Abstract
Background
The recent upsurge in interest about pediatric bipolar disorder (BD) has spurred the need for greater understanding of its neurobiology. Structural and functional magnetic resonance imaging (MRI) studies have implicated fronto-temporal dysfunction in pediatric BD. However, recent data suggest that task-dependent neural changes account for a small fraction of the brain’s energy consumption. We now report the first use of task-independent spontaneous resting state functional connectivity (RSFC) to study the neural underpinnings of pediatric BD.
Methods
We acquired a task-independent RSFC blood oxygen level-dependent fMRI scans while participants were at rest and also a high-resolution anatomical image (both at 3 Tesla) in BD and control youths (N=15 of each). Based on prior research, we focused on the left dorsolateral prefrontal cortex (DLPFC), amygdala, and accumbens. Image processing and group-level analyses followed that of prior work.
Results
Our primary analysis showed that pediatric BD participants had significantly greater negative RSFC between the left DLPFC and the right superior temporal gyrus (STG) versus controls. Secondary analyses using partial correlation showed that BD and control youths had opposite phase relationships between spontaneous RSFC fluctuations in the left DLPFC and right STG.
Conclusions
Our data indicate that pediatric BD is characterized by altered task-independent functional connectivity in a fronto-temporal circuit that is also implicated in working memory and learning. Further study is warranted to determine the effects of age, sex, development, and treatment on this circuit in pediatric BD.
Keywords: Bipolar Disorder, Child, Adolescent, Magnetic Resonance Imaging, Frontal Lobe, Temporal Lobe
INTRODUCTION
Although it was once thought that mania could not occur in children, the past decade has witnessed an upsurge in research and clinical interest about pediatric bipolar disorder (BD). Recent data suggest that pediatric BD’s incidence has risen 40-fold in just a decade, with over 20% of all minors discharged from psychiatric hospitals now diagnosed with BD (1;2). Determining the validity of this dramatic increase is problematic because current psychiatric nosology is based entirely on clinical history that is considerably more difficult to elicit from children and adolescents than from adults. Thus, there is a pressing need for greater neurobiological understanding of pediatric BD.
Towards that end, structural magnetic resonance imaging (MRI) studies have implicated fronto-temporal alterations in pediatric BD. As summarized in a recent meta-analysis (3), the most consistent neuroanatomical finding in pediatric BD is decreased amygdala volume vs. typically-developing healthy controls (HC), now shown in 7 of 9 cross-sectional studies including our own (4–10), but not in 2 others (11;12). In Dickstein et al. 2005, we used voxel-based morphometry (VBM) to compare gray matter volume in 20 age- and sex-matched pediatric BD vs. HC participants. Our primary analyses showed that BD youths had decreased left dorsolateral prefrontal cortex (DLPFC, Brodmann area 9 [BA9]) volume, and decreased left amygdala and left accumbens volume in secondary analyses using small volume correction within individual a priori regions of interest (ROIs). Other studies have shown decreased superior temporal gyrus (STG) and anterior cingulate cortex (ACC) volume in pediatric BD (13;14).
Functional MRI (fMRI) studies have also demonstrated altered fronto-temporal neural activity in pediatric BD. Thus far, such studies have used a variety of cognitive tasks, including processing of emotionally-valenced pictures and faces (10;15–17). However, such task-dependent changes in neural activation may represent a small fraction (perhaps <5%) of the brain’s total activity (18;19).
To fully understand the neural pathophysiology of pediatric BD, it may be fruitful to examine how the brain allocates the majority of its resources—i.e., task-independent, spontaneous neural activity. This innovative approach evaluates spontaneous fluctuations in the blood-oxygen level dependent (BOLD) fMRI signal recorded without a specific task—i.e., while participants are at rest. The consistent observation of significant temporal correlations between spatially distinct loci has been termed “resting state functional connectivity” (RSFC) (18;19). RSFC analyses have revealed abnormalities in the intrinsic connectivity networks in several psychiatric disorders, but not in pediatric BD (20–22).
We report the first use of this novel approach to test the hypothesis that pediatric BD involves fronto-temporal alterations in spontaneous RSFC. We focused on the three a priori ROIs identified by our prior structural MRI study (left DLPFC, amygdala, and accumbens). We then conducted secondary analyses to elucidate the potential temporal relationships among our fronto-temporal ROIs.
METHODS
Participants
7–17 year olds were enrolled in a study conducted at, and IRB approved by, Bradley Hospital and Brown University. After study explanation, written informed consent/assent were obtained from parents/children. Recruitment included advertisements in physicians’ offices, local newspapers, and support groups’ websites.
BD (N=15) inclusion criteria were: (1) meeting Diagnostic and Statistical Manual 4th Edition Text Revision (DSM-IV-TR) criteria for BD, including at least one episode meeting full DSM-IV-TR criteria for hypomania (≥4 days) or mania (≥7 days) (23); and (2) ongoing psychiatric treatment. Exclusion criteria were BD not otherwise specified, IQ ≤70, autistic or Asperger’s disorder; medical illness that was unstable or could cause psychiatric symptoms; pregnancy; or substance abuse within ≤2 months of participation.
