Abstract
Acute amyloid-β peptide (Aβ) deposition has been observed in young traumatic brain injury (TBI) patients, leading to the hypothesis that elevated extracellular Aβ levels could underlie the increased risk of dementia following TBI. However, a recent microdialysis-based study in human brain injury patients found that extracellular Aβ dynamics correlate with changes in neurological status. Because neurological status is generally diminished following injury, this correlation suggested the alternative hypothesis that soluble extracellular Aβ levels may instead be reduced after TBI relative to baseline. We have developed a methodologically novel mouse model that combines experimental controlled cortical impact TBI with intracerebral microdialysis. In this model, we found that Aβ levels in microdialysates were immediately decreased by 25–50% in the ipsilateral hippocampus following TBI. This result was found in PDAPP, Tg2576, and Tg2576-ApoE2 transgenic mice producing human Aβ plus wild-type animals. Changes were not due to altered probe function, edema, changes in APP levels, or Aβ deposition. Similar decreases in Aβ were observed in phosphate buffered saline-soluble tissue extracts. Hippocampal electroencephalographic activity was also decreased up to 40% following TBI, and correlated with reduced microdialysate Aβ levels. These results support the alternative hypothesis that post-injury extracellular soluble Aβ levels are acutely decreased relative to baseline. Reduced neuronal activity may contribute, though the underlying mechanisms have not been definitively determined. Further work will be needed to assess the dynamics of insoluble and oligomeric Aβ after TBI.
Keywords: Amyloid-beta, Traumatic brain injury, Microdialysis, EEG, Alzheimer s disease, Dementia, Mouse
INTRODUCTION
Moderate to severe TBI is a well-documented environmental risk factor for the later development of dementia of the Alzheimer type (Bazarian et al., 2009; Guo et al., 2000; Plassman et al., 2000; Van Den Heuvel et al., 2007). The amyloid-β peptide (Aβ) is believed to play a central role in both familial and late-onset Alzheimer s disease (AD), and may also be involved in TBI-related dementia. Histologically apparent Aβ deposits have been detected in young TBI patients as early as 2–4 hours after injury (Ikonomovic et al., 2004; Roberts et al., 1994). However, deposits occur only in 20–30% of human TBI patients coming to autopsy or requiring decompressive surgery (Ikonomovic et al., 2004; Roberts et al., 1994).
In contrast to neuropathological studies, intracerebral microdialysis permits dynamic sampling of soluble, extracellular Aβ in the interstitial fluid (ISF) (Brody et al., 2008; Cirrito et al., 2008; Cirrito et al., 2003; Cirrito et al., 2005; Elvang et al., 2009; Kang et al., 2007; Kang et al., 2009; Marklund et al., 2009). In the brains of awake, behaving mice, microdialysis studies have uncovered a clear relationship between neuronal activity and ISF Aβ concentrations (Cirrito et al., 2005). In a subsequent study, ISF Aβ levels were shown to depend in large part on synaptically-coupled endocytosis (Cirrito et al., 2008). Physiological modulations of neuronal activity have been shown to similarly affect Aβ levels (Kang et al., 2007; Kang et al., 2009).
Recently, our group measured the dynamics of Aβ by intracerebral microdialysis in acutely brain-injured patients (Brody et al., 2008). We found that ISF Aβ levels generally rose over time, and that these changes were positively correlated with changes in neurological status as assessed by the Glasgow Coma Score (GCS). Because we could not measure pre-injury levels in our human subjects, the true relationship of post-injury to pre-injury levels was unknown (Suppl. Fig. S1A). Additionally, the relationship of ISF Aβ to levels in other tissue compartments could not be assessed in the human study; this is an important consideration, as the extent of equilibration between pools of Aβ (Suppl. Fig. S1B) is largely unknown.
To address these gaps, we developed a novel mouse model that combined a standardized experimental traumatic brain injury (Brody et al., 2007) with intracerebral microdialysis in awake, behaving mice (Fig. 1). While similar methods have been used in rats (Bell et al., 1998; Krishnappa et al., 1999; Palmer et al., 1993; Rose et al., 2002) this mouse model allowed the study of both wild-type and transgenic animals expressing human-sequence Aβ.
Fig. 1. Combined microdialysis-controlled cortical impact TBI mouse model for assessment of Aβ dynamics.
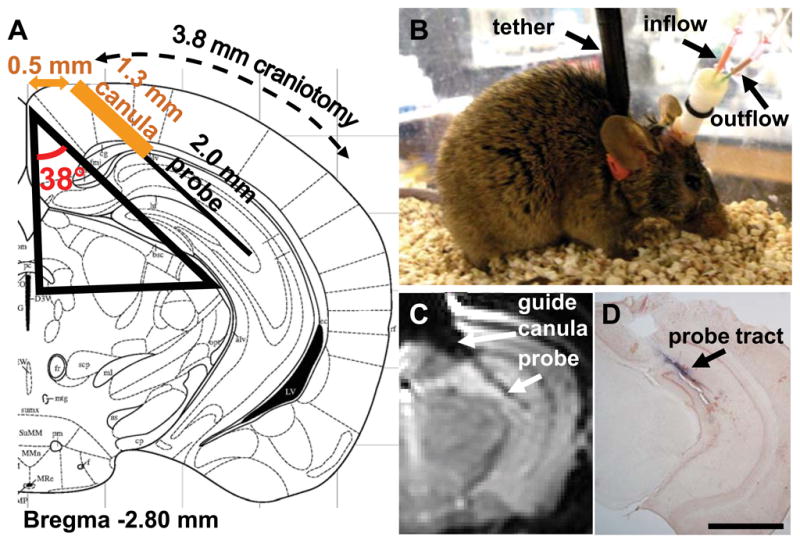
(A) Design of stereotaxic surgery for microdialysis probe placement and craniotomy for controlled cortical impact TBI. A rigid guide canula was inserted via a stereotaxically placed burr hole. Then, the microdialysis probe was placed through the guide canula into the left hippocampus. A 3.8 mm-diameter craniotomy was performed to allow controlled cortical impact TBI. Modified from Franklin and Paxinos (Franklin and Paxinos, 2001). (B) Photograph of awake, moving mouse with implanted canula and probe affixed with dental cement. Mice were tethered to an electronic swivel system to prevent tangling of the microdialysis tubing. (C) In vivo T2-weighted magnetic resonance image of a living mouse with implanted canula and probe. (D) Post-mortem staining of probe tract with Evans blue dye and counterstained with Neutral Red. Scale bar, 2.0 mm.
Using this model, we found that Aβ levels were reduced immediately after TBI in 4 genotypes of mice and in a dose-of-injury dependent fashion. There was a quantitative correlation between the extent of reductions in ISF Aβ levels and in local electroencephalographic (EEG) activity after injury. This supports the hypothesis that ISF Aβ levels are reduced acutely following TBI, but leaves unresolved the question of why TBI increases the later risk of dementia of the Alzheimer type.
METHODS
Mice
Most experiments used male and female PDAPP+/− mice (Games et al., 1995) on a C57Bl6 background at 3–6 months of age. These mice were originally obtained from Eli Lilly and Co., and were bred at Washington University to C57Bl/6J wild-type mice from Jackson Labs. They were genotyped by PCR (Cirrito et al., 2003). Wild-type mice used were C57Bl6 littermates of the PDAPP+/− mice. Tg2576+/− (Hsiao et al., 1996) mice were originally obtained from Dr. K. Ashe, University of Minnesota. They were bred to C57Bl6/SJL wild-type mice (Taconic Farms, Germantown, NY) and genotyped by PCR as described (Cirrito et al., 2005). Tg2576+/− / hApoE2+/+ mice were a kind gift from Dr. David Holtzman, and were bred as described (Fryer et al., 2005).
The mice were housed at 3–5 mice per cage under standard laboratory conditions prior to experiments. All experiments were approved by the Animal Studies Committee at Washington University in accordance with IACUC and NIH guidelines for the ethical treatment of animals.
