Abstract
Tobacco smoke exposure during development can result in lasting alterations in sensory processing and attention. This suggests that some constituent of smoke, such as the primary addictive component, nicotine, alters neurodevelopment. Although many effects of developmental nicotine exposure have been identified in humans and animal models, very few mechanistic studies have identified the molecular and anatomical basis for a defined behavioral consequence of developmental exposure. We show in this study that a mouse model of developmental nicotine exposure results in hypersensitive passive avoidance in adulthood. We have used transgenic mice in which β2 subunit containing nicotinic acetylcholine receptors (β2* nAChRs) are expressed exclusively on corticothalamic neurons (β2 tr(CT) mice) to identify the receptor subtypes involved and also to define the circuit level site of action responsible for this persistent, nicotine-induced behavioral phenotype. Further characterization of the native nAChRs expressed in this circuit indicates that both (α4)2(β2)3 and (α4)2(β2)2α5 nAChR subtypes are present in corticothalamic projections. Consistent with a role for (α4)2(β2)2α5 nAChRs in mediating the effect of developmental nicotine exposure on adult passive avoidance behavior, constitutive deletion of the α5 nAChR subunit also alters this behavior. A critical period for this developmental consequence of nicotine exposure was defined by limiting exposure to the early post-natal period. Taken together, these studies identify a novel consequence of developmental nicotine exposure in the mouse, define the nAChR subtypes and neural circuit involved in this behavioral change and delimit the neurodevelopmental period critical for vulnerability to a behavioral alteration that persists into adulthood.
Keywords: nicotinic acetylcholine receptors, development, nicotine, thalamus, cortex
INTRODUCTION
Recent epidemiological data estimate that between 10.7 and 12.4% of pregnant women in the United States smoke during pregnancy (Martin et al, 2007). Children exposed to tobacco smoke exhibit persistent impairments in a variety of cognitive tasks, as well as altered processing of sensory stimuli, suggesting that early tobacco exposure alters neurodevelopment (Heath and Picciotto, 2009). For example, developmental tobacco exposure alters auditory processing (Fried and Makin, 1987; McCartney et al, 1994) with no effect on stimulus detection or auditory brainstem responses (Trammer et al, 1992). Although there are likely effects of gestational tobacco exposure on the higher cortical areas responsible for attention and cognitive function (Jacobsen et al, 2006, 2007b), alterations may also occur in circuits responsible for early processing and cortical relay of sensory stimuli, such as the thalamocortical and corticothalamic neurons connecting thalamic sensory nuclei to primary sensory cortex (Heath and Picciotto, 2009; Metherate and Hsieh, 2003).
A major psychoactive component of tobacco is nicotine (Stolerman and Jarvis, 1995) which acts through nicotinic acetylcholine receptors (nAChRs) to exert profound effects on neurodevelopment, including the maturation of γ-amino butyric acid (GABA)ergic (Liu et al, 2006) and glutamatergic neurons (Maggi et al, 2004). In rodents, nicotine exposure during a critical period corresponding to the third trimester of human pregnancy (Dobbing and Sands, 1979) alters maturation of thalamocortical neurons in the auditory system and impairs behavior in a task dependent on auditory stimuli (Aramakis et al, 2000; Aramakis and Metherate, 1998; Liang et al, 2006). Similarly, expression of α4β2-containing (α4β2*) nAChRs on developing corticothalamic neurons is required for normal performance in passive avoidance, a somatosensory stimulus-dependent task (King et al, 2003; Picciotto et al, 1995).
In this study, we show that developmental nicotine exposure results in hypersensitive passive avoidance behavior. This phenotype is characterized by a significantly increased latency to enter a chamber in which a mild footshock was previously administered. In addition, we identify the native nAChR subtypes and the neuronal circuit upon which nicotine acts during development to induce hypersensitive passive avoidance behavior in adulthood. To identify the circuit altered by developmental nicotine exposure, we tested passive avoidance performance in developmental nicotine-treated transgenic mice with α4β2* nAChR expression exclusively in corticothalamic neurons (β2 tr(CT)). We then performed a detailed biochemical characterization of the native nAChR subtypes expressed in the corticothalamic projections of these animals and identified the relatively rare (α4)2(β2)2α5 nAChR as a predominant subtype expressed in these neurons. The α5 nAChR subunit alters nAChR conductance, affinity and desensitization kinetics (Girod et al, 1999; Kuryatov et al, 2008; Ramirez-Latorre et al, 1996). To identify a functional role for these α5* nAChRs we also tested passive avoidance performance of α5 nAChR subunit knockout (KO) mice. Finally, to determine the critical period during which nicotine exposure acts to induce this persistent behavioral phenotype, we conducted a cross-fostering study to limit nicotine exposure to either the prenatal or early postnatal period.
Taken together, this study describes a novel consequence of developmental nicotine exposure in mice which persists long after nicotine exposure has ceased, a characteristic that strongly parallels the deleterious effects observed in humans exposed to tobacco smoke in utero (Jacobsen et al, 2006, 2007b). Furthermore, these experiments identify both the neuronal circuit and the nAChR subtypes underlying this developmental effect of nicotine exposure and describe a window of vulnerability for the induction of this phenotype.
MATERIALS AND METHODS
Mice
All procedures were approved by the Yale University Institutional Animal Care and Use Committee and/or the University of Colorado Animal Utilization Committee and conformed to the standards of the NIH Guide for Care and Use of Laboratory Animals. C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) used in the developmental nicotine exposure studies acclimated for 1 week, had ad libitum food and water and a 12-h light–dark cycle (lights on 0700 hours). β2 tr(CT) mice expressing the β2 nAChR subunit in corticothalamic neurons (King et al, 2003), β2 KO mice (Picciotto et al, 1995) and α5 KO mice (Salas et al, 2003), all of which have been backcrossed onto the C57BL/6J background for at least 15 generations, were bred from heterozygous (HET) breeding pairs.
Developmental Nicotine Exposure
After vaginal plug identification, gestating mice were singly housed with ad libitum food and water containing 200 μg/ml nicotine hydrogen tartrate (calculated as free base) in 2% (w/v) saccharin or pH-matched 2% saccharin with 0.2% (v/v) tartaric acid (Sigma-Aldrich, St Louis, MO). Solutions were prepared twice a week. At 21 days, offspring were weaned and left undisturbed until behavioral testing except for routine husbandry.
Passive Avoidance Behavior
Passive avoidance testing was conducted using mice >90 days old in a step-through, two chamber apparatus (Ugo Basile, Comerio, Italy) using a 3-day paradigm (King et al, 2003). Day 1: mice explored the apparatus for 5 min. Day 2: mice were placed in the light chamber and received a mild, inescapable footshock (0.5 mA, 2 s) after dark chamber entry. Day 3: mice were placed in the light chamber and time to cross into the dark chamber was measured. Latency to cross was measured on days 2 (train) and 3 (test), with a 5 min maximum. On day 3, if mice placed their front paws in the dark chamber but failed to cross before the door between the compartments closed, time of approach was recorded, the door was immediately re-opened and the session continued until dark chamber entry or until the 5 min limit was reached.
Shock Reactivity Threshold
Unconditioned response to footshock was measured as described (Caldarone et al, 2000). Mice received 2 s stimuli ranging from 0.05 to 1.0 mA at 20 s intervals in ascending 0.05 mA steps (0.1 mA steps for α5 KO mice and their wild-type (WT) controls). Mice were scored for flinch (any observable reaction), vocalization, run and jump responses. The test was terminated when all four reactions had been observed or if 1.0 mA intensity was reached.
nAChR Subunit In Situ Hybridization
In situ hybridization for nAChR subunits was performed as described (Marks et al, 1992). Details of the methods for hybridization and quantitation can be found in the Supplementary Information.
Crude Synaptosomal Preparation
Cortical and thalamic synaptosomes were prepared as described (Gotti et al, 2008). See Supplementary Information for more details.
[86Rb+] Uptake
Synaptosomal [86Rb+] uptake was performed as described (Gotti et al, 2008). See Supplementary Information for more details.
Synaptosomal ACh-Stimulated [86Rb+] Efflux
Filters with [86Rb+]-loaded synaptosomes were placed on an open-air platform and superfused at 22°C with buffer (135 mM NaCl, 1.5 mM KCl, 5 mM CsCl, 2 mM CaCl2, 1 mM MgSO4, 20 mM glucose, 1 μM atropine, 50 nM tetrodotoxin, 0.1% bovine serum albumin and 25 mM HEPES hemisodium, pH 7.5). Buffer was applied to the top of the filter at a rate of 2.5 ml/min by a Minipuls 3 peristaltic pump (Gilson, Middleton, WI) and removed from the bottom by a second pump at a rate of 3.2 ml/min to actively remove buffer and, therefore, prevent pooling. Radioactivity was continuously monitored by pumping the effluent through a 200 μl Cherenkov cell in a β-Ram HPLC detector (IN/US Systems, Tampa, FL).
Data collection began after 5 min of the filter being superfused to ensure basal efflux stability. Concentration-effect curves were generated by exposing filters to a single ACh concentration (0.1–1000 μM) for 5 s and curves were generated for both cortex and thalamus from each mouse used.