Healthy control (HC; N=15) inclusion criteria were a negative history of psychiatric illness in the control and their first-degree relatives. Exclusion criteria were: IQ ≤70; ongoing medical or neurological illness; pregnancy; or past/present psychiatric or substance disorder.
All participants were evaluated by the same board-certified child/adolescent psychiatrist (DPD) using the Child Schedule for Affective Disorders Present and Lifetime version (K-SADS-PL) administered to parents and children separately (24). Comorbid diagnoses for BD youths were assessed by inquiring about symptoms during a time of relative euthymia to ensure that BD symptoms were not double-counted.
All participants completed the Wechsler Abbreviated Scale of Intelligence (WASI) as an overall measure of cognitive ability. BD participants completed the Young Mania Rating Scale (YMRS) (25), Children’s Depression Rating Scale (CDRS) (26), and Children’s Global Assessment Scale (CGAS) (27).
MRI Data Acquisition
Scans were acquired on a Siemens Trio 3.0 Tesla scanner with a 12-channel head coil. The RSFC scan contained 256 continuous BOLD volumes (TRepetition=2000msec, TEcho=25ms, flip angle=90°, slices=35, field of view=192mm, voxels=3×3×3mm, duration=8.36minutes). During the scan, participants were instructed to rest with their eyes open while the word “relax” was back-projected via LCD projector. A high-resolution T1-weighted MPRAGE anatomical image was also acquired for normalization and localization (TRepetition=2250ms, TEcho=2.98ms, T1=900ms, flip angle=90°, slices=160, field of view=256mm, voxels=1×1×1mm, duration=7.36min).
Image Preprocessing and Nuisance Signal Regression
As described elsewhere (28), data were processed using both AFNI (Analysis of Functional NeuroImages, v.AFNI_2007_05_29_1644) and FSL (FMRIB Software Library, v.4.1.2). AFNI preprocessing included: (1) slice timing correction (for interleaved acquisitions), (2) motion correction (x, y, z, roll, pitch, yaw), and (3) despiking of extreme time series outliers and temporal band-pass filtering (0.005–0.1Hz). FSL preprocessing included: (1) mean-based intensity normalization (scaling) of all volumes by the same factor, (2) spatial smoothing with full-width half-maximum=6mm Gaussian kernel, (3) FSL’s Linear Registration Tool (FLIRT) to register the anatomical scan to the Montreal Neurological Institute (MNI) 152 template with (2mm3 resolution), and (4) FLIRT registration of each participant’s RSFC time series to the same space as the anatomical scan.
To control the effects of physiological processes (e.g., cardiac/respiratory cycle fluctuations) and motion, we regressed each participant’s 4-dimensional (4-D) volume on nine predictors to model nuisance signals from white matter, cerebrospinal fluid, global brain signal, and six motion parameters (28). Correction for time series autocorrelation (pre-whitening) was performed. This nuisance signal regression produced a 4-D residuals volume for each participant. Each voxel’s time series was scaled by its standard deviation to ensure that the resultant RSFC estimates represented partial correlations, rather than regression parameter estimates. Finally, each participant’s 4-D volume was spatially normalized by applying the previously computed transformation to MNI152 template.
Seed Selection
We created spherical seeds (diameter=6mm) based on our work showing decreased gray matter in the left DLPFC (x=−32 y=42 z=32), amygdala (x=−24 y=5 z=−15), and accumbens (x=−6 y=9 z=−7) (9). Talairach coordinates for these ROIs were transformed into MNI space using the icbm2tal conversion (29).
Participant-Level Analyses
For each participant and each seed, we performed a separate multiple regression analysis in which we regressed the participant’s 4-D residuals volume on the seed time series, using FSL’s Expert Analysis Tool (FEAT). These analyses produced individual participant-level maps of all voxels that were positively and negatively correlated with the seed time series.
Primary Group-Level Analyses
Group-level mixed-effects analysis was conducted with FSL’s Local Analysis of Mixed-Effects (FLAME). The model included mean positive and negative vectors for the BD and HC groups separately, BD greater than HC, HC greater than BD, plus de-meaned values for age, full-scale IQ, and sex as nuisance covariates. To correct for multiple comparisons, group-level statistical maps were first thresholded at Z>2.3, and then Gaussian random field theory was used to determine the significance of clusters surviving this threshold, with p<0.05 whole-brain corrected as the criteria for cluster-wise significance.
To further characterize the underlying neurocircuitry, we created 6mm “iterative seeds” centered at the peak of any ROI exhibiting significant between-group differences (whole-brain corrected) in RSFC with our three primary ROIs. We repeated the above participant-level and primary group-level analyses for any iterative seeds and included them in all secondary analyses. Post-hoc analyses were conducted using extracted RSFC data from our primary analyses to determine the potential impact of age, Tanner pubertal stage, medication, global signal correction (GSC), and ROI selection using Statistical Package for Social Sciences (v.17).