Combination microdialysis and controlled cortical impact (CCI) model of TBI
To allow for baseline Aβ microdialysis measurements, mice underwent an initial surgery for implantation of a microdialysis guide canula (MD-2250, BASi) and craniotomy placement 24 hours prior to CCI. Isoflurane anesthesia was induced at 5% and maintained between 1.5 – 2% during the procedure. Following anesthesia, mice were placed on a stereotactic frame (David Kopf) on a thermoregulated heating pad kept at 37º C for the duration of the 60-minute surgery. The scalp was shaved, 10% povidone-iodine applied to the skin and allowed to dry, and the skin opened to expose the skull. The mouse s head was fixed in place by cupped head holders (David Kopf) mounted on the stereotactic frame. The skull was leveled along the anterior-posterior and lateral axes to a tolerance of 0.10 mm with a digital stereotactic device (Benchmark Digital).
For microdialysis guide canula implantation, a 0.7-mm burr bit mounted an on an electric drill (Foredom) was used to create a groove centered at 2.5 mm posterior to bregma suture, 0.0 mm midline through 1.0 mm left of midline, at a depth to visualize but not breach dura mater and the sagittal sinus. Three additional holes were placed for bone screw anchors at 1.0 mm right of midline, 0.75 mm posterior to bregma; 3.0 mm right, 0.75 mm posterior to bregma; and 3 mm right, 3.5 mm posterior to bregma. Finally, fiduciary markers for a 3.8 mm-diameter craniotomy were made at 3.6 mm left of midline, 1.1 mm anterior to lamboid suture and 1.7 mm left of midline, 3.0 mm anterior to lamboid suture.
The left craniotomy required for CCI-TBI was performed during the same surgical procedure as guide canula implantation (Fig. 1A). Control experiments indicated that there was a small decrease in the microdialysis concentration of Aβ1-x after craniotomy (Suppl. Fig. S6). Although this difference was not statistically significant at the 95% confidence level (p=0.0556, Mann-Whitney U-test), we included the craniotomy as part of the baseline procedure to separate its effect on levels of Aβ1-x from those due to the CCI-TBI. The craniotomy was created with a cylindrical, air-vent cooled, 3.8 mm-diameter micro-trephine (Xemax Surgical) angled between 12–15º to create an even, circular groove through the skull. Once the skull bone under the trephine became translucent, the bone flap was carefully removed using a 1.0 mm cup rongeur and spatula. A plastic cap was immediately placed over the exposed dura. The central part of the cap touching the dura was coated with petrolatum-based veterinary ointment (Purelube) and the peripheral part was secured to the skull with veterinary adhesive (VetBond, 3M).
Bone screw anchors were inserted into the previously drilled holes at the minimum depth required for secure placement, typically between one-half and one full turn. The bone screw anchors provided necessary support for the dental cement (DuraLay inlay pattern resin, Reliance) crown to secure the guide canula to the skull.
A microdialysis guide canula (MD 2250, BASi) was mounted on the right arm of the stereotactic frame, and the arm was positioned at 38º relative to the sagittal plane (Fig. 1A). The guide canula was introduced into the left cortex to a depth of 1.3 mm through the groove at 0.5 mm left of midline, centered at 2.5 mm posterior to bregma (Bregma −2.5 mm). The depth was measured from where the rightmost (bottom) edge of the guide canula contacted the dura. Since the guide canula radius is 0.3 mm, it extends from Bregma −2.2 to Bregma −2.8 mm. Then, the guide canula was secured using dental cement. A ½-circumference cap from a 1.7 mL microcentrifuge tube was placed just to the left of the canula to protect the craniotomy skull cap from the dental cement crown. Dental cement was carefully placed around the canula and bone screw anchors for maximum security and allowed to dry 10–15 minutes. Once dry, the head holders were released and skin sutured around the resultant cement crown using 4-0 interrupted nylon suture. Triple antibiotic ointment was applied to the entire area.
The guide canula stylet was removed and a primed, 2 mm microdialysis probe (MD2200 BR-2, BASi) was carefully inserted by hand with visualization of the guide canula tract. Mice were then removed from the stereotaxic frame, placed in Rat Turn electronic swivel cages (BASi) and allowed to wake from anesthesia. Most mice were alert and moving within 20 minutes. An adjustable plastic collar was loosely placed around the neck, and attached to the suspended tether of the automatic swivel arm (Fig. 1B). Microdialysis tubing was secured to the tether arm to prevent tangling. Standard cob bedding, mouse chow pellets, and fresh water were provided daily. Mice were kept in constant light conditions. This reduces the confound of circadian Aβ fluctuations (Kang et al, 2008) and is similar to the constant light conditions of the intensive care unit where human TBI patients are treated. The mice slept, ate, drank and moved around in the swivel cages during the pre-injury and post-injury periods, though they were often lethargic after injury.
Approximately 24 hours later, mice underwent a single, moderate left lateral controlled cortical impact with craniotomy, as described previously (Brody et al., 2007). Briefly, mice were anesthetized with isoflurane, placed in the stereotaxic frame and maintained at 37°C as before. Head holders were used to secure the skull in a level position. The sutures were opened, plastic skull cap removed to expose underlying dura, and impactor tip aligned in the center of the craniotomy. Just prior to impact, microdialysis probes were removed and immersed in CMA perfusion fluid to avoid damage to the probes or additional tissue shearing during impact. The probes were replaced approximately 5 minutes later into the same location as prior to injury. Brief removal of the probe from the guide canula does not affect microdialysis levels of Aβ1-x (Fig. 2A, C, D, data for sham groups). Mice were subjected to controlled cortical impact (CCI) in which a 3 mm-diameter, flat metal tip impounder was driven by an electromagnetic device at a velocity of 5 m/s to various depths (1.0, 1.5, or 2.0 mm) into the cortex. In part because this electromagnetic device does not overshoot the way some pneumatic controlled cortical impact devices can, these impact depths produce less severe injuries than those produced by some pneumatic CCI devices at the same nominal depth (Brody et al., 2007). Sham-injured mice underwent identical procedures, except the impactor tip was discharged into the air and did not contact the dura. The wound was then cleaned by gentle irrigation with sterile-filtered phosphate-buffered saline (PBS), and a plastic skull cap replaced and again secured with VetBond. Skin was resutured around the cement crown using 4-0 interrupted nylon sutures and triple antibiotic ointment applied to the entire area. As before, mice were allowed to wake and recover in the electronic swivel cages where they were monitored by microdialysis for an additional 24 hours or longer.
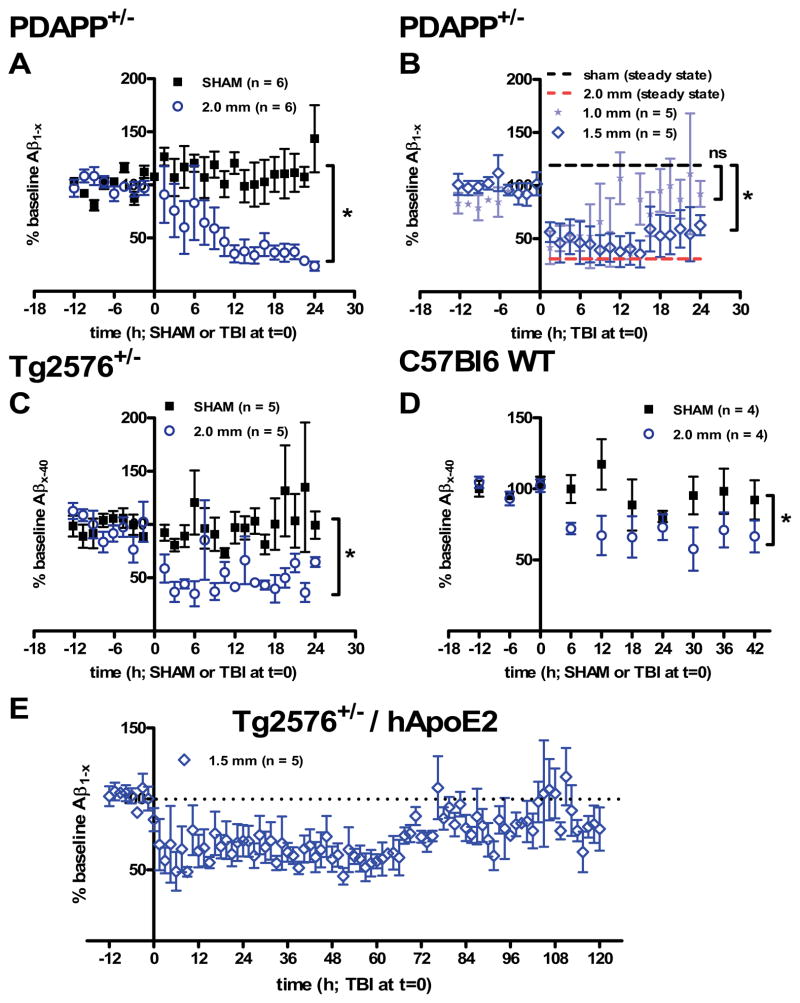
Fig. 2. ISF Aβ dynamics following controlled cortical impact TBI in mice.