Sample Preparation for [125I]-Epibatidine Binding
Samples were prepared as described (Marks et al, 2004). See Supplementary Information for more details.
[125I]-Epibatidine Binding
Frozen pellets were resuspended in overlying hypotonic buffer (Marks et al, 2004) and centrifuged at 20 000 g for 20 min. The supernatant was discarded and pellets were then suspended in ice-cold water. The volume used was adjusted such that less than 10% of the [125I]-epibatidine was bound to the protein at the highest ligand concentration. Samples were incubated for 3 h at room temperature in 96-well polystyrene plates in a final volume of 30 μl. After incubation, 200 μl ice-cold wash buffer was added to each sample and the diluted samples were then filtered through glass fiber filters (top=MFS type B; bottom=Gelman A/E) treated with 0.5% polyethelenimine under 0.2-atmosphere vacuum. Samples were collected by an Inotech Cell Harvester (Inotech Biosystems, Rockville, MD) and washed with ice-cold buffer five times. Filters containing the samples were placed in glass culture tubes and radioactivity was measured at 80% efficiency by a Packard Cobra Auto-Gamma Counter (Packard Instruments, Downers Grove, IL). For all experiments, 100 μM nicotine was added to measure non-specific binding.
Saturation binding curves were generated for cortex and thalamus by measuring specific binding at eight [125I]-epibatidine concentrations. Protein concentration was measured using the method of Lowry (Lowry et al, 1951) with bovine serum albumin standards.
Antibody Production and Characterization
The nAChR subunit-specific rabbit polyclonal antibodies (Abs) used were produced by immunization with peptides derived from the rat, mouse or human subunit intracytoplasmic loop or C-terminal domain sequences. To determine the contribution of the α5 subunit to the nAChRs investigated in this study, we generated an antiserum specifically directed against a mouse α5 subunit cytoplasmic peptide (DRYFTQREEAEKDGGPKSRNTLEAALDC) that was used in parallel with the anti-α5 rat-directed antisera. This new antiserum was tested for specificity in extracts obtained from cortex and hippocampus of α5 KO mice and failed to immunoprecipitate significant amounts (less than 1%) of [3H]-epibatidine-labeled receptors. All these antibodies were affinity purified and have been characterized previously (Champtiaux et al, 2003; Gotti et al, 2005a, 2005b; Moretti et al, 2004; Zoli et al, 2002).
Antibody specificity and immunoprecipitation capacity was examined by immunoprecipitation or immunopurification of nAChR subunits from brain tissue collected from WT and various nAChR subunit KO mice. Specificity was also verified by Western blotting. Verification of specificity is required because this characteristic is both sequence- (a single peptide can generate Abs with different levels of specificity between rabbits) and time-related (specificity can vary within the same rabbit).
Membrane and 2% Triton X-100 Extract Preparation
Membrane and 2% Triton X-100 extracts were prepared as described (Gotti et al, 2005a, 2005b, 2008). See Supplementary Information for further details.
[3H]-Epibatidine Binding
All experiments were conducted in the presence of 2 μM α-bungarotoxin to prevent α7* nAChR binding. Cortical and thalamic homogenates were incubated overnight with 2 nM [3H]-epibatidine at 4°C. Non-specific binding was determined in parallel by including 100 nM unlabeled epibatidine in the incubation. Following incubation, samples were filtered through a 0.5% polyethylenimine-treated GFC filter, washed with 15 ml buffer (10 mM sodium-phosphate, pH 7.4 and 50 mM NaCl) and radioactivity was counted in a beta counter.
Triton X-100 extracts were also labeled with 2 nM [3H]-epibatidine and binding was performed using DE52 ion-exchange resin (Whatman, Maidstone, UK) as described (Vailati et al, 1999).
Quantitative Immunoprecipitation of [3H]-Epibatidine-Labeled Receptors by Subunit-Specific Antibodies
Cortical and thalamic extracts (100–150 μl) were labeled with 2 nM [3H]-epibatidine, and were then incubated overnight with saturating concentrations of affinity-purified anti-subunit immunoglobulin G. Immunoprecipitates were recovered by incubation with anti-rabbit goat immunoglobulin G beads (Technogenetics, Milan, Italy). The amount of immunoprecipitation with each antibody is expressed as the percentage of [3H]-epibatidine labeled receptors precipitated (the initial amount present in the Triton X-100 extract taken as 100%) or as femtomoles of immunoprecipitated receptor per milligram protein.
Western Blotting
Western blotting was performed as described (Gotti et al, 2008). See Supplementary Information for more details.
β2 nAChR Subunit Binding
Brains from β2 tr(CT) transgenic mice and their WT siblings (P1, P7 and P14) and β2 nAChR subunit KO mice (P14) were frozen on dry ice and stored at –80°C. 12 μm sections were cut on a cryostat, thaw mounted on chrom-alum-coated slides (0.5% chromium (III) phosphate/0.5% gelatin), dried at room temperature for 20 min and stored at −80°C. Sections were thawed at room temperature and incubated with [125I]-epibatidine for 30 min in 50 mM Tris-HCl pH7.4, washed twice in the same buffer, dried and exposed to [3H]-Hyperfilm for 2–7 days.
Statistical Analysis
Analyses of pre- and post-natal drug treatment data in C57BL/6J mice were performed between litters to minimize potential litter effects (Holson and Pearce, 1992). Litters with a minimum of four mice were used for testing (two of each sex). For the cross-fostering experiment, we required at least one member of each representative group to be included (male/female, non-cross fostered/cross-fostered). Passive avoidance data were analyzed by analysis of variance; shock reactivity data were analyzed by Mann–Whitney U-test. In situ hybridization, radioligand binding and quantitative immunoprecipitation data were analyzed by One-Way analysis of variance followed by Duncan post hoc test. A p-value of <0.05 was considered significant. Data are presented as means±standard error of the mean.
RESULTS
Nicotine Exposure During Development Induces Hypersensitivity in the Passive Avoidance Paradigm through Corticothalamic β2* nAChRs
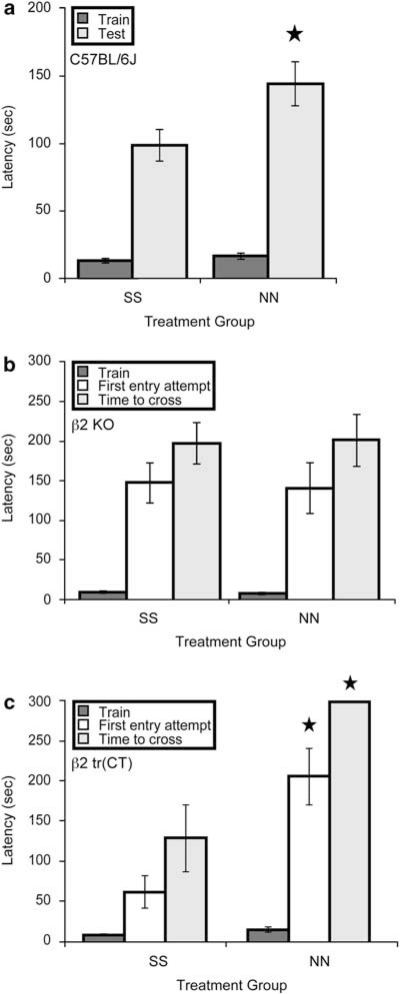
Adult C57BL/6J mice exposed to nicotine from conception until weaning at 21 days of age exhibited hypersensitive passive avoidance behavior, as shown by their increased latency to enter the chamber in which the shock was administered, compared with saccharin-exposed mice (Figure 1a). As β2 nAChR subunit KO mice also show hypersensitive passive avoidance (Picciotto et al, 1995), this suggests that chronic nicotine exposure may interfere with the function of β2* nAChRs during development, either through receptor desensitization or interference with the temporal dynamics of acetylcholine signaling at the receptors, to result in this phenotype in adulthood. Developmental nicotine exposure had no significant effect on latency to enter the dark chamber on training day (Figure 1a).
Figure 1.
Nicotine exposure during development induces hypersensitive passive avoidance through β2* nAChRs expressed by corticothalamic neurons. (a) C57BL/6J mice exposed to nicotine throughout gestation until weaning (NN) have a significantly longer latency to enter the dark chamber in response to a low level foot shock relative to saccharin-exposed controls (SS) (F(1, 12)=5.096, p=0.043; n=7 litters per treatment). (b) β2 nAChR subunit knockout (KO) mice exposed to nicotine throughout gestation until weaning (NN) are indistinguishable (p>0.05) from saccharin-exposed controls (SS) when latency to first entry attempt and latency to successful dark chamber entry are compared (n=15–24 per group). (c) Mice expressing α4β2* nAChRs exclusively on corticothalamic neurons (β2 tr(CT)) and exposed to nicotine throughout gestation until weaning (NN) have a significantly longer time to first entry attempt (F(1, 13)=11.857, p=0.004) and total time to successful dark chamber entry (F(1, 13)=18.885, p=0.001) relative to saccharin-exposed controls (SS) (n=7–8 per treatment). ★P<0.05.
