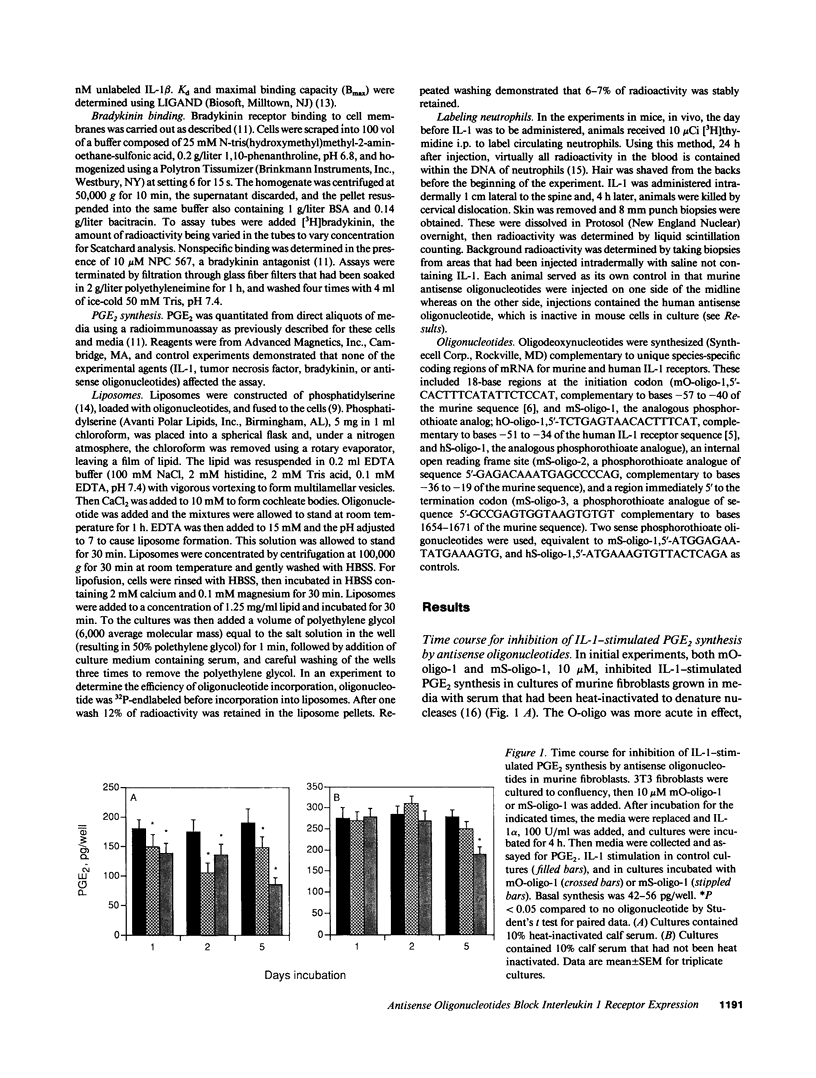
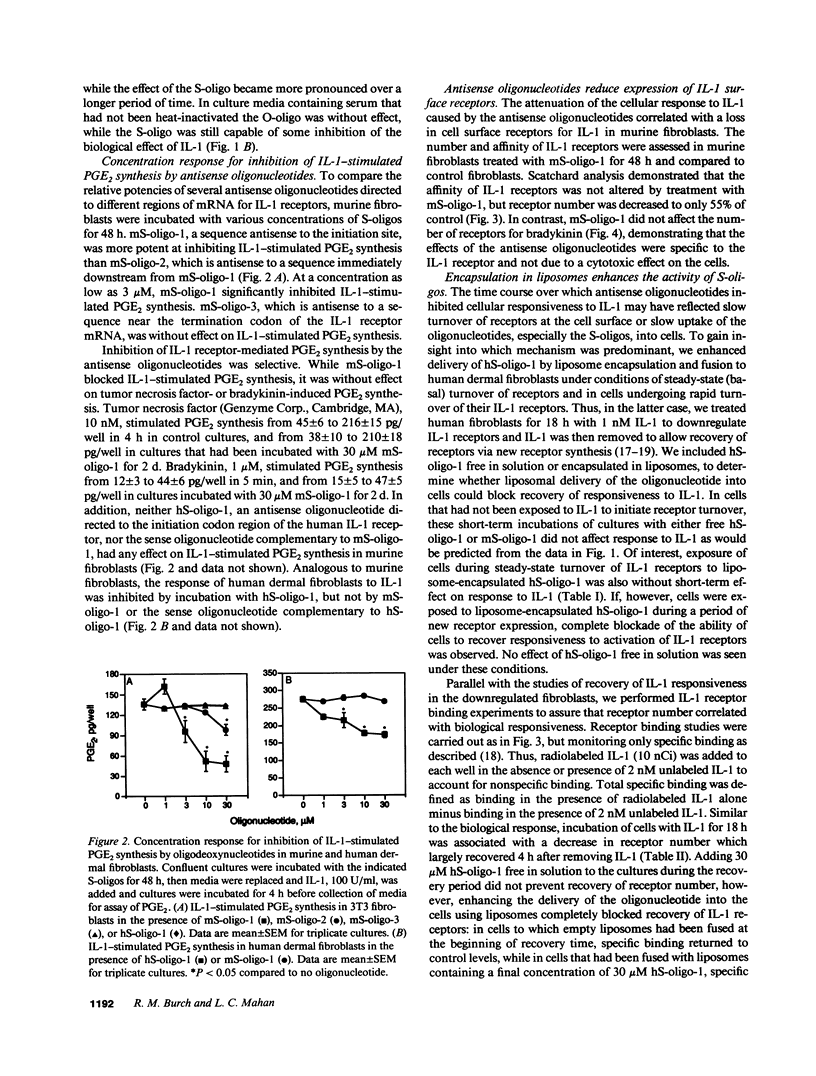
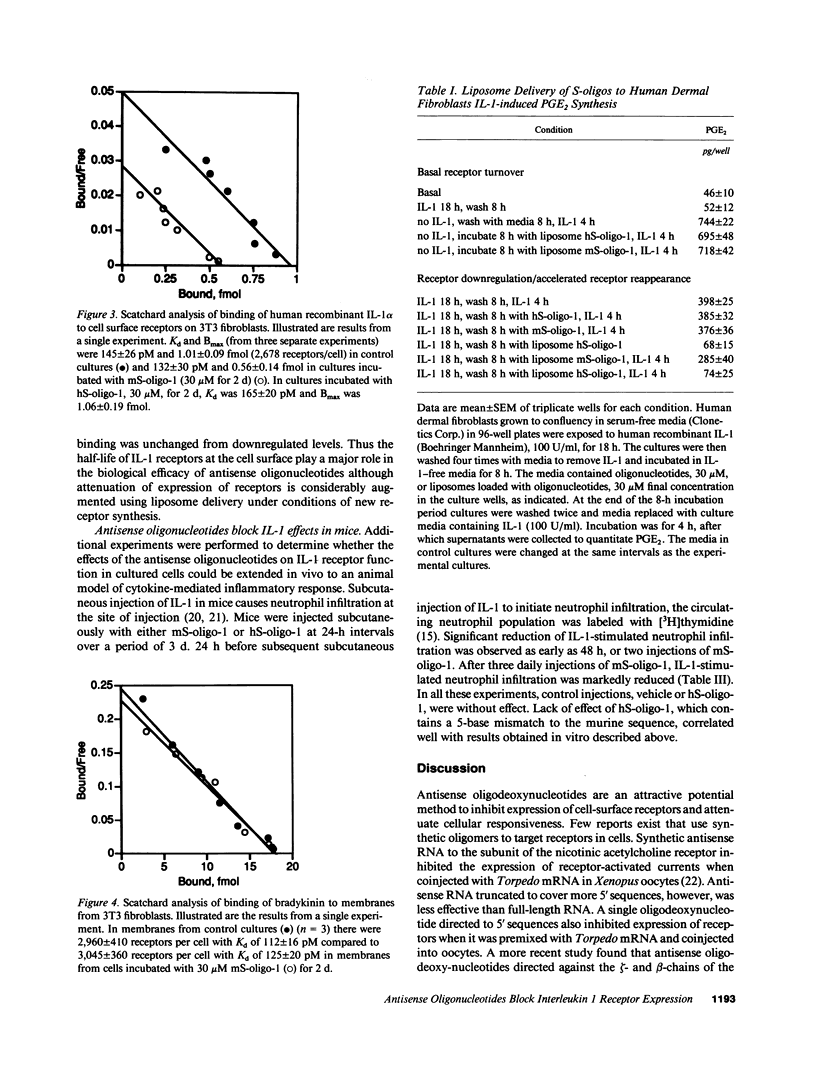
Abstract
Phosphodiester and phosphorothioate oligodeoxynucleotides (18 mers) were constructed antisense to sequences of the recently cloned murine and human IL-1 receptors. Murine antisense oligonucleotides inhibited IL-1-stimulated PGE2 synthesis by murine fibroblasts in culture in a time (days) and concentration-dependent (3 microM-30 microM) fashion. Murine sense oligonucleotide and an oligonucleotide antisense to human IL-1 receptor were without effect. Moreover, murine antisense oligonucleotides did not affect tumor necrosis factor- or bradykinin-stimulated PGE2 synthesis by murine fibroblasts. Similarly, antisense oligonucleotides to the human, but not the murine, IL-1 receptor inhibited IL-1-stimulated PGE2 synthesis by cultured human fibroblasts. The attenuation of the cellular response to IL-1 caused by the antisense oligonucleotides correlated with a loss in cell surface receptors for IL-1, without any change in the number of bradykinin receptors on these cells. When antisense oligonucleotides were encapsulated in liposomes, they blocked completely the appearance of newly synthesized IL-1 receptors and IL-1-stimulated PGE2 synthesis. In mice, subcutaneous injection with an oligonucleotide antisense to the murine IL-1 receptor markedly inhibited the infiltration of neutrophils in response to subsequent injection of IL-1. These data suggest that antisense oligodeoxynucleotides may share a role in the design of antiinflammatory therapeutics.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akahoshi T., Oppenheim J. J., Matsushima K. Interleukin 1 stimulates its own receptor expression on human fibroblasts through the endogenous production of prostaglandin(s). J Clin Invest. 1988 Oct;82(4):1219–1224. doi: 10.1172/JCI113719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiziau C., Kurfurst R., Cazenave C., Roig V., Thuong N. T., Toulmé J. J. Inhibition of translation initiation by antisense oligonucleotides via an RNase-H independent mechanism. Nucleic Acids Res. 1991 Mar 11;19(5):1113–1119. doi: 10.1093/nar/19.