(A) Aβ1-x levels were stable at baseline and after sham injury, but reduced after 2.0 mm impact depth controlled cortical impact TBI in 3–6 month old PDAPP mice. Microdialysis samples were collected every 90 minutes and assessed for Aβ by ELISA. An average baseline value was computed for each mouse by taking the mean of all pre-injury samples beginning approximately 12 hours prior to injury. All samples were then normalized to baseline and expressed as “% baseline Aβ.” Error bars represent standard errors of the mean. *p = 0.0073, one-way ANOVA with Bonferroni correction for multiple comparisons. (B) Less severe injuries result in less profound reduction in ISF Aβ levels. ISF Aβ levels began to rise at later times following these less severe injuries. *p = 0.005 for 1.5 mm impact depth vs. sham. Not significant for 1.0 mm impact depth injuries. (C) Reduction in Aβx-40 levels in young, injured Tg2576+/− mice. *p = 0.0152, one-tailed Mann-Whitney U-test. (D) Reduction in murine Aβx-40 levels in C57Bl6 wild-type mice following TBI. *p = 0.0286, one-tailed Mann-Whitney U-test. For wild-type mice, Aβ was measured in 6-hour intervals. (E) Aβ1-x levels measured in Tg2576+/− mice with targeted replacement of the mouse ApoE gene by human ApoE2 allele (n = 5). Aβ levels began to rise around 60 h, recovering towards but not exceeding baseline levels between 80–120 h.
Microdialysis
Microdialysis probes (MD2200, Br-2, BASi) had a 2 mm-long, 320 μm outer diameter, 38 kDa molecular-weight cutoff membrane and were connected to an infusion syringe pump (KDS 101, KD Scientific). The inlet tract of the probes were attached to the syringe pump via a 1.0 m-long, Teflon (FEP) tubing (inner diameter, 0.12 mm; SciPro) and primed for 1–2 hours with 0.15% sterile human albumin (diluted from 25% Human Albumin, Grifols) in sterile, isotonic saline solution (147 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, and 0.85 mM MgCl2; CNS perfusion fluid, CMA, Sweden). Samples from transgenic mice were collected for 90 minutes at a flow rate of 1.5 μL/min to yield a volume of 135 μL per sample. Samples from wild-type mice were collected for 90 minutes at a rate of 0.3 μL/min to yield a volume of 27 μL per sample. The outlet tract of the probe was connected to a second 1.0 m-long piece of FEP tubing. The outflow was collected in low-protein-binding polypropylene tubes housed in a 4ºC-refrigerated, automated fraction collector (Univentor 820 Microsampler, SciPro). For the initial baseline sampling period, sampling was begun after the dead volume had cleared the outflow line. The dead volume was allowed 8–10 minutes to clear through an outflow line with a capacity of 12 μL at a flow rate of 1.5 μL/min. The initial 6–12 hours of microdialysates were not used, and baseline measurements were started 12 hours before injury.
Zero-flow extrapolation
Flow rates were varied systematically and the concentration of Aβ was measured at each flow rate using the Aβ1-x ELISA (Suppl. Fig. S7A). For each animal, a flow rate of 1.5 μl/min was used for the first 4.5 hours (3 samples) after probe insertion. An equilibration period of 4–5 hours has previously been described for microdialysis measurement of Aβ in the hippocampi of PDAPP+/− mice (Cirrito et al., 2003). Then, the flow rates were changed in a systematic fashion to 0.1, 0.3 and 0.5 μl/min. These particular flow rates were used because they yielded the most efficient fit to an exponential curve among a set of 8 test flow rates in pilot experiments (not shown). After each flow rate change, the dead volume (12 μl) was allowed to clear the tubing before the sample was collected for analysis. This required 8 minutes at 1.5 μl/min and 120 minutes at 0.1 μl/min. Samples were collected for 90–540 minutes, and ELISA dilution factors were varied between 3–8. The same pattern of flow rate variation was followed in all animals. After TBI, an identical set of flow rate changes and ELISA dilutions were performed. Except for flow-rate variation, these experiments were conducted in an identical manner to other combined CCI-microdialysis experiments.
All Aβ concentrations were normalized to the 0.1 μL/min pre-TBI concentration for each mouse ([Aβ]/pre-TBI [Aβ] 0.1 μL/min), and the resultant ratios were averaged to derive a group mean for each flow rate before and after TBI. The concentration as a function of flow rate was fit to a decaying exponential relationship (Jacobson et al., 1985) using the Excel Solver tool:
| (Equation 1) |
where C is the concentration at a given flow rate, C0 is the zero-flow concentration, K0 is the mass transfer coefficient, A is the microdialysis probe membrane surface area (160.85 μm2) and F is the flow rate.
For comparison of pre- and post-TBI mass transfer coefficients, the natural logs of the mean ratios were plotted against their corresponding flow rates and a linear regression of the relationship was performed with a statistical analysis package (GraphPad Prism 5.0). The best-fit slope of this semi-log plot is equal to the -K0A term in Equation 1. The 95% confidence intervals of the best-fit slope were used to determine the significance of differences between pre- and post-TBI mass transfer coefficients.
Retrodialysis
An N-terminal-biotinylated, synthetic, human-sequence Aβ1–40 peptide (rPeptide) was continually infused through the inlet port before and after CCI-TBI in wild-type mice at a concentration of 3000 pg/mL in the standard 0.15% albumin-CMA CNS perfusion solution (Suppl. Fig. S7B). To ensure that a loss of infused peptide could be reliably detected when comparing the infused and outflow concentrations, we used a slower flow rate of 1.0 μL/min. Microdialysis samples were analyzed by ELISA using m266 as the capture antibody and incubation with SA-HRP20 for detection of biotinylated Aβ1–40. Percentage loss was calculated as the ratio of the concentration in each sample to the concentration in the infused substrate ([Aβ]/[Aβ]IN). Both infused and outflow samples were measured on the same ELISA plates.
Urea assay
After the volume required for Aβ quantification was removed from the microdialysis collection tubes, urea concentration was measured in the remaining volume using a commercially-available kit (Suppl. Fig. S7C) (Quanti-Chrom Urea Assay Kit, BioAssay Systems). If an experiment showed fluctuating or significantly decreased urea levels > 20%, it was excluded on the basis of probe malfunction. Approximately 1 in 15 experiments was excluded due to abnormal urea levels.
Wet-dry method for estimation of water content
Three groups of wild-type mice (n = 5 each) were either sham or 2.0 mm-injured, and sacrificed at either 2 h or 24 h (Suppl. Fig. S8). Brains were quickly removed without perfusion. Ipsilateral and contralateral hippocampi and cortices were dissected on an ice-cold glass plate, placed on a pre-weighed piece of aluminum foil, and weighed immediately on a Mettler-Toledo XS64 analytical (digital) balance to determine the wet weight. Tissues were immediately placed in a laboratory drying oven at 100º C for 48 h and reweighed to determine the dry weight. Water content was calculated by taking the ratio of the difference between wet and dry weights, and the wet weight (Equation 2).
| (Equation 2) |
Additional details are included in the Supporting Information Online.
