Abstract
Urothelium-specific overexpression of NGF in the urinary bladder of transgenic mice stimulates neuronal sprouting or proliferation in the urinary bladder, produces urinary bladder hyperreflexia and results in increased referred somatic hypersensitivity. Additional NGF-mediated changes might contribute to the urinary bladder hyperreflexia and pelvic hypersensitivity observed in these transgenic mice such as upregulation of neuropeptide/receptor systems. Chronic overexpression of NGF in the urothelium was achieved through the use of a highly urothelium-specific, uroplakin II promoter. In the present study, we examined PACAP, VIP and associated receptors (PAC1, VPAC1, VPAC2) transcripts or protein expression in urothelium and detrusor smooth muscle and lumbosacral DRG in NGF-overexpressing and littermate wildtype mice using real-time quantitative reverse transcription-polymerase chain reaction and immunohistochemical approaches. Results demonstrate upregulation of PAC1 receptor transcript and PAC1-immunoreactivity in urothelium of NGF-OE mice whereas PACAP transcript and PACAP-immunoreactivity were decreased in urothelium of NGF-OE mice. In contrast, VPAC1 receptor transcript was decreased in both urothelium and detrusor smooth muscle of NGF-OE mice. VIP transcript expression and immunostaining was not altered in urinary bladder of NGF-OE mice. Changes in PACAP, VIP and associated receptors transcripts and protein expression in micturition pathways resemble some, but not all, changes observed after induction of urinary bladder inflammation known to involve NGF production.
Index entries: NGF, urinary bladder, neuropeptides, urothelium
Introduction
Nerve growth factor (NGF) has been suggested to play a role in urinary bladder dysfunction by mediating inflammation as well as morphological and functional changes in sensory and sympathetic neurons innervating the urinary bladder. Many previous studies in rodents have demonstrated the importance of NGF in bladder sensory function and the development of referred hyperalgesia in response to bladder inflammation (Jaggar et al., 1999; Zvara and Vizzard, 2007; Guerios et al., 2008; Arms et al., 2009). We recently examined the role of NGF in urinary bladder dysfunction by generating a mouse model of urinary bladder hypersensitivity based on the hypothesis that chronic urothelial NGF overexpression would induce sensory neuronal hypersensitivity and increased urinary bladder reflex function (Schnegelsberg et al., 2009). Chronic overexpressing of NGF in the urothelium was achieved through the use of a highly urothelium-specific, uroplakin II promoter (Lin et al., 1995; Liang et al., 2005). Our studies (Schnegelsberg et al., 2009) revealed that urothelium-specific overexpression of NGF in the urinary bladder of transgenic mice (1) stimulates neuronal sprouting or proliferation in the urinary bladder; (2) produces local inflammatory changes in the urinary bladder; (3) produces urinary bladder hyperreflexia; and (4) results in increased referred somatic hypersensitivity. Elevated levels of neurotrophins have also been detected in the urine of women with interstitial cystitis (IC)/bladder pain syndrome (BPS) (77) and in the urothelium of individuals with IC/BPS or other painful bladder conditions (63). More recently, it was demonstrated that urinary NGF levels are increased in patients with overactive bladder (OAB) symptoms associated with detrusor overactivity (DO), stress urinary incontinence, or bladder outlet obstruction (BOO) (47; 48; 57–62; 77; 103).
Additional NGF-mediated changes might contribute to the urinary bladder hyperreflexia and pelvic hypersensitivity observed in these mice (Schnegelsberg et al., 2009), such as stimulation/recruitment of bladder mast cells, modulation of local neuroinflammatory responses, upregulation of neuropeptide/receptor systems and ion channels as well as changes in the expression of other neurotrophins and associated receptors. NGF-mediated changes in urinary bladder function and altered referred somatic sensitivity (Nicol and Vasko, 2007) may involve changes in expression of nociception-related molecules, including brain-derived neurotrophic factor, tropomyosin-related receptor tyrosine kinases, TrkA and TrkB, as well as p75NTR (Vizzard, 2000d; Vizzard, 2001; Braas et al., 2006; de Groat and Yoshimura, 2009).
In the present study, we examined PACAP, VIP and associated receptors transcripts or protein expression in urothelium and detrusor smooth muscle in NGF-overexpressing (OE) and littermate wildtype mice using real-time quantitative reverse transcription-polymerase chain reaction (Q-PCR) and immunohistochemical approaches.
Materials and Methods
Animals
NGF-OE transgenic mice were generated at Roche Palo Alto (material transfer agreement with Roche Palo Alto and Dr. Debra Cockayne) in collaboration with Dr. Henry Sun at New York University Medical School as previously described (Cheppudira et al., 2008; Schnegelsberg et al., 2009). Animal genotype was confirmed by Southern and/or PCR analyses; all mice have the inbred genetic C57BL/6J background and were derived from F2 to F4 generations maintained through a hemizygous backcross strategy with C57BL/6J wildtype mice. Female mice used in this study were bred locally at The University of Vermont College of Medicine. The litters were of normal size and weight and behaviors (feeding, drinking, activity patterns) appeared normal. All experimental protocols involving animal use were approved by the University of Vermont Institutional Animal Care and Use Committee (IACUC # 08-085). Animal care was under the supervision of the University of Vermont’s Office of Animal Care Management in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health guidelines. All efforts were made to minimize the potential for animal pain, stress or distress.
Measurement of urinary bladder NGF by ELISA
Determination of NGF content in the urinary bladder and dorsal root ganglia (DRG; L1-S1) of NGF-OE transgenic mice and WT littermate controls was determined using enzyme-linked immunoassays (ELISAs) as previously described (Vizzard, 2000c; Cheppudira et al., 2008; Schnegelsberg et al., 2009). Microtiter plates (R & D Systems, Minneapolis, MN) were coated with a mouse anti-rat NGF antibody (R & D Systems, Minneapolis, MN). Sample and standard solutions were run in duplicate. A horseradish peroxidase-streptavidin conjugate was used to detect the antibody complex. Tetramethyl benzidine was the substrate and the enzyme activity was measured by the change in optical density. The NGF standard provided with this protocol generated a linear standard curve from 15 to 1000 pg/ml (R2 = 0.998, P ≤ 0.0001) for tissue samples. The absorbance values of standards and samples were corrected by subtraction of the background absorbance due to nonspecific binding. No samples fell below the minimum detection limits of the assay and no samples were diluted prior to use. Curve fitting of standards and evaluation of NGF content of samples was performed using a least squares fit as previously described (Vizzard, 2000c; Schnegelsberg et al., 2009).
Euthanasia and Tissue Harvest
Wildtype (WT) and NGE-OE littermate (n = 5 – 7 for each; 5–6 weeks of age) mice were deeply anesthetized with isoflurane (3–4%) and then euthanized via thoracotomy. The urinary bladder and lumbosacral DRG were quickly dissected under RNase-free conditions. The bladder was cut open along the midline and pinned to a sylgard-coated dish and the urothelium was removed with the aid of fine forceps and a dissecting microscope and all tissues were snap-frozen on dry ice prior to processing as previously described (Zvarova and Vizzard, 2005; Arms et al., 2009). The urothelium has suburothelial structures associated with it; the term urothelium in this paper refers to both urothelial and suburothelial structures. DRG were identified and isolated as previously described (Vizzard, 1997; Vizzard, 2000a; Vizzard, 2000d). Lumbosacral DRG (L6, S1) were specifically chosen for real-time quantitative reverse transcription-polymerase chain reaction (Q-PCR) analysis based upon the previously determined segmental representation of urinary bladder circuitry (Donovan et al., 1983; Keast and de Groat, 1992; Nadelhaft and Vera, 1995). Bladder afferents are not distributed within the L4–L5 DRG (Donovan et al., 1983; Keast and de Groat, 1992) that contain only somatic afferents nor are neurons that are involved in urinary bladder function observed in the L4–L5 spinal segments (Nadelhaft and Vera, 1995). Harvest of DRG was restricted to L6-S1 because previous studies have demonstrated the presence of PAC1 and VPAC2 receptors in these tissues using non-quantitative reverse-transcription polymerase chain reaction (Braas et al., 2006).
Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction (Q-PCR)
Total RNA was extracted using the STAT-60 total RNA/mRNA isolation reagent (Tel-Test‘B’, Friendswood, TX, USA) as previously described (Girard et al., 2002; Klinger et al., 2008). One to 2 mg of RNA per sample (urinary bladder or DRG) was used to synthesize complementary DNA using SuperScript II reverse transcriptase and a mix of random hexamer and oligo dT primers with the SuperScript II Preamplification System (Invitrogen, Carlsbad, CA, USA) in a 20 ml final reaction volume.
The quantitative PCR standards for all transcripts were prepared with the amplified PAC1, VPAC1, VPAC2, PACAP, VIP, NGF and 18S cDNA products ligated directly into pCR2.1 TOPO vector using the TOPO TA cloning kit (Invitrogen). The nucleotide sequences of the inserts were verified by automated fluorescent dideoxy dye terminator sequencing (Vermont Cancer Center DNA Analysis Facility). To estimate the relative expression of the receptor transcripts, 10-fold serial dilutions of stock plasmids were prepared as quantitative standards. The range of standard concentrations was determined empirically.
Real-time quantitative PCR was performed using SYBR Green I detection (Girard et al., 2002; Klinger et al., 2008; Arms et al., 2009). Complementary DNA templates, diluted 5-fold to minimize the inhibitory effects of the reverse transcription reaction components, were assayed using SYBR Green I JumpStart™. Taq ReadyMix™ (Sigma, St. Louis, MO, USA) containing 5 mM MgCl2, 200 mM dATP, dGTP, dCTP and dTTP, 0.64 U Taq DNA polymerase and 300 nM of each primer in a final 25-ml reaction volume. The real-time quantitative PCR was performed on an Applied Biosystems 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA, USA) (Girard et al., 2002; Klinger et al., 2008; Arms et al., 2009) using the following standard conditions: (i) serial heating at 50 °C for 2 min and 94 °C for 2 min; (ii) amplification over 40 cycles at 94 °C for 15 s and 60 to 64 °C depending on primers set for 40 s.
The amplified product from these amplification parameters was subjected to SYBR Green I melting analysis by ramping the temperature of the reaction samples from 60°C to 95°C. A single DNA melting profile was observed under these dissociation assay conditions demonstrating amplification of a single unique product free of primer dimers or other anomalous products. Oligonucleotide primer sequences for PACAP (Girard et al., 2002), VIP (Girard et al., 2002), PAC1(Braas and May, 1999), VPAC1(Girard et al., 2006), VPAC2(Girard et al., 2006), NGF (Schnegelsberg et al., 2009) and 18S (Girard et al., 2002; Klinger et al., 2008) used in these studies have been previously described.
For data analyses, a standard curve was constructed by amplification of serially diluted plasmids containing the target sequence. Data were analyzed at the termination of each assay using the Sequence Detection Software version 1.3.1 (Applied Biosystems, Norwalk, CT). In standard assays, default baseline settings were selected. The increase in SYBR Green I fluorescence intensity (DRn) was plotted as a function of cycle number and the threshold cycle was determined by the software as the amplification cycle at which the DRn first intersects the established baseline. All data are expressed as the relative quantity of the gene of interest normalized to the relative quantity of the housekeeping gene 18S. WT samples are set equal to 100%.
Immunohistochemistry
Bladder cryosections (10 μm) from wildtype (n = 5) and NGF-OE (n = 5) mice were prepared for immunohistochemistry using an on-slide processing technique (Klinger et al., 2008; Klinger and Vizzard, 2008; Arms et al., 2009). For immunohistochemical processing, the cryosections were incubated overnight at room temperature with rabbit anti-PAC1 (1:2000) (Braas and May, 1999), rabbit anti-PACAP (1:500; Bachem Americas, Inc., Torrance, CA) or rabbit anti-VIP (1:1000; Immunostar, Inc., Hudson, WI) diluted in 0.1M potassium phosphate buffered saline (KPBS) containing 1% goat serum. After washing, the preparations were incubated with a Cy3-conjugated species-specific secondary antibody (1:500) for 2 h at room temperature, rinsed and mounted with antifade medium (Citifluor Ltd., London) for fluorescent microscopy. Methodological and procedural controls were performed including preabsorption of PAC1, PACAP or VIP antisera with appropriate immunogen (1–3 μg/ml) that reduced staining to background levels.
Visualization and semi-quantitative analysis of PAC1, PACAP, or VIP in urothelium
PAC1, PACAP or VIP staining in bladder sections was visualized and images were captured using an Olympus fluorescence photomicroscope. The filter was set with an excitation range of 560–569 nm and emission range of 610–655 nm for visualization of Cy3. Images were captured, acquired in tiff format and imported into Meta Morph image analysis software (version 4.5r4; University Imaging, Downingtown, PA) (Klinger et al., 2008; Arms et al., 2009). The free hand drawing tool was used to select the urothelium and the urothelium was measured in total pixels area (Klinger et al., 2008; Arms et al., 2009). A threshold encompassing an intensity range of 100–250 grayscale values was applied to the region of interest in the least brightly stained condition first. The threshold was adjusted for each experimental series using concomitantly processed negative controls as a guide for setting background fluorescence. The same threshold was subsequently used for all images. Immunoreactivity was considered to be positive only when the staining for the marker of interest (PAC1, PACAP, VIP) exceeded the established threshold. Percent marker expression above threshold in the total area selected was calculated.
Visualization and semi-quantitative analysis of PAC1, PACAP or VIP in detrusor smooth muscle
Visualization of PAC1, PACAP and VIP-IR in detrusor smooth muscle of cryostat sections was identical to that described for the urothelium (above). Semi-quantification of PAC1, PACAP or VIP-IR in detrusor smooth muscle was performed as previously described (Klinger et al., 2007; Cheppudira et al., 2008) and modified from Brady et al. (Brady et al., 2004). Grayscale images acquired in tiff format were imported into Image J (Abramoff et al., 2004) and images were thresholded. Images of detrusor smooth muscle were acquired from the dome, body and neck region of the urinary bladder in WT and NGF-OE mice. A rectangle of fixed dimension (500 × 500 pixels) was placed on the section according to a random selection of x and y coordinates. This process was repeated seven times for each image of detrusor. The average optical density of PAC1, PACAP or VIP-IR in detrusor smooth muscle was then calculated. PAC1, PACAP and VIP-IR in the detrusor exhibited equivocal expression throughout detrusor of the dome, body and neck of the urinary bladder; thus, data from each region are pooled and presented as PAC1, PACAP or VIP-IR in detrusor smooth muscle.
Digital images were obtained using a CCD camera (MagnaFire SP; Optronics; Optical Analysis Corp., Nashua, NH) and LG-3 frame grabber (Scion Corp; Frederick, MD). Exposure times, brightness and contrast were held constant when acquiring images from experimental or control animals processed and analyzed on the same day. Images were imported into Adobe Photoshop 7.0 (Adobe Systems Incorporated, San Jose, CA) assemblage and labeling.
Statistical Analyses
One-way analysis of variance was used to evaluate differences among groups for Q-PCR. Percentage data from image analysis were arcsin transformed to meet the requirements of this statistical test. Animals, processed and analyzed on the same day, were tested as a block in the ANOVA. Differences were considered statistically significant if p ≤ 0.05. When F ratios exceeded the critical value (p ≤ 0.05), the Newman-Keul’s post-hoc test was used to compare the experimental means.
Results
NGF expression in urothelium, detrusor and lumbosacral DRG of WT and NGF-OE mice
Consistent with our previous studies (Cheppudira et al., 2008; Schnegelsberg et al., 2009), NGF transcript and protein expression was significantly (p ≤ 0.01) increased in urothelium of NGF OE mice; no changes were observed in detrusor smooth muscle between NGF OE and littermate WT mice (data not shown). Similarly, no changes in NGF transcript were detected in any DRG level examined (L1-S1) between NGF OE and littermate WT mice (data not shown).
