Abstract
Rationale
Vascular smooth muscle cell (SMC) migration is an important pathological process in several vascular occlusive diseases, including atherosclerosis and restenosis, both of which are accelerated by diabetes mellitus.
Objective
To determine the mechanisms of abnormal vascular SMC migration in type-2 diabetes, the obese Zucker rat (ZO), a model of obesity and insulin resistance, was studied.
Methods and Results
In culture, ZO aortic SMCs showed a significant increase in Nox4 mRNA and protein levels compared with the control lean Zucker rat (ZL). The sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) nitrotyrosine-294,295 and cysteine-674 (C674)-SO3H were increased in ZO SMCs, indicating oxidant stress. Unlike ZL SMC, nitric oxide (NO) failed to inhibit serum-induced SMC migration in ZO. Transfection of Nox4 small interference RNA or overexpression of SERCA2b wild type, but not C674S mutant SERCA, restored the response to NO. Knockdown of Nox4 also decreased SERCA oxidation in ZO SMCs. In addition, transforming growth factor β1 (TGF-β1) via Smad2 was necessary and sufficient to upregulate Nox4, oxidize SERCA, and block the anti-migratory action of NO in ZO SMCs. Corresponding to the results in cultured SMCs, immunohistochemistry confirmed that Nox4 and SERCA C674-SO3H were significantly increased in ZO aorta. After common carotid artery injury, knockdown of Nox4 by adenoviral Nox4 short hairpin RNA decreased Nox4 and SERCA C674-SO3H staining and significantly decreased injury-induced neointima.
Conclusion
These studies indicate that the upregulation of Nox4 by TGF-β1 in ZO SMCs is responsible for the impaired response to NO by a mechanism involving the oxidation of SERCA C674. Knockdown of Nox4 inhibits oxidation of SERCA as well as neointima formation after ZO common carotid artery injury.
Keywords: Nitric oxide, cell migration, NADPH oxidase, obese Zucker rat, TGF-β1, Smad2
Introduction
Diabetic patients have a much higher morbidity and mortality from cardiovascular diseases including atherosclerosis and restenosis compared with other patients. Vascular smooth muscle cell (SMC) migration contributes significantly to these pathological processes. Generally, SMCs stay quiescent in the vasculature, but when the endothelial layer is disrupted the underlying SMCs migrate from media to intima and form the neointima. This process is accelerated by diabetes mellitus. Numerous clinical studies have indicated that diabetic patients have a higher incidence of restenosis after percutaneous coronary interventions compared with patients without diabetes 1–3. Nitric oxide (NO), the biologically active component of endothelium-derived relaxing factor, has critical roles in the maintenance of vascular homeostasis. The sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) plays a very important role in maintaining intracellular calcium levels by taking up calcium into SR/ER. Previous studies showed that NO decreases intracellular calcium, which causes SMC relaxation and inhibits growth and migration. Our previous studies showed that NO upregulates SERCA activity by S-glutathiolation of the most reactive thiol on cysteine-674 (C674) and thereby inhibits SMC migration 4. Impaired NO-induced relaxation of atherosclerotic arteries or inhibition of migration of cultured SMCs exposed to high glucose was due to irreversible oxidation of SERCA C674 which prevents the S-glutathiolation and increase in SERCA activity required for the response to NO 5. A recent report that vascular injury which is normally inhibited by NO is unaffected in protein kinase G deficient mice 6 supports the concept that cyclic GMP-independent mechanisms are important in the response to NO, and their impairment may serve as a mechanism for disease.
Increased production of superoxide anion (O2−.) both reacts with and decreases the biological activity of NO in diseased arteries. Potential sources of vascular O2−. production include NADPH oxidases, xanthine oxidase, lipoxygenase, mitochondrial electron transport, and NO synthases (NOS). NADPH oxidases appear to be the principal source of O2−. in several animal models of vascular disease, including diabetes. NADPH oxidase is a multi-component enzyme that is comprised of membrane components p22phox and gp91phox (Nox2 or its homologues Nox1, 3–5), and cytoplasmic components p47phox, p67phox and the small G protein, rac1, which plays a role in activating NADPH oxidase. SMCs mainly express the Nox4 isoform, and together with p22phox are the major components of the active Nox4-based NADPH oxidase complex 7, 8. There is a continuous low-level of Nox4-derived ROS production in cardiovascular cells, the activity of which does not require rac1, p67phox or p47phox 9–11.
The obese Zucker rat (ZO) is a leptin-receptor deficient model, exhibiting obesity, insulin resistance and hyperinsulinemia. It has significantly higher body and liver weight, as well as plasma levels of insulin, lactate, cholesterol, triglyceride and tumor necrosis factor-alpha (TNF-α) compared to the lean Zucker rat (ZL) 12. By about 13 weeks of age, ZO rats have increased fasting plasma glucose and systolic blood pressure compared with ZL 13, 14. O2˙− levels and NADPH oxidase activity in aortic segments and renal cortex are significantly increased in ZO compared with ZL 15. Administration of the superoxide scavenger, Tempol, or the NADPH oxidase inhibitor, apocynin, restored aortic endothelium-dependent relaxation in ZO 15. Aortic neointima after endothelial balloon injury was much greater in ZO than in ZL due to an increase in SMC number within the intima 16. Here, we studied the ZO model to further understand the mechanisms responsible for the abnormal SMC migration and injury-induced neointimal growth in diabetes.
Research Design and Methods (please refer to online supplement for details)
Cell Culture
Aortic SMCs from 11 week old male ZL or ZO were cultured as previously described 17. Four pairs of ZL and ZO aortic SMCs were isolated separately. SMCs were confirmed by α-smooth muscle actin positive staining. Cells from passages 1 to 4 were used.
Amplex red assay for ROS production 18
The H2O2-dependent oxidation of amplex red was measured by a microplate fluorimeter (excitation 540 nm, emission 580 nm).
Detection of mRNA levels for NADPH oxidase components by real time quantitative PCR 18
The sequences of primers used here are listed in Online Table I.
RNA interference 18
Cells were transfected by siRNA (60 nmol/L) in DMEM without serum and antibiotics for 6h before switching to DMEM containing 0.2%FBS for 3 days.
Application of transforming growth factor-β1 (TGF-β1) in cultured aortic ZL SMCs
Human TGF-β1 (0.5 ng/mL, Sigma) was applied to cells in DMEM containing 0.2% FBS; isotype matched irrelevant IgG was administered as a control. Three days later, cells were lysed for immuno-blotting or undergone migration assay. In separate experiments, ZL SMCs were transfected with Nox4 siRNA for 6 h as mentioned above and then were switched to DMEM containing 0.2% FBS and TGF-β1 for 3 days for migration assay.
Application of anti-TGF-β1 or SB203580 to cultured ZO aortic SMCs
ZO SMCs were seeded into 6-well cell culture plates in DMEM with 10% FBS. When cells were 80% confluent, anti-TGF-β1 monoclonal antibody (0.5 µg/mL, Sigma) or SB203580 (5 µM, Promega) was applied in DMEM with 0.2% FBS for 3 days. As a control treatment, isotype matched irrelevant IgG was administered instead of anti-TGF-β1, and DMSO (0.1%) was applied instead of SB203580.
Adenovirus application19
ZO SMCs were infected with lacZ, SERCA wild type (WT), or SERCA C674S adenovirus (1–10 pfu/cell) in DMEM with 0.2% FBS for 3 days. The SERCA C674S and WT adenoviral constructs were published 4.
Wounded monolayer migration assay in SMCs
The detailed methods were published 19. DETA NONOate (300 µmol/L) was used as NO donor. Cells were allowed to migrate for 6 h after wounding.
Western blot analysis
Total SERCA (IID8 919, Affinity Bioreagent), SERCA C674SO3H (Bethyl laboratory, Inc) 20, SERCA-294, 295 nY (Bethyl laboratory, Inc) 21, Nox4 (Novus), TGF-β1 precursor (Sigma), TGF-β1 (Novus), Smad2/3 (Cell signaling) and phospho-Smad2 (Ser245/250/255, Cell signaling) were detected. Alpha-actin (Sigma) or GAPDH (Santa Cruz Biotechnology) were assessed for loading controls.
