456. doi: 10.1196/annals.1317.060. [DOI] [PubMed] [Google Scholar]
- Moro O, Lerner EA. Maxadilan, the vasodilator from sand flies, is a specific pituitary adenylate cyclase activating peptide type I receptor agonist. J Biol Chem. 1997;272:966–970. doi: 10.1074/jbc.272.2.966. [DOI] [PubMed] [Google Scholar]
- Moro O, Wakita K, Ohnuma M, et al. Functional characterization of structural alterations in the sequence of the vasodilatory peptide maxadilan yields a pituitary adenylate cyclase-activating peptide type 1 receptor-specific antagonist. J Biol Chem. 1999;274:23103–23110. doi: 10.1074/jbc.274.33.23103. [DOI] [PubMed] [Google Scholar]
- Nicot A, Otto T, Brabet P, et al. Altered social behavior in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. J Neurosci. 2004;24:8786–8795. doi: 10.1523/JNEUROSCI.1910-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Das M, Legradi G. Behavioral effects of local microinfusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN) Regul Pept. 2005;128:33–41. doi: 10.1016/j.regpep.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell M, Garippa RJ, Rinaldi N, et al. Ro 25-1553: a novel, long-acting vasoactive intestinal peptide agonist. Part I: In vitro and in vivo bronchodilator studies. J Pharmacol Exp Ther. 1994;270:1282–1288. [PubMed] [Google Scholar]
- Ogi K, Kimura C, Onda H, et al. Molecular cloning and characterization of cDNA for the precursor of rat pituitary adenylate cyclase activating polypeptide (PACAP) Biochem Biophys Res Commun. 1990;173:1271–1279. doi: 10.1016/s0006-291x(05)80924-6. [DOI] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, et al. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res. 2001;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- Pantaloni C, Brabet P, Bilanges B, et al. Alternative splicing in the N-terminal extracellular domain of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor modulates receptor selectivity and relative potencies of PACAP-27 and PACAP-38 in phospholipase C activation. J Biol Chem. 1996;271:22146–22151. doi: 10.1074/jbc.271.36.22146. [DOI] [PubMed] [Google Scholar]
- Pavelock KA, Girard BM, Schutz KC, et al. Bone morphogenetic protein down-regulation of neuronal pituitary adenylate cyclase-activating polypeptide and reciprocal effects on vasoactive intestinal peptide expression. J Neurochem. 2007;100:603–616. doi: 10.1111/j.1471-4159.2006.04293.x. [DOI] [PubMed] [Google Scholar]
- Pego JM, Morgado P, Pinto LG, et al. Dissociation of the morphological correlates of stress-induced anxiety and fear. Eur J Neurosci. 2008;27:1503–1516. doi: 10.1111/j.1460-9568.2008.06112.x. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Stamp JA, Burns J, et al. Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdala of the rat. J Comp Neurol. 1996;376:278–294. doi: 10.1002/(SICI)1096-9861(19961209)376:2<278::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Roman CW, May V, Kocho-Schellenberg M, et al. Activation of adenylate cyclase activating polypeptide (PACAP) in the bed nucleus of the stria terminalis (BNST) mediates increases in anxiety-like behavior following chronic stressor exposure. Program No 4696 Neuroscience 2009. Chicago, IL: Society for Neuroscience; 2009. Online. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sauvage M, Brabet P, Holsboer F, et al. Mild deficits in mice lacking pituitary adenylate cyclase-activating polypeptide receptor type 1 (PAC1) performing on memory tasks. Brain Res Mol Brain Res. 2000;84:79–89. doi: 10.1016/s0169-328x(00)00219-9. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Shen CP, Tsimberg Y, Salvadore C, et al. Activation of Erk and JNK MAPK pathways by acute swim stress in rat brain regions. BMC Neurosci. 2004;5:36. doi: 10.1186/1471-2202-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 2000;21:619–670. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- Simon NM. Generalized anxiety disorder and psychiatric comorbidities such as depression, bipolar disorder and substance abuse. J Clin Psychiatry. 2009;70 Suppl 2:10–14. doi: 10.4088/jcp.s.7002.02. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, et al. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Stout SC, Mortas P, Owens MJ, et al. Increased corticotropin-releasing factor concentrations in the bed nucleus of the stria terminalis of anhedonic rats. Eur J Pharmacol. 2000;401:39–46. doi: 10.1016/s0014-2999(00)00412-x. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Stroth N, Eiden LE. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.11.023. [Epub ahead of print] PMID: 19931358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, et al. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, e, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste G, Robberecht P, Lefebvre RA. Inhibitory pathways in the circular muscle of rat jejunum. Br J Pharmacol. 2004;143:107–118. doi: 10.1038/sj.bjp.0705918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol. 1997;383:305–325. [PubMed] [Google Scholar]
- Villalba M, Bockaert J, Journot L. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J Neurosci. 1997;17:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Light-enhanced startle: further pharmacological and behavioral characterization. Psychopharmacology (Berl) 2002a;159:304–310. doi: 10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Quantifying fear potentiated startle using absolute versus proportional increase scoring methods: implications for the neurocircuitry of fear and anxiety. Psychopharmacology (Berl) 2002b;164:318–328. doi: 10.1007/s00213-002-1213-0. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versusthe amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Mai JK, Lanta L, et al. Differential distribution of immunohistochemical markers in the bed nucleus of the stria terminalis in the human brain. J Chem Neuroanat. 1991;4:281–298. doi: 10.1016/0891-0618(91)90019-9. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Region-specific regulation of neuropeptide mRNAs in rat limbic forebrain neurones by aldosterone and corticosterone. J Physiol. 1995;484:721–736. doi: 10.1113/jphysiol.1995.sp020698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Hey DY, Phamluong K, et al. Pituitary adenylate cyclase activating polypeptide (PACAP1-38) enhances N-methyl-D-aspartate receptor function and brain derived neurotrophic factor expression via RACK1. J Biol Chem. 2003;278:9630–9638. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]
- Zink M, Otto C, Zörner B, et al. Reduced expression of brain-derived neurotrophic factor in mice deficient for pituitary adenylate cyclase activating polypeptide type-I-receptor. Neurosci Lett. 2004;360:106–108. doi: 10.1016/j.neulet.2004.01.030. [DOI] [PubMed] [Google Scholar]