PAC1, VPAC1, and VPAC2 receptor transcript expression in urothelium and detrusor of WT and NGE-OE mice
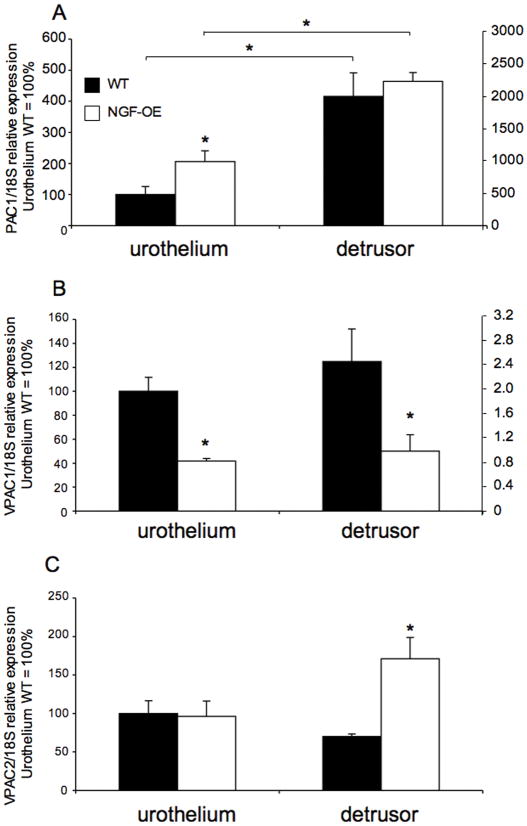
Consistent with previous studies in rats (Braas et al., 2006; Girard et al., 2008), PAC1, VPAC1 and VPAC2 receptor transcripts were expressed in the urothelium and detrusor smooth muscle of mouse urinary bladder (Fig. 1A–C). In urothelium of NGF-OE mice, PAC1 receptor transcript exhibited a significant (p ≤ 0.05) increase in expression whereas no changes were exhibited in detrusor smooth muscle between wildtype (WT) and NGF-OE mice (Fig. 1A). PAC1 receptor transcript expression was also significantly greater in detrusor smooth muscle compared to urothelium in both WT and NGF-OE mice (Fig. 1A). In urothelium and detrusor smooth muscle of NGF-OE mice, VPAC1 receptor transcript expression was significantly (p ≤ 0.01) decreased compared to WT with no changes in receptor transcript expression observed between tissues (urothelium vs. detrusor) (Fig. 1B). In detrusor smooth muscle of NGF OE mice, VPAC2 receptor transcript expression was significantly (p ≤ 0.01) increased whereas no changes were observed in the urothelium or between detrusor and urothelium in WT and NGF-OE mice (Fig. 1C).
Figure 1.
Regulation of PAC1, VPAC1 and VPAC2 receptor transcript levels in littermate wildtype (WT) and NGF overexpressing (NGF-OE) mice in urothelium and detrusor smooth muscle. Relative expression of the urothelium and detrusor receptor transcripts are expressed as a percentage of WT urothelium and normalized to the relative expression of the housekeeping gene, 18S. A: PAC1 mRNA expression. B: VPAC1 mRNA expression. C: VPAC2 mRNA expression. Samples size are n of 5 – 7; *, p ≤ 0.01 versus control.
PAC1-Immunoreactivity (IR) in urothelium and detrusor of WT and NGE-OE mice
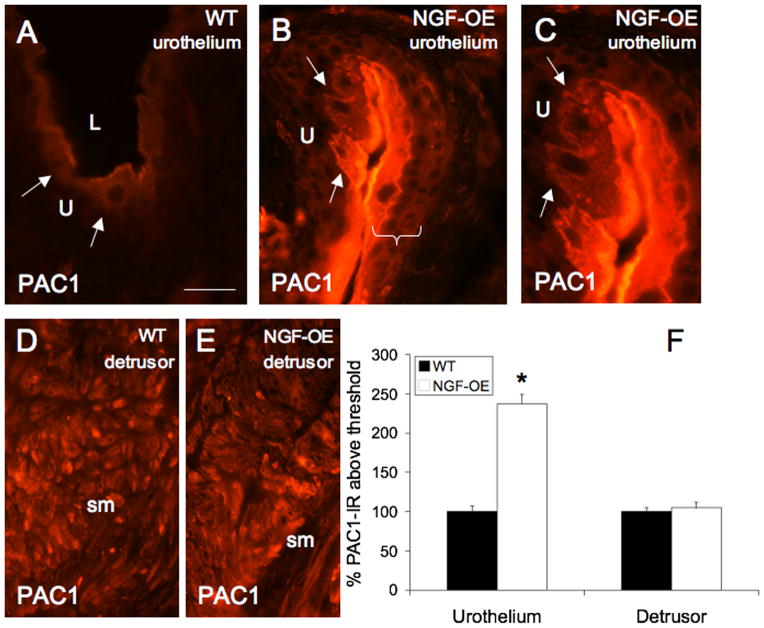
Changes in PAC1-IR in tissue sections paralleled changes in PAC1 receptor transcript expression. PAC1-IR was significantly increased in urothelium of NGF-OE compared to WT (Fig. 2A–C, F). PAC1-IR was observed in WT urothelium, primarily in apical cells, consistent with previous demonstrations of PAC1-IR in rat urothelium (Braas et al., 2006). In urothelium of NGF-OE mice, PAC1-IR was increased in apical urothelial cells (Fig. 2B–C) and PAC1-IR was also observed in other urothelial layers (intermediate and basal). No changes in PAC1-IR were observed in detrusor smooth muscle between WT and NGF-OE mice (Fig. 2D–F).
Figure 2.
PAC1-immunoreactivity in urothelium (U) (A–C) and detrusor smooth muscle (sm) (D–E) of littermate wildtype (WT) and NGF overexpressing (NGF-OE) mice. Faint PAC1-IR was present in apical urothelial cells of WT urothelium (A, arrows). In NGF-OE mice, PAC1-IR was increased in apical urothelial cells (B–C, arrows) but was also expressed in all urothelial cell layers (B, bracket). PAC1-IR was expressed in detrusor smooth muscle of WT and NGF-OE mice (D–E). F. Summary histogram of PAC1 expression in the U and detrusor sm in WT and NGF-OE mice. Values are mean ± SEM; (n = 5). *, p ≤ 0.01. L, lumen. Calibration bar equals 50 μm.
PACAP, VIP transcript expression in urothelium and detrusor of WT and NGE-OE mice
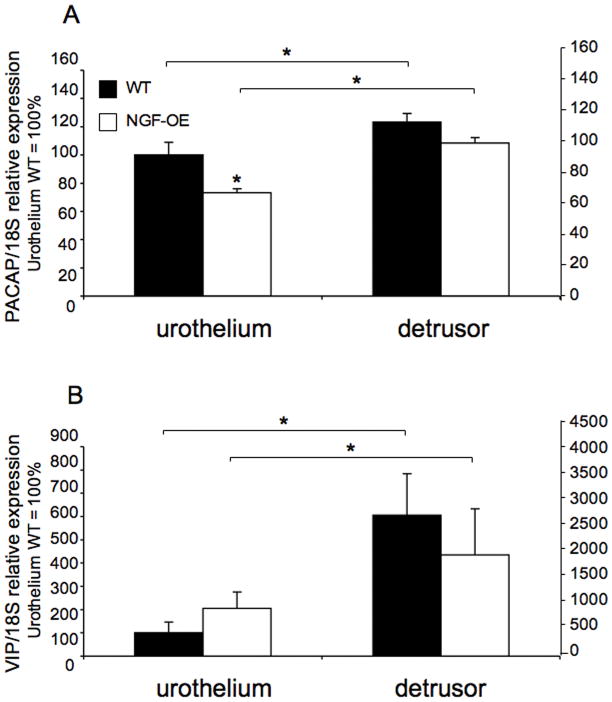
PACAP transcript expression significantly (p ≤ 0.05) decreased in the urothelium of NGF-OE mice compared to WT (Fig. 3A). PACAP transcript expression was significantly (p ≤ 0.05) greater in detrusor smooth muscle compared to urothelium in both WT and NGF-OE mice (Fig. 3A). No changes in VIP transcript expression were detected in urothelium or detrusor of WT or NGF-OE mice (Fig. 3B). However, VIP transcript expression was significantly (p ≤ 0.01) greater in detrusor smooth muscle compared to urothelium in both WT and NGF-OE mice (Fig. 3B).