RESULTS
Levels of ISF Aβ decrease immediately after controlled cortical impact TBI
We stereotaxically implanted microdialysis probes into the left hippocampus of young adult mice at a 38º angle so that subsequent controlled cortical impact (CCI) TBI could be performed (Fig. 1A). In vivo MRI and post-mortem histology confirmed the placement of the catheters (Fig. 1C-D). The protocol modifications due to implantation of the microdialysis probe did not substantially change the pathological characteristics of the injury. First, we consistently observed a 10–20% weight loss within the first 24 hours after CCI injury with or without microdialysis. Second, the CCI injury consistently results in neuronal cell loss in the CA3 region of the hippocampus in PDAPP mice. We found no significant difference in cell loss in the inferior blade of the CA3 region between injury with (24.5%) and without microdialysis (30.9%; p=0.572, unpaired, two-tailed t-test comparing cell counts; Suppl. Figs. S2–3). The CA3 cell loss in CCI-injured PDAPP mice was concordant with previous results (Hartman et al., 2002; Smith et al., 1998b). Third, we found no difference in astroglial marker expression in the hippocampus of sham-injured mice with and without microdialysis catheter placement (Suppl. Fig. S4). Implantation of the guide canula does cause some injury to medial portions of the cortex and superficial hippocampus at the implantation site. This injury is slightly greater in mice subjected to CCI injury than those subjected to sham injury (Suppl. Fig. S5). The additional injury may be due to tissue shearing injury around the guide canula at the time of impact. This additional injury was typically minor, and did not fundamentally change the characteristics of the model.
Baseline ISF Aβ levels were stable over 12 hours before injury, as measured by ELISA (Fig. 2A-E). Before normalization to individual baselines, the average baseline concentrations of microdialysate Aβ in each genotype were as follows (mean ± standard deviation): 100 ± 57 pg/mL in PDAPP+/− mice, 335 ± 200 pg/mL in Tg2576+/− mice, and 62 ± 20 pg/mL in wild-type mice. These values have not been corrected for fractional recovery and do not directly indicate in situ Aβ levels. The main focus of all subsequent experiments was on Aβ dynamics, rather than absolute levels. Sham injury, including anesthesia and placement of the mice in a stereotaxic frame, had no significant effect on ISF Aβ levels in young PDAPP mice (Fig. 2A), Tg2576 mice (Fig. 2C) or wild-type mice (Fig. 2D). Craniotomies were performed along with microdialysis probe implantation due to a small effect of this procedure on ISF Aβ levels (Suppl. Fig. S6). Thus, the effects of craniotomy were included in the baseline and did not confound assessments of later Aβ dynamics.
A moderately severe CCI TBI (2 mm impact depth using an electromagnetic impactor (Brody et al., 2007)) caused a statistically significant, sustained reduction in ISF Aβ1-x levels in PDAPP mice (Fig. 2A, p = 0.0073). There was no evidence for an acute spike in ISF Aβ in these or any of the other experiments. Less severe injuries (1.0 and 1.5 mm impact depth) caused more modest reductions in ISF Aβ1-x levels (Fig. 2B). After less severe injuries, Aβ1-x levels began to return towards baseline over 24 hours. There was a significant difference between 1.5 mm and sham groups by the same analysis (p = 0.005), but not between 1.0 mm and sham groups.
Reductions in Aβ following TBI were not unique to PDAPP mice. CCI TBI in young Tg2576 mice caused a similar lowering of ISF Aβ x-40 levels as compared to sham (Fig. 2C, p = 0.0152 one-tailed Mann-Whitney U-test).
Aβ levels were reduced in wild-type mice after TBI as well (Fig. 2D). Both PDAPP and Tg2576 mice produce human sequence Aβ due to exogenous promoter-driven mutant APP overexpression. Thus, Aβ dynamics related to injury-induced regulation of endogenous mouse APP expression might not be captured in studies of these transgenic animals. ISF Aβ levels were less profoundly, but still statistically significantly reduced following TBI in C57Bl6 wild-type mice (p = 0.0286, one-tailed Mann-Whitney U-test). Wild-type mice have substantially lower ISF Aβ levels at baseline than PDAPP and Tg2576 mice and the ELISA methods used to assess murine Aβ are less sensitive than those used to assess human Aβ (Cirrito et al., 2003), so we performed measurements every 6 hours on pooled samples instead of every 90 minutes. Thus, the reduction in ISF Aβ after TBI does not appear to be an artifact of the transgenic regulation of APP, nor unique to human sequence Aβ vs. murine Aβ.
An important feature of the human microdialysis studies of Aβ dynamics after brain injury was that ISF Aβ levels rose in concert with clinical recovery over 72 hours (Brody et al., 2008). To obtain comparable prolonged microdialysis measurements after experimental TBI, we assessed several mouse genotypes under many injury and microdialysis conditions. This was technically challenging, but in Tg2576+/− mice with the human ApoE2 gene knocked into the mouse locus (Fryer et al., 2005) we were able to obtain consistently high-quality microdialysis measurements for 120 hours after TBI. The baseline concentration of microdialysate Aβ was 700 ± 156 pg/mL in these Tg2576-ApoE2 mice. ISF Aβ levels were reduced after injury in Tg2576-ApoE2 mice (Fig. 2E), as for the other 3 genotypes. In these prolonged microdialysis measurements, ISF Aβ levels recovered toward baseline but did not rise above pre-injury values (Fig. 2E).
We did not find any differences between male and female mice in terms of the effects of TBI on ISF Aβ levels (two-way ANOVA, p = 0.588 for group by gender interaction). Our group (Brody et al., 2007) and others (Hall et al., 2005) have previously found no differences between male and female mice in behavioral or histological outcome following CCI TBI.
Measurements of Aβ1-x likely reflect physiological sequelae of TBI
A key assumption underlying the reliability of measurements made by microdialysis is that the probe is functioning normally throughout the experiment. For example, the probe should never be clogged or otherwise blocked from free exchange with the surrounding interstitial fluid (ISF). To address this concern, we used three approaches to test for consistent probe function: zero flow extrapolation, retrodialysis, and measurement of urea.
We performed zero-flow extrapolations during baseline periods and again during post-TBI periods in 4 PDAPP+/− mice undergoing 2.0 mm CCI injury (Suppl. Fig. S7A). The zero-flow extrapolation method involves varying the flow rate over time and assumes that the underlying ISF concentration remains constant. Because the levels of post-TBI Aβ decreased by 4.5–6 hours after injury and remained relatively stable over the next 18 hours (Fig. 2A), we were able to vary the flow rate during the post-injury period. We plotted the Aβ levels before and after TBI as a function of flow rate, and fit this data to decaying exponential curves. The mass transfer coefficients for these exponential curves were not significantly different before and after TBI (K0, pre-TBI = 9.5 ± 2.2 mL/(min*mm2) and K0, post-TBI = 10.2 ± 1.6 (mL/min*mm2) as indicated by overlapping 95% confidence intervals. This result indicates that there was no significant change in the fractional recovery of Aβ before and after TBI. Normalized to the respective zero-flow concentrations, the mean fractional recoveries at 1.5 μL/min were 0.0527 ± 0.00129 (pre-TBI) and 0.0642 ± 0.00286 (post-TBI); these were not significantly different (two-tailed Mann-Whitney U-test, p = 0.1333).
Next, probe function was evaluated using retrodialysis of infused Aβ. For simple diffusion, Aβ diffusion rates from the microdialysis probe into the brain should be similar to diffusion rates from the brain into the microdialysis probe. Wild-type mice were subjected to the combined CCI-microdialysis procedure with perfusion of 3000 pg/ml of synthetic, biotinylated Aβ1–40 through the inlet tubing. There was an average Aβ loss of approximately 20–25% both before and after a 2.0 mm CCI injury at a flow rate of 1.0 μL/min (Suppl. Fig. S7B). Pre and post-injury levels were not significantly different (p=0.1944, paired t-test). This experiment further indicates that there were no changes in the diffusion of Aβ across the microdialysis probe after TBI.