Animal surgical procedure
11 week old male obese Zucker rats were purchased from Charles River Laboratories (Boston, MA). Balloon catheter injury of the left common carotid artery (CCA) was accomplished by denuding the endothelium with a 2Fr Fogarty balloon catheter (Edwards Lifesciences) that was introduced through a skin incision and via the external carotid artery. Inflation and retraction of the balloon catheter were repeated three times. Adenoviral Nox4 short hairpin RNA (shRNA) or GFP (5×108 pfu) was introduced into the lumen, and the CCA was incubated for 20 minutes without blood flow. Then the external carotid artery was tied off and viral solution was flushed out when blood flow was resumed. The right CCA underwent the same procedure without balloon catheter injury and adenovirus application. Vascular remodeling and immunohistochemistry was evaluated following euthanasia of the animal.
Tissue processing and Immunohistochemistry
Aorta and CCA were fixed in 10% buffered formalin acetate and embedded in paraffin. For CCA injured groups, about 0.5 cm length of the middle part of the whole injured artery were used for data analysis. The sham and injured CCA were embedded together for each animal. Primary antibodies against smooth muscle 22α actin (SM22α actin), total SERCA, SERCA C674-SO3H, TGF-β1 and Nox4 were used. The appropriate IgG isotype acted as negative control. A biotinylated anti-mouse or anti-rabbit IgG secondary antibody was used at 1:200. Vector Red alkaline phosphatase substrate or DAB peroxidase substrate (Vector Laboratory) was used to visualize positive immuno-reactivity. Staining of aortic and CCA smooth muscle was scored on a scale of 0–4 by 3 experienced people that were blinded to sample identity. The staining of injured CCA was normalized by its sham CCA of each animal. Hematoxylin/Eosin (HE) staining was routinely done to evaluate the lesion thickness. The intima and media thickness were measured separately along three cross lines (2, 4 and 6 o’clock) and the mean values were used for analysis.
Data analysis
Data are expressed as means ± SEM. Statistics were analyzed with SPSS 13.0 as indicated for each experiment, and statistical significance was accepted for a P value less than 0.05.
Results
Migration of ZO SMCs is not inhibited by NO, but overexpression of SERCA 2b WT restores the NO response
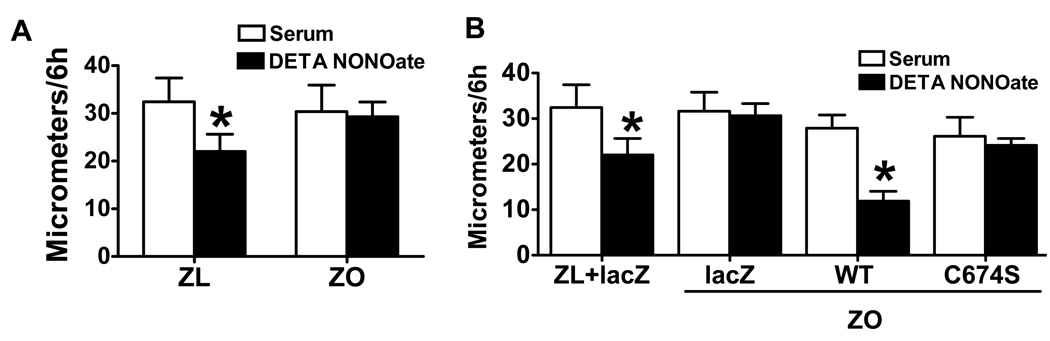
Serum-induced SMC migration was measured in the presence or absence of NO donor, DETA NONOate. SMCs from ZO and ZL migrated similarly in response to serum. However, DETA NONOate failed to inhibit cell migration in ZO SMCs, although it did so in SMCs from ZL (Fig. 1A), indicating the abnormal response to NO in ZO SMCs.
Figure 1.
Migration of obese Zucker rat (ZO) smooth muscle cells (SMCs) is not inhibited by nitric oxide (NO), but overexpression of SERCA 2b wild type (WT) restores NO function. A. The NO donor, DETA NONOate, failed to inhibit serum-induced SMC migration in ZO. *P<0.05 compared with serum. N=6, Student t-test. B. Overexpression of WT but not C674S mutant SERCA 2b restored the inhibition of migration by NO in ZO SMCs. *P<0.05 compared with serum alone, N=10, one-way ANOVA. ZL, lean Zucker rat.
Though NO failed to inhibit ZO SMC migration induced by serum, overexpression of WT but not C674S mutant SERCA 2b restored the inhibition of SMC migration by DETA NONOate (Fig. 1B), indicating that SERCA and the reactive cysteine-674 thiol is crucial to restore the NO response of migrating ZO SMCs.
ZO SMCs have increased oxidant stress and SERCA oxidation
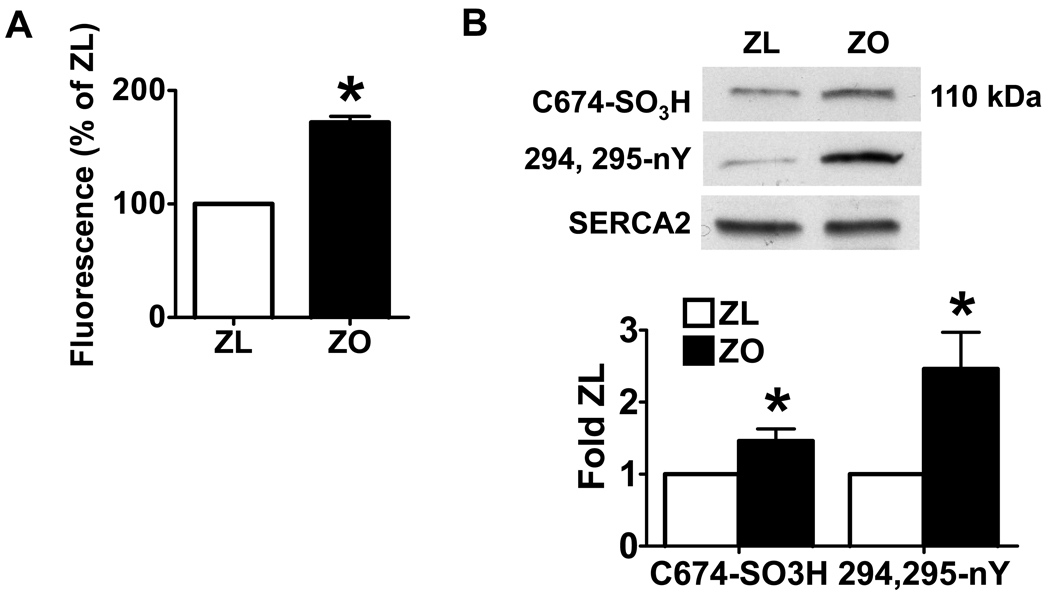
H2O2 levels were measured by Amplex Red which includes both H2O2 produced and that dismutated from O2−.. As shown in Fig. 2A, there was more than a 50% increase in reactive oxygen species (ROS) production in ZO SMCs compared with ZL SMCs, which is similar to other studies in which O2−. levels and NADPH oxidase activity were significantly increased in ZO compared with ZL aorta 22.
Figure 2.
Increased oxidant production and SERCA oxidation in ZO SMCs. A. Increased reactive oxygen species production measured by Amplex Red in ZO SMCs compared with ZL SMCs. *P<0.05 compared with ZL SMCs. N=3, Student t-test. B. ZO SMCs have significantly increased SERCA C674SO3H (N=4) and SERCA nY (N=7) staining compared with ZL SMCs. The upper panel shows a representative western blot. The lower panel shows a summary of band density. *P<0.05 compared with ZL SMCs, Student t-test.
3-nitrotyrosine (nY) modification of proteins serves as a marker for the biological formation of increased levels of reactive nitrogen species (RNS), including peroxynitrite. Using sequence-specific antibodies to a peptide containing a pair of nY (294, 295) located on the inside of the SERCA Ca2+ pore 21, shown in Fig. 2B, there was more SERCA nY staining in ZO SMCs compared with ZL. A sequence-specific antibody towards the peptide 669CLNARC(SO3H)FARV678 containing the C674 thiol in the oxidized sulfonic acid form was used to detect the irreversible oxidation C674 20. ZO SMCs showed more SERCA C674-SO3H staining than ZL (Fig. 2B). In contrast, there was no difference in total SERCA protein in ZO and ZL SMCs. These data indicate that SERCA is significantly more oxidized in ZO SMCs.
