Abstract
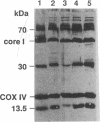
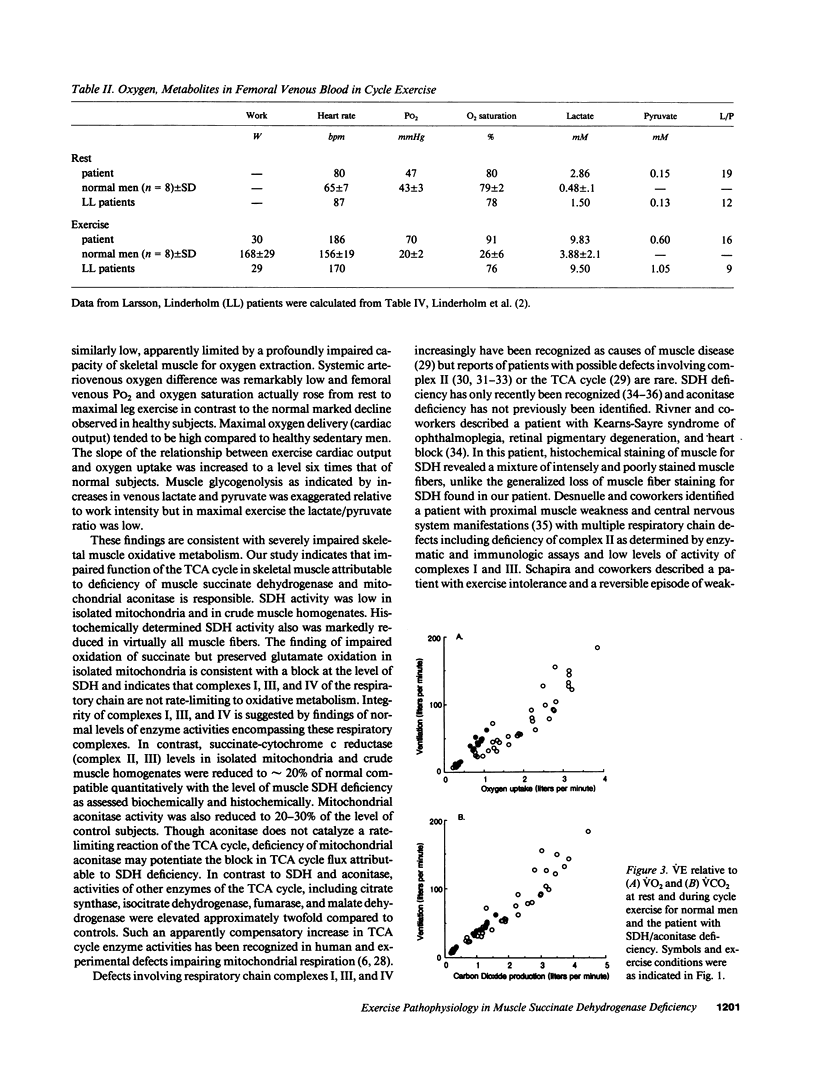
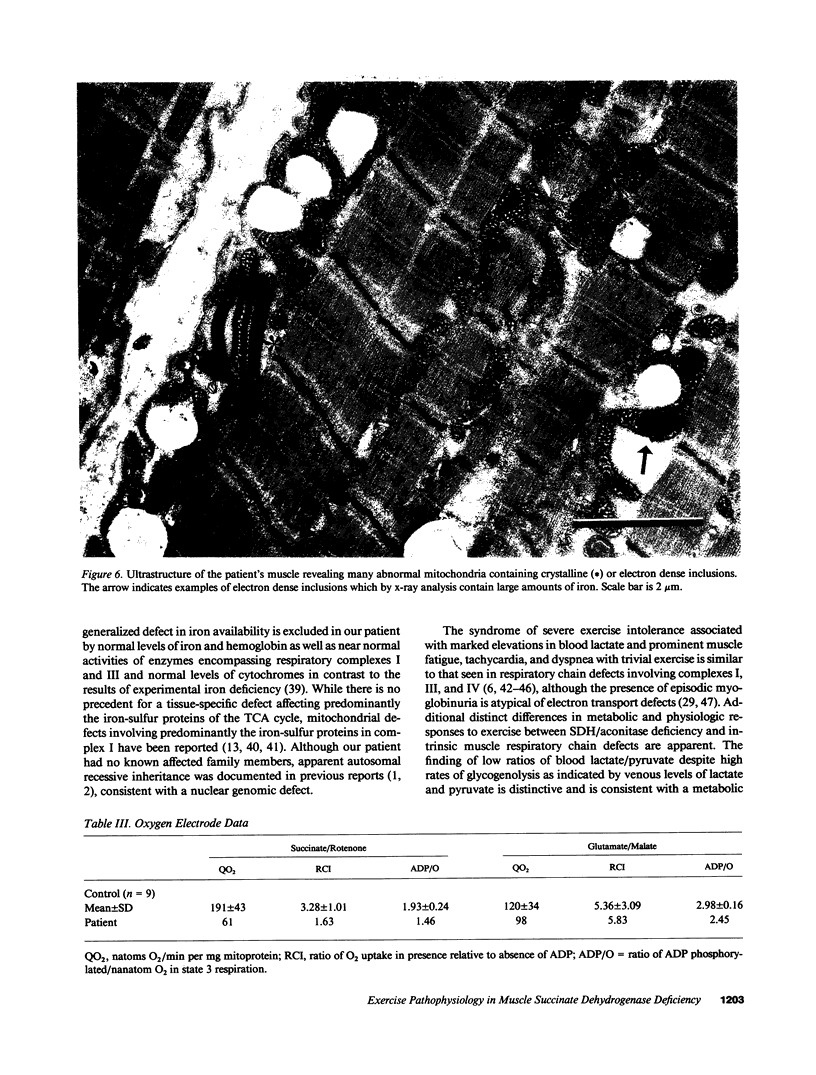
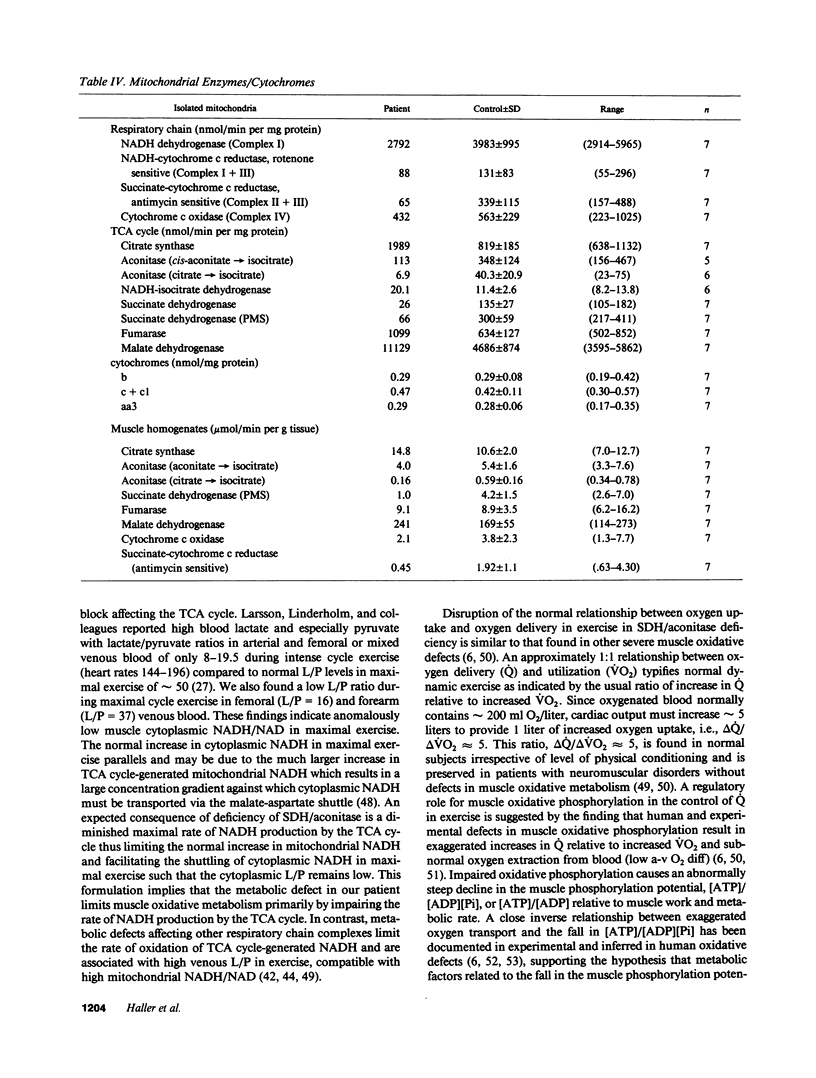
We evaluated a 22-yr-old Swedish man with lifelong exercise intolerance marked by premature exertional muscle fatigue, dyspnea, and cardiac palpitations with superimposed episodes lasting days to weeks of increased muscle fatigability and weakness associated with painful muscle swelling and pigmenturia. Cycle exercise testing revealed low maximal oxygen uptake (12 ml/min per kg; healthy sedentary men = 39 +/- 5) with exaggerated increases in venous lactate and pyruvate in relation to oxygen uptake (VO2) but low lactate/pyruvate ratios in maximal exercise. The severe oxidative limitation was characterized by impaired muscle oxygen extraction indicated by subnormal systemic arteriovenous oxygen difference (a-v O2 diff) in maximal exercise (patient = 4.0 ml/dl, normal men = 16.7 +/- 2.1) despite normal oxygen carrying capacity and Hgb-O2 P50. In contrast maximal oxygen delivery (cardiac output, Q) was high compared to sedentary healthy men (Qmax, patient = 303 ml/min per kg, normal men 238 +/- 36) and the slope of increase in Q relative to VO2 (i.e., delta Q/delta VO2) from rest to exercise was exaggerated (delta Q/delta VO2, patient = 29, normal men = 4.7 +/- 0.6) indicating uncoupling of the normal approximately 1:1 