Secondary Analyses: Modeling Temporal Relationships
To elucidate the relationships among our three primary seeds and any iterative seeds, we conducted secondary analyses employing two complementary methods: Partial Cross Correlation (PCC) and Multivariate Autoregressive Modeling (MAR).
PCC is a model-free method for studying cross-relationships between neural activation occurring simultaneously across several ROIs. PCC gives a measure of direct linear connectivity between each pair of ROIs to answer the question “Are the spontaneous RSFC in two ROIs simultaneously related at the same time point t?” Thus, PCC is ideal for multiple ROIs that may be interdependent—i.e., PCC is ‘0’ even for a pair of ROIs that are highly cross-correlated if the correlation is being driven by another ROI.
PCC values were Fisher-z transformed to yield normalized variance-stabilized values via Yijs =0.5 log[(1 + Xijs)/(1 − Xijs)] where Xijs is the partial cross-correlation value for subject s, between ROIs i and j. For each of the six pairs of ROIs (HC minus BD), joint 95% confidence intervals (Bonferroni-corrected) were calculated. P-values from the two-independent sample t-test were calculated (30). Diagnostic procedures (Q-Q plots for the normality assumption and a formal test for equality of variance) confirmed that the assumptions (normality and equal variances) required for computing confidence intervals and t-tests were valid.
PCC examines simultaneous relationships, but PCC does not capture dynamic (time lagged) relationships among ROIs. Instead, MAR modeling is a potential means to address the question “Is the BOLD signal in one ROI associated with the past BOLD signal in other regions?”(31). MAR modeling has been used to identify Granger-causality relationships—i.e., RSFC BOLD signal in one ROI predicted that in another. The distinction between physiological causality and Granger-causality has always been important, with the Granger-causality being a mathematical model used to explore sequential relationship between BOLD signal peaks whose inference at the neuronal level remains unknown (32;33). In particular, researchers have begun to appreciate alternative conditions under which significant MAR-based relationships may arise (e.g., regional differences in hemodynamic lag, regional differences in rise to peak for the hemodynamic response) (32). Thus, to balance the need for completeness with the rapidly evolving debate about the interpretations of MAR modeling, we present the full methods, results, and discussion of our MAR analyses in the Supplement.
RESULTS
Participants
The groups did not differ significantly in age, sex, Tanner pubertal stage, or FSIQ. The BD sample consisted of 15 participants with type I BD; none had type II BD, although it was not excluded. As a group, our BD participants were euthymic by mood ratings (YMRS 8.9±5.0, CDRS 33.4±15.4), and they were mildly impaired (CGAS 60.0±20.1; non-clinical >70), though none were acutely symptomatic at the time of the scan. All BD participants were receiving psychopharmacological treatment, including anti-manic medications such as lithium (N=6 [40%]) or atypical neuroleptics (N=13 [87%]). Five BD participants (33%) had at least one first-degree relative with BD.
Primary Analysis: Fronto-Temporal Functional Connectivity
Using our primary left DLPFC seed, we found significantly decreased RSFC between BD vs. control youth in the right superior temporal gyrus (STG) (BA 22, x=54, y=−44, z=8; Voxels=775, pcorrected=0.04). This was due to greater negative RSFC (anti-correlation) in the BD group than controls. For this same left DLPFC seed, we did not identify any regions where BD youth had greater RSFC than controls. We did not identify significant between-group RSFC differences using the left amygdala or left accumbens seeds (Figures 1 and 2).
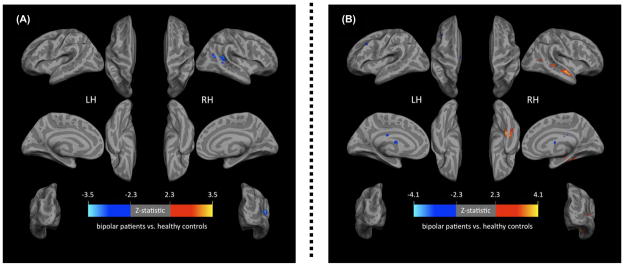
Figure 1. Whole-brain corrected significant between-group differences in resting state functional connectivity (RSFC) in Pediatric Bipolar Disorder (BD, N=15) vs. Typically-Developing Healthy Controls (HC, N=15).
(A) Significantly Decreased RSFC between Left Dorsolateral Prefrontal Cortex (DLPFC) and Right Superior Temporal Gyrus (STG) in Pediatric BD vs. Controls (BA 22, x=54 y=−44 z=8; Voxels=775, pcorrected=0.04).
(B) Significantly Altered RSFC between Right STG and Fronto-Temporal Regions in Pediatric BD vs. Controls.
Note: (B) Significantly decreased (blue) RSFC activity in BD vs. control youths between the right STG and: (1) left middle frontal gyrus (BA9, x=−48 y=36 z=28; Voxels=953, pcorrected=0.009), (2) right superior frontal gyrus (BA9, x=38 y=58 z=22; Voxels=809, pcorrected=0.02), and (3) left thalamus/caudate body (x=−16 y=−12 z=18; Voxels=688, pcorrected=0.05). Significantly increased (orange) RSFC between STG and right parahippocampal gyrus (BA36, x=38 y=−32 z=−20; Voxels=1931, pcorrected=0.00007).