To confirm that β2* nAChRs mediate the effects of perinatal nicotine exposure on passive avoidance performance, KO mice lacking β2 nAChR subunit expression were also exposed to nicotine from conception until weaning and tested for passive avoidance as adults. No significant effects of developmental nicotine exposure on passive avoidance were observed in these mice (Figure 1b). It is unlikely that a ceiling effect explains the absence of a nicotine exposure-induced increase in passive avoidance latency in β2 KO mice as the maximum latency permitted in this task was 300 s and the average latency in both treatment groups was well below this threshold (Figure 1b). This suggests that β2 subunit containing nAChRs are required for passive avoidance modulation by perinatal nicotine exposure.
We have shown previously that the expression of α4β2* nAChRs on corticothalamic neurons during development rescues the hypersensitive passive avoidance phenotype observed in β2 nAChR subunit KO mice (King et al, 2003); we, therefore, hypothesized that the hypersensitive passive avoidance behavior observed after developmental nicotine exposure is mediated through effects on α4β2* nAChRs exclusively on corticothalamic neurons. To test this hypothesis, β2 tr(CT) transgenic mice that express α4β2* nAChRs exclusively on corticothalamic neurons were exposed to nicotine throughout gestation and postnatally until weaning and tested in passive avoidance as adults. This nicotine exposure resulted in hypersensitive passive avoidance performance in the β2 tr(CT) mice suggesting that expression of α4β2* nAChRs on corticothalamic neurons is sufficient to mediate the effects of developmental nicotine on adult passive avoidance behavior (Figure 1c).
Developmental Nicotine Exposure does not Induce Consistent Alterations in Foot Shock Sensitivity
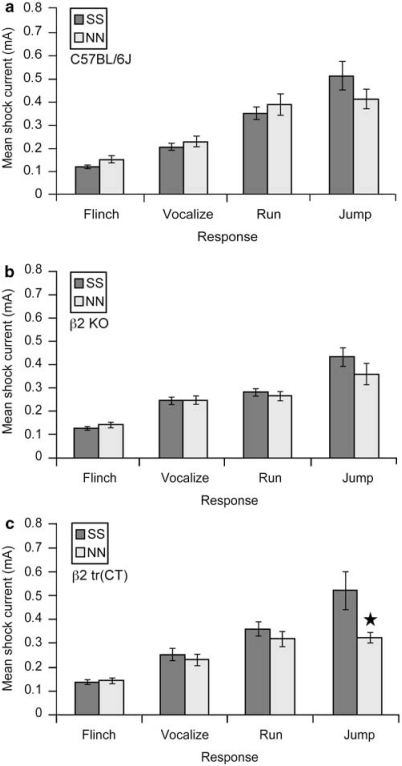
To confirm that the changes in passive avoidance behavior observed were not a consequence of altered sensitivity to the foot shock stimulus used, the shock current thresholds required to elicit a number of behavioral responses were determined for developmental nicotine-exposed C57BL/6J, β2 KO and β2 tr(CT) mice and their respective controls.
No significant differences in any of the shock reactivity parameters assessed were observed between nicotine- and saccharin-exposed C57BL/6J (Figure 2a) or β2 KO (Figure 2b) mice. A small, but significant decrease in the threshold for jump responses (Mann–Whitney U=9.000; p=0.027; n=7–8 per group) was observed in the nicotine-exposed β2 tr(CT) group relative to the saccharin-exposed controls (Figure 2c). All other shock reactivity parameters tested were identical between these groups (Figure 2c). These data suggest that the effects of developmental nicotine exposure on passive avoidance behavior are not a consequence of changes in the detection of sensory stimuli.
Figure 2.
Nicotine exposure during development does not induce consistent differences in the shock reactivity thresholds of C57BL/6J, β2 KO or β2 tr(CT) mice. (a) C57BL/6J mice exposed to nicotine throughout gestation until weaning (NN) exhibit flinch, run, vocalize and jump foot shock responses at currents that were not significantly different (p>0.05) from saccharin-exposed controls (SS) (n=15–16 per group). (b) No significant differences (p>0.05) in flinch, run, vocalize or jump foot shock response thresholds were detected between nicotine exposed (NN) KO mice and saccharin-exposed controls (SS) (n=15–24 per group). (c) Nicotine exposed (NN) β2 tr(CT) mice exhibit flinch, run and vocalize foot shock responses at currents not significantly different (p>0.05) from saccharin-exposed controls (SS). A significant decrease in the NN jump response threshold (Mann–Whitney U=9.000; p=0.027; n=7–8 per group) was detected. ★P<0.05.
(α4)2(β2)3 and (α4)2(β2)2α5 nAChRs are Expressed in the Thalamo-Cortico-Thalamic Circuitry of β2 tr(CT) Mice
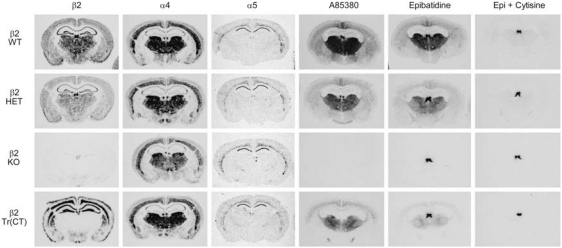
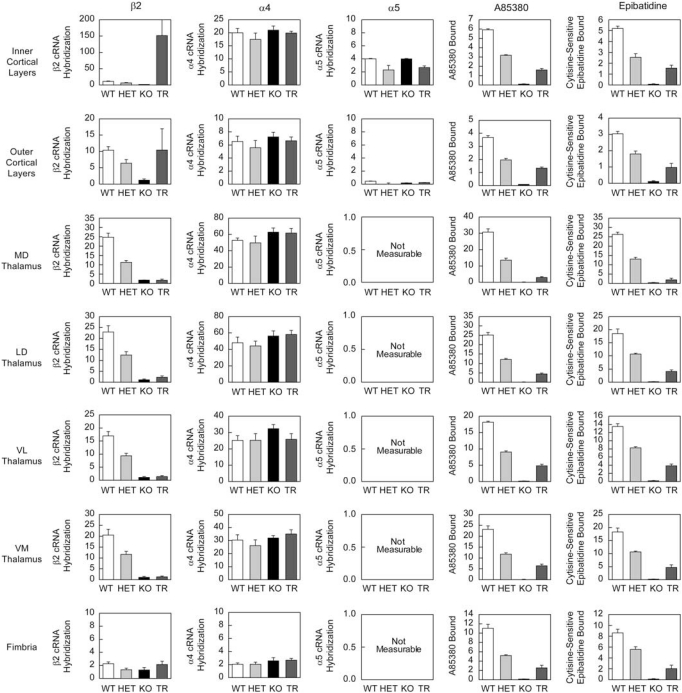
As β2 tr(CT) mice demonstrate sensitivity to the effects of developmental nicotine on passive avoidance performance, it was important to identify the nAChR subtypes re-expressed in these animals more precisely and to define their neuroanatomical location and sites of function. The images in Figure 3 show the expression of mRNAs encoding the β2, α4 and α5 nAChR subunits by in situ hybridization to illustrate the effects of β2 subunit deletion in HET and KO mice and restoration in β2 tr(CT) transgenic mice. Deletion of the β2 subunit resulted in a gene-dosage dependent reduction in the amount of β2 mRNA with virtual elimination of message in the brains of β2 KO mice (Figure 4). Deletion of the β2 subunit had no significant effect on the expression of mRNAs encoding either the α4 or α5 subunits in the brain areas quantitated. This analysis also confirmed that the pattern of re-expression of the mRNA encoding the β2 nAChR subunit in β2 tr(CT) mice corresponds to that previously reported (King et al, 2003). Quantitation of mRNA levels revealed that little or no re-expression occurred in thalamic nuclei or fimbria. However, mRNA levels in the outer cortical layers were restored to WT levels and expression in the inner cortical layers, primarily layer VI, was greater than that of WT mice. It should be noted that mRNA encoding both α4 and α5 subunits are co-localized with mRNA encoding β2 in the deep cortical layers. As heteromeric nAChRs are limited in number by assembly of multiple subunits, the number of assembled nAChRs in layer VI of β2 tr(CT) mice is likely to be limited by the expression of the α4 and α5 subunits.
Figure 3.
nAChR subunit mRNA expression patterns and radioligand binding identifies assembly of α5 subunit containing α4β2 nAChRs in the thalamo-cortico-thalamic circuitry of the mouse. In-situ hybridization for β2, α4 and α5 nAChR subunit expression in comparable coronal sections taken from wild type (WT), heterozygous (HET), β2 nAChR subunit knockout (KO) and corticothalamic transgenic (Tr(CT)) mice. HET, KO and Tr(CT) mice exhibit alterations in β2 mRNA expression with no significant changes in the α4 or α5 subunits. A85380 and epibatidine binding on comparable coronal sections from these animals also show differences in the numbers of assembled nAChRs in these mice. Epibatidine binding is eliminated in the presence of cytisine (Epi+cytisine) and corresponds to the binding of A-85380 in these brain regions. Representative sections at a level of approximately −1.6 mm Bregma are shown.
Figure 4.