Figure 3.
Regulation of PACAP and VIP transcript levels in littermate wildtype (WT) and NGF overexpressing (NGF-OE) mice in urothelium and detrusor smooth muscle. Relative expression of the urothelium and detrusor receptor transcripts are expressed as a percentage of WT urothelium and normalized to the relative expression of the housekeeping gene, 18S. A: PACAP mRNA expression. B: VIP mRNA expression. Samples size are n of 5 – 7; *, p ≤ 0.01 versus control.
PACAP, VIP-immunoreactivity (IR) in urothelium and detrusor of WT and NGE-OE mice
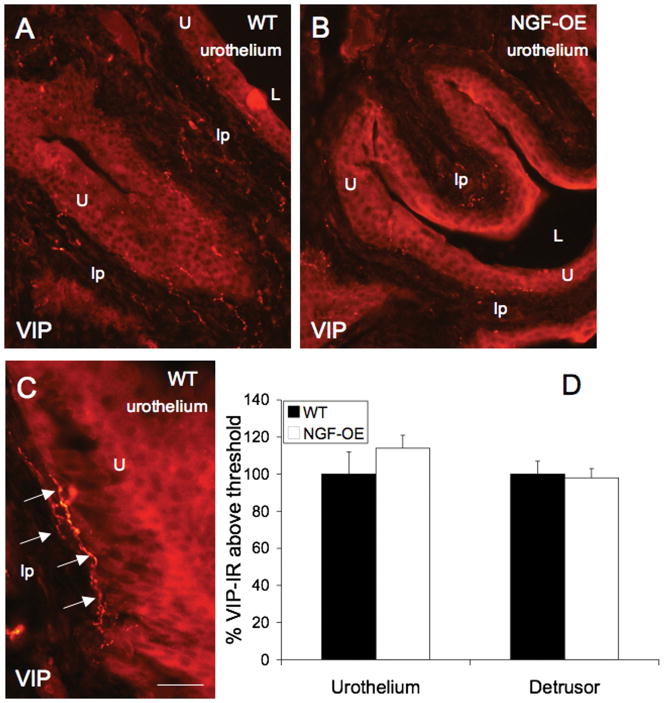
Changes in PACAP-IR in tissue sections paralleled changes in PACAP receptor transcript expression. PACAP-IR was significantly (p ≤ 0.01) decreased in urothelium of NGF-OE compared to WT (Fig. 4A–C). PACAP-IR was observed in WT urothelium in all cell layers (apical, intermediate and basal) (Fig. 4A, arrows). Some evidence of PACAP-IR in putative suburothelial nerves was also observed in WT mice (Fig. 4A). In urothelium of NGF-OE mice, PACAP-IR was decreased throughout the urothelium (Fig. 4B). No changes in PACAP-IR were observed in detrusor smooth muscle between WT and NGF-OE mice (Fig. 4C). No changes in VIP-IR were observed in urothelium or detrusor smooth muscle in WT and NGF-OE mice (Fig. 5A–D). In urothelium from WT and NGF-OE mice, VIP-IR was expressed throughout all urothelial cell layers (Fig. 5A, B). VIP-IR in putative suburothelial nerves was observed in urinary bladder sections from both WT and NGF-OE mice (Fig. 5A–C, arrows) consistent with previous studies (Fahrenkrug et al., 1998; Lasanen et al., 1992).
Figure 4.
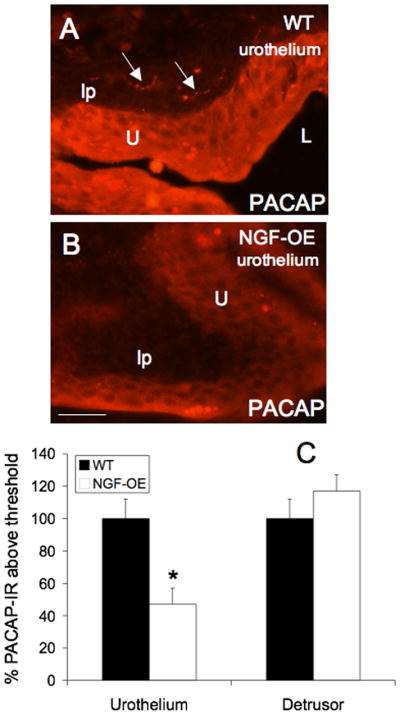
PACAP-immunoreactivity in urothelium (U) (A–C) and detrusor smooth muscle (sm) (C) of littermate wildtype (WT) and NGF overexpressing (NGF-OE) mice. Robust PACAP-IR was present throughout the WT urothelium (A). Some evidence of PACAP-IR in presumptive suburothelial nerve fibers was observed (A, arrows). In NGF-OE mice, PACAP-IR was decreased in all urothelial cell layers (B). C. Summary histogram of PACAP expression in the U and detrusor sm in WT and NGF-OE mice. No changes in PACAP-IR were observed in WT or NGF-OE sm. Values are mean ± SEM; (n = 5). *, p ≤ 0.01. L, lumen; lp, lamina propria. Calibration bar equals 50 μm.
Figure 5.
VIP-immunoreactivity in urothelium (U) (A–C) and detrusor smooth muscle (sm) (C) of littermate wildtype (WT) and NGF overexpressing (NGF-OE) mice. VIP-IR was present throughout the WT urothelium (A, C) and NGF-OE (B) urothelium. VIP-IR in presumptive suburothelial nerve fibers was observed in WT and NGF-OE urinary bladder (C, arrows). C. Summary histogram of VIP expression in the U and detrusor sm in WT and NGF-OE mice. No changes in VIP-IR were observed in WT or NGF-OE U or sm. Values are mean ± SEM; (n = 5). *, p ≤ 0.01. L, lumen; lp, lamina propria. Calibration bar equals 100 μm in A, B and 30 μm in C.
PAC1, VPAC1, and VPAC2 receptor transcript expression in L6-S1 dorsal root ganglia (DRG) of WT and NGE-OE mice
Consistent with previous rat studies (Braas et al., 2006; Girard et al., 2008), PAC1, VPAC1 and VPAC2 receptor transcripts are expressed in mouse lumbosacral (L6-S1) DRG (data not shown). No changes in PAC1, VPAC1 or VPAC2 receptor transcript expression was observed between WT and NGF-OE mice or between L6 and S1 DRG (data not shown)
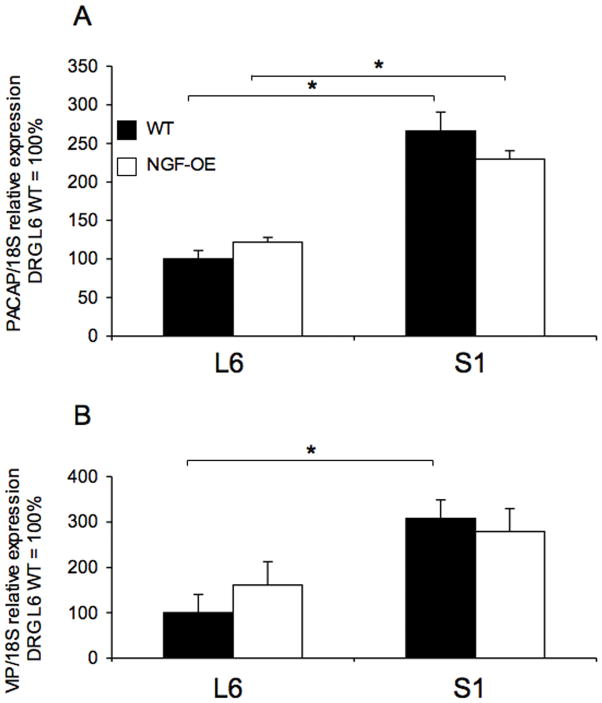
PACAP and VIP in L6-S1 dorsal root ganglia (DRG) of WT and NGE-OE mice
No changes in PACAP or VIP transcript expression were observed between WT and NGF-OE mice in L6 or S1 DRG (Fig. 6A–B). In S1 DRG, PACAP transcript expression was significantly greater in both WT and NGF-OE mice compared to L6 levels (Fig. 6A). In S1 DRG, VIP transcript expression was significantly greater in WT mice compared to L6 levels (Fig. 6B).