Upregulated Nox4-based NADPH oxidase causes increased ROS production in ZO SMCs
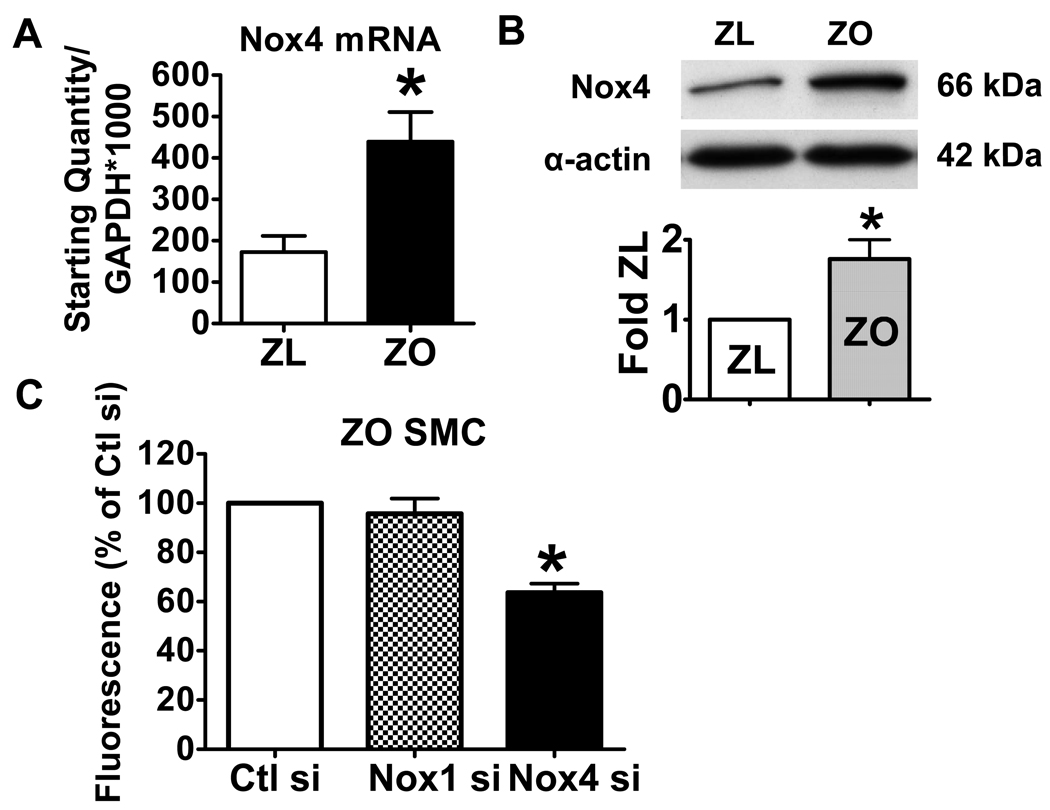
The above results suggest that increased oxidants in ZO SMCs are responsible for the oxidation of SERCA which prevents NO from inhibiting SMC migration. As in primary cultured normal rat aortic SMCs 18, Nox4 mRNA was more than 100-fold more abundant than Nox1 (data not shown) in ZL, and there was a 2-fold increase in Nox4 mRNA in ZO compared with ZL. The increase in Nox4 mRNA was also reflected in Nox4 protein levels by immuno-blot (Fig. 3A and B). The mRNA levels for other NADPH oxidase components (Nox1, p22phox, p47phox, p67phox, rac1) were not different between ZO and ZL SMCs. Nox2 mRNA was not detectable.
Figure 3.
The upregulation of Nox4 NADPH oxidase in ZO SMCs. A. Nox4 mRNA level in ZL and ZO SMCs. *P<0.05 compared with the ZL SMCs, N=4, Student t-test. B. Nox4 protein levels in ZL and ZO SMCs. The upper panel shows a representative western blot. The bar graph in the lower panel shows a summary of band density. *P<0.05 compared with the ZL SMCs, N=3, Student t-test. C. Knockdown of Nox4 by Nox4 siRNA, but not by control siRNA or Nox1 siRNA decreased ROS production in ZO SMCs. *P<0.05 compared with control siRNA, N=3, one-way ANOVA.
Furthermore, siRNA was used to knock down the expression of Nox4 to test its role in ROS production, and control siRNA and Nox1 siRNA served as controls. As expected, Nox1 and Nox4 mRNA levels were decreased by their respective siRNAs, but not by control siRNA in ZO SMCs (Online Figure IA and B), and neither affected the expression of the other isoform indicating that the siRNAs were specific to their targets. Transfection of Nox4 siRNA, but not control or Nox1 siRNA, decreased ROS production (Fig. 3C). These results indicate that the upregulated Nox4 in ZO SMCs is responsible for the increased ROS production.
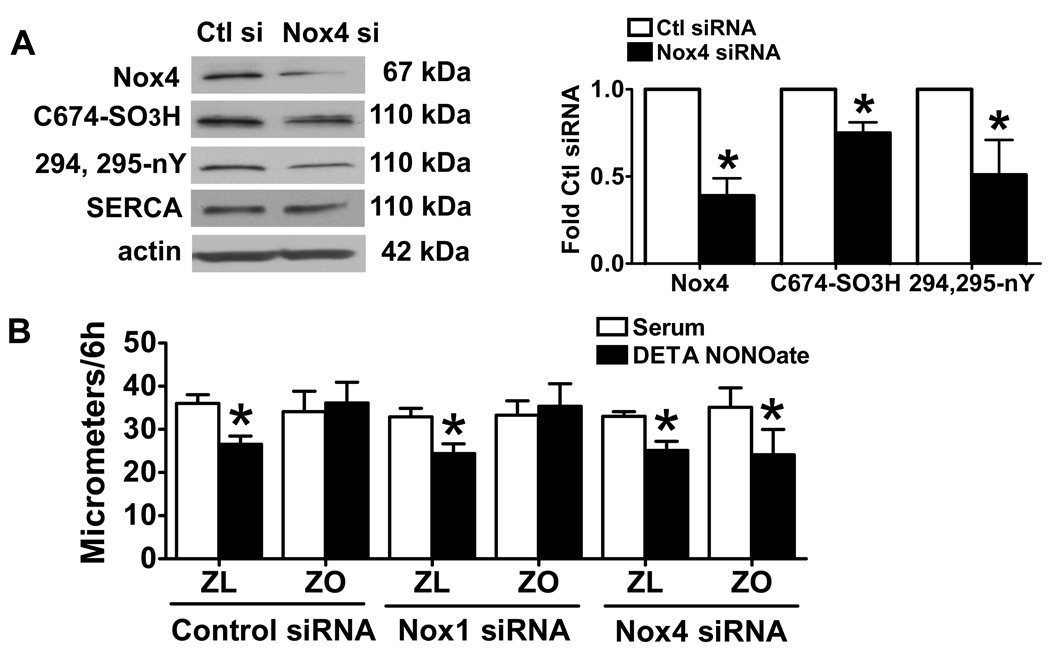
Knockdown of Nox4 decreases SERCA oxidation and restores the inhibition of cell migration by NO in cultured ZO SMCs
To test whether the upregulated Nox4-based NADPH oxidase is responsible for the oxidation of SERCA and accounts for the failure of NO to inhibit SMC migration, we used Nox4 siRNA to knock down Nox4 in ZO SMCs. As shown in Fig. 4A, knockdown of Nox4 by siRNA, but not by control or Nox1 siRNA (data not shown) decreased the protein levels of Nox4 as well as SERCA C674-SO3H and SERCA nY294,295, but not total SERCA in ZO SMCs. Furthermore, transfection of Nox4 siRNA, but not control or Nox1 siRNA, restored the ability of DETA NONOate to inhibit serum-induced migration of ZO SMCs (Fig. 4B). These results indicate that knockdown of Nox4 decreases SERCA oxidation and restores NO function.
Figure 4.