Finally, the recovery of urea was used as a third control for probe function. Urea is a small molecule produced in the liver that rapidly equilibrates throughout all tissue compartments, and is frequently used as a control for probe function in human cerebral microdialysis (Brody et al., 2008; Hillered et al., 2005; Ronne-Engstrom et al., 2001). Urea levels were comparable in all microdialysis samples (Suppl. Fig. S7C). There were no significant differences between pre and post-TBI urea levels (p=0.1747, paired t-test).
Taken together, these control experiments indicate that the reductions in ISF Aβ following TBI are likely of physiological origin.
Decreases in Aβ are not due to tissue edema
To address the possibility that increased extracellular water content could dilute ISF Aβ, we performed CCI-TBI in a separate set of wild-type mice sacrificed 2 or 24 hours after 2.0 mm impact depth TBI, and 24 hours after sham-injury.
TBI caused a small increase in tissue edema, as measured using the wet/dry method (Suppl. Fig. S8). Tissue water content in the hippocampus increased from 79.5 ± 0.6% in sham-injured mice to 81.7 ± 0.7% two hours after 2.0 mm impact depth TBI and 81.5 ± 0.5% 24 hours after injury. Tissue water contents at 2 hours and 24 hours were indistinguishable, whereas ISF Aβ levels were considerably lower at 24 hours than in the first few hours after 2.0 mm injury in PDAPP mice (Fig. 2A). Thus, while we cannot rule out a small contribution of dilution due to edema, this does not appear to be the primary mechanism underlying the acute reduction in extracellular Aβ following TBI.
Decreases in microdialysate levels of Aβ1-x are reflected in PBS-soluble tissue lysates
Post-TBI changes in microdialysate levels of Aβ might reflect changes in overall tissue levels or be specific to ISF Aβ. To assess these alternative hypotheses, we used a serial tissue extraction protocol in a separate set of PDAPP+/− mice sacrificed 2 hours after 2.0 mm impact depth TBI. The injuries with and without microdialysis were similar, based on stereological quantification of ipsilateral hippocampal CA3 cell counts (Suppl. Figs. S2–3).
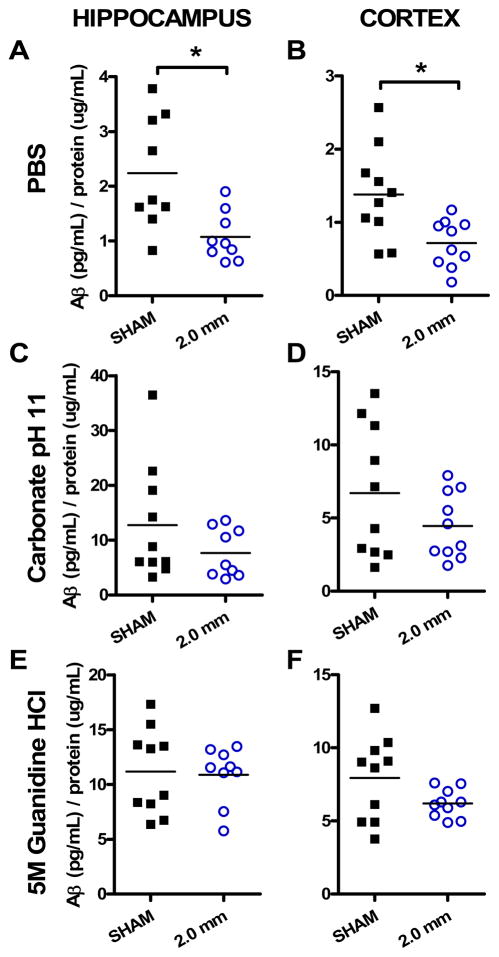
In 2.0 mm-injured PDAPP+/− mice, Aβ1-x levels in PBS-soluble hippocampus and cortex tissue lysates were reduced relative to sham-injured mice (Fig. 3). In the ipsilateral hippocampus, levels were reduced by approximately 50% in the injured group (Fig. 3A; two-tailed Mann-Whitney test, p = 0.0078). In ipsilateral cortex, significant differences were also measured in the PBS-soluble pool of Aβ1-x between sham and 2.0 mm-injured groups (Fig. 3B; p = 0.0052). As in hippocampus, cortical levels were reduced to approximately 50% of that found in sham-injured animals. This change is of the same magnitude as was measured by microdialysis in these mice (Fig. 2A). PBS-soluble lysates contain largely, but not exclusively, extracellular material. Aβ1-x measurements in carbonate and guanidine-soluble extracts of ipsilateral hippocampus and cortex were not significantly different between sham and either injured group at the 2-hour timepoint (Fig. 3C-F). Carbonate-soluble extracts contain intracellular material, and guanidine is known to extract otherwise insoluble pools of Aβ. These results indicate that the reduction in Aβ following TBI is likely limited to the extracellular space. The absence of a spike in Aβ levels in any of the extracts contrasts with some previous reports, though substantial methodological differences preclude a direct comparison across studies (Suppl. Tables S1–2, and see discussion).
Fig. 3. PBS-soluble pool of Aβ reduced after injury.
(A, B) Aβ levels were significantly reduced in 2.0 mm-injured PDAPP+/− mice in PBS-soluble extracts from ipsilateral hippocampus (*p = 0.0078) and cortex (*p = 0.0052, one-tailed Mann-Whitney U-tests). Separate groups of injured and sham mice were assessed. Each symbol represents 1 mouse. (C, D) Aβ levels were not significantly different in 2.0 injured mice compared to sham in carbonate-soluble extracts. (E, F) No differences between groups in guanidine-soluble extracts.
No acute changes in APP levels or Aβ deposition to account for reduced ISF levels
Amyloid precursor protein (APP) levels in tissue lysates were not significantly affected by TBI. Western blotting following by quantitative densitometry revealed no effect of TBI on APP levels in ipsilateral and contralateral hippocampus and cortex (Suppl. Figs. S9–10). Similar results at acute timepoints have been reported previously in CCI-injured rats (Ciallella et al., 2002) and mice (Abrahamson et al., 2006). Previous studies (Gentleman et al., 1993; Mac Donald et al., 2007a; Mac Donald et al., 2007b; Stone et al., 2000) have demonstrated immunohistochemical evidence for increased APP accumulation in neuronal perinuclear regions and in axonal varicosities. This may represent concentration of APP at these locations, rather than an absolute increase in the amount of APP in the brain as a whole. Certainly, there was no evidence that a reduction in APP levels accounted for the reductions in ISF and PBS tissue lysate Aβ levels.
Likewise, there was no evidence for deposition of Aβ into insoluble plaques or aggregates following TBI in young PDAPP mice (Suppl. Fig. S11). This is consistent with the finding that there was no effect of TBI on carbonate or guanidine tissue extracts (Fig. 3). The mice in the microdialysis experiments were 3–6 months of age, whereas Aβ deposition in PDAPP+/− mice typically begins around 6–9 months of age ((Games et al., 1995) and Suppl. Fig. S11E-G). Thus, reduction in the solubility of Aβ following TBI does not appear to be the explanation for the reduction in ISF Aβ levels in these experiments.