Figure 6.
Regulation of PACAP and VIP mRNA expression in lumbosacral (L6-S1) dorsal root ganglia (DRG) in littermate wildtype (WT) and NGF overexpressing (NGF-OE) mice. Relative expression of the L6 and S1 receptor transcripts are expressed as a percentage of WT L6 DRG and normalized to the relative expression of the housekeeping gene, 18S. A: PACAP mRNA expression. B: VIP mRNA expression. Samples size are no 5 to 7; *, p ≤ 0.01 versus control.
Discussion
We examined PACAP, VIP and associated receptors (PAC1, VPAC1, VPAC2) transcripts or protein expression in urothelium and detrusor smooth muscle and lumbosacral DRG in NGF-overexpressing (OE) and littermate wildtype mice using real-time quantitative reverse transcription-polymerase chain reaction and immunohistochemical approaches. PAC1 receptor transcript and PAC1-immunoreactivity was significantly increased in urothelium of NGF-OE mice whereas PACAP transcript and PACAP-immunoreactivity were decreased in urothelium of NGF-OE mice. In contrast, VPAC1 receptor transcript was decreased in both urothelium and detrusor smooth muscle of NGF-OE mice. VPAC2 receptor transcript was significantly increased in the detrusor smooth muscle in NGF-OE mice. VIP transcript expression and immunostaining was not altered in urinary bladder of NGF-OE mice. Changes in PACAP, VIP and associated receptors transcripts and protein expression in micturition pathways resemble some, but not all, changes observed after induction of urinary bladder inflammation (Girard et al., 2008) known to involve NGF production (Oddiah et al., 1998; Vizzard, 2000c; Bjorling et al., 2001; Dupont et al., 2001; Murray et al., 2004; Guerios et al., 2008; Klinger and Vizzard, 2008).
Increased urinary bladder NGF content may underlie many of the sensory changes that occur in patients with OAB symptoms or IC/BPS, including irritative voiding symptoms and pain in the case of IC/BPS (Okragly et al., 1999; Kim et al., 2005; Kim et al., 2006; Liu and Kuo, 2007; Liu and Kuo, 2008a; Liu and Kuo, 2008b). Altered NGF content is associated with urinary bladder inflammation and dysfunction in rodents (Vizzard, 2001; Zvarova et al., 2004; Braas et al., 2006; Klinger and Vizzard, 2008; Schnegelsberg et al., 2009). IC/BPS is a chronic inflammatory bladder disease of unknown etiology characterized by urinary frequency, urgency, and suprapubic/pelvic pain (Driscoll and Teichman, 2001; Sant and Hanno, 2001). Pain and altered bladder/visceral hypersensitivity in IC/BPS patients may involve organizational or functional changes in peripheral bladder afferents and central pathways such that bladder afferent neurons become sensitized and hyper-responsive to normally innocuous stimuli such as bladder filling (Driscoll and Teichman, 2001; Sant and Hanno, 2001). A few studies have demonstrated increased innervation of the bladder suburothelial and detrusor layers by PGP9.5-positive, substance P-positive or sympathetic nerve fibers in IC/BPS patients (Christmas et al., 1990; Lundeberg et al., 1993; Hoyle et al., 1998; Peeker et al., 2000). In NGF-OE mice, there was a marked increase in the density of CGRP- and substance P-positive C-fiber sensory afferents, neurofilament 200 (NF200) myelinated sensory afferents and tyrosine hydroxylase-positive sympathetic nerve fibers within the suburothelial nerve plexus of the urinary bladder (Schnegelsberg et al., 2009). In this study, we have begun to determine whether chronic NGF-OE in the urothelium causes additional neurochemical changes in expression of PACAP, VIP and associated receptors (PAC1, VPAC1, VPAC2) in the urinary bladder and lumbosacral DRG that might contribute to the bladder hyperreflexia and pelvic hypersensitivity in NGF-OE mice.
Pituitary adenylate cyclase activating polypeptide (PACAP) peptides have diverse functions in the endocrine, nervous, gastrointestinal and cardiovascular systems (Braas and May, 1996; Arimura, 1998) and differential effects on nociception (Sandor et al., 2009) through PAC1, VPAC1 and VPAC2 G protein-coupled receptors. High levels of PACAP and VIP expression have been identified in many central nervous system neurons and in sensory and autonomic ganglia (Arimura et al., 1991; Sundler et al., 1996; Moller et al., 1997a; Moller et al., 1997b; Arimura, 1998; Braas et al., 1998). Both PACAP- and VIP-immunoreactivity have been identified in urinary bladder (Fahrenkrug and Hannibal, 1998; Mohammed et al., 2002). PACAP and VIP peptides regulate smooth muscle function, either directly or by facilitating cholinergic and nitric oxide mechanisms, in a tissue- and species-specific manner (Mizumoto et al., 1992; Onaga et al., 1998; Fox-Threlkeld et al., 1999; Seebeck et al., 2002; Zizzo et al., 2004). A number of studies have implicated PACAP in lower urinary tract function (Vizzard, 2000d; Zvarova et al., 2005; Braas et al., 2006; Herrera et al., 2006).
In the present study using mice with chronic overexpression of NGF in the urothelium, we have demonstrated increased PAC1 receptor transcript expression and immunostaining in the urothelium. These data are consistent with previous studies demonstrating that NGF increases PAC1 mRNA levels in rat PC12 cells (Jamen et al., 2000; Jamen et al., 2002) and that reduced PAC1 receptor mRNA expression is present in NGF null embryos (Andres et al., 2008). In this study, VPAC1 receptor transcript expression was reduced in both the urothelium and detrusor smooth muscle in NGF-OE mice and no changes in VPAC2 receptor transcript expression were observed in either urothelium or detrusor in NGF-OE mice. PACAP transcript expression and immunostaining was decreased in urothelium of NGF-OE mice whereas VIP transcript expression and immunostaining was not altered in urinary bladder of NGF-OE mice. In contrast, enhanced target-derived NGF availability has been shown to increase PACAP expression in small nociceptive neurons in DRG (Vizzard, 2000d; Jongsma Wallin et al., 2001; Jongsma Wallin et al., 2003). In experimental inflammation paradigms, the expression of several sensory peptides including PACAP is increased while VIP levels, by contrast, are unchanged (Donaldson et al., 1992; Lu et al., 2005). NGF does not increase VIP expression in DRG that may be consistent with the target tissue neurotrophic model to selectively augment PACAP expression in DRG (Mulderry and Lindsay, 1990) (V. May and K. Braas, unpublished observations). Although many studies have demonstrated similarities in receptors and channels expressed and neurochemical properties between urothelial cells and DRG cells (Birder, 2005; Birder, 2006; Birder, 2009), the present study demonstrates differences in NGF regulation of PACAP expression between these cell types.