Knockdown of Nox4 decreased SERCA oxidation in ZO SMCs and restores the inhibition of cell migration by NO. A. The left panel shows a representative western blot. The right panel shows a summary of band density. *P<0.05 compared with control siRNA. N=3 of Nox4, N=5 of SERCA C674SO3H/total SERCA, N=6 of 294, 295-nY SERCA/total SERCA, Student t-test. B. Knockdown of Nox4 by Nox4 siRNA but not by control siRNA or Nox1 siRNA restores the inhibition of ZO SMC migration by NO donor, DETA NONOate. *P<0.05 compared with control siRNA, N=4, two-way ANOVA.
The upregulation of Nox4 by TGF-β1 and Smad2 is responsible for the oxidation of SERCA and the failure of NO to inhibit migration of ZO SMCs
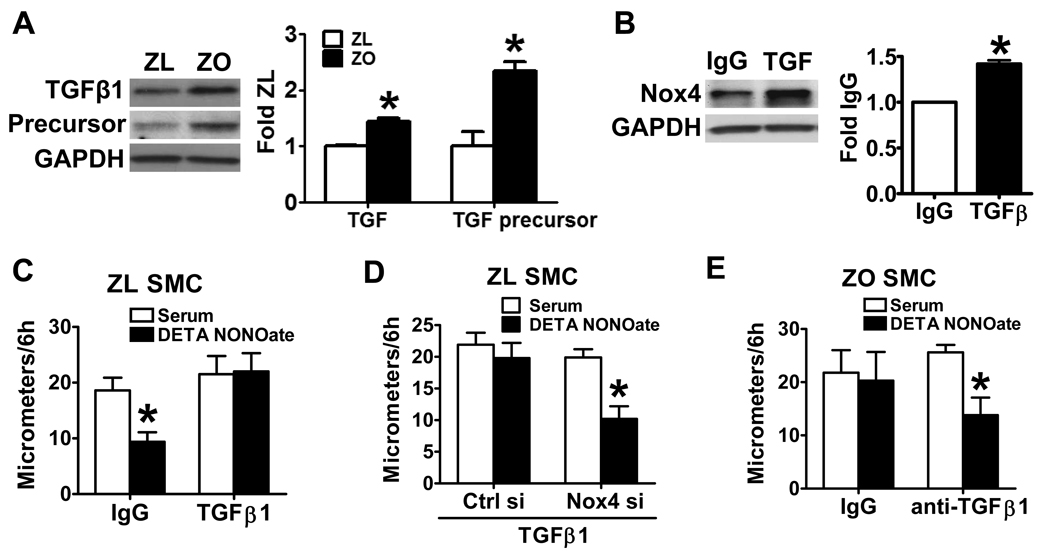
To explore the mechanisms that cause Nox4 upregulation in ZO SMCs, we tested several potential factors, and found that the expression levels of both TGF-β1 and its precursor were increased in ZO SMCs compared with ZL (Fig. 5A). Furthermore, in ZL SMCs, application of recombinant TGF-β1 for 3 d significantly increased Nox4 protein level (Fig. 5B), indicating that TGF-β1 might be the cause of Nox4 upregulation in ZO SMCs.
Figure 5.
The upregulation of Nox4 by TGF-β1 is responsible for the abnormal response to NO in ZO SMCs. A. The expression levels of both TGF-β1(12.5 kDa) and its precursor (45 kDa) are significantly increased in ZO SMCs. The left panel shows a representative western blot. The right panel shows a summary of band density. *P<0.05 compared with the ZL SMCs, N=4, Student t-test. B. Application of TGF-β1 in ZL SMCs increased Nox4 protein level. The left panel shows a representative western blot. The right panel shows a summary of band density. *P<0.05 compared with IgG control, N=3, paired t-test. C. Application of TGF-β1 blocked the inhibition of ZL SMC migration by DETA NONOate. P<0.05 compared with serum alone, N=6, Student t-test. D. Knockdown of Nox4 counteracted the effect of TGF-β1 on NO-induced inhibition of ZL SMC migration. *P<0.05 compared with serum alone, N=6, Student t-test. E. Blockade of TGF-β1 by anti-TGF-β1 antibody restored the inhibition of ZO SMC migration by DETA NONOate. *P<0.05 compared with serum alone, N=6, Student t-test.
To test whether TGF-β1 is responsible for the abnormal response to NO in ZO SMC migration, TGF-β1 was applied to ZL SMCs to determine whether it induces resistance to NO like that was seen in ZO SMCs. As shown in Fig. 5C, the application of TGF-β1, but not the IgG control, blocked the inhibition of ZL SMC migration by NO. Furthermore, application of TGF-β1 increased ROS production in ZL SMCs (Online Figure IIA), which corresponded to the increased Nox4 protein level induced by TGF-β1. To directly test if the effect of TGF-β1 is through upregulation of Nox4 NADPH oxidase, TGF-β1 was applied for 3 days to ZL SMCs in which Nox4 was knocked down with siRNA. The transfection of Nox4, but not control siRNA preserved the inhibition of migration by DETA NONOate in ZL SMCs treated with TGF-β1 (Fig. 5D). To directly test whether TGF-β1 mediated the increase in ROS production and impaired NO function in ZO SMCs, anti-TGF-β1 antibody was applied to neutralize TGF-β1 produced by ZO SMCs. As shown in Figure 5E, blockade of TGF-β1 by anti-TGF-β1 antibody not only restored the anti-migratory response to the NO donor, but also decreased ROS production in ZO SMCs compared with the IgG control (Online Figure IIB). These results indicate that increased TGF-β1 is responsible for the failure of NO to inhibit ZO SMC migration.
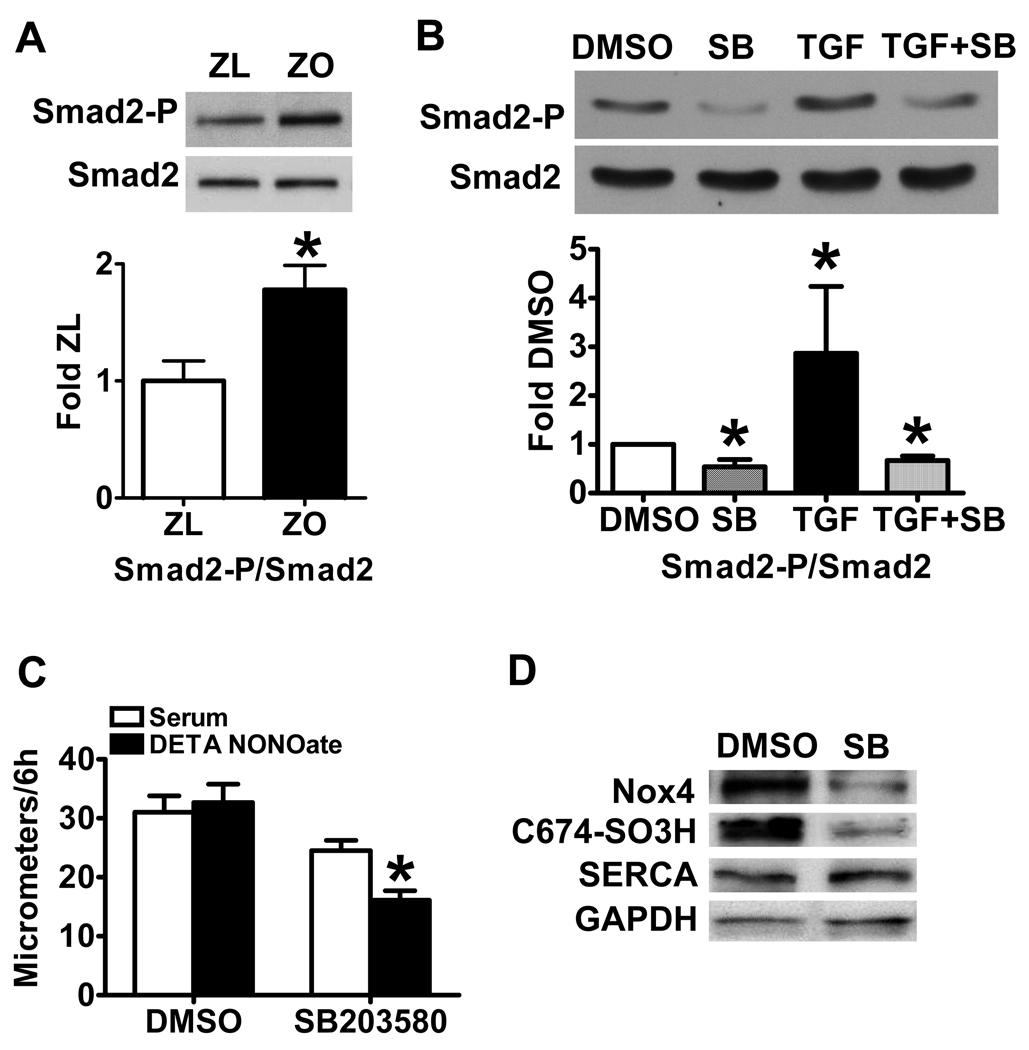