Quantitation of nAChR subunit mRNA expression and radioligand binding in the thalamo-cortico-thalamic circuitry of the mouse. In-situ hybridization for β2, α4 and α5 nAChR subunit expression in comparable coronal sections taken from wild type (WT), heterozygous (HET) and β2 nAChR subunit knock out (KO) mice. HET and KO mice exhibit a marked reduction in β2 mRNA expression with no significant alteration in the α4 or α5 subunits. Analysis of subunit expression in the corticothalamic transgenic mice (TR) indicates that β2 mRNA expression is enhanced in deep cortical layers, at WT levels in superficial layers and at KO levels in all other areas analyzed. α4 and α5 expression is at approximately WT levels in the transgenic animals. A85380 and epibatidine binding on comparable coronal sections from these animals indicates a partial recovery of assembled nAChRs in the TR mice, relative to β2 knockout animals. Epibatidine binding is eliminated in the presence of cytisine (Epi+cytisine) and corresponds to the binding of A-85380 in these brain regions.
The effect of β2 subunit re-expression in β2 tr(CT) mice on nAChR binding sites was measured with both [125I]-A85380 and [125I]-epibatidine, ligands that recognize assembled nAChR receptors. Images in Figure 3 illustrate the effects of modifying β2 gene expression on these binding sites. Quantitation of these effects is summarized in Figure 4. Deletion of the β2 subunit resulted in a gene-dosage dependent reduction in [125I]-A85380 binding. No specific binding was observed in brains of β2 KO mice. A similar gene-dosage dependent reduction was observed for [125I]-epibatidine binding with the exception of labeling in the medial habenula in these sections. The residual habenular binding sites that also persist in the presence of 50 nM cytisine represent β4* nAChRs. Both [125I]-A85380 and [125I]-epibatidine binding sites are partially restored in the inner and outer cortical layers of β2 tr(CT) mice, in which β2 mRNA is re-expressed. Furthermore, there was significant re-expression of [125I]-A85380 and [125I]-epibatidine binding sites in each thalamic region analyzed, as well as in the fimbria, regions in which β2 mRNA was undetectable in the β2 tr(CT) mice. These results are also consistent with those reported previously for the β2 subunit (King et al, 2003), and the binding sites that represent assembled nAChRs located on corticothalamic projections.
As noted above, in situ hybridization demonstrated that mRNA encoding the α5 subunit has a much more restricted expression than mRNAs encoding either β2 or α4 (Figure 3); however, α5 mRNA is expressed in cortical layer VI in which both β2 and α4 are also expressed and where intense re-expression of β2 mRNA occurs in the β2 tr(CT) mice. The co-expression of α4, α5 and β2 mRNA in cortical layer VI suggests that a mixed population of native α4β2* nAChRs with different subunit composition and/or stoichiometries exists in the cortical cell bodies or on the corticothalamic terminals. The composition of native nAChRs in the thalamo-cortico-thalamic circuit was, therefore, evaluated by quantitative immunoprecipitation of cortical and thalamic [3H]-epibatidine binding sites using a panel of nAChR subunit-specific antibodies (Champtiaux et al, 2003; Gotti et al, 2005a, 2005b; Moretti et al, 2004; Zoli et al, 2002). As [3H]-epibatidine binding was minimal in the β2 KO mice, tissue from these animals was not examined by immunoprecipitation.
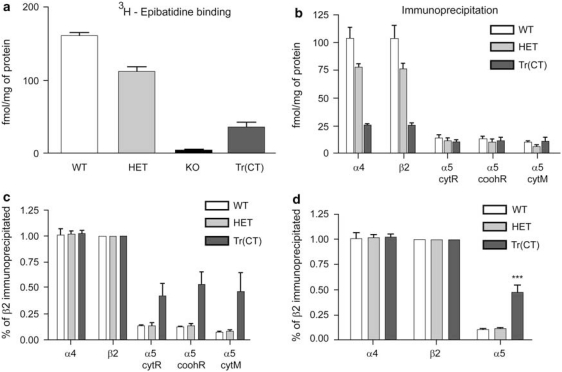
Consistent with the requirement of β2 expression for the assembly of receptors measured by [3H]-epibatidine binding, immunoprecipitation of nAChRs from cortex and thalamus of WT mice demonstrated that [3H]-epibatidine binding sites in the cortico-thalamic loops were quantitatively precipitated by α4- and β2-subunit selective antisera. A small, but significant, fraction of both the thalamic (10% Figure 5) and cortical (15% Figure 6) nAChRs were precipitated by three different α5-subunit selective antibodies.
Figure 5.
Quantitative immunoprecipitation of thalamic-epibatidine binding sites with nAChR subunit-specific antibodies indicates a preferential recovery of α5 containing nAChRs in tr(CT) mice. (a) [3H]-epibatidine thalamic binding in wild type (WT), heterozygous (HET), β2 knockout (KO) and tr(CT) mice indicates partial recovery in the tr(CT) line relative to KO. (b) Immunoprecipitation of α4, β2 and α5 nAChR subunits from thalamic samples of each genotype expressed as femtomoles of immunoprecipitated [3H]-epibatidine-labeled nAChR per milligram of protein. Antibodies raised against the cytoplasmic loop of the rat (cytR), carboxyl terminal region of the rat (coohR) and cytoplasmic loop of the mouse (cytM) α5 nAChR subunit were used. (c) Immunoprecipitation results expressed with β2 subunit levels taken as 100%. (d) Immunoprecipitation results expressed with β2 subunit levels taken as 100% and with data from the three α5 subunit antibodies combined, which indicates a significantly (***p<0.001) higher proportion of β2* nAChRs in the tr(CT) thalamus also contain the α5 subunit relative to the other genotypes.
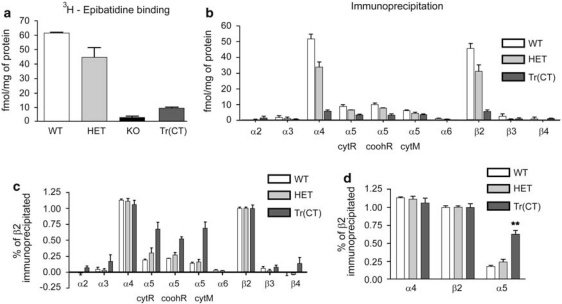
Figure 6.
Quantitative immunoprecipitation of cortical-epibatidine binding sites with nAChR subunit-specific antibodies indicates a preferential recovery of α5 containing nAChRs in tr(CT) mice. (a) Cortical [3H]-epibatidine binding in wild type (WT), heterozygous (HET), β2 knockout (KO) and tr(CT) mice indicates partial recovery of binding in the tr(CT) line relative to KO. (b) Immunoprecipitation of a panel of nAChR subunits from cortical samples of each genotype expressed as femtomoles of immunoprecipitated [3H]-epibatidine-labeled nAChR per milligram of protein. Antibodies raised against the cytoplasmic loop of the rat (cytR), carboxyl terminal region of the rat (coohR) and cytoplasmic loop of the mouse (cytM) α5 nAChR subunit were used. (c) Immunoprecipitation results expressed with β2 subunit levels taken as 100%. (d) Immunoprecipitation results expressed with β2 subunit levels taken as 100% and with data from the three α5 subunit antibodies combined, which indicates a significantly (**p<0.001) higher proportion of β2* nAChRs in the tr(CT) cortex also contain the α5 subunit relative to the other genotypes.
In the β2 HET mice, total [3H]-epibatidine binding was significantly reduced. As was the case with tissue from WT mice, all [3H]-epibatidine binding sites in the cortex and thalamus of the β2 HET mice were completely precipitated by the β2- and α4-subunit selective antibodies and the fraction of sites precipitated by the α5-subunit selective antibodies was the same as that for WT tissue.
Examination of native nAChRs from β2 tr(CT) transgenic mice using this approach indicated that cortical and thalamic [3H]-epibatidine binding sites were indistinguishable from those observed in WT animals and contained the α4 and β2 nAChR subunits (Figures 5 and 6). This analysis also revealed that a significantly larger proportion of [3H]-epibatidine binding sites in β2 tr(CT) mice contained the α5 nAChR subunit (∼50% of nAChRs in both cortex and thalamus), suggesting that the β2 subunit was selectively rescued in α5-expressing neurons from β2 tr(CT) mice (Figures 5 and 6).
The expression of the α5 nAChR subunit in deep layers of cortex along with the α4 and β2 subunits (Figures 3 and 4) and the results from the immunoprecipitation experiments (Figures 5 and 6) suggested that a mixed population of native α4β2* nAChR stoichiometries might be present in the corticothalamic projection neurons of WT, HET and β2 tr(CT) mice. To assess the characteristics of the nAChRs expressed in the thalamo-cortico-thalamic circuitry further and to determine the effect of β2 nAChR subunit genotype on the terminal nAChRs in cortical and thalamic regions, we collected acetylcholine (ACh)-stimulated [86Rb+] efflux data from thalamic (Figure 7a) and cortical (Figure 8a) synaptosomes.