Method: 3 Tesla Siemens Tim Trio BOLD scan (TRepetition=2000msec, TEcho=25ms, flip angle=90°, slices=35, field of view=192mm, voxels=3×3×3mm, duration=8.36minutes) acquired while participant was at rest. To correct for multiple comparisons, group-level statistical maps were first thresholded at Z>2.3, and then Gaussian random field theory was used to determine the significance of clusters surviving this threshold, with p<0.05 whole-brain corrected as the criteria for cluster-wise significance.
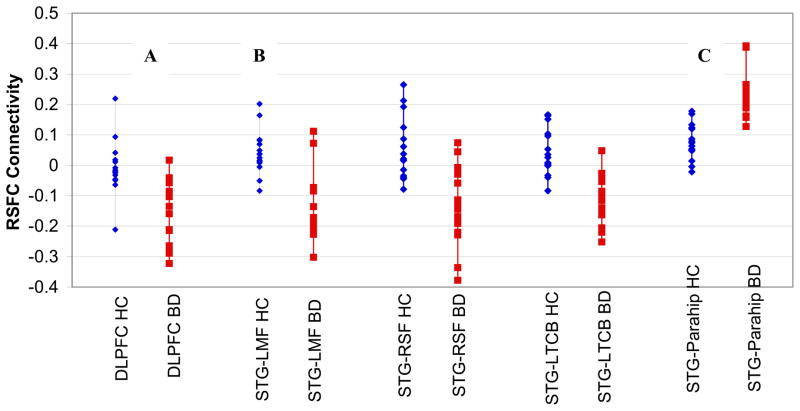
Figure 2. Significantly Decreased Fronto-Temporal Resting State Functional Connectivity (RSFC) in Pediatric Bipolar Disorder (BD, N=15, red) vs. Typically-Developing Healthy Controls (HC, N=15, blue).
(A) Primary analysis showed significantly decreased RSFC activity between the left DLPFC and the right superior temporal gyrus (STG) in BD vs. control youth (BA 22, x=54 y=−44 z=8; Voxels=775, pcorrected=0.04).
(B) Then, using this iterative right STG seed, we found significantly decreased RSFC in BD vs. control youths in the left middle frontal gyrus (BA9, x=−48 y=36 z=28; Voxels=953, pcorrected=0.009), right superior frontal gyrus (BA9, x=38 y=58 z=22; Voxels=809, pcorrected=0.02), and left thalamus/caudate body (x=−16 y=−12 z=18; Voxels=688, pcorrected=0.05), and (C) increased RSFC in BD vs. control youths between the iterative right STG seed and the parahippocampal gyrus (BA36, x=38 y=−32 z=−20; Voxels=1931, pcorrected=0.0007)
Method: 3 Tesla Siemens Tim Trio BOLD scan (TRepetition=2000msec, TEcho=25ms, flip angle=90°, slices=35, field of view=192mm, voxels=3×3×3mm, duration=8.36minutes) acquired while participant was at rest.
Our iterative regression analysis used the right STG identified in our primary analysis as a seed. We found significantly decreased RSFC in BD vs. controls in the left middle frontal gyrus (BA9, x=−48, y=36, z=28; Voxels=953, pcorrected=0.009), right superior frontal gyrus (BA9, x=38, y=58, z=22; Voxels=809, pcorrected=0.02), and left thalamus/caudate body (x=−16, y=−12, z=18; Voxels=688, pcorrected=0.05). We also found significantly greater RSFC in BD vs. controls between the right STG and the right parahippocampal gyrus (BA36, x=38, y=−32, z=−20; Voxels=1931, pcorrected=0.00007).
Within the BD group, there were no significant correlations between RSFC in the right STG or the four regions identified by our iterative analysis and mood (YMRS, CDRS) or overall functioning (CGAS).
Secondary Analyses: Modeling Temporal Relationships
PCC analyses tested the pair-wise relationship in RSFC, showing a significant between-group difference for the DLPFC-STG (Figure 3). Specifically, whereas the PCC was positive for controls (mean=0.084±0.151), the PCC was negative for BD youths (mean= −0.148±0.161; p<0.001). In other words, among controls, increased spontaneous BOLD signal in the left DLPFC was associated with simultaneous increases in the right STG and vice versa (positive correlation), whereas the opposite was true for BD youths—i.e., increased spontaneous activity in the left DLPFC was associated with simultaneous decreases in the right STG (anti-correlation). Among BD youths, there were no significant correlations between the DLPFC-STG PCC measures and measures of mood (YMRS, CDRS) or function (CGAS).
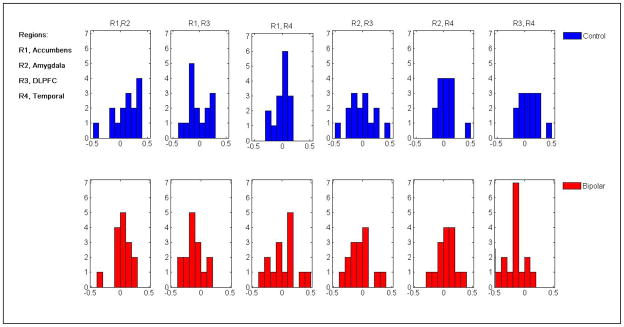
Figure 3.