Reduced EEG activity in the injured hippocampus correlates with reduced ISF Aβ
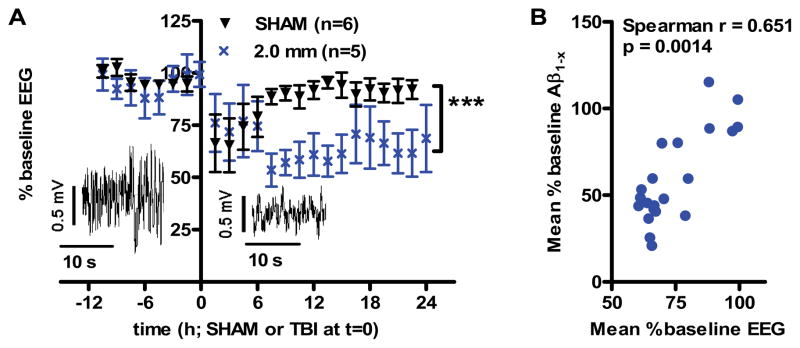
Using intraparenchymal EEG, we asked whether the changes seen in microdialysis levels of Aβ in our model might be associated with changes in local neuronal activity. Two electrodes were affixed to the guide canula (Suppl. Fig. S12A) for continuous recording of field potentials in the ipsilateral hippocampus during baseline and post-injury periods (Alves et al., 2005; Cirrito et al., 2005). Root-mean squared (RMS) EEG amplitude was stable during baseline, and returned to normal quickly after sham-injury (Fig. 4A). However, after 2.0 mm impact depth TBI, the EEG amplitude was significantly decreased compared to baseline (Fig. 4A, p < 0.0005). This indicates a reduction in neuronal activity following TBI.
Fig. 4. Reduced local neuronal activity after injury correlates with reduced Aβ levels.
(A) Local neuronal activity was measured by hippocampal depth electrode recordings (EEG) in PDAPP+/− mice before and after either sham or 2.0 mm injury. Root mean squared amplitude of EEG tracings were measured during noise-free periods and binned into 90 minute epochs. Insets: sample tracings before and after injury. Neuronal activity was significantly reduced after TBI compared to sham (***p < 0.0005, repeated-measures ANOVA, main effect of group). (B) Correlation between changes in EEG amplitude and changes in ISF Aβ in injured PDAPP mice. Each symbol represents group-averaged data for each 90 minute sample interval beginning 6 hours prior to injury through 24 hours post-injury (r = 0.651, p = 0.0014 by Spearman rank-order correlation).
Furthermore, there was a strong correlation between the extent of EEG amplitude reduction and the extent of ISF Aβ reduction following TBI (Fig 4B). This correlation was based on matched 90 minute intervals with both artifact-free EEG recordings and valid ISF Aβ measurements in PDAPP mice following 2.0 mm impact depth TBI. The correlation was highly statistically significant (p=0.0014, Spearman r = 0.651).
Taken together with previous studies of extracellular Aβ dynamics and neuronal activity (Cirrito et al., 2008; Cirrito et al., 2005; Kamenetz et al., 2003), these findings suggest that reduced neuronal activity could be responsible for the observed reductions in ISF Aβ following experimental TBI. Additional mechanisms are possible, and further experiments will be necessary to definitively address this issue.
DISCUSSION
In summary, we developed a combined CCI-microdialysis method to assess the effects of experimental TBI on brain ISF Aβ dynamics. In 4 different genotypes of mice, ISF Aβ levels were reduced after TBI, and the extent of the reduction was related to the severity of the injury across 3 impact depths. Over time, Aβ levels stayed stable or recovered toward baseline as the animals recovered from injury. There was no evidence for a spike in ISF Aβ levels at very early time points after injury, nor a rise above baseline levels in the subsequent 1–5 days. The changes in the Aβ levels measured in microdialysates were not due to changes in microdialysis catheter function, but instead most likely reflect true changes in brain extracellular fluid soluble Aβ levels. There was a similar reduction of Aβ levels in PBS-soluble brain extracts following TBI. Neither tissue edema, changes in APP levels, nor deposition of Aβ into insoluble forms explained these changes. Instead, ISF Aβ dynamics correlated well with changes in local EEG amplitude, suggesting a relationship with neuronal activity.
These experimental results complement the findings from recent clinical microdialysis studies of Aβ dynamics in human brain injury patients (Brody et al., 2008; Marklund et al., 2009). First, pre-injury Aβ levels were measured prior to experimental TBI, whereas this could not be performed in humans. These baseline measurements allowed us to clearly determine that ISF Aβ levels were reduced following injury. Second, Aβ levels were measured at very early times following injury, and these were also found to be decreased within the first 90 minutes. In human patients, microdialysis catheters were typically placed 12–24 hours after injury, thus very early Aβ measurements were not possible. Third, Aβ levels in tissue compartments other than the ISF pool sampled by microdialysis were assessed in comparably injured animals. Levels of Aβ in PBS-soluble extracts were also found to be decreased at the 2-hour timepoint, but there were no changes in carbonate or guanidine-soluble extracts. Taken together, the experimental and human studies indicate that soluble brain extracellular Aβ levels are most likely reduced immediately following injury and then increase as both mice and human patients recover (Suppl. Fig. S13). We recognize that other interpretations are possible, and discuss these in detail elsewhere (Magnoni, in press).
The most parsimonious explanation for this set of observations is that changes in synaptic activity underlie both the correlation between Aβ dynamics and EEG amplitude in mice and the correlation between Aβ dynamics and neurological status in humans (Suppl. Fig. S14). Reduced synaptic activity in the setting of TBI is likely due to a combination of neuronal cell death and physiological dysfunction of the surviving neurons. Previous experimental results indicate that ISF Aβ production is directly linked to synaptic activity-related endocytosis (Cirrito et al., 2008). It is highly likely that acute brain injuries reduce synaptic activity and hence ISF Aβ levels (Magnoni, in press). However, experimental evidence for a causal relationship between reduced synaptic activity and reduced ISF Aβ levels in TBI will require additional studies. It is also possible that neuronal cell death was actually higher than assessed using our stereological cell counting approach, and that neuronal cell death plus a small contribution from edema were responsible for the overall reduction in ISF Aβ. Likewise, the chronic effects of injury on brain ISF Aβ levels are unknown.
A substantial body of work has indicated that alterations in small molecule metabolites such a lactate, pyruvate, glucose and glutamate measured by microdialysis provide important insights into the physiology of the injured brain (Hillered et al., 2005). While these metabolites were not measured in this experimental study, Aβ levels were shown to correlate positively with glucose and negatively with lactate/pyruvate ratio in human brain injury (Brody, Magnoni et al., 2008). Thus, Aβ measurements may provide an additional indicator of cerebral physiology following injury.
Caveats associated with extrapolation from experimental mouse models to human studies warrant consideration here. Differences in Aβ production in mutant human APP isoforms might yield mutation-specific post-injury Aβ dynamics. However, the reduction of post-injury ISF Aβ in wild-type and three different lines of transgenic mice indicates that the observed dynamics are unlikely to be specific for a particular APP or Aβ sequence. Likewise, the biomechanical aspects of the injury are quite different in mice, compared to humans. Future Aβ microdialysis experiments in pig models of TBI (Alessandri et al., 2003) would be of great interest. Similarly, the severity of injuries in human TBI patients treated in the ICU is likely to be considerably greater than the injuries produced in laboratory experiments.
Without careful consideration, our results may seem inconsistent with previous descriptions of post-injury Aβ dynamics in tissue homogenates (Abrahamson et al., 2006; Loane et al., 2009; Smith et al., 1998a). However, many tissue homogenates reflect a mixture of different pools of Aβ, as opposed to microdialysis in which only the extracellular soluble pool is sampled (Suppl. Fig. S1B). Several important methodological differences between studies preclude a direct comparison of the results. These differences are outlined in Suppl. Tables S1–2.
An overview of the literature in this field indicates that there are at least three distinct Aβ dynamic phenomena that have been described following acute brain injury. The first phenomenon is the acute increase in Aβ deposition in a minority of human patients (Ikonomovic et al., 2004; Loane et al., 2009; Roberts et al., 1991; Roberts et al., 1994). The drivers of immunohistochemically apparent insoluble deposits may not be reflected in microdialysis measurements of soluble Aβ in the interstitial fluid; for example, initial intracellular Aβ accumulation and aggregation may seed extracellular deposits (Chen et al., 2004). This acute deposition has been reproduced in a pig model of TBI (Smith et al., 1999; Smith et al., 2000), but not in small animal studies published to date (Van Den Heuvel et al., 2007).