In exploring the mechanisms that cause the upregulation of Nox4 by TGF-β1 in ZO SMCs, the role of Smad proteins, which are part of the TGF-β1 receptor signaling complex, were considered. Phosphorylation of Smad2 at serine 245/250/255 was increased in ZO SMCs (Fig. 6A), though total Smad2 protein levels were unchanged between ZL and ZO SMCs, and Smad3 was undetectable. Furthermore, whether Smad2 phosphorylation was involved in the impaired response to NO donor of migrating ZO SMCs was determined using a p38 mitogen-activated protein kinase inhibitor, SB203580, which was shown previously to inhibit the phosphorylation of endogenous Smad2 by TGFβ receptor I and to affect a TGFβ-specific transcriptional response 23. In ZO SMCs, application of SB203580 significantly decreased both the endogenous and TGF-β1-induced Smad2 phosphorylation (Fig. 6B) compared with solvent control DMSO. Application of SB203580, but not DMSO for 3 d to ZO SMCs restored the response of migrating ZO SMCs to NO donor (Fig. 6C). The cell lysates pooled from migration assays showed that both Nox4 and SERCA C674-SO3H were decreased in SMCs treated with SB203580 (Fig. 6D). To further test if Smad2 mediates TGF-β1-induced signal transduction pathway, we transfected HEK293T cells with a SMAD luciferase reporter, which indicates the translocation of Smad2 to the nucleus and the activation of Smad2 responsive genes. As shown in Online Figure III, SB203580 decreased both endogenous and TGF-β1-induced Smad2 responsive gene expression. These results indicate that Smad2 is involved in the induction of Nox4 and ROS production by TGF-β1.
Figure 6.
Blockade of Smad2 phosphorylation restores the inhibition of cell migration by DETA NONOate and decreases Nox4 and SERCA oxidation. A. The phospho-Smad 2 (Ser245/250/255, 60 kDa) is significantly increased in ZO SMCs compared with ZL SMCs. The upper panel shows a representative western blot. The lower panel shows a summary of band density. *P<0.05 compared with the ZL SMCs, N=4, Student t-test. B. Application of Smad2 phosphorylation inhibitor SB203580 in ZO SMCs decreases both the endogenous and TGF-β1-induced Smad2 phosphorylation. *P<0.05 compared with DMSO, N=3. C. Application of SB203580 in ZO SMCs restores the inhibition of cell migration by DETA NONOate. *P<0.05 compared with serum alone, N=3 in DMSO group and N=6 in SB group, Student t-test. D. Application of SB203580 in ZO SMCs decreases Nox4 and SERCA oxidation. Blot of SMC lysates pooled after the migration assay shown in Figure 6C.
TGF-β1, Nox4 and SERCA-C674SO3H staining are increased in ZO aorta compared with that of ZL
To test whether the SMC characteristics observed in primary cell culture are present in vivo, immunohistochemistry was performed to determine the levels of TGF-β1, Nox4, and SERCA in ZO and ZL aorta. As shown in Online Figure IV, the levels of TGF-β1 and Nox4 were increased in ZO compared with ZL aorta. Oxidation of SERCA detected by the SERCA-C674SO3H antibody was also increased, but the total SERCA expression was unaltered. These results indicate upregulation of TGF-β1 and Nox4 NADPH oxidase, as well as SERCA oxidation in the ZO aorta in vivo.
Knockdown of Nox4 inhibits the neointima formation after ZO common carotid artery injury
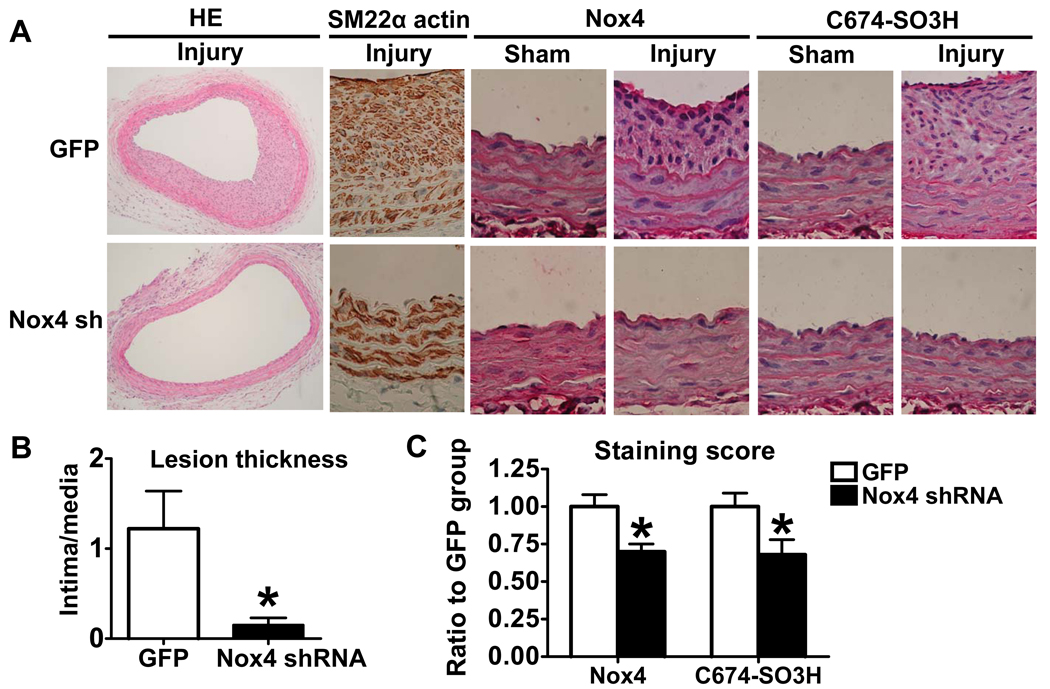
Because Nox4 was shown in the above studies to be responsible for the abnormal response to NO of migrating ZO SMCs, the role of Nox4 was tested in SMC expansion into balloon catheter-induced neointima in vivo. Adenoviral Nox4 shRNA and GFP were tested first in ZO SMCs. As shown in Online Figure V, the infection of Nox4 shRNA decreased Nox4 protein level in cultured ZO SMCs by half compared with GFP control. Then adenoviral Nox4 shRNA was used to knockdown Nox4 in vivo. Preliminary studies indicated there was no difference in the neointimal thickness in GFP infected artery compared with arteries injured alone without adenovirus (data not shown). HE staining showed a significant decrease in the neointima in Nox4 shRNA infected arteries compared with GFP infected arteries as shown in Fig. 7A. The ratio of intima to media is summarized in Fig. 7B. Almost all cells in the neoinitima are SMCs stained by SM22α actin antibody (Fig. 7A), indicating that SMC are the major cellular component. Furthermore, infection of adenoviral Nox4 shRNA significantly decreased Nox4 staining by immunohistochemistry (Fig. 7A and C). Knockdown of Nox4 also decreased oxidants as indicated by a decrease in SERCA C674-SO3H staining (Fig. 7A and C).
Figure 7.