relationship between oxygen delivery and utilization in dynamic exercise. Studies of isolated skeletal muscle mitochondria in our patient revealed markedly impaired succinate oxidation with normal glutamate oxidation implying a metabolic defect at the level of complex II of the mitochondrial respiratory chain. A defect in Complex II in skeletal muscle was confirmed by the finding of deficiency of succinate dehydrogenase as determined histochemically and biochemically. Immunoblot analysis showed low amounts of the 30-kD (iron-sulfur) and 13.5-kD proteins with near normal levels of the 70-kD protein of complex II. Deficiency of succinate dehydrogenase was associated with decreased levels of mitochondrial aconitase assessed enzymatically and immunologically whereas activities of other tricarboxylic acid cycle enzymes were increased compared to normal subjects. The exercise findings are consistent with the hypothesis that this defect impairs muscle oxidative metabolism by limiting the rate of NADH production by the tricarboxylic acid cycle.
Full text
PDF

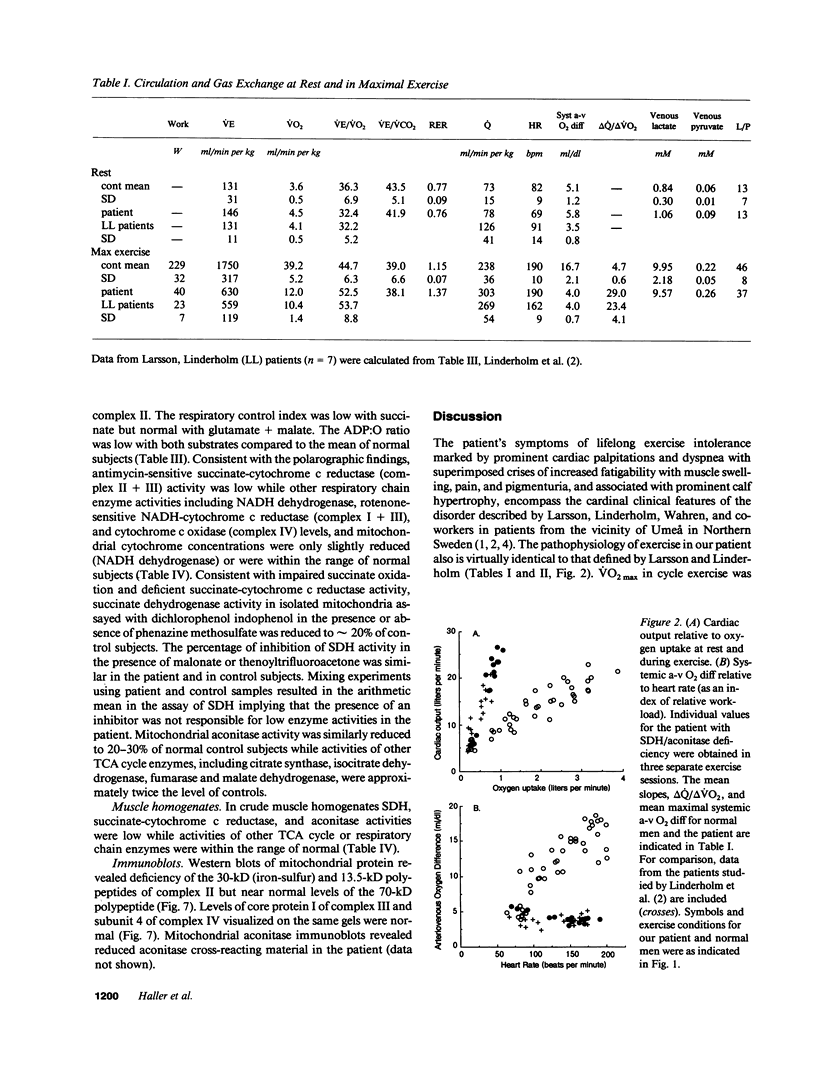



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behbehani A. W., Goebel H., Osse G., Gabriel M., Langenbeck U., Berden J., Berger R., Schutgens R. B. Mitochondrial myopathy with lactic acidosis and deficient activity of muscle succinate cytochrome-c-oxidoreductase. Eur J Pediatr. 1984 Nov;143(1):67–71. doi: 10.1007/BF00442753. [DOI] [PubMed] [Google Scholar]