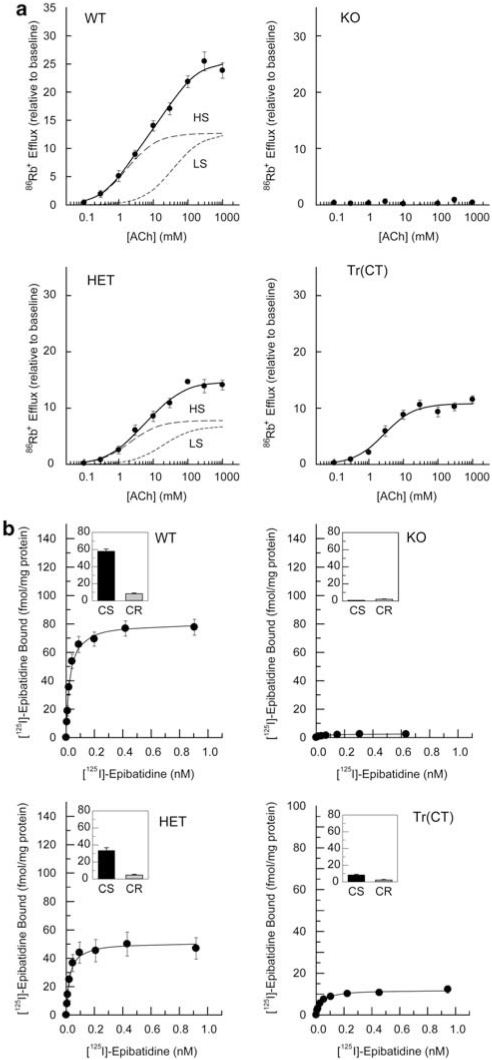
Figure 7.
ACh stimulated [86Rb+] efflux from thalamic synaptosomes and [125I]-epibatidine binding to particulate fractions. (a) Crude thalamic synaptosomes were prepared from wild-type (WT), heterozygous (HET), β2 subunit knockout (KO) and tr(CT) mice. Concentration effect curves for [86Rb+] efflux were biphasic for both WT and HET mice and absent in the KO animals. tr(CT) samples exhibited partial functional recovery relative to the KO, with high sensitivity (HS) to ACh. This HS tr(CT) component is comparable to that derived from WT animals. The high ACh sensitivity component (HS) is indicated by a long dashed line and the low ACh sensitivity component (LS) is indicated by a short dashed line. (b) [125I]-epibatidine binding to particulate fractions derived from thalamic synaptosomes from each of the genotypes indicates a progressive decline in cytisine-sensitive (CS) epibatidine binding between WT and HET mice, an absence of binding in KO samples and a partial recovery in the tr(CT) mice.
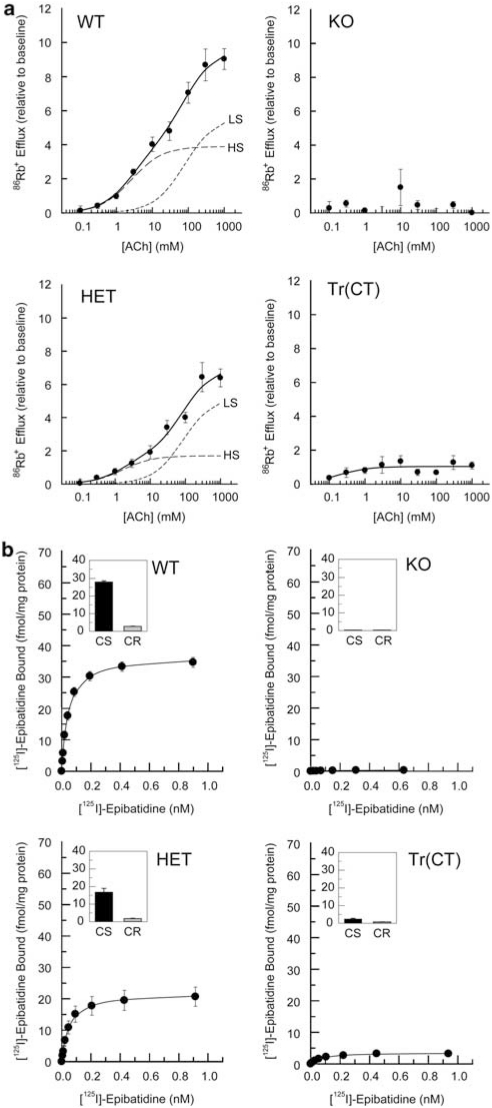
Figure 8.
ACh stimulated [86Rb+] efflux from cortical synaptosomes and [125I]-epibatidine binding to particulate fractions. (a) Crude cortical synaptosomes were prepared from wild type (WT), heterozygous (HET), β2 subunit knockout (KO) and tr(CT) mice. Concentration effect curves for [86Rb+] efflux were biphasic for both WT and HET mice and absent in the KO animals. tr(CT) samples exhibited partial functional recovery relative to the KO, with high sensitivity (HS) to ACh. This HS tr(CT) component is approximately 20% of that derived from WT animals. The high ACh-sensitivity component (HS) is indicated by a long dashed line and the low ACh sensitivity component (LS) is indicated by a short dashed line. (b) [125I]-epibatidine binding to particulate fractions derived from cortical synaptosomes from each of the genotypes indicates a progressive decline in cytisine-sensitive (CS) epibatidine binding between WT and HET mice, an absence of binding in KO samples and a partial recovery in the tr(CT) mice.
Thalamic synaptosomes prepared from WT mice yielded a biphasic ACh concentration-response curve, with estimated EC50 values of 1.7 and 56 μM for the components with higher sensitivity and lower sensitivity for ACh stimulation, respectively (Figure 7a). Thalamic synaptosomes from β2 HET mice also yielded a biphasic concentration-response curve with EC50 values of 2.5 μM (higher sensitivity) and 58 μM (lower sensitivity), but the maximal efflux observed was significantly lower than that of WT mice (Figure 7a). ACh-stimulated efflux was virtually eliminated in thalamic synaptosomes prepared from β2 nAChR subunit KO mice (Figure 7a).
In contrast to the concentration–response curves for WT and HET thalamic synaptosomes, the concentration–response curve for β2 tr(CT) thalamic synaptosomes exhibited only an higher sensitivity ACh component (EC50=2.8 μM) (Figure 7a), consistent with the expression of nAChR subtypes activated by relatively low ACh concentrations (Brown et al, 2007; Gotti et al, 2008; Marks et al, 2007), including the (α4)2(β2)3 and (α4)2(β2)2α5 nAChR subtypes, in corticothalamic projection neurons. Further, the maximal higher sensitivity-mediated efflux from the β2 tr(CT) thalamic synaptosomes (10.8 units) was comparable to that observed in WT synaptosomes (12.7 units), indicating that the highest sensitivity class of nAChRs rescued in the corticothalamic neurons of the β2 tr(CT) transgenic mice was nearly as active as the high sensitivity class in WT mice (Figure 7a).
Cortical synaptosomes derived from WT and HET mice also yielded biphasic ACh concentration–effect curves, with EC50 values of 2.6 and 80 μM calculated for the WT components with higher and lower ACh sensitivity, respectively, and with corresponding EC50 values of 1.6 and 87 μM for the HET components (Figure 8a). Efflux was eliminated in β2 KO cortical synaptosomes (Figure 8a). Consistent with selective expression of the β2 nAChR subunit in corticothalamic projection neurons, [86Rb+] efflux from β2 tr(CT) cortical synaptosomes indicated that there was little rescue (<20%) of nAChR function in neurons with terminals in the cortical regions of these mice (Figure 8a), although this rescued activity displayed high sensitivity to ACh stimulation (EC50=0.3 μM).
[125I]-epibatidine binding analysis of particulate fractions derived from the thalamic and cortical synaptosomes used in these [86Rb+] efflux experiments (Figures 7b and 8b) confirmed that the relative levels of nAChRs present in these preparations were similar to those determined by both binding in coronal sections (Figures 3 and 4) and in the solubilized receptors used for immunoprecipitation between the genotypes examined (Figures 5 and 6), emphasizing that there were no significant changes in the properties of nAChRs derived from tissue slices as compared with brain homogenates or synaptosome preparations.
Elimination of α5 nAChR Subunit Expression can Modulate Passive Avoidance Behavior
The finding that nAChR expression in β2 tr(CT) mice is rescued in α5 subunit-expressing cortical neurons was of interest as the α5 nAChR subunit modulates the functional properties of the nAChRs with which it co-assembles (Girod et al, 1999; Kuryatov et al, 2008; Ramirez-Latorre et al, 1996) and is important for corticothalamic α4β2* nAChR function. Significantly, [86Rb+] efflux from thalamic synaptosomes is greatly decreased in α5 subunit KO mice (Brown et al, 2007). Further, a nicotinic current in layer VI pyramidal neurons peaks during early postnatal development and was associated with α4β2α5 nAChRs (Kassam et al, 2008). As our hypothesis is that ACh-mediated stimulation of nAChRs on corticothalamic neurons is critical during development for appropriate passive avoidance performance, we evaluated the functional contribution of the α5 nAChR subunit to this behavior. We hypothesized that the absence of the (α4)2(β2)2α5 nAChRs in corticothalamic projections would induce an increase in passive avoidance latency, although not to the extent observed in β2 nAChR subunit KO animals, in which both (α4)2(β2)3 and (α4)2(β2)2α5 nAChRs are absent.