Knockdown of Nox4 by adenovirus Nox4 shRNA inhibits neointima formation after common carotid artery balloon catheter injury. A. Immunohistochemistry of injured and sham common carotid artery. Hematoxylin/Eosin (HE) staining (100×) and others (400×). B. Infection of adenoviral Nox4 shRNA decreases the intima to media ratio. *P<0.05 compared with GFP group, N=5, Student t-test. C. Infection of adenoviral Nox4 shRNA decreases the Nox4 and SERCA C674-SO3H staining. *P<0.05 compared with GFP, N=5, Student t-test.
Discussion
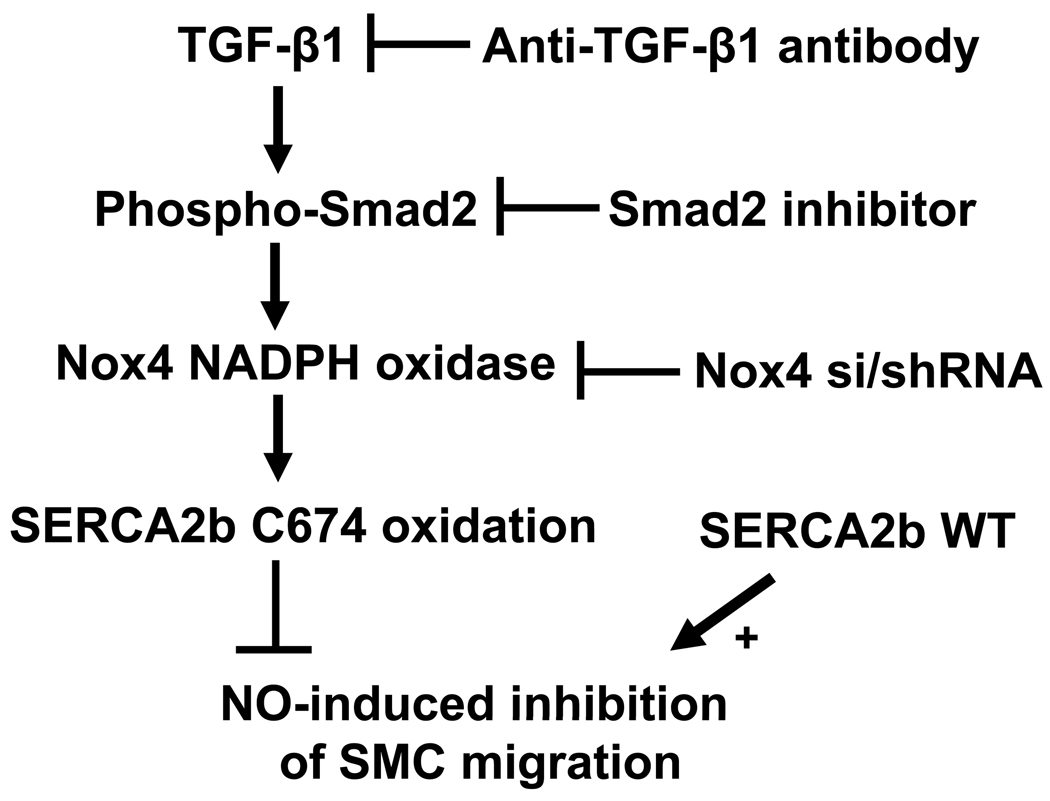
Our studies indicate that increases in Nox4 expression, oxidant production, and impaired NO responsiveness of the ZO aorta are maintained during cell culture. Our studies in cultured ZO SMCs indicate that upregulation of TGF-β1 and Nox4 are associated with oxidation of SERCA, and that the oxidation of SERCA prevents inhibition of SMC migration by NO. In ZO SMCs, the increase in Nox4 was attributed to the activation of Smad2 by TGF-β1. The proposed mechanisms involved in the abnormal response to NO in ZO SMCs by redox regulation of SERCA are shown in Fig.8. Increased TGF-β1 in ZO SMCs activates the phosphorylation of Smad2 which upregulates Nox4 NADPH oxidase and causes SERCA oxidation. The oxidation of SERCA prevents inhibition of SMC migration by NO. Blockade of TGF-β1 or Smad2, or knockdown of Nox4 reversed the abnormal regulation of the SMCs by NO indicating their key roles. The fact that TGF-β1 and Nox4 are increased and SERCA-C674 is irreversibly oxidized in ZO aorta in vivo, may help to explain the increased neointima formation after arterial injury in the ZO 24. As shown in previous studies in normal SMC exposed to high glucose, the lack of response to NO indicates that a high percentage of SERCA C674 thiol is oxidized 19. As indicated by the nitration of two susceptible SERCA tyrosines (294, 295), it is likely that many other amino acid residues also are oxidized. These two tyrosines border the calcium pore of SERCA, so they might be exposed to oxidants. Mutants of these two tyrosines have not yet been studied, and it is unclear whether they would accurately mimic nitration. Mass spectrometry and antibody studies indicate that these tandem tyrosines are widely nitrated under the same conditions in which C674 is oxidized 20, 21. Oxidation is also likely to be widespread on other proteins. However, our studies also show that WT, but not C674S mutant SERCA can mediate the anti-migratory response to NO 4, 19. Given the fact that WT and C674S mutant SERCA have normal calcium uptake activity, and that the mutant only lacks the stimulation of activity by NO, our results indicate that oxidation of this single thiol on SERCA is sufficient for SMCs to be insensitive to NO, and that its restitution can restore the response.
Figure 8.
The proposed mechanisms involved in the abnormal response to NO in ZO SMCs by oxidation of SERCA. Increased TGF-β1 in ZO SMCs activates the phosphorylation of Smad2 which upregulates Nox4-based NADPH oxidase and causes SERCA oxidation. The oxidation of SERCA, especially of the most reactive cysteine 674, inhibits the NO-induced stimulation of SERCA activity and blocks NO-induced inhibition of SMC migration. Blockade of TGF-β1 or Smad2, or knockdown of Nox4, or overexpression of SERCA WT can maintain the ability of NO to inhibit SMC migration.
In preliminary studies, we studied other factors potentially involved in impaired function of ZO SMCs including insulin, TNF-α, and palmitate, before we found that TGF-β1 was the major factor involved in maintaining Nox4 expression, oxidants, and impaired NO responsiveness. TGF-β1 is a pro-fibrotic cytokine that is involved in the induction of arterial intimal thickening. Up-regulation of TGF-β1 after arterial injury results in the activation of various downstream pathways which stimulate the proliferation and migration of SMCs, as well as the production of local extracellular matrix proteins. Recent evidence suggests that antagonizing TGF-β1 with direct or indirect inhibitors may attenuate or prevent intimal thickening 25. In aorta, the mRNA for TGF-β1 was significantly increased in ZO compared with ZL 26. Our immunohistochemistry confirmed that TGF-β1 is increased both in the aortic media and endothelium 26, and our studies show that an increase in TGF-β1 protein in ZO SMCs is maintained in cell culture. The mechanism of upregulation of TGF-β1 in ZO needs to be further explored. TNF-α and/or insulin, which are both increased in ZO serum compared with ZL 12, are potential factors that upregulate TGF-β1 in ZO aorta. Adenoviral transfer of cDNA for TNF-α to rat lung induced TGF-β1 expression in bronchoalveolar fluids 27. Also, application of insulin for 12–16 h to cultured SMCs from ZL aorta significantly increased TGF-β1 mRNA 26, suggesting these two factors.