- Beinert H. Recent developments in the field of iron-sulfur proteins. FASEB J. 1990 May;4(8):2483–2491. doi: 10.1096/fasebj.4.8.2185975. [DOI] [PubMed] [Google Scholar]
- Bookelman H., Trijbels J. M., Sengers R. C., Janssen A. J. Measurement of cytochromes in human skeletal muscle mitochondria, isolated from fresh and frozen stored muscle specimens. Biochem Med. 1978 Jun;19(3):366–373. doi: 10.1016/0006-2944(78)90037-6. [DOI] [PubMed] [Google Scholar]
- Dahlström U., Atterhög J. H., Jorfeldt L. Hemodynamics and leg muscle metabolism at rest and during exercise in young healthy men after prenalterol. Clin Pharmacol Ther. 1983 Jun;33(6):701–709. doi: 10.1038/clpt.1983.96. [DOI] [PubMed] [Google Scholar]
- Desnuelle C., Birch-Machin M., Pellissier J. F., Bindoff L. A., Ackrell B. A., Turnbull D. M. Multiple defects of the respiratory chain including complex II in a family with myopathy and encephalopathy. Biochem Biophys Res Commun. 1989 Sep 15;163(2):695–700. doi: 10.1016/0006-291x(89)92279-1. [DOI] [PubMed] [Google Scholar]
- Elliot D. L., Buist N. R., Goldberg L., Kennaway N. G., Powell B. R., Kuehl K. S. Metabolic myopathies: evaluation by graded exercise testing. Medicine (Baltimore) 1989 May;68(3):163–172. [PubMed] [Google Scholar]