VPAC1 and VPAC2 receptor transcript expression was differentially affected in detrusor smooth muscle of NGF-OE mice. VPAC1 receptor transcript expression was reduced whereas VPAC2 receptor expression was increased in detrusor smooth muscle in NGF-OE mice. Changes in transcript expression in detrusor in mice with chronic overexpression of NGF in the urothelium suggest urothelial-mesenchymal interactions whereby urothelial NGF expression may influence detrusor smooth muscle properties as previously demonstrated for development and differentiation of detrusor smooth muscle (Liu et al., 2000; Baskin et al., 2001). In contrast to observed changes in VIP/PACAP and receptor systems in the urinary bladder of NGF-OE mice, no changes were observed in lumbosacral (L6-S1) DRG of NGF-OE mice. This was not surprising given the absence of increased NGF content in these DRG as demonstrated with ELISAs and suggests absence of retrograde NGF transport from urinary bladder to DRG in these mice.
It is clear from the present studies that chronic overexpression of NGF in the urothelium results in some, but not all of the same changes in VIP, PACAP and receptors transcript or protein expression in micturition pathways demonstrated in rodent models of CYP-induced bladder inflammation (Vizzard, 2000d; Girard et al., 2008). We have previously demonstrated involvement of many chemical mediators (e.g., neurotrophins, cytokines, chemokines, neuropeptides) produced in micturition reflex pathways with cystitis that may underlie changes in neurochemical (Vizzard, 2001; Zvarova et al., 2004; Braas et al., 2006; Klinger and Vizzard, 2008), organizational (Vizzard, 1999; Vizzard, 2000b) and electrophysiological (Yoshimura and de Groat, 1999) properties of micturition pathways and referred somatic sensitivity (Guerios et al., 2006; Guerios et al., 2008). Although changes in PAC1 receptor transcript and protein in NGF-OE mice paralleled changes observed in the rodent model of CYP-induced cystitis, VPAC1, VPAC2 and PACAP transcript and protein expression did not change or changed in an opposite manner compared to the CYP-induced bladder inflammation model. These results suggest that chemical mediators upregulated with CYP-induced bladder inflammation other than or in addition to NGF (e.g., neurotrophins (Vizzard, 2000c), cytokines (Malley and Vizzard, 2002), chemokines (Yuridullah et al., 2006; Arms et al., 2009), neuropeptides (Vizzard, 2001; Braas et al., 2006) contribute to altered VPAC1, VPAC2 and PACAP transcript and protein expression in micturition pathways following CYP-induced cystitis. Given the increased PAC1 receptor transcript and protein expression in urothelium of NGF-OE mice, current studies are addressing the contribution of PACAP/PAC1 signaling to demonstrated bladder hyperreflexia and referred pelvic hypersensitivity in NGF-OE mice (Schnegelsberg et al., 2009).
Acknowledgments
The authors thank Dr. Debra Cockayne, Roche Palo Alto, for the generous gift of NGF-OE mouse breeders used in the present study. The authors gratefully acknowledge the technical expertise and support provided by the VT Cancer Center DNA Analysis Facility.
Grants
This work was funded by NIH grants DK051369, DK060481, and DK065989. NIH Grant Number P20 RR16435 from the COBRE Program of the National Center also supported the project for research resources.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11(7):36–42. [Google Scholar]
- Andres R, Herraez-Baranda LA, Thompson J, Wyatt S, Davies AM. Regulation of sympathetic neuron differentiation by endogenous nerve growth factor and neurotrophin-3. Neurosci Lett. 2008;431(3):241–246. doi: 10.1016/j.neulet.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Arimura A. Pituitary adenylate cyclase activating polypeptide (PACAP): discovery and current status of research. Regul Pept. 1992;37(3):287–303. [PubMed] [Google Scholar]
- Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48(5):301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129(5):2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- Arms L, Girard BM, Vizzard MA. Expression and Function of CXCL12/CXCR4 in Rat Urinary Bladder with Cyclophosphamide (CYP) - Induced Cystitis. Am J Physiol Renal Physiol. 2009;298(3):F589–600. doi: 10.1152/ajprenal.00628.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin L, DiSandro M, Li Y, Li W, Hayward S, Cunha G. Mesenchymal-epithelial interactions in bladder smooth muscle development: effects of the local tissue environment. J Urol. 2001;165(4):1283–1288. [PubMed] [Google Scholar]
- Birder LA. More than just a barrier: urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol. 2005;289(3):F489–495. doi: 10.1152/ajprenal.00467.2004. [DOI] [PubMed] [Google Scholar]
- Birder LA. Urinary bladder urothelium: molecular sensors of chemical/thermal/mechanical stimuli. Vascul Pharmacol. 2006;45(4):221–226. doi: 10.1016/j.vph.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Birder LA. Urothelial signaling. Auton Neurosci. 2009;153(1–2):33–40. doi: 10.1016/j.autneu.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorling DE, Jacobsen HE, Blum JR, et al. Intravesical Escherichia coli lipopolysaccharide stimulates an increase in bladder nerve growth factor. BJU Int. 2001;87(7):697–702. doi: 10.1046/j.1464-410x.2001.02138.x. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides, PACAP-38 and PACAP-27, regulation of sympathetic neuron catecholamine, and neuropeptide Y expression through activation of type I PACAP/VIP receptor isoforms. Ann N Y Acad Sci. 1996;805:204–216. doi: 10.1111/j.1749-6632.1996.tb17484.x. discussion 217–208. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. J Biol Chem. 1999;274(39):27702–27710. doi: 10.1074/jbc.274.39.27702. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci. 1998;18(23):9766–9779. doi: 10.1523/JNEUROSCI.18-23-09766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braas KM, May V, Zvara P, et al. Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R951–962. doi: 10.1152/ajpregu.00734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady CM, Apostolidis AN, Harper M, et al. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int. 2004;93(6):770–776. doi: 10.1111/j.1464-410X.2003.04722.x. [DOI] [PubMed] [Google Scholar]
- Cheppudira BP, Girard BM, Malley SE, et al. Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2008;295(3):F826–836. doi: 10.1152/ajprenal.90305.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmas TJ, Rode J, Chapple CR, Milroy EJ, Turner-Warwick RT. Nerve fibre proliferation in interstitial cystitis. Virchows Arch A Pathol Anat Histopathol. 1990;416(5):447–451. doi: 10.1007/BF01605152. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol. 2009;(194):91–138. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson LF, Harmar AJ, McQueen DS, Seckl JR. Increased expression of preprotachykinin, calcitonin gene-related peptide, but not vasoactive intestinal peptide messenger RNA in dorsal root ganglia during the development of adjuvant monoarthritis in the rat. Brain Res Mol Brain Res. 1992;16(1–2):143–149. doi: 10.1016/0169-328x(92)90204-o. [DOI] [PubMed] [Google Scholar]
- Donovan MK, Winternitz SR, Wyss JM. An analysis of the sensory innervation of the urinary system of the rat. Brain Res Bull. 1983;11:321–324. doi: 10.1016/0361-9230(83)90168-5. [DOI] [PubMed] [Google Scholar]
- Driscoll A, Teichman JMH. How do patients with interstitial cystitis present? J Urol. 2001;166(6):2118–2120. [PubMed] [Google Scholar]
- Dupont MC, Spitsbergen JM, Kim KB, Tuttle JB, Steers WD. Histological and neurotrophic changes triggered by varying models of bladder inflammation. J Urol. 2001;166(3):1111–1118. [PubMed] [Google Scholar]