TGF-β1 and its receptor subtypes I and II activate several pathways, including Smad-dependent and Smad-independent pathways 28. Among the potential mechanisms involved in the activation of Nox4 by TGF-β1, the phosphorylation of Smad2 was increased in ZO SMCs and blockade of Smad2 phosphorylation decreased Nox4 production and SERCA oxidation, and also restored NO function, suggesting that the increased TGF-β1 in ZO SMCs activates Smad2 to upregulate Nox4. There are eight distinct Smad proteins, constituting three functional classes: receptor-regulated Smad (R-Smad), Co-mediator Smad (Co-Smad), and inhibitory Smad (I-Smad) 29. Smad2 belongs to R-Smads which can be directly phosphorylated and activated by the type I receptor kinases and undergo homotrimerization and formation of heteromeric complexes with the Co-Smad. The activated Smad complexes are translocated into the nucleus and in conjunction with other nuclear cofactors, regulate the transcription of target genes29, including Nox4 30, 31. Smad2 and Smad3 can be specifically immobilized near the cell surface by the Smad anchor for receptor activation, or SARA, through the interactions between a peptide sequence of SARA and an extended hydrophobic surface area on Smad2/Smad3 32. TGF-β receptor complex and SARA show a characteristically punctate membrane distribution 33, which is the hallmark of the staining pattern of caveolin-1, a principal component of caveolae. TGF-β receptor I, and Smad2, but not Smad4 fractionate with caveolin-1 in caveolae enriched microdomains 34. The p38 mitogen-activated protein kinase inhibitor, SB203580, used in studies of ZO SMCs in culture dramatically decreased Smad2 phosphorylation, Nox4 production, inhibited SERCA oxidation, and restored the responsiveness to NO. Due to the low efficiency of Smad reporter gene transfection in ZO SMCs, we showed in HEK293T cells,that Smad2 mediated gene transcription is increased by TGF-β1 and inhibited by SB203580. These results indicate that the phosphorylation of Smad 2 is the key mediator in TGF-β1 induced Nox4 production and SERCA oxidation.
Application of TGF-β1 in ZL SMCs increased Nox4 expression. In ZO SMCs, si RNA knockdown of Nox4 decreased Nox4 protein expression and SERCA oxidation, indicating that there is a direct link between increased Nox4 levels and SERCA oxidation. Confirmation that Nox4 is implicated in SERCA oxidation in vivo provides further evidence that there is a direct link between Nox4 and SERCA oxidation. TGF-β1 activates Nox4 in embryonic stem cells 35 and human pulmonary artery SMCs 36. In cultured human airway SMCs, TGF-β1-induced Nox4 was localized within the endoplasmic reticulum (ER) and nucleus, implying a role for Nox4 in regulation of both the cell cycle and protein synthesis 37. In data not shown here, we found no co-immunoprecipitation of SERCA and Nox4. However, other recent studies indicate that Nox4 is located in the ER membrane 38–40, where SERCA is located. This might result in subcellular concentration of Nox4-derived oxidants which can oxidize SERCA. Earlier studies showed abnormalities in Ca2+ regulation by SERCA in ZO red blood cells and SMC 41, 42, but did not link them to oxidants or to the impaired function of NO noted since then in this model of type 2 diabetes. After common carotid artery injury, the downregulation of Nox4 decreased the oxidation of SERCA C674, confirming it as a significant source oxidants in vivo. Our in vitro studies indicate that maintaining redox responsiveness of SERCA is key to the inhibition of cell migration by NO, suggesting that this mechanism can contribute to the dramatic decrease in neointima formation caused by Nox4 shRNA. Thus, increases in Nox4 which occur after arterial injury 43, may be exaggerated in the insulin-resistant ZO, and account for greater oxidation and neointima. To our knowledge, this is the first paper showing that knockdown of Nox4 decreases restenosis after carotid artery injury.
In summary, ZO aortic SMCs have significantly increased Nox4 expression, oxidant production and SERCA oxidation compared with those from ZL which can account for the defect in the ability of NO to stimulate SERCA and inhibit SMC migration, and may also help to explain earlier observations of abnormal calcium and NO response in ZO SMCs 15, 44. Although NO synthase uncoupling or oxidant-mediated decreases in NO bioactivity may be other factors involved, our studies indicate that impaired NO responsiveness of ZO SMC due to Nox4 and SERCA oxidation is important. The fact that WT SERCA restores the response of SMC suggests that the responsiveness of SERCA is of key importance. The upregulation of TGF-β1 and Nox4 in ZO aorta are potential therapeutic targets to improve NO function in insulin-resistant states.
Novelty and Significance
What Is Known?
Vascular smooth muscle cell (SMC) migration contributes to neointimal growth and may underlie the higher morbidity and mortality of atherosclerosis and restenosis in patients with diabetes mellitus.
Aortic neointima induced by endothelial balloon injury is much greater in obese Zucker rats, a model of type 2 diabetes, compared with control lean Zucker rats.
What New Information Does This Article Contribute?
In aortic SMC from obese Zucker rats Nox4-derived oxidants selectively impair the ability of nitric oxide (NO) to inhibit migration by oxidizing a key reactive cysteine thiol of the sarco/endoplasmic reticulum Ca2+ATPase (SERCA).
Transforming growth factor β1 (TGF-β1) and Smad2 are upstream activators of Nox4 in SMC from obese Zucker rats
Summary
Abnormal SMC migration contributes to the higher morbidity of atherosclerosis and restenosis in diabetes mellitus, while the mechanisms involved are not fully understood. Our studies indicate that there is significantly increased Nox4 expression and oxidant levels, and that a key cysteine thiol of SERCA is oxidized in aortic SMC from obese Zucker rats, which can account for the defect in the ability of NO to inhibit SMC migration. TGF-β1 via Smad2 is necessary and sufficient to upregulate Nox4, oxidize SERCA, and block the anti-migratory action of NO in SMC from obese rats. In these rats knockdown of Nox4 inhibits the oxidation of SERCA as well as neointima formation after common carotid artery injury. We report for the first time that knockdown of Nox4 inhibits protein oxidation and decreases restenosis after carotid artery injury. Our studies indicate that maintaining redox responsiveness of SERCA is key to the inhibition of cell migration by NO. TGF-β1 and Nox4 in aorta are potential therapeutic targets for improving NO function in type 2 diabetes.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by American Diabetes Association award 7-09-JF-69 and NIH grants: R01 HL031607, PO1 HL081587, R01 AG27080, P01 HL068758.
Non-standard Abbreviations and Acronyms
- CCA
common carotid artery
- ER
endoplasmic reticulum
- HE
hematoxylin/eosin
- NO
nitric oxide
- nY
nitrotyrosine
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- shRNA
short hairpin RNA
- siRNA
small interference RNA
- SMC
smooth muscle cell
- SERCA
sarco/endoplasmic reticulum Ca2+ ATPase
- O2−.