Partial Cross Correlation (PCC) Analysis of Resting State Functional Connectivity (RSFC) in Fronto-Temporal Regions of Interest between Pediatric Bipolar Disorder (BD) (Bottom, Red) and Typically-Developing Healthy Controls (HC) (Top, Blue)
Note: Significant PCC between Left Dorsolateral Prefrontal Cortex (R3, DLPFC) and Right Superior Temporal Gyrus (R4, STG) indicating these regions are correlated in controls (blue, top)—i.e., increases in DLPFC lead to increases in STG—whereas they are anti-correlated in pediatric BD (red, bottom)—i.e., increases in DLPFC lead to decreases in STG (p<0.001).
Significant group-differences were detected using MAR. See Supplement for presentation of findings.
Post-hoc Analyses
Post-hoc analyses examined the potential impact of development, medication, global signal correction (GSC), and ROI selection on the results of our primary analysis (see Supplement). We did not find medication effects, but there was a suggestion of developmental differences in correlations between right STG-right parahippocampal RSFC and age that were in opposite directions in the BD vs. HC groups (BD Pearson 0.51 p=0.05, HC Pearson= −0.58 p=0.02). Re-analyzing our data without GSC incorporated as a nuisance variable showed that GSC successfully reduced inter-subject variability without disproportionately affecting one group. Re-analyzing our data with anatomical ROIs from the Harvard-Oxford Brain Atlas confirmed that our primary analyses’ failure to find significant differences in the left amygdala or left accumbens was not due to our use of coordinate-based seeds.
DISCUSSION
Our examination of RSFC in youths with pediatric BD has three main findings. First, our primary analysis revealed a significant between-group difference in RSFC between the left DLPFC and right STG. In particular, pediatric BD participants had significantly greater negative RSFC between the left DLPFC and the right STG vs. controls. Subsequent iterative analyses showed that pediatric BD participants had significantly decreased RSFC between the right STG and bilateral PFC (BA 9), left thalamus/caudate, and increased RSFC between the right STG and the right parahippocampal gyrus. Second, PCC analysis showed that, when relationships with the other ROIs were taken into account, BD and control youths had opposite phase relationships between spontaneous BOLD fluctuations in the left DLPFC and right STG—i.e., whereas RSFC was in-phase for controls, it was 180 degrees out of phase (anti-correlated) for BD youths. Third, MAR analyses identified significantly different relationships among patterns of spontaneous fluctuation between BD and control youth in our circuits of interest, but caution is urged in interpreting these MAR analyses given potential contributions of physiological variables, such as regional differences in the hemodynamic response, to the relationships observed (32).
The results from our primary analyses, showing that BD youths have altered RSFC between the DLPFC and STG, and iteratively, between the STG and frontal, striatal, and parahippocampal areas, align with prior studies implicating working memory in BD (34;35). For example, BD youths have impaired working memory vs. controls (36–38). Working memory deficits in BD youths have been associated with increased PFC and temporal fMRI activation (39). Similar fronto-temporal alterations have been shown in BD youths using emotionally-valenced picture and face tasks (10;15–17).
Our results align with the small connectivity literature in BD. The only task-independent RSFC study in BD involved BD adults (n=11), showing decreased RSFC between the pregenual ACC and bilateral amygdala and thalamus (40). Using task-dependent data from a face processing paradigm, BD youths have decreased functional connectivity between the left amygdala and the right posterior cingulate/precuneus and fusiform/parahippocampal gyri (41). Therefore, our work is an important first step towards understanding how some of this fronto-temporal dysfunction may be intrinsic to pediatric BD, rather than contingent upon a particular cognitive task or process.
Our data also suggests the importance of development in the pathophysiology of pediatric BD. Specifically, top-down control of cognitive processes, including declarative memory, requires interplay between the frontal cortex, especially DLPFC, and the striatum and temporal cortex (42–44). Longitudinal neuroimaging studies have shown that typical development involves the progressive maturation of phylogenetically older brain areas, like the temporal cortex and striatum, prior to newer ones, like the DLPFC (45). Some posit that typical adolescent characteristics, including identity formation and risk-taking, depend on the dynamic balance between the earlier maturing striatum and amygdala responsible for bottom-up reward processing, and the later maturing PFC responsible for top-down cognitive control (46).
Three studies have robustly demonstrated developmental maturation effects on RSFC, showing that healthy adults have stronger, more focused within-network RSFC vs. children or adolescents (47–49). Our data show that BD and control participants have the opposite correlation between age and RSFC between the right STG and parahippocampal gyrus. Given power issues, such post-hoc comparisons should be interpreted with caution. Future longitudinal neuroimaging studies will be required to ascertain the developmental trajectories of spontaneous neural activity in pediatric BD, with sufficient power to address issues inherent in BD research, including potential neuromodulatory effects of medications and comorbidity.