- Fahrenkrug J, Hannibal J. Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience. 1998;83(4):1261–1272. doi: 10.1016/s0306-4522(97)00474-0. [DOI] [PubMed] [Google Scholar]
- Fizanne L, Sigaudo-Roussel D, Saumet JL, Fromy B. Evidence for the involvement of VPAC1 and VPAC2 receptors in pressure-induced vasodilatation in rodents. J Physiol. 2004;554(Pt 2):519–528. doi: 10.1113/jphysiol.2003.053835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox-Threlkeld JA, McDonald TJ, Woskowska Z, Iesaki K, Daniel EE. Pituitary adenylate cyclase-activating peptide as a neurotransmitter in the canine ileal circular muscle. J Pharm Exp Therap. 1999;290(1):66–75. [PubMed] [Google Scholar]
- Girard BA, Lelievre V, Braas KM, et al. Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. J Neurochem. 2006;99(2):499–513. doi: 10.1111/j.1471-4159.2006.04112.x. [DOI] [PubMed] [Google Scholar]
- Girard BM, May V, Bora SH, Fina F, Braas KM. Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regul Pept. 2002;109(1–3):89–101. doi: 10.1016/s0167-0115(02)00191-x. [DOI] [PubMed] [Google Scholar]
- Girard BM, Wolf-Johnston A, Braas KM, Birder LA, May V, Vizzard MA. PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci. 2008;36(1–3):310–320. doi: 10.1007/s12031-008-9104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerios SD, Wang ZY, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett. 2006;392(3):193–197. doi: 10.1016/j.neulet.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R111–22. doi: 10.1152/ajpregu.00728.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GM, Braas KM, May V, Vizzard MA. PACAP enhances mouse urinary bladder contractility and is upregulated in micturition reflex pathways after cystitis. Ann N Y Acad Sci. 2006;1070:330–336. doi: 10.1196/annals.1317.040. [DOI] [PubMed] [Google Scholar]
- Hoyle GW, Graham RM, Finkelstein JB, Nguyen KP, Gozal D, Friedman M. Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol. 1998;18(2):149–157. doi: 10.1165/ajrcmb.18.2.2803m. [DOI] [PubMed] [Google Scholar]
- Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Reg Integr Comp Physiol. 2003;284(2):R574–R585. doi: 10.1152/ajpregu.00465.2002. [DOI] [PubMed] [Google Scholar]
- Jaggar SI, Scott HCF, Rice ASC. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth. 1999;83(3):442–448. doi: 10.1093/bja/83.3.442. [DOI] [PubMed] [Google Scholar]
- Jamen F, Bouschet T, Laden JC, Bockaert J, Brabet P. Up-regulation of the PACAP type-1 receptor (PAC1) promoter by neurotrophins in rat PC12 cells and mouse cerebellar granule cells via the Ras/mitogen-activated protein kinase cascade. J Neurochem. 2002;82(5):1199–1207. doi: 10.1046/j.1471-4159.2002.01124.x. [DOI] [PubMed] [Google Scholar]
- Jamen F, Laden JC, Bouschet T, Rodriguez-Henche N, Bockaert J, Brabet P. Nerve growth factor upregulates the PAC1 promoter by activating the MAP kinase pathway in rat PC12 cells. Ann N Y Acad Sci. 2000;921:390–394. doi: 10.1111/j.1749-6632.2000.tb07002.x. [DOI] [PubMed] [Google Scholar]
- Jongsma Wallin H, Danielsen N, Johnston JM, Gratto KA, Karchewski LA, Verge VMK. Exogenous NT-3 and NGF differentially modulate PACAP expression in adult sensory neurons, suggesting distinct roles in injury and inflammation. Eur J Neurosci. 2001;14:267–282. doi: 10.1046/j.0953-816x.2001.01641.x. [DOI] [PubMed] [Google Scholar]
- Jongsma Wallin H, Pettersson LM, Verge VM, Danielsen N. Effect of anti-nerve growth factor treatment on pituitary adenylate cyclase activating polypeptide expression in adult sensory neurons exposed to adjuvant induced inflammation. Neuroscience. 2003;120(2):325–331. doi: 10.1016/s0306-4522(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Keast JR, de Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- Kim JC, Park EY, Hong SH, Seo SI, Park YH, Hwang TK. Changes of urinary nerve growth factor and prostaglandins in male patients with overactive bladder symptom. Int J Urol. 2005;12(10):875–880. doi: 10.1111/j.1442-2042.2005.01140.x. [DOI] [PubMed] [Google Scholar]
- Kim JC, Park EY, Seo SI, Park YH, Hwang TK. Nerve growth factor and prostaglandins in the urine of female patients with overactive bladder. J Urol. 2006;175(5):1773–1776. doi: 10.1016/S0022-5347(05)00992-4. discussion 1776. [DOI] [PubMed] [Google Scholar]
- Klinger MB, Dattilio A, Vizzard MA. Expression of cyclooxygenase-2 in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2007;293(2):R677–685. doi: 10.1152/ajpregu.00305.2007. [DOI] [PubMed] [Google Scholar]
- Klinger MB, Girard B, Vizzard MA. p75(NTR) expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. J Comp Neurol. 2008;507(3):1379–1392. doi: 10.1002/cne.21627. [DOI] [PubMed] [Google Scholar]
- Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2008;295(6):F1778–1789. doi: 10.1152/ajprenal.90501.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasanen LT, Tammela TL, Liesi P, Waris T, Polak JM. The effect of acute distension on vasoactive intestinal polypeptide (VIP), neuropeptide Y (NPY) and substance P (SP) immunoreactive nerves in the female rat urinary bladder. Urol Res. 1992;20(4):259–263. doi: 10.1007/BF00300255. [DOI] [PubMed] [Google Scholar]
- Liang FX, Bosland MC, Huang H, et al. Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J Cell Biol. 2005;171(5):835–844. doi: 10.1083/jcb.200505035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Zhao H, Sun TT. A tissue-specific promoter that can drive a foreign gene to express in the suprabasal urothelial cells of transgenic mice. Proc Natl Acad Sci USA. 1995;92(3):679–683. doi: 10.1073/pnas.92.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden A, Cardell LO, Yoshihara S, Nadel JA. Bronchodilation by pituitary adenylate cyclase-activating peptide and related peptides. Eur Respir J. 1999;14(2):443–451. doi: 10.1034/j.1399-3003.1999.14b34.x. [DOI] [PubMed] [Google Scholar]
- Liu HT, Kuo HC. Intravesical botulinum toxin A injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology. 2007;70(3):463–468. doi: 10.1016/j.urology.2007.04.038. [DOI] [PubMed] [Google Scholar]
- Liu HT, Kuo HC. Urinary nerve growth factor level could be a potential biomarker for diagnosis of overactive bladder. J Urol. 2008a;179(6):2270–2274. doi: 10.1016/j.juro.2008.01.146. [DOI] [PubMed] [Google Scholar]
- Liu HT, Kuo HC. Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. Urology. 2008b;72(1):104–108. doi: 10.1016/j.urology.2008.01.069. [DOI] [PubMed] [Google Scholar]
- Liu W, Li Y, Cunha S, Hayward G, Baskin L. Diffusable growth factors induce bladder smooth muscle differentiation. In Vitro Cell Dev Biol Anim. 2000;36(7):476–484. doi: 10.1290/1071-2690(2000)036<0476:dgfibs>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lu CL, Pasricha PJ, Hsieh JC, et al. Changes of the neuropeptides content and gene expression in spinal cord and dorsal root ganglion after noxious colorectal distension. Regul Pept. 2005;131(1–3):66–73. doi: 10.1016/j.regpep.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Lundeberg T, Liedberg H, Nordling L, Theodorsson E, Owzarski A, Ekman P. Interstitial cystitis: correlation with nerve fibres, mast cells and histamine. Br J Urol. 1993;71(4):427–429. doi: 10.1111/j.1464-410x.1993.tb15986.x. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Lecci A, Santicioli P, Del Bianco E, Giuliani S. Cyclophosphamide cystitis in rats: involvement of capsaicin-sensitive primary afferents. J Auton Nerv Syst. 1992;38(3):201–208. doi: 10.1016/0165-1838(92)90031-b. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Lecci A, Santicioli P, Del Bianco E, Giuliani S. Cyclophosphamide-induced cystitis in rats: involvement of capsaicin-sensitive primary afferents. Agents Actions. 1993;38:C28–C30. doi: 10.1007/BF01991127. [DOI] [PubMed] [Google Scholar]