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsztyk K., Sims J. E., Stanton T. H., Slack J., McMahan C. J., Valentine M. A., Dower S. K. Evidence for different interleukin 1 receptors in murine B- and T-cell lines. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8034–8038. doi: 10.1073/pnas.86.20.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Connor J. R., Axelrod J. Interleukin 1 amplifies receptor-mediated activation of phospholipase A2 in 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6306–6309. doi: 10.1073/pnas.85.17.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D. B., Deibel M. R., Jr, Dunn C. J., Tomich C. S., Laborde A. L., Slightom J. L., Berger A. E., Bienkowski M. J., Sun F. F., McEwan R. N. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990 Apr 12;344(6267):633–638. doi: 10.1038/344633a0. [DOI] [PubMed] [Google Scholar]
- Chizzonite R., Truitt T., Kilian P. L., Stern A. S., Nunes P., Parker K. P., Kaffka K. L., Chua A. O., Lugg D. K., Gubler U. Two high-affinity interleukin 1 receptors represent separate gene products. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8029–8033. doi: 10.1073/pnas.86.20.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua A. O., Gubler U. Sequence of the cDNA for the human fibroblast type interleukin-1 receptor. Nucleic Acids Res. 1989 Dec 11;17(23):10114–10114. doi: 10.1093/nar/17.23.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Dower S. K., Wignall J. M., Schooley K., McMahan C. J., Jackson J. L., Prickett K. S., Lupton S., Cosman D., Sims J. E. Retention of ligand binding activity by the extracellular domain of the IL-1 receptor. J Immunol. 1989 Jun 15;142(12):4314–4320. [PubMed] [Google Scholar]
- Dunn C. J., Hardee M. M., Staite N. D. Acute and chronic inflammatory responses to local administration of recombinant IL-1 alpha, IL-beta, TNF alpha, IL-2 and Ifn gamma in mice. Agents Actions. 1989 Jun;27(3-4):290–293. doi: 10.1007/BF01972801. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. P., Evans R. J., Arend W. P., Verderber E., Brewer M. T., Hannum C. H., Thompson R. C. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990 Jan 25;343(6256):341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- Forsyth K. D., Levinsky R. J. Role of the LFA-1 adhesion glycoprotein in neutrophil adhesion to endothelium and plastic surfaces. Clin Exp Immunol. 1989 Feb;75(2):265–268. [PMC free article] [PubMed] [Google Scholar]
- Krieg A. M., Gause W. C., Gourley M. F., Steinberg A. D. A role for endogenous retroviral sequences in the regulation of lymphocyte activation. J Immunol. 1989 Oct 15;143(8):2448–2451. [PubMed] [Google Scholar]
- Lee J. C., Griswold D. E., Votta B., Hanna N. Inhibition of monocyte IL-1 production by the anti-inflammatory compound, SK&F 86002. Int J Immunopharmacol. 1988;10(7):835–843. doi: 10.1016/0192-0561(88)90007-0. [DOI] [PubMed] [Google Scholar]
- Loke S. L., Stein C. A., Zhang X. H., Mori K., Nakanishi M., Subasinghe C., Cohen J. S., Neckers L. M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke S. L., Stein C., Zhang X., Avigan M., Cohen J., Neckers L. M. Delivery of c-myc antisense phosphorothioate oligodeoxynucleotides to hematopoietic cells in culture by liposome fusion: specific reduction in c-myc protein expression correlates with inhibition of cell growth and DNA synthesis. Curr Top Microbiol Immunol. 1988;141:282–289. doi: 10.1007/978-3-642-74006-0_38. [DOI] [PubMed] [Google Scholar]
- Luscinskas F. W., Cybulsky M. I., Kiely J. M., Peckins C. S., Davis V. M., Gimbrone M. A., Jr Cytokine-activated human endothelial monolayers support enhanced neutrophil transmigration via a mechanism involving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. J Immunol. 1991 Mar 1;146(5):1617–1625. [PubMed] [Google Scholar]
- Marcus-Sekura C. J. Techniques for using antisense oligodeoxyribonucleotides to study gene expression. Anal Biochem. 1988 Aug 1;172(2):289–295. doi: 10.1016/0003-2697(88)90447-2. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Yodoi J., Tagaya Y., Oppenheim J. J. Down-regulation of interleukin 1 (IL 1) receptor expression by IL 1 and fate of internalized 125I-labeled IL 1 beta in a human large granular lymphocyte cell line. J Immunol. 1986 Nov 15;137(10):3183–3188. [PubMed] [Google Scholar]