The second phenomenon is the acute decrease in extracellular, soluble (ISF) Aβ that we describe in this mouse model. This phenomenon fits well with the human Aβ microdialysis dynamics discussed above. These findings similarly fit well with a previous study that described a marked decrease in amyloid plaque burden in PDAPP mice injured at 4 months of age and assessed 5 and 8 months after injury (Nakagawa et al., 1999). They are also consistent with the reduced Aβ deposition in the dentate gyrus observed following perforant pathway transection (Lazarov et al., 2002). In light of our current results, we suggest that reduced extracellular concentrations of Aβ after these injuries may have resulted in the diminished deposition at chronic timepoints.
Finally, there appears to be at least one more phenomenon: a variable spike or gradual rise of Aβ levels above baseline seen in some experimental studies (Abrahamson et al., 2006; Abrahamson et al., 2009; Chen et al., 2004; Loane et al., 2009; Smith et al., 1998a). These observations were made using brain tissue homogenates, so the exact pool of Aβ responsible cannot be determined. Co-accumulation of APP with the secretases that cleave it to form Aβ (Chen et al., 2004), caspase activation (Abrahamson et al., 2006), and changes in cholesterol metabolism (Abrahamson et al., 2009) have all been implicated in these changes. As some forms of soluble Aβ appear to have neurotoxic properties (Hardy and Selkoe, 2002; Shankar et al., 2008), elevated levels of Aβ have been hypothesized to contribute to neuronal cell death after TBI (Smith et al., 1998a). Our findings suggest that this spike or gradual rise of Aβ after TBI may not be a universal finding, and therefore the contribution of Aβ–related neurotoxicity in TBI remains an open question.
Returning to the issue of TBI and the risk of AD, our experimental and clinical results do not add support to the hypothesis that TBI acutely increases total extracellular soluble Aβ levels. However, this study examined only the immediate dynamics of ISF Aβ in an acute period, from 24 hours to 5 days. These experiments were not designed to assess delayed effects of TBI on extracellular Aβ levels. Future investigations could be performed to measure ISF Aβ in the subacute and chronic post-injury periods.
While astrogliosis does not appear to be prominent in the first 24 hours after CCI, it could contribute to the effects seen over the subsequent days. In addition, other aspects of inflammation such as soluble cytokine and chemokine responses (Bye et al., 2007; Harting et al., 2008; Semple et al., 2010) could affect Aβ dynamics. Microdialysis using high molecular weight cutoff catheters could be used monitor ISF cytokine and chemokine levels following TBI in our experimental mouse model, as has been done in initial human (Hillman et al., 2007; Hutchinson et al., 2007; Winter et al., 2002; Winter et al., 2004) and rat (Folkersma et al., 2008) studies. This would be especially interesting in the context of more prolonged microdialysis studies, during which the correlations of astrogliosis and microglial reactions with ISF Aβ dynamics could be assessed (Bye et al., 2007; Di Giovanni et al., 2005; Sastre et al., 2008).
Other critical questions regarding the role of Aβ in the increased risk of AD after TBI remain unresolved. What is the oligomerization state of Aβ in the brain after TBI? How do genetic factors such as ApoE status affect Aβ dynamics following TBI (Hartman et al., 2002; Nicoll et al., 1995)? How do sleep/wake cycle alterations (Kang et al., 2009) influence the effects of TBI? Does the mechanism of injury alter the relationship between trauma and Aβ levels, i.e. is there a major contribution of traumatic axonal injury (Chen et al., 2004)? We argue that microdialysis assessment of Aβ dynamics in the brain extracellular fluid is a relevant approach to all of these questions, given the potential for human and experimental studies. It is, of course, possible that changes in extracellular Aβ levels are in fact unrelated to the increase risk of dementia of the Alzheimer type. Reduced cognitive reserve, intracellular Aβ, or other, as yet undefined factors may play major roles (Magnoni, in press).
In conclusion, these combined microdialysis-TBI methods in mice may allow many further experiments involving a wide variety of transgenic animals. Clearly, further studies in both experimental models and human TBI patients will be required to address the mechanisms underlying the observed Aβ dynamics and elucidate the reasons why TBI increases the risk of AD.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH NS049237 (DLB), AG13956 (DMH), a Burroughs Wellcome Career Award in the Biomedical Sciences (DLB), Thrasher Research Fund (DLB), and Cure Alzheimer s Fund (DMH). We are grateful to Eli Lilly and Co. for providing antibodies and the founders of our PDAPP mouse colony. The authors have no conflicts of interest to disclose.
Footnotes
Author Contributions: KES, JRC, DMH and DLB designed research. KES, TJE and CLM performed research, KES and DLB analyzed data. KES and DLB wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamson EE, Ikonomovic MD, Ciallella JR, Hope CE, Paljug WR, Isanski BA, et al. Caspase inhibition therapy abolishes brain trauma-induced increases in Abeta peptide: implications for clinical outcome. Exp Neurol. 2006;197:437–50. doi: 10.1016/j.expneurol.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Abrahamson EE, Ikonomovic MD, Dixon CE, DeKosky ST. Simvastatin therapy prevents brain trauma-induced increases in beta-amyloid peptide levels. Ann Neurol. 2009;66:407–14. doi: 10.1002/ana.21731. [DOI] [PubMed] [Google Scholar]
- Alessandri B, Heimann A, Filippi R, Kopacz L, Kempski O. Moderate controlled cortical contusion in pigs: effects on multi-parametric neuromonitoring and clinical relevance. J Neurotrauma. 2003;20:1293–305. doi: 10.1089/089771503322686094. [DOI] [PubMed] [Google Scholar]
- Alves OL, Bullock R, Clausen T, Reinert M, Reeves TM. Concurrent monitoring of cerebral electrophysiology and metabolism after traumatic brain injury: an experimental and clinical study. J Neurotrauma. 2005;22:733–49. doi: 10.1089/neu.2005.22.733. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24:439–51. doi: 10.1097/HTR.0b013e3181c15600. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Kochanek PM, Carcillo JA, Mi Z, Schiding JK, Wisniewski SR, et al. Interstitial adenosine, inosine, and hypoxanthine are increased after experimental traumatic brain injury in the rat. J Neurotrauma. 1998;15:163–70. doi: 10.1089/neu.1998.15.163. [DOI] [PubMed] [Google Scholar]
- Brody DL, Mac Donald C, Kessens CC, Yuede C, Parsadanian M, Spinner M, et al. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J Neurotrauma. 2007;24:657–73. doi: 10.1089/neu.2006.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N, et al. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–4. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bye N, Habgood MD, Callaway JK, Malakooti N, Potter A, Kossmann T, et al. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp Neurol. 2007;204:220–33. doi: 10.1016/j.expneurol.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Chen XH, Siman R, Iwata A, Meaney DF, Trojanowski JQ, Smith DH. Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am J Pathol. 2004;165:357–71. doi: 10.1016/s0002-9440(10)63303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciallella JR, Ikonomovic MD, Paljug WR, Wilbur YI, Dixon CE, Kochanek PM, et al. Changes in expression of amyloid precursor protein and interleukin-1beta after experimental traumatic brain injury in rats. J Neurotrauma. 2002;19:1555–67. doi: 10.1089/089771502762300229. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, et al. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, May PC, O’Dell MA, Taylor JW, Parsadanian M, Cramer JW, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844–53. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–22. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Di Giovanni S, Movsesyan V, Ahmed F, Cernak I, Schinelli S, Stoica B, et al. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci U S A. 2005;102:8333–8. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvang AB, Volbracht C, Pedersen LO, Jensen KG, Karlsson JJ, Larsen SA, et al. Differential effects of gamma-secretase and BACE1 inhibition on brain Abeta levels in vitro and in vivo. J Neurochem. 2009;110:1377–87. doi: 10.1111/j.1471-4159.2009.06215.x. [DOI] [PubMed] [Google Scholar]