- Malley SE, Vizzard MA. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiological Genomics. 2002;9(1):5–13. doi: 10.1152/physiolgenomics.00117.2001. [DOI] [PubMed] [Google Scholar]
- Mizumoto A, Fujimura M, Ohtawa M, et al. Pituitary adenylate cyclase activating polypeptide stimulates gallbladder motility in conscious dogs. Regul Pept. 1992;42(1–2):39–50. doi: 10.1016/0167-0115(92)90022-m. [DOI] [PubMed] [Google Scholar]
- Mohammed H, Hannibal J, Fahrenkrug J, Santer R. Distribution and regional variation of pituitary adenylate cyclase activating polypeptide and other neuropeptides in the rat urinary bladder and ureter: effects of age. Urol Res. 2002;30(4):248–255. doi: 10.1007/s00240-002-0261-6. [DOI] [PubMed] [Google Scholar]
- Moller K, Reimer M, Ekblad E, et al. The effects of axotomy and preganglionic denervation on the expression of pituitary adenylate cyclase activating peptide (PACAP), galanin and PACAP type 1 receptors in the rat superior cervical ganglion. Brain Res. 1997a;775(1–2):166–182. doi: 10.1016/s0006-8993(97)00923-2. [DOI] [PubMed] [Google Scholar]
- Moller K, Reimer M, Hannibal J, Fahrenkrug J, Sundler F, Kanje M. Pituitary adenylate cyclase-activating peptide (PACAP) and PACAP type 1 receptor expression in regenerating adult mouse and rat superior cervical ganglia in vitro. Brain Res. 1997b;775(1–2):156–165. doi: 10.1016/s0006-8993(97)00937-2. [DOI] [PubMed] [Google Scholar]
- Mulderry PK, Lindsay RM. Rat dorsal root ganglion neurons in culture express vasoactive intestinal polypeptide (VIP) independently of nerve growth factor. Neurosci Lett. 1990;108(3):314–320. doi: 10.1016/0304-3940(90)90660-2. [DOI] [PubMed] [Google Scholar]
- Murray E, Malley SE, Qiao LY, Hu VY, Vizzard MA. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J Urol. 2004;172(6 Pt 1):2434–2439. doi: 10.1097/01.ju.0000143549.29867.4e. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL. Central nervous system neurons infected by pseudorabies virus injected into the rat urinary bladder following unilateral transection of the pelvic nerve. J Comp Neurol. 1995;359:443–456. doi: 10.1002/cne.903590307. [DOI] [PubMed] [Google Scholar]
- Nicol GD, Vasko MR. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol Interv. 2007;7(1):26–41. doi: 10.1124/mi.7.1.6. [DOI] [PubMed] [Google Scholar]
- Oddiah D, Anand P, McMahon SB, Rattray M. Rapid increase of NGF, BDNF and NT-3 mRNAs in inflamed bladder. Neuroreport. 1998;9(7):1455–1458. doi: 10.1097/00001756-199805110-00038. [DOI] [PubMed] [Google Scholar]
- Okragly AJ, Niles AL, Saban R, et al. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol. 1999;161:438–442. [PubMed] [Google Scholar]
- Onaga T, Harada Y, Okamoto K. Pituitary adenylate cyclase-activating polypeptide (PACAP) induces duodenal phasic contractions via the vagal cholinergic nerves in sheep. Regul Pept. 1998;77(1–3):69–76. doi: 10.1016/s0167-0115(98)00046-9. [DOI] [PubMed] [Google Scholar]
- Peeker R, Enerback L, Fall M, Aldenborg F. Recruitment, distribution and phenotypes of mast cells in interstitial cystitis. J Urol. 2000;163(3):1009–1015. [PubMed] [Google Scholar]
- Sandor K, Bolcskei K, McDougall JJ, et al. Divergent peripheral effects of pituitary adenylate cyclase-activating polypeptide-38 on nociception in rats and mice. Pain. 2009;141(1–2):143–150. doi: 10.1016/j.pain.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Sant G, Hanno PM. Interstitial cystitis: current issues and controversies in diagnosis. Urology. 2001;57:82. doi: 10.1016/s0090-4295(01)01131-1. [DOI] [PubMed] [Google Scholar]
- Schnegelsberg B, Sun TT, Cain G, et al. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol. 2009 Dec 23; doi: 10.1152/ajpregu.00367.2009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schworer H, Clemens A, Katsoulis S, Kohler H, Creutzfeldt W, Schmidt WE. Pituitary adenylate cyclase-activating peptide is a potent modulator of human colonic motility. Scand J Gastroenterol. 1993;28(7):625–632. doi: 10.3109/00365529309096101. [DOI] [PubMed] [Google Scholar]
- Seebeck J, Lowe M, Kruse ML, et al. The vasorelaxant effect of pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in isolated rat basilar arteries is partially mediated by activation of nitrergic neurons. Regul Pept. 2002;107(1–3):115–123. doi: 10.1016/s0167-0115(02)00072-1. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 2000;21(6):619–670. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- Steenstrup BR, Ottesen B, Jorgensen M, Jorgensen JC. Pituitary adenylate cyclase activating polypeptide induces vascular relaxation and inhibits non-vascular smooth muscle activity in the rabbit female genital tract. Acta Physiologica Scandinavica. 1994;152(2):129–136. doi: 10.1111/j.1748-1716.1994.tb09792.x. [DOI] [PubMed] [Google Scholar]
- Sundler F, Ekblad E, Hannibal J, et al. Pituitary adenylate cyclase-activating peptide in sensory and autonomic ganglia: localization and regulation. Ann NY Acad Sci. 1996;805:410–426. doi: 10.1111/j.1749-6632.1996.tb17501.x. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharm Rev. 2000;52(2):269–324. [PubMed] [Google Scholar]
- Vizzard MA. Increased expression of neuronal nitric oxide synthase in bladder afferent and spinal neurons following spinal cord injury. Dev Neurosci. 1997;19(3):232–246. doi: 10.1159/000111212. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Alterations in growth-associated protein (GAP-43) expression in lower urinary tract pathways following chronic spinal cord injury. Somatosensory Motor Res. 1999;16(4):369–381. doi: 10.1080/08990229970429. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol. 2000a;278(4):R1027–1039. doi: 10.1152/ajpregu.2000.278.4.R1027. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Alterations in spinal Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol. 2000b;278:R1027–R1039. doi: 10.1152/ajpregu.2000.278.4.R1027. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000c;161(1):273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol. 2000d;420(3):335–348. [PubMed] [Google Scholar]
- Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat. 2001;21(2):125–138. doi: 10.1016/s0891-0618(00)00115-0. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19(11):4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuridullah R, Corrow KA, Malley SE, Vizzard MA. Expression of fractalkine and fractalkine receptor in urinary bladder after cyclophosphamide (CYP)-induced cystitis. Auton Neurosci. 2006;126–127:380–389. doi: 10.1016/j.autneu.2006.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizzo MG, Mule F, Serio R. Interplay between PACAP and NO in mouse ileum. Neuropharmacology. 2004;46(3):449–455. doi: 10.1016/j.neuropharm.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol. 2007;7(1):9. doi: 10.1186/1472-6793-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvarova K, Dunleavy JD, Vizzard MA. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp Neurol. 2005;192(1):46–59. doi: 10.1016/j.expneurol.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Zvarova K, Murray E, Vizzard MA. Changes in galanin immunoreactivity in rat lumbosacral spinal cord and dorsal root ganglia after spinal cord injury. J Comp Neurol. 2004;475(4):590–603. doi: 10.1002/cne.20195. [DOI] [PubMed] [Google Scholar]
- Zvarova K, Vizzard MA. Distribution and fate of cocaine- and amphetamine-regulated transcript peptide (CARTp)-expressing cells in rat urinary bladder: a developmental study. J Comp Neurol. 2005;489(4):501–517. doi: 10.1002/cne.20657. [DOI] [PMC free article] [PubMed] [Google Scholar]