Regarding the functional significance of negative (anti-phase, anti-correlated) DLPFC/STG RSFC in BD participants, and positive RSFC in controls, we note the continuing controversy in the RSFC literature surrounding negative connectivity (50;51). Authors agree that negative correlations can and do exist in the brain (51;52), but recent work has suggested that procedures—e.g., GSC—may exaggerate them by shifting “zero” correlations into negative ones (53). However, re-processing and re-analyzing our data without GSC confirmed that incorporating GSC reduced inter-subject variability, thus enhancing our ability to detect between-group differences, rather than either altering negative correlations or disproportionately affecting one group vs. another. This aligns with both mathematical and empirical investigations of GSC’s effect on RSFC, showing that GSC resulted in improved ability to detect system-specific correlations in RSFC and improved the correspondence between RSFC and anatomy (51;54). We conclude that our data indicate that pediatric BD involves a greater degree of segregation between the DLPFC and STG than was observed in controls.
Three issues related to our ROI approach bear further comment. First, both structural (55–57) and functional (58) ROIs have been used to guide RSFC analyses. In our current study, we chose to follow up on structural ROIs identified by our prior work, but functional ROIs would be equally important for future studies. Second, our failure to fully replicate our prior VBM results does not diminish the relevance of our ROIs because they have been consistently implicated by other pediatric BD neuroimaging studies (17;59;60). Moreover, we note several important methodological differences between our original study and our current post-hoc VBM analyses, including: (1) greater power to detect gray matter volume differences in our original study (20 vs. 15 participants per group), (2) lack of gender matching as in our original study, (3) different MRI manufacturers and field strengths (original=GE 1.5 Tesla vs. current=Siemens 3 Tesla), (4) different structural scan sequences (original=SPGR [TRepetition=24ms, TEcho=5.0ms, slices=124]; current=MPRAGE [TRepetition=2250ms, TEcho=2.98ms, slices=160]), (5) different statistical software (SPM vs. FSL). Third, while our post-hoc analyses using an anatomic left amygdala ROI confirmed our lack of amygdala findings from our primary analysis, larger samples are necessary to evaluate RSFC alterations in other ROIs among BD youths.
Our study has several additional limitations, including psychotropic medications, comorbidity, and sample size. First, our post-hoc analyses suggest that medications do not confound our current results. However, all BD participants were taking their usual psychotropic medications with the exception of psychostimulants, which were withheld for a minimum of five drug half-lives. The rationale was that psychostimulants are commonly held for drug holidays—e.g., school vacations—in clinical care, and they affect the BOLD signal (61;62), whereas it would be unethical to withhold anti-manic, anti-depressant, or anti–anxiety medications for research purposes alone, and brief discontinuations would be ineffective given their longer half-lives. Recent BD research suggests that such medications may not influence BOLD signal in fMRI studies such as ours (63). Nevertheless, future work, perhaps with non-human primates is warranted to evaluate the effect of pharmacologic treatment on fronto-temporal RSFC during development.
Secondly, our BD participants had rates of comorbid psychopathology corresponding to other pediatric and adult BD studies. Yet, future studies are needed to deterimine the specificity of these RSFC alterations, including comparisons to those with primary ADHD or anxiety (20). Third, while our BD and control samples are on par with the current literature in pediatric BD, we note the need for even larger studies with sufficient power to meaningfully explore the potential effects of age, sex, puberty, and treatment.
CONCLUSION
Our study is the first to evaluate spontaneous RSFC activity in pediatric BD. Our data indicate that pediatric BD is characterized by altered task-independent RSFC in fronto-temporal regions also implicated in working memory and learning. Further study is warranted to determine the effects of age, sex, development, and treatment on this circuit in pediatric BD.
Supplementary Material
Table 1.