superoxide anion
- TNF-α
Tumor necrosis factor-α
- TGF-β1
transforming growth factor-β1
- WT
wild type
- ZL
lean Zucker rat
- ZO
obese Zucker rat.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
Reference List
- 1.Elezi S, Kastrati A, Pache J, Wehinger A, Hadamitzky M, Dirschinger J, Neumann FJ, Schomig A. Diabetes mellitus and the clinical and angiographic outcome after coronary stent placement. J Am Coll Cardiol. 1998;32:1866–1873. doi: 10.1016/s0735-1097(98)00467-7. [DOI] [PubMed] [Google Scholar]
- 2.Schofer J, Schluter M, Rau T, Hammer F, Haag N, Mathey DG. Influence of treatment modality on angiographic outcome after coronary stenting in diabetic patients: a controlled study. J Am Coll Cardiol. 2000;35:1554–1559. doi: 10.1016/s0735-1097(00)00574-x. [DOI] [PubMed] [Google Scholar]
- 3.Van BE, Bauters C, Hubert E, Bodart JC, Abolmaali K, Meurice T, McFadden EP, Lablanche JM, Bertrand ME. Restenosis rates in diabetic patients: a comparison of coronary stenting and balloon angioplasty in native coronary vessels. Circulation. 1997;96:1454–1460. doi: 10.1161/01.cir.96.5.1454. [DOI] [PubMed] [Google Scholar]
- 4.Ying J, Tong X, Pimentel DR, Weisbrod RM, Trucillo MP, Adachi T, Cohen RA. Cysteine-674 of the sarco/endoplasmic reticulum calcium ATPase is required for the inhibition of cell migration by nitric oxide. Arterioscler Thromb Vasc Biol. 2007;27:783–790. doi: 10.1161/01.ATV.0000258413.72747.23. [DOI] [PubMed] [Google Scholar]
- 5.Adachi T, Matsui R, Weisbrod RM, Najibi S, Cohen RA. Reduced sarco/endoplasmic reticulum Ca2+ uptake activity can account for the reduced response to NO, but not sodium nitroprusside, in hypercholesterolemic rabbit aorta. Circ. 2001;104:1040–1045. doi: 10.1161/hc3501.093798. [DOI] [PubMed] [Google Scholar]
- 6.Lukowski R, Weinmeister P, Bernhard D, Feil S, Gotthardt M, Herz J, Massberg S, Zernecke A, Weber C, Hofmann F, Feil R. Role of smooth muscle cGMP/cGKI signaling in murine vascular restenosis. Arterioscler Thromb Vasc Biol. 2008;28:1244–1250. doi: 10.1161/ATVBAHA.108.166405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a Novel Regulator of Nox4 and Cytoskeletal Integrity in Vascular Smooth Muscle Cells. Circulation Research. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 10.Martyn KD, Frederick LM, von LK, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Meng D, Lv DD, Fang J. Insulin-like growth factor-I induces reactive oxygen species production and cell migration through Nox4 and Rac1 in vascular smooth muscle cells. Cardiovasc Res. 2008;80:299–308. doi: 10.1093/cvr/cvn173. [DOI] [PubMed] [Google Scholar]
- 12.Raju J, Bird RP. Alleviation of hepatic steatosis accompanied by modulation of plasma and liver TNF-alpha levels by Trigonella foenum graecum (fenugreek) seeds in Zucker obese (fa/fa) rats. Int J Obes (Lond) 2006;30:1298–1307. doi: 10.1038/sj.ijo.0803254. [DOI] [PubMed] [Google Scholar]
- 13.Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui AH, Ali Q, Hussain T. Protective role of angiotensin II subtype 2 receptor in blood pressure increase in obese Zucker rats. Hypertension. 2009;53:256–261. doi: 10.1161/HYPERTENSIONAHA.108.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Standley PR, Ram JL, Sowers JR. Insulin attenuation of vasopressin-induced calcium responses in arterial smooth muscle from Zucker rats. Endocrinology. 1993;133:1693–1699. doi: 10.1210/endo.133.4.8404611. [DOI] [PubMed] [Google Scholar]
- 16.Ridray S. Hyperinsulinemia and smooth muscle cell proliferation. Int J Obes Relat Metab Disord. 1995;19 Suppl 1:S39–S51. [PubMed] [Google Scholar]
- 17.Grainger DJ, Hesketh TR, Metcalfe JC, Weissberg PL. A large accumulation of non-muscle myosin occurs at first entry into M phase in rat vascular smooth-muscle cells. Biochem J. 1991;277(Pt 1):145–151. doi: 10.1042/bj2770145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong X, Schroder K. NADPH oxidases are responsible for the failure of nitric oxide to inhibit migration of smooth muscle cells exposed to high glucose. Free Radic Biol Med. 2009;47:1578–1583. doi: 10.1016/j.freeradbiomed.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong X, Ying J, Pimentel DR, Trucillo M, Adachi T, Cohen RA. High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J Mol Cell Cardiol. 2008;44:361–369. doi: 10.1016/j.yjmcc.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ying J, Sharov V, Xu S, Jiang B, Gerrity R, Schoneich C, Cohen RA. Cysteine-674 oxidation and degradation of sarcoplasmic reticulum Ca(2+) ATPase in diabetic pig aorta. Free Radic Biol Med. 2008;45:756–762. doi: 10.1016/j.freeradbiomed.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu S, Ying J, Jiang B, Guo W, Adachi T, Sharov V, Lazar H, Menzoian J, Knyushko TV, Bigelow D, Schoneich C, Cohen RA. Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am J Physiol Heart Circ Physiol. 2006;290:H2220–H2227. doi: 10.1152/ajpheart.01293.2005. [DOI] [PubMed] [Google Scholar]
- 22.Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2008;294:H1658–H1666. doi: 10.1152/ajpheart.01206.2007. [DOI] [PubMed] [Google Scholar]
- 23.Yakymovych I, Engstrom U, Grimsby S, Heldin CH, Souchelnytskyi S. Inhibition of transforming growth factor-beta signaling by low molecular weight compounds interfering with ATP- or substrate-binding sites of the TGF beta type I receptor kinase. Biochemistry. 2002;41:11000–11007. doi: 10.1021/bi025936u. [DOI] [PubMed] [Google Scholar]
- 24.Pei H, Gu J, Thimmalapura PR, Mison A, Nadler JL. Activation of the 12-lipoxygenase and signal transducer and activator of transcription pathway during neointima formation in a model of the metabolic syndrome 1. Am J Physiol Endocrinol Metab. 2006;290:E92–E102. doi: 10.1152/ajpendo.00133.2005. [DOI] [PubMed] [Google Scholar]
- 25.Khan R, Agrotis A, Bobik A. Understanding the role of transforming growth factor-beta1 in intimal thickening after vascular injury. Cardiovasc Res. 2007;74:223–234. doi: 10.1016/j.cardiores.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Sista AK, O'Connell MK, Hinohara T, Oommen SS, Fenster BE, Glassford AJ, Schwartz EA, Taylor CA, Reaven GM, Tsao PS. Increased aortic stiffness in the insulin-resistant Zucker fa/fa rat. Am J Physiol Heart Circ Physiol. 2005;289:H845–H851. doi: 10.1152/ajpheart.00134.2005. [DOI] [PubMed] [Google Scholar]
- 27.Sime PJ, Marr RA, Gauldie D, Xing Z, Hewlett BR, Graham FL, Gauldie J. Transfer of tumor necrosis factor-alpha to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-beta1 and myofibroblasts. Am J Pathol. 1998;153:825–832. doi: 10.1016/s0002-9440(10)65624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 29.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 30.Carmona-Cuenca I, Roncero C, Sancho P, Caja L, Fausto N, Fernandez M, Fabregat I. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J Hepatol. 2008;49:965–976. doi: 10.1016/j.jhep.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta 1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 32.Wu G, Chen YG, Ozdamar B, Gyuricza CA, Chong PA, Wrana JL, Massague J, Shi Y. Structural basis of Smad2 recognition by the Smad anchor for receptor activation. Science. 2000;287:92–97. doi: 10.1126/science.287.5450.92. [DOI] [PubMed] [Google Scholar]
- 33.Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell. 1998;95:779–791. doi: 10.1016/s0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- 34.Razani B, Zhang XL, Bitzer M, von GG, Bottinger EP, Lisanti MP. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem. 2001;276:6727–6738. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- 35.Xiao Q, Luo Z, Pepe AE, Margariti A, Zeng L, Xu Q. Embryonic stem cell differentiation into smooth muscle cells is mediated by Nox4-produced H2O2. Am J Physiol Cell Physiol. 2009;296:C711–C723. doi: 10.1152/ajpcell.00442.2008. [DOI] [PubMed] [Google Scholar]
- 36.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol. 2009;296:L489–L499. doi: 10.1152/ajplung.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sturrock A, Huecksteadt TP, Norman K, Sanders K, Murphy TM, Chitano P, Wilson K, Hoidal JR, Kennedy TP. Nox4 mediates TGF-beta1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1543–L1555. doi: 10.1152/ajplung.00430.2006. [DOI] [PubMed] [Google Scholar]
- 38.Helmcke I, Heumuller S, Tikkanen R, Schroder K, Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal. 2009;11:1279–1287. doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- 39.Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal. 2006;8:1473–1484. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- 40.van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 41.Zemel MB, Sowers JR, Shehin S, Walsh MF, Levy J. Impaired calcium metabolism associated with hypertension in Zucker obese rats. Metabolism. 1990;39:704–708. doi: 10.1016/0026-0495(90)90104-k. [DOI] [PubMed] [Google Scholar]
- 42.Zemel MB. Insulin resistance vs. hyperinsulinemia in hypertension: insulin regulation of Ca2+ transport and Ca2+-regulation of insulin sensitivity. J Nutr. 1995;125:1738S–1743S. doi: 10.1093/jn/125.suppl_6.1738S. [DOI] [PubMed] [Google Scholar]
- 43.Szocs K, Lassegue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- 44.Russo I, Del MP, Doronzo G, Mattiello L, Viretto M, Bosia A, Anfossi G, Trovati M. Resistance to the nitric oxide/cyclic guanosine 5'-monophosphate/protein kinase G pathway in vascular smooth muscle cells from the obese Zucker rat, a classical animal model of insulin resistance: role of oxidative stress. Endocrinology. 2008;149:1480–1489. doi: 10.1210/en.2007-0920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.