- Mizel S. B., Kilian P. L., Lewis J. C., Paganelli K. A., Chizzonite R. A. The interleukin 1 receptor. Dynamics of interleukin 1 binding and internalization in T cells and fibroblasts. J Immunol. 1987 May 1;138(9):2906–2912. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Palaszynski E. W. Synthetic C-terminal peptide of IL-1 functions as a binding domain as well as an antagonist for the IL-1 receptor. Biochem Biophys Res Commun. 1987 Aug 31;147(1):204–211. doi: 10.1016/s0006-291x(87)80107-9. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Jacobson K., Poste G. Cochleate lipid cylinders: formation by fusion of unilamellar lipid vesicles. Biochim Biophys Acta. 1975 Jul 3;394(3):483–491. doi: 10.1016/0005-2736(75)90299-0. [DOI] [PubMed] [Google Scholar]
- Rampart M., Williams T. J. Evidence that neutrophil accumulation induced by interleukin-1 requires both local protein biosynthesis and neutrophil CD18 antigen expression in vivo. Br J Pharmacol. 1988 Aug;94(4):1143–1148. doi: 10.1111/j.1476-5381.1988.tb11632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyne J. A., Mizel S. B., Taylor R. G., Chedid M., McCall C. E. Characterization of the human interleukin 1 receptor on human polymorphonuclear leukocytes. Clin Immunol Immunopathol. 1988 Sep;48(3):354–361. doi: 10.1016/0090-1229(88)90029-3. [DOI] [PubMed] [Google Scholar]
- Scapigliati G., Ghiara P., Bartalini M., Tagliabue A., Boraschi D. Differential binding of IL-1 alpha and IL-1 beta to receptors on B and T cells. FEBS Lett. 1989 Jan 30;243(2):394–398. doi: 10.1016/0014-5793(89)80169-3. [DOI] [PubMed] [Google Scholar]
- Sims J. E., Acres R. B., Grubin C. E., McMahan C. J., Wignall J. M., March C. J., Dower S. K. Cloning the interleukin 1 receptor from human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8946–8950. doi: 10.1073/pnas.86.22.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J. E., March C. J., Cosman D., Widmer M. B., MacDonald H. R., McMahan C. J., Grubin C. E., Wignall J. M., Jackson J. L., Call S. M. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science. 1988 Jul 29;241(4865):585–589. doi: 10.1126/science.2969618. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Johnson J. A., Loeser R., Turner R. A. Evaluation of Tenidap (CP-66,248) on human neutrophil arachidonic acid metabolism, chemotactic potential and clinical efficacy in the treatment of rheumatoid arthritis. Agents Actions. 1990 Aug;31(1-2):102–109. doi: 10.1007/BF02003228. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K., Miledi R. Repression of nicotinic acetylcholine receptor expression by antisense RNAs and an oligonucleotide. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1302–1306. doi: 10.1073/pnas.85.4.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany C. W., Hoefler S., Moser H. W., Burch R. M. Arachidonic acid metabolism in fibroblasts from patients with peroxisomal diseases: response to interleukin 1. Biochim Biophys Acta. 1990 Nov 14;1096(1):41–46. doi: 10.1016/0925-4439(90)90010-m. [DOI] [PubMed] [Google Scholar]
- Walder R. Y., Walder J. A. Role of RNase H in hybrid-arrested translation by antisense oligonucleotides. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5011–5015. doi: 10.1073/pnas.85.14.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. M., Spires D. A., Bedord C. J., Wagner B., Ballaron S. J., De Young L. M. The mouse ear inflammatory response to topical arachidonic acid. J Invest Dermatol. 1984 Apr;82(4):367–371. doi: 10.1111/1523-1747.ep12260709. [DOI] [PubMed] [Google Scholar]
- Zheng H., Sahai B. M., Kilgannon P., Fotedar A., Green D. R. Specific inhibition of cell-surface T-cell receptor expression by antisense oligodeoxynucleotides and its effect on the production of an antigen-specific regulatory T-cell factor. Proc Natl Acad Sci U S A. 1989 May;86(10):3758–3762. doi: 10.1073/pnas.86.10.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol A. R., Mol J. N., Stuitje A. R. Modulation of eukaryotic gene expression by complementary RNA or DNA sequences. Biotechniques. 1988 Nov-Dec;6(10):958–976. [PubMed] [Google Scholar]