Participant Demographics in Pediatric Bipolar Disorder (BD, N=15) Versus Typically-Developing Healthy Controls (HC, N=15)
| Group: | BD | HC | Stats | |
|---|---|---|---|---|
| Age | 13.7 ± 3.3 | 14.0 ± 3.1 | p= 0.84, t=−0.21 | |
| Tanner pubertal stage | Genitals | 2.9 ± 1.6 | 3.5+1.7 | p=0.3, t=−1.0 |
| Pubic hair | 3.1 ± 1.6 | 3.7+1.7 | p=−0.7, t=−1.1 | |
| Verbal IQ (VIQ) | 113.0 ± 10.0 | 118.6 ± 10.6 | p=0.15, t=−1.49 | |
| Performance IQ (PIQ) | 104.1 ± 10.7 | 106.5 ± 11.9 | p=0.57, t=−0.58 | |
| Full-Scale IQ (FSIQ) | 109.6 ± 9.0 | 114.0 ± 8.8 | p= 0.19, t=−1.35 | |
| Sex | Male | 10 | 7 | Pearson Χ2=0.56 p= 0.46 |
| Female | 5 | 8 | ||
| Ethnicity: | Non-Hispanic/Latino | 14 (93.3%) | 14 (93.3%) | Pearson Χ2=0.00 p= 1.0 |
| Not Reporting | 1 (6.7%) | 1 (6.7%) | ||
| Race: | Black | 2 (13.3%) | 1 (6.7%) | Pearson Χ2=1.7 p= 0.64 |
| White | 11 (77.3%) | 12 (80%) | ||
| >1 Race | 2 (13.3%) | 1 (6.7%) | ||
| Young Mania Rating Scale Score | 8.9 ± 5.0 | |||
| Children’s Depression Rating Scale Score | 33.4 ± 15.4 | |||
| Children’s Global Assessment Scale Score | 60.0 ± 20.1 | |||
| Current Comorbid KSADS Diagnoses | ||||
| Attention Deficit/Hyperactivity Disorder | 11 (73.3%) | |||
| Oppositional Defiant Disorder | 13 (86.7%) | |||
| Obsessive-Compulsive Disorder | 1 (6.7%) | |||
| Generalized Anxiety Disorder | 3 (20.0%) | |||
| Specific Phobia | 3 (20.0%) | |||
| Separation Anxiety | 2 (13.3%) | |||
| Social Phobia | 2 (13.3%) | |||
| Psychosis | 1 (6.7%) | |||
| Transient Tic Disorder | 1 (6.7%) | |||
| Medications: Number and Percent of BD participants | ||||
| Lithium | 9 (60%) | |||
| Atypical Neuroleptics | 13 (86.7%) | |||
| Antidepressants | 1 (6.7%) | |||
| Stimulant Medications * | 6 (40%) | |||
| Benzodiazepines | 1 (6.7%) | |||
N=6/15 BD participants were taking stimulant medications for ADHD (N=4 on methylphenidate, N=2 on dextroamphetamine). However, to minimize their impact on magnetic resonance imaging (MRI) data, all 6 held these medications for a minimum of 5 drug half-lives prior to their MRI scan in consultation with their treating physician.
Table 2.
Significant Between-Group Differences in Resting State Functional Connectivity (RSFC) in Pediatric Bipolar Disorder Participants (BD, N=15) vs. Typically-Developing Healthy Controls (HC, N=15)
| Seed | Between-Group Difference | Region | Cluster Size | X | Y | Z | Pcorrected |
|---|---|---|---|---|---|---|---|
| Left DLPFC | HC>BD | Right Superior Temporal Gyrus (STG; BA22) | 775 | 54 | −44 | 8 | 0.04 |
| Left Amygdala | --- | ||||||
| Left Accumbens Area | --- | ||||||
| Iterative Right STG seed from primary analysis | HC>BD | Left Middle Frontal Gyrus (BA9) | 953 | −48 | 36 | 28 | 0.009 |
| HC>BD | Right Superior Frontal Gyrus (BA9) | 809 | 38 | 58 | 22 | 0.02 | |
| HC>BD | Left Thalamus and Caudate Body | 688 | −16 | −12 | 18 | 0.05 | |
| BD>HC | Right Parahippocampal Gyrus(BA36) | 1931 | 38 | −32 | −20 | 0.00007 |
Note: To correct for multiple comparisons, group-level statistical maps were first thresholded at Z>2.3, and then Gaussian random field theory was used to determine the significance of clusters surviving this threshold, with p<0.05 whole-brain corrected as the criteria for cluster-wise significance.
Acknowledgments
This project was supported in part by departmental funds from E.P. Bradley Hospital, the National Institute of Mental Health (K22MH074945 and R01MH087513, PI DP Dickstein), a NARSAD Young Investigator Award (PI DP Dickstein). We are truly grateful for the time and effort of all participants and their families.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Blader JC, Carlson GA. Increased Rates of Bipolar Disorder Diagnoses Among U.S. Child, Adolescent, and Adult Inpatients, 1996–2004. Biol Psychiatry. 2007;62:107–114. doi: 10.1016/j.biopsych.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64:1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- 3.Pfeifer JC, Welge J, Strakowski SM, Adler CM, DelBello MP. Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:1289–1298. doi: 10.1097/CHI.0b013e318185d299. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 5.DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen BK, Sassi R, Axelson D, Hatch JP, Sanches M, Nicoletti M, et al. Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biol Psychiatry. 2004;56:399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg HP, Fredericks CA, Wang F, Kalmar JH, Spencer L, Papademetris X, et al. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disorders. 2005;7:570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 9.Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney DS, Pine DS, et al. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005;62:734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 10.Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, et al. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frazier JA, Hodge SM, Breeze JL, Giuliano AJ, Terry JE, Moore CM, et al. Diagnostic and sex effects on limbic volumes in early-onset bipolar disorder and schizophrenia. Schizophr Bull. 2008;34:37–46. doi: 10.1093/schbul/sbm120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Larson M, Michael ES, Terry JE, Breeze JL, Hodge SM, Tang L, et al. Subcortical differences among youths with attention-deficit/hyperactivity disorder compared to those with bipolar disorder with and without attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:31–39. doi: 10.1089/cap.2008.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen HH, Nicoletti MA, Hatch JP, Sassi RB, Axelson D, Brambilla P, et al. Abnormal left superior temporal gyrus volumes in children and adolescents with bipolar disorder: a magnetic resonance imaging study. Neurosci Lett. 2004;363:65–68. doi: 10.1016/j.neulet.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 14.Kaur S, Sassi RB, Axelson D, Nicoletti M, Brambilla P, Monkul ES, et al. Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am J Psychiatry. 2005;162:1637–1643. doi: 10.1176/appi.ajp.162.9.1637. [DOI] [PubMed] [Google Scholar]