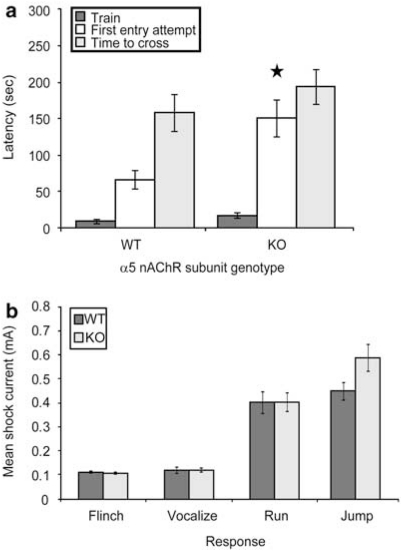
Adult α5 nAChR subunit KO mice took significantly longer to attempt a cross into the dark chamber on testing day (defined as the time at which a mouse first placed its paws in the dark chamber) (Figure 9a). No differences in overall latency to successfully cross into the dark chamber or in shock reactivity were observed for these mice (Figure 9a and b). As expected, this phenotype was more moderate than that seen in β2 subunit KO mice, consistent with α5 acting as an accessory nAChR subunit and, therefore, modulating corticothalamic nAChR function.
Figure 9.
The α5 nAChR subunit modulates passive avoidance behavior. (a) α5 nAChR subunit knockout mice exhibit significantly longer latency to attempt to cross into the dark chamber (F(1, 47)=9.529, p=0.003; n=24–25 per genotype). (b) α5 nAChR subunit knockout mice (KO) exhibit flinch, vocalize, run and jump foot shock responses at currents that were not significantly different (p>0.05) from wild-type controls (WT) (n=24–25 per group). ★p<0.05.
Maternal Nicotine Exposure During a Defined Developmental Period is Sufficient to Induce Hypersensitive Passive Avoidance
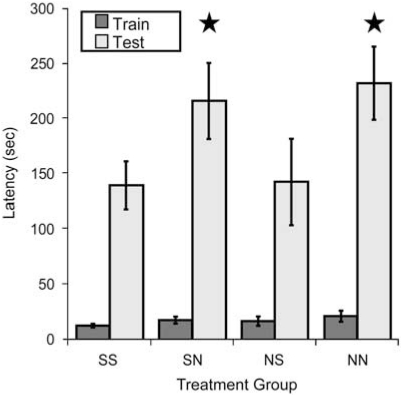
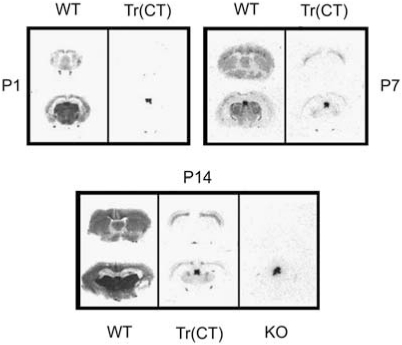
The previous sets of studies demonstrated that developmental nicotine exposure results in hypersensitive passive avoidance performance and identified (α4)2(β2)3 and (α4)2(β2)2α5 nAChRs on corticothalamic neurons as sufficient for this behavioral effect of nicotine, but did not identify the critical period. Nicotine can alter synaptic plasticity in the auditory system during the second postnatal week in rats (Aramakis et al, 2000), a period of synaptic maturation in the thalamo-cortico-thalamic system (Aramakis et al, 2000; Katz and Shatz, 1996; Maffei and Turrigiano, 2008). To determine whether a similar critical period underlies nicotine-induced passive avoidance hypersensitivity, pups were cross-fostered between nicotine- and saccharin-exposed dams at birth. Pups were exposed to nicotine only until birth (NS), between birth and weaning at 21 days (SN), throughout gestation until 21 days (NN) or to saccharin exclusively (SS) and were tested for passive avoidance as adults. There was a significant effect of postnatal nicotine exposure on latency to enter the dark chamber on testing day with no effect of prenatal exposure (Figure 10). In contrast, exposure to nicotine on postnatal days 34–46 (defined as rodent adolescence) did not result in hypersensitive passive avoidance behavior (Test day mean latencies; Saccharin=90.31±27.18 s; Nicotine=80.53±25.05 s; p>0.05; n=13–16 per group; data not shown), suggesting that the timing of exposure to nicotine during perinatal development is critical and that this is a period in which the thalamo-cortico-thalamic system is uniquely vulnerable to nicotine exposure. To provide convergent data from the β2 tr(CT) mice identifying the critical period for nAChR expression in hypersensitive passive avoidance behavior, we used [125I]-epibatidine to identify the time course of assembled β2* nAChR expression in these mice (Figure 11). β2 tr(CT) mice showed little expression of β2* nAChRs at P1, but had progressively greater expression at P7 and P14. In addition, the pattern of expression at P7 and P14 resembled the adult pattern (Figure 3) and was only apparent in the corticothalamic pathway. This period of development (P0–P21) is also coincident with the period of highest activity of α5* nAChRs in this circuit (Kassam et al, 2008).
Figure 10.
Nicotine exposure during early postnatal development is sufficient to induce hypersensitive passive avoidance performance. C57BL/6J mice were cross-fostered between nicotine- and saccharin-exposed dams at birth and tested for passive avoidance as adults. Mice were exposed to nicotine throughout gestation and postnatal development to 21 days of age (NN), between birth and 21 days of age (SN), only during gestation (NS) or were exposed only to saccharin (SS). A significant main effect of postnatal nicotine exposure was detected on the test day (F(1, 14)=7.511, p=0.016; n=8 per group). No main effect of prenatal exposure and no interaction between pre- and postnatal exposure was detected. ★p<0.05.
Figure 11.
Corticothalamic transgenic mice do not express α4β2* nAChRs at birth, but show an adult pattern of expression starting at postnatal day 7. Expression of β2* nAChRs was determined by [125I]-epibatidine binding. At postnatal day 1 (P1), the expression of β2* nAChRs in tr(CT) mice was largely absent compared with wild type (WT). By P7, β2* nAChRs were expressed by tr(CT) layer VI corticothalamic neurons and this expression level was increased further at P14. β2* nAChRs were not expressed by β2 nAChR subunit knockout (KO) mice at any time point (P14 shown here). Residual [125I]-epibatidine binding in the medial habenula seen in KO tissue represents β4* nAChRs.
DISCUSSION
We show in this study that exposure to nicotine during a critical period of thalamic and cortical synapse maturation results in persistent hypersensitive passive avoidance behavior in adulthood. Developmental nicotine exposure induces the same phenotype in β2 tr(CT) transgenic mice that express the β2 nAChR subunit exclusively on corticothalamic neurons, identifying nicotinic modulation of these neurons and the descending branch of the thalamo-cortico-thalamic circuit as the site of action for developmental nicotine exposure on passive avoidance performance. Among the many possible native nAChR subunit combinations that could mediate the effect of nicotine on these neurons, we have used a combination of in situ hybridization, equilibrium binding, selective immunoprecipitation and ACh-stimulated rubidium efflux from synaptosomes to identify the (α4)2(β2)3 and (α4)2(β2)2α5 nAChR variants as those critical for hypersensitive passive avoidance as a defined behavioral consequence of developmental nicotine exposure. Furthermore, the increased proportion of corticothalamic α4β2* nAChRs associated with the α5 subunit in β2 tr(CT) mice suggests that β2 subunit expression is selectively rescued in α5 subunit-expressing cortical neurons. Consistent with a modulatory effect of the α5 subunit on nAChR function in this circuit, the absence of (α4)2(β2)2α5 nAChRs in α5 nAChR subunit KO mice results in a more modest passive avoidance hypersensitivity than is seen in β2 subunit KO mice or developmental nicotine-treated WT mice. Finally, we have identified the early postnatal period in the mouse as a window of vulnerability in which nicotine exposure can induce this persistent behavioral phenotype.
This study complements previous experiments indicating that developing, ascending thalamocortical neurons are sensitive to nicotine by showing for the first time that developing, descending corticothalamic neurons are also vulnerable to nicotine. In particular, nicotine exposure during the early postnatal period alters the N-methyl-D-aspartate (NMDA) receptor-mediated component of thalamocortical excitatory post-synaptic potentials (EPSPs) by the activation of α7 nAChRs expressed by these neurons (Aramakis et al, 2000; Aramakis and Metherate, 1998; Liang et al, 2006). Persistent alterations in the mRNA encoding the NR2A and NR2B NMDA receptor subunits in the auditory cortex and associated thalamus have also been identified following nicotine exposure (Hsieh et al, 2002). Taken together, these data indicate that the development of thalamo-cortico-thalamic connectivity is profoundly sensitive to nicotine exposure.
In contrast to the role for the α7 nAChR in nicotine-dependent changes in thalamocortical neurons, we have implicated the (α4)2(β2)3 and (α4)2(β2)2α5 nAChR variants as critical mediators of the effects of nicotine exposure on developing corticothalamic neurons. The α5 nAChR subunit is of particular interest, given its ability to potentiate nAChR function as an accessory subunit (Brown et al, 2007; Gotti et al, 2009) and the significant association between the human α5 nAChR subunit gene and nicotine dependence (Saccone et al, 2007). The current study shows that expression of (α4)2(β2)3 and (α4)2(β2)2α5 nAChRs on developing corticothalamic neurons is sufficient for the persistent effect of developmental nicotine exposure on passive avoidance performance. Further, studies in genetically manipulated mice demonstrate that corticothalamic neurons must express β2 and α5 nAChR subunits for normal passive avoidance performance.