- Folkersma H, Breve JJ, Tilders FJ, Cherian L, Robertson CS, Vandertop WP. Cerebral microdialysis of interleukin (IL)-1beta and IL-6: extraction efficiency and production in the acute phase after severe traumatic brain injury in rats. Acta Neurochir (Wien) 2008;150:1277–84. doi: 10.1007/s00701-008-0151-y. discussion 1284. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Elsevier/Academic Press; San Diego; London: 2001. [Google Scholar]
- Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, et al. Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005;25:2803–10. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–7. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW. Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci Lett. 1993;160:139–44. doi: 10.1016/0304-3940(93)90398-5. [DOI] [PubMed] [Google Scholar]
- Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–23. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- Hall ED, Gibson TR, Pavel KM. Lack of a gender difference in post-traumatic neurodegeneration in the mouse controlled cortical impact injury model. J Neurotrauma. 2005;22:669–79. doi: 10.1089/neu.2005.22.669. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harting MT, Jimenez F, Adams SD, Mercer DW, Cox CS., Jr Acute, regional inflammatory response after traumatic brain injury: Implications for cellular therapy. Surgery. 2008;144:803–13. doi: 10.1016/j.surg.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, Laurer H, Longhi L, Bales KR, Paul SM, McIntosh TK, et al. Apolipoprotein E4 influences amyloid deposition but not cell loss after traumatic brain injury in a mouse model of Alzheimer’s disease. J Neurosci. 2002;22:10083–7. doi: 10.1523/JNEUROSCI.22-23-10083.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillered L, Vespa PM, Hovda DA. Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J Neurotrauma. 2005;22:3–41. doi: 10.1089/neu.2005.22.3. [DOI] [PubMed] [Google Scholar]
- Hillman J, Aneman O, Persson M, Andersson C, Dabrosin C, Mellergard P. Variations in the response of interleukins in neurosurgical intensive care patients monitored using intracerebral microdialysis. J Neurosurg. 2007;106:820–5. doi: 10.3171/jns.2007.106.5.820. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, O’Connell MT, Rothwell NJ, Hopkins SJ, Nortje J, Carpenter KL, et al. Inflammation in human brain injury: intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J Neurotrauma. 2007;24:1545–57. doi: 10.1089/neu.2007.0295. [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VM, et al. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190:192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Jacobson I, Sandberg M, Hamberger A. Mass transfer in brain dialysis devices--a new method for the estimation of extracellular amino acids concentration. J Neurosci Methods. 1985;15:263–8. doi: 10.1016/0165-0270(85)90107-4. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, et al. APP processing and synaptic function. Neuron. 2003;37:925–37. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc Natl Acad Sci U S A. 2007;104:10673–8. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-{beta} Dynamics Are Regulated by Orexin and the Sleep-Wake Cycle. Science. 2009 doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnappa IK, Contant CF, Robertson CS. Regional changes in cerebral extracellular glucose and lactate concentrations following severe cortical impact injury and secondary ischemia in rats. J Neurotrauma. 1999;16:213–24. doi: 10.1089/neu.1999.16.213. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Lee M, Peterson DA, Sisodia SS. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J Neurosci. 2002;22:9785–93. doi: 10.1523/JNEUROSCI.22-22-09785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Pocivavsek A, Moussa CE, Thompson R, Matsuoka Y, Faden AI, et al. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat Med. 2009;15:377–9. doi: 10.1038/nm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007a;27:11869–76. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol. 2007b;205:116–31. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnoni S, Brody DL. New perspectives on amyloid-β dynamics following acute brain injury: moving between experimental approaches and studies in the human brain. Archives of Neurology. doi: 10.1001/archneurol.2010.214. in press. [DOI] [PubMed] [Google Scholar]
- Marklund N, Blennow K, Zetterberg H, Ronne-Engström E, Enblad P, Hillered L. Monitoring of Brain Interstitial Total Tau and β Amyloid Proteins by Microdialysis in Patients with Traumatic Brain Injury. Journal of Neurosurgery. 2009;110:1227–37. doi: 10.3171/2008.9.JNS08584. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Nakamura M, McIntosh TK, Rodriguez A, Berlin JA, Smith DH, et al. Traumatic brain injury in young, amyloid-beta peptide overexpressing transgenic mice induces marked ipsilateral hippocampal atrophy and diminished Abeta deposition during aging. J Comp Neurol. 1999;411:390–8. [PubMed] [Google Scholar]
- Nicoll JA, Roberts GW, Graham DI. Apolipoprotein E epsilon 4 allele is associated with deposition of amyloid beta-protein following head injury. Nat Med. 1995;1:135–7. doi: 10.1038/nm0295-135. [DOI] [PubMed] [Google Scholar]
- Palmer AM, Marion DW, Botscheller ML, Swedlow PE, Styren SD, DeKosky ST. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J Neurochem. 1993;61:2015–24. doi: 10.1111/j.1471-4159.1993.tb07437.x. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–66. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Gentleman SM, Lynch A, Graham DI. beta A4 amyloid protein deposition in brain after head trauma. Lancet. 1991;338:1422–3. doi: 10.1016/0140-6736(91)92724-g. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Gentleman SM, Lynch A, Murray L, Landon M, Graham DI. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1994;57:419–25. doi: 10.1136/jnnp.57.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronne-Engstrom E, Cesarini KG, Enblad P, Hesselager G, Marklund N, Nilsson P, et al. Intracerebral microdialysis in neurointensive care: the use of urea as an endogenous reference compound. J Neurosurg. 2001;94:397–402. doi: 10.3171/jns.2001.94.3.0397. [DOI] [PubMed] [Google Scholar]
- Rose ME, Huerbin MB, Melick J, Marion DW, Palmer AM, Schiding JK, et al. Regulation of interstitial excitatory amino acid concentrations after cortical contusion injury. Brain Res. 2002;943:15–22. doi: 10.1016/s0006-8993(02)02471-x. [DOI] [PubMed] [Google Scholar]
- Sastre M, Walter J, Gentleman SM. Interactions between APP secretases and inflammatory mediators. J Neuroinflammation. 2008;5:25. doi: 10.1186/1742-2094-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2−/− mice. J Cereb Blood Flow Metab. 2010;30:769–82. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Chen XH, Nonaka M, Trojanowski JQ, Lee VM, Saatman KE, et al. Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. J Neuropathol Exp Neurol. 1999;58:982–92. doi: 10.1097/00005072-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Smith DH, Nakamura M, McIntosh TK, Wang J, Rodriguez A, Chen XH, et al. Brain trauma induces massive hippocampal neuron death linked to a surge in beta-amyloid levels in mice overexpressing mutant amyloid precursor protein. Am J Pathol. 1998a;153:1005–10. doi: 10.1016/s0002-9440(10)65643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Nakamura M, McIntosh TK, Wang J, Rodriguez A, Chen XH, et al. Brain trauma induces massive hippocampal neuron death linked to a surge in beta-amyloid levels in mice overexpressing mutant amyloid precursor protein. Am J Pathol. 1998b;153:1005–10. doi: 10.1016/s0002-9440(10)65643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Nonaka M, Miller R, Leoni M, Chen XH, Alsop D, et al. Immediate coma following inertial brain injury dependent on axonal damage in the brainstem. J Neurosurg. 2000;93:315–22. doi: 10.3171/jns.2000.93.2.0315. [DOI] [PubMed] [Google Scholar]
- Stone JR, Singleton RH, Povlishock JT. Antibodies to the C-terminus of the beta-amyloid precursor protein (APP): a site specific marker for the detection of traumatic axonal injury. Brain Res. 2000;871:288–302. doi: 10.1016/s0006-8993(00)02485-9. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer’s disease: a review. Prog Brain Res. 2007;161:303–16. doi: 10.1016/S0079-6123(06)61021-2. [DOI] [PubMed] [Google Scholar]
- Winter CD, Iannotti F, Pringle AK, Trikkas C, Clough GF, Church MK. A microdialysis method for the recovery of IL-1beta, IL-6 and nerve growth factor from human brain in vivo. J Neurosci Methods. 2002;119:45–50. doi: 10.1016/s0165-0270(02)00153-x. [DOI] [PubMed] [Google Scholar]
- Winter CD, Pringle AK, Clough GF, Church MK. Raised parenchymal interleukin-6 levels correlate with improved outcome after traumatic brain injury. Brain. 2004;127:315–20. doi: 10.1093/brain/awh039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.