- 15.Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 16.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 19.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 20.Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fornito A, Bullmore ET. What can spontaneous fluctuations of the blood oxygenation-level-dependent signal tell us about psychiatric disorders? Curr Opin Psychiatry. 2010 doi: 10.1097/YCO.0b013e328337d78d. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 4th Edition Text Revision (DSM-IV-TR) Washington, D.C.: American Psychiatric Association; 2000. [Google Scholar]
- 24.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 26.Poznanski E, Freeman LNMH. Children’s Depression Rating Scale-Revised. Psychopharmacol Bull. 1985;21(4):979–984. [Google Scholar]
- 27.Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A children’s global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 28.Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, et al. L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci. 2009;29:7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark E, Drori R, Abeles M. Partial cross-correlation analysis resolves ambiguity in the encoding of multiple movement features. J Neurophysiol. 2006;95:1966–1975. doi: 10.1152/jn.00981.2005. [DOI] [PubMed] [Google Scholar]
- 31.Goebel R, Roebroeck A, Kim DS, Formisano E. Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magn Reson Imaging. 2003;21:1251–1261. doi: 10.1016/j.mri.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roosendaal SD, Schoonheim MM, Hulst HE, Sanz-Arigita EJ, Smith SM, Geurts JJ, et al. Resting state networks change in clinically isolated syndrome. Brain. 2010;133:1612–1621. doi: 10.1093/brain/awq058. [DOI] [PubMed] [Google Scholar]
- 34.Blumberg HP, Charney DS, Krystal JH. Frontotemporal neural systems in bipolar disorder. Semin Clin Neuropsychiatry. 2002;7:243–254. doi: 10.1053/scnp.2002.35220. [DOI] [PubMed] [Google Scholar]
- 35.DelBello MP, Adler CM, Strakowski SM. The neurophysiology of childhood and adolescent bipolar disorder. CNS Spectr. 2006;11:298–311. doi: 10.1017/s1092852900020794. [DOI] [PubMed] [Google Scholar]
- 36.Glahn DC, Bearden CE, Caetano S, Fonseca M, Najt P, Hunter K, et al. Declarative memory impairment in pediatric bipolar disorder. Bipolar Disord. 2005;7:546–554. doi: 10.1111/j.1399-5618.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 37.Pavuluri MN, Schenkel LS, Aryal S, Harral EM, Hill SK, Herbener ES, et al. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. Am J Psychiatry. 2006;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- 38.Bearden CE, Glahn DC, Caetano S, Olvera RL, Fonseca M, Najt P, et al. Evidence for disruption in prefrontal cortical functions in juvenile bipolar disorder. Bipolar Disord. 2007;9(Suppl 1):145–159. doi: 10.1111/j.1399-5618.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- 39.Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6:540–549. doi: 10.1111/j.1399-5618.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- 40.Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. 2009;171:189–198. doi: 10.1016/j.pscychresns.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rich BA, Fromm SJ, Berghorst LH, Dickstein DP, Brotman MA, Pine DS, et al. Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. J Child Psychol Psychiatry. 2008;49:88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.den Ouden HE, Frith U, Frith C, Blakemore SJ. Thinking about intentions. Neuroimage. 2005;28:787–796. doi: 10.1016/j.neuroimage.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirwan CB, Wixted JT, Squire LR. Activity in the medial temporal lobe predicts memory strength, whereas activity in the prefrontal cortex predicts recollection. J Neurosci. 2008;28:10541–10548. doi: 10.1523/JNEUROSCI.3456-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- 49.Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Popa D, Popescu AT, Pare D. Contrasting activity profile of two distributed cortical networks as a function of attentional demands. J Neurosci. 2009;29:1191–1201. doi: 10.1523/JNEUROSCI.4867-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Stark DE, Margulies DS, Shehzad ZE, Reiss P, Kelly AM, Uddin LQ, et al. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci. 2008;28:13754–13764. doi: 10.1523/JNEUROSCI.4544-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci U S A. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- 60.Nelson EE, Vinton DT, Berghorst L, Towbin KE, Hommer RE, Dickstein DP, et al. Brain systems underlying response flexibility in healthy and bipolar adolescents: an event-related fMRI study. Bipolar Disord. 2007;9:810–819. doi: 10.1111/j.1399-5618.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 61.Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci U S A. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teicher MH, Anderson CM, Polcari A, Glod CA, Maas LC, Renshaw PF. Functional deficits in basal ganglia of children with attention-deficit/hyperactivity disorder shown with functional magnetic resonance imaging relaxometry. Nat Med. 2000;6:470–473. doi: 10.1038/74737. [DOI] [PubMed] [Google Scholar]
- 63.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.