Layer VI corticothalamic neurons are the primary source of reciprocal cortical feedback and thus a major modulatory input to sensory thalamic relay neurons (Llano and Sherman, 2009; Reichova and Sherman, 2004) and allow the cortex to adjust the parameters of thalamic processing, and, therefore, the salience of any stimulus it subsequently receives, gating cortical stimulus input (Briggs and Usrey, 2008; Jones, 2009). Activity of these neurons enhances the relay of stimuli to the cortex by altering the gain and responsiveness of the postsynaptic relay cells and by modulating the tuning and/or receptive field properties of these neurons (Briggs and Usrey, 2008). Feedback from corticothalamic neurons is thought to be critical for modulation of thalamic activity by attention and to enhance the reliability of stimulus-derived thalamic responses (Briggs and Usrey, 2008). Thus, alterations in the synaptic maturation of these neurons by developmental nicotine exposure would induce a phenotype in the adult that may involve changes related to sensory processing and sensory attention.
The vulnerability of the thalamo-cortico-thalamic circuitry to developmental nicotine exposure suggests that the hypersensitive passive avoidance phenotype may be a consequence of altered processing of sensory information. For example, mice that remain in the illuminated chamber for longer may have found the training footshock significantly more aversive. Although changes in passive avoidance performance could also be interpreted to indicate alterations in learning and memory processes, such changes may be an indirect consequence of nicotine exposure-mediated changes in thalamo-cortico-thalamic circuitry. For instance, the insular cortex is involved in passive avoidance training acquisition and consolidation (Miranda and McGaugh, 2004), possesses reciprocal connectivity with the thalamus (Saper, 1982) and has been classified as a somatosensory area (Miranda and McGaugh, 2004). As developmental nicotine exposure affects nAChRs on corticothalamic neurons and results in altered passive avoidance behavior, nicotine exposure-induced changes in the thalamo-insular cortical circuitry could lead to altered input processing in this brain region and contribute to the passive avoidance phenotype observed.
Consistent with a sensory processing alteration, previous studies have reported the effects of developmental nicotine exposure on pre-pulse inhibition and acoustic startle responses (Gaworski et al, 2004; Popke et al, 1997). In contrast, relatively inconsistent effects of developmental nicotine exposure have been reported in tasks that assess learning and memory (Heath and Picciotto, 2009). In addition, 3-month-old β2 nAChR subunit KO mice exhibit hypersensitive passive avoidance and yet no differences in performance in the Morris water maze (Picciotto et al, 1995). This suggests that functional ablation of the β2 nAChR subunit, either through genetic KO or nAChR desensitization induced by chronic developmental nicotine exposure does not induce a global change in learning and memory and, therefore, this cannot explain the hypersensitive passive avoidance phenotype.
The absence of a consistent decrease across all four measures of footshock reactivity evaluated in the developmental nicotine exposed animals in this study also suggests that this exposure affects sensory processing as opposed to stimulus detection, although the small, but significant, change in the jump response in developmental nicotine-treated β2 tr(CT) mice could indicate some effect of nicotine exposure during development on stimulus sensitivity. Previous studies of sensory function following nicotine exposure of rats during development have shown no auditory detection deficits (Liang et al, 2006) and although humans exposed to tobacco smoke during gestation also demonstrate persistent deficits in performance of cognitive tasks involving the auditory system (Fried and Makin, 1987; Fried et al, 1992, 1997, 1998, 2003; Jacobsen et al, 2007b; Kristjansson et al, 1989; McCartney et al, 1994; Picone et al, 1982; Saxton, 1978), there is no evidence of deficits in the detection of auditory stimuli (Trammer et al, 1992).
Consistent with the hypothesis that alterations in thalamo-cortico-thalamic connectivity could underlie these effects, tobacco exposure during development in humans alters the development of white matter in the internal capsule, the tract containing corticothalamic and thalamocortical axons, and this alteration is correlated with performance on an auditory attention task (Jacobsen et al, 2007a).
The hypersensitive passive avoidance phenotype observed in this study can be induced by nicotine exposure exclusively between birth and weaning, which is consistent with the window of vulnerability described for nicotine exposure of thalamocortical neurons (Aramakis et al, 2000; Aramakis and Metherate, 1998; Liang et al, 2006). As this early postnatal epoch is the period in rodent development when the synapses of the thalamo-cortico-thalamic circuitry are being refined, mechanisms that underlie modulation of synaptic properties, such as the physiological alterations observed in the thalamocortical neurons (Aramakis et al, 2000; Aramakis and Metherate, 1998; Liang et al, 2006) are likely to underlie this effect. The possibility that nAChRs modulate synaptic refinement in the early postnatal period is further supported by the identification of a current mediated by α5* nAChRs in layer VI projection neurons that peaks during the early postnatal period in rodents and can be significantly reduced by previous nicotine exposure (Kassam et al, 2008). The experiments presented in this study demonstrate that lack of this nAChR in α5 KO mice alters passive avoidance behavior, suggesting that this current is important in the normal maturation of the corticothalamic circuit. This study suggests that the precise physiological alterations in corticothalamic neurons induced by developmental nicotine exposure should be examined further.
In conclusion, we have identified hypersensitive passive avoidance behavior as a novel and persistent consequence of developmental nicotine exposure in the mouse. We have also identified a particular set of nAChR subtypes in the corticothalamic circuit that are responsible for hypersensitive passive avoidance behavior following early nicotine exposure. Indeed, most nAChR subunits are expressed early in neurodevelopment (Heath and Picciotto, 2009), suggesting a broad variety of neuronal subtypes and circuits are sensitive to developmental nicotine exposure; however, the current set of experiments identifies a defined neural circuit vulnerable to nicotine exposure mediated through β2* nAChRs during a discrete developmental epoch, which can induce significant and lasting alterations in behavioral reactivity to a very mild stressor in adulthood. The persistent alterations in passive avoidance in the mouse may parallel the persistent sensory processing-related changes observed in human subjects exposed to tobacco smoke in utero (Jacobsen et al, 2006, 2007b). The current study also emphasizes the prominent behavioral teratogenic effect of the nicotine within tobacco smoke. Although there are numerous differences in the timing of human and rodent neurodevelopment, human thalamo-cortico-thalamic connectivity is refined during the third trimester of pregnancy; therefore, the potential effect of nicotine on corticothalamic refinement could also add to the debate concerning the timing and the best type of smoking cessation aid for pregnant smokers.
Acknowledgments
This work was supported by grants DA10455 and DA00436 from the National Institutes of Health to MRP and the State of Connecticut, Department of Mental Health and Addiction Services. SLK was supported by grants IRG029145 from the European Commission, G0802715 from the Medical Research Council and a research grant from the Royal Society. CG was supported by EC grant 202088 Neurocypres and by the Italian PRIN 20072BTSR2. MJM was supported by grants DA003194 and DA015663 from the National Institutes of Health. We thank Dr Milena Moretti for help with the immunoprecipitation experiments.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Aramakis V, Hsieh C, Leslie F, Metherate R. A critical period for nicotine-induced disruption of synaptic development in rat auditory cortex. J Neurosci. 2000;20:6106–6116. doi: 10.1523/JNEUROSCI.20-16-06106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramakis V, Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J Neurosci. 1998;18:8485–8495. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey W. Emerging views of corticothalamic function. Curr Opin Neurobiol. 2008;18:403–407. doi: 10.1016/j.conb.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R, Collins A, Lindstrom J, Whiteaker P. Nicotinic alpha5 subunit deletion locally reduces high-affinity agonist activation without altering nicotinic receptor numbers. J Neurochem. 2007;103:204–215. doi: 10.1111/j.1471-4159.2007.04700.x. [DOI] [PubMed] [Google Scholar]
- Caldarone B, George T, Zachariou V, Picciotto M. Gender differences in learned helplessness behavior are influenced by genetic background. Pharmacol Biochem Behav. 2000;66:811–817. doi: 10.1016/s0091-3057(00)00271-9. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David D, Przybylski C, Léna C, et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Fried P, Makin J. Neonatal behavioural correlates of prenatal exposure to marihuana, cigarettes and alcohol in a low risk population. Neurotoxicol Teratol. 1987;9:1–7. doi: 10.1016/0892-0362(87)90062-6. [DOI] [PubMed] [Google Scholar]
- Fried P, O'Connell C, Watkinson B. 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: cognitive and language assessment. J Dev Behav Pediatr. 1992;13:383–391. [PubMed] [Google Scholar]
- Fried P, Watkinson B, Gray R. Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 1998;20:293–306. doi: 10.1016/s0892-0362(97)00091-3. [DOI] [PubMed] [Google Scholar]
- Fried P, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2003;25:427–436. doi: 10.1016/s0892-0362(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Fried P, Watkinson B, Siegel L. Reading and language in 9- to 12-year olds prenatally exposed to cigarettes and marijuana. Neurotoxicol Teratol. 1997;19:171–183. doi: 10.1016/s0892-0362(97)00015-9. [DOI] [PubMed] [Google Scholar]
- Gaworski C, Carmines E, Faqi A, Rajendran N. In utero and lactation exposure of rats to 1R4F reference cigarette mainstream smoke: effect on prenatal and postnatal development. Toxicol Sci. 2004;79:157–169. doi: 10.1093/toxsci/kfh083. [DOI] [PubMed] [Google Scholar]
- Girod R, Crabtree G, Ernstrom G, Ramirez-Latorre J, McGehee D, Turner J, et al. Heteromeric complexes of alpha 5 and/or alpha 7 subunits. Effects of calcium and potential role in nicotine-induced presynaptic facilitation. Ann N Y Acad Sci. 1999;868:578–590. doi: 10.1111/j.1749-6632.1999.tb11331.x. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, et al. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Clementi F, Riganti L, McIntosh J, Collins A, et al. Expression of nigrostriatal alpha 6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by beta 3 subunit gene deletion. Mol Pharmacol. 2005a;67:2007–2015. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Meinerz N, Clementi F, Gaimarri A, Collins A, et al. Partial deletion of the nicotinic cholinergic receptor alpha 4 or beta 2 subunit genes changes the acetylcholine sensitivity of receptor-mediated 86Rb+ efflux in cortex and thalamus and alters relative expression of alpha 4 and beta 2 subunits. Mol Pharmacol. 2008;73:1796–1807. doi: 10.1124/mol.108.045203. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Zanardi A, Gaimarri A, Champtiaux N, Changeux J, et al. Heterogeneity and selective targeting of neuronal nicotinic acetylcholine receptor (nAChR) subtypes expressed on retinal afferents of the superior colliculus and lateral geniculate nucleus: identification of a new native nAChR subtype alpha3beta2(alpha5 or beta3) enriched in retinocollicular afferents. Mol Pharmacol. 2005b;68:1162–1171. doi: 10.1124/mol.105.015925. [DOI] [PubMed] [Google Scholar]
- Heath C, Picciotto M. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56 (Suppl 1:254–262. doi: 10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson R, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Hsieh C, Leslie F, Metherate R. Nicotine exposure during a postnatal critical period alters NR2A and NR2B mRNA expression in rat auditory forebrain. Brain Res Dev Brain Res. 2002;133:19–25. doi: 10.1016/s0165-3806(01)00314-5. [DOI] [PubMed] [Google Scholar]
- Jacobsen L, Picciotto M, Heath C, Frost S, Tsou K, Dwan R, et al. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J Neurosci. 2007a;27:13491–13498. doi: 10.1523/JNEUROSCI.2402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen L, Slotkin T, Mencl W, Frost S, Pugh K. Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007b;32:2453–2464. doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- Jacobsen L, Slotkin T, Westerveld M, Mencl W, Pugh K. Visuospatial memory deficits emerging during nicotine withdrawal in adolescents with prenatal exposure to active maternal smoking. Neuropsychopharmacology. 2006;31:1550–1561. doi: 10.1038/sj.npp.1300981. [DOI] [PubMed] [Google Scholar]
- Jones E. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann NY Acad Sci. 2009;1157:10–23. doi: 10.1111/j.1749-6632.2009.04534.x. [DOI] [PubMed] [Google Scholar]
- Kassam S, Herman P, Goodfellow N, Alves N, Lambe E. Developmental excitation of corticothalamic neurons by nicotinic acetylcholine receptors. J Neurosci. 2008;28:8756–8764. doi: 10.1523/JNEUROSCI.2645-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L, Shatz C. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- King S, Marks M, Grady S, Caldarone B, Koren A, Mukhin A, et al. Conditional expression in corticothalamic efferents reveals a developmental role for nicotinic acetylcholine receptors in modulation of passive avoidance behavior. J Neurosci. 2003;23:3837–3843. doi: 10.1523/JNEUROSCI.23-09-03837.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson E, Fried P, Watkinson B. Maternal smoking during pregnancy affects children's vigilance performance. Drug Alcohol Depend. 1989;24:11–19. doi: 10.1016/0376-8716(89)90003-3. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol. 2008;74:132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- Liang K, Poytress B, Chen Y, Leslie F, Weinberger N, Metherate R. Neonatal nicotine exposure impairs nicotinic enhancement of central auditory processing and auditory learning in adult rats. Eur J Neurosci. 2006;24:857–866. doi: 10.1111/j.1460-9568.2006.04945.x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Neff R, Berg D. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Llano D, Sherman S. Differences in Intrinsic properties and local network connectivity of identified layer 5 and layer 6 adult mouse auditory corticothalamic neurons support a dual corticothalamic projection hypothesis. Cereb Cortex. 2009;19:2810–2826. doi: 10.1093/cercor/bhp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maffei A, Turrigiano G. The age of plasticity: developmental regulation of synaptic plasticity in neocortical microcircuits. Prog Brain Res. 2008;169:211–223. doi: 10.1016/S0079-6123(07)00012-X. [DOI] [PubMed] [Google Scholar]
- Maggi L, Sola E, Minneci F, Le Magueresse C, Changeux J, Cherubini E. Persistent decrease in synaptic efficacy induced by nicotine at Schaffer collateral-CA1 synapses in the immature rat hippocampus. J Physiol. 2004;559 (Part 3:863–874. doi: 10.1113/jphysiol.2004.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M, Meinerz N, Drago J, Collins A. Gene targeting demonstrates that alpha4 nicotinic acetylcholine receptor subunits contribute to expression of diverse [3H]epibatidine binding sites and components of biphasic 86Rb+ efflux with high and low sensitivity to stimulation by acetylcholine. Neuropharmacology. 2007;53:390–405. doi: 10.1016/j.neuropharm.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M, Pauly J, Gross S, Deneris E, Hermans-Borgmeyer I, Heinemann S, et al. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M, Rowell P, Cao J, Grady S, McCallum S, Collins A. Subsets of acetylcholine-stimulated 86Rb+ efflux and [125I]-epibatidine binding sites in C57BL/6 mouse brain are differentially affected by chronic nicotine treatment. Neuropharmacology. 2004;46:1141–1157. doi: 10.1016/j.neuropharm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Martin J, Hamilton B, Sutton P, Ventura S, Menacker F, Kirmeyer S, et al. Births: final data for 2005. National Vital Statistics Reports. 2007;56:1–104. [PubMed] [Google Scholar]
- McCartney J, Fried P, Watkinson B. Central auditory processing in school-age children prenatally exposed to cigarette smoke. Neurotoxicol Teratol. 1994;16:269–276. doi: 10.1016/0892-0362(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Metherate R, Hsieh C. Regulation of glutamate synapses by nicotinic acetylcholine receptors in auditory cortex. Neurobiol Learn Mem. 2003;80:285–290. doi: 10.1016/s1074-7427(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Miranda M, McGaugh J. Enhancement of inhibitory avoidance and conditioned taste aversion memory with insular cortex infusions of 8-Br-cAMP: involvement of the basolateral amygdala. Learn Mem. 2004;11:312–317. doi: 10.1101/lm.72804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti M, Vailati S, Zoli M, Lippi G, Riganti L, Longhi R, et al. Nicotinic acetylcholine receptor subtypes expression during rat retina development and their regulation by visual experience. Mol Pharmacol. 2004;66:85–96. doi: 10.1124/mol.66.1.85. [DOI] [PubMed] [Google Scholar]
- Picciotto M, Zoli M, Léna C, Bessis A, Lallemand Y, Le Novère N, et al. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- Picone T, Allen L, Olsen P, Ferris M. Pregnancy outcome in North American women. II. Effects of diet, cigarette smoking, stress, and weight gain on placentas, and on neonatal physical and behavioral characteristics. Am J Clin Nutr. 1982;36:1214–1224. doi: 10.1093/ajcn/36.6.1214. [DOI] [PubMed] [Google Scholar]
- Popke E, Tizabi Y, Rahman M, Nespor S, Grunberg N. Prenatal exposure to nicotine: effects on prepulse inhibition and central nicotinic receptors. Pharmacol Biochem Behav. 1997;58:843–849. doi: 10.1016/s0091-3057(97)98985-1. [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu C, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Reichova I, Sherman S. Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Saccone S, Hinrichs A, Saccone N, Chase G, Konvicka K, Madden P, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide R, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- Saper C. Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol. 1982;210:163–173. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- Saxton D. The behaviour of infants whose mothers smoke in pregnancy. Early Hum Dev. 1978;2:363–369. doi: 10.1016/0378-3782(78)90063-4. [DOI] [PubMed] [Google Scholar]
- Stolerman I, Jarvis M.1995The scientific case that nicotine is addictive Psychopharmacology (Berl) 1172–10.discussion 14–20. [DOI] [PubMed] [Google Scholar]
- Trammer R, Aust G, Köster K, Obladen M. Narcotic and nicotine effects on the neonatal auditory system. Acta Paediatr. 1992;81:962–965. doi: 10.1111/j.1651-2227.1992.tb12154.x. [DOI] [PubMed] [Google Scholar]
- Vailati S, Hanke W, Bejan A, Barabino B, Longhi R, Balestra B, et al. Functional alpha6-containing nicotinic receptors are present in chick retina. Mol Pharmacol. 1999;56:11–19. doi: 10.1124/mol.56.1.11. [DOI] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh J, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.