- Fischer J. C., Ruitenbeek W., Berden J. A., Trijbels J. M., Veerkamp J. H., Stadhouders A. M., Sengers R. C., Janssen A. J. Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta. 1985 Nov 29;153(1):23–36. doi: 10.1016/0009-8981(85)90135-4. [DOI] [PubMed] [Google Scholar]
- Haller R. G., Lewis S. F., Cook J. D., Blomqvist C. G. Hyperkinetic circulation during exercise in neuromuscular disease. Neurology. 1983 Oct;33(10):1283–1287. doi: 10.1212/wnl.33.10.1283. [DOI] [PubMed] [Google Scholar]
- Haller R. G., Lewis S. F., Estabrook R. W., DiMauro S., Servidei S., Foster D. W. Exercise intolerance, lactic acidosis, and abnormal cardiopulmonary regulation in exercise associated with adult skeletal muscle cytochrome c oxidase deficiency. J Clin Invest. 1989 Jul;84(1):155–161. doi: 10.1172/JCI114135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- Hayes D. J., Lecky B. R., Landon D. N., Morgan-Hughes J. A., Clark J. B. A new mitochondrial myopathy. Biochemical studies revealing a deficiency in the cytochrome b-c1 complex (complex III) of the respiratory chain. Brain. 1984 Dec;107(Pt 4):1165–1177. doi: 10.1093/brain/107.4.1165. [DOI] [PubMed] [Google Scholar]
- Ichiki T., Tanaka M., Kobayashi M., Sugiyama N., Suzuki H., Nishikimi M., Ohnishi T., Nonaka I., Wada Y., Ozawa T. Disproportionate deficiency of iron-sulfur clusters and subunits of complex I in mitochondrial encephalomyopathy. Pediatr Res. 1989 Feb;25(2):194–201. doi: 10.1203/00006450-198902000-00023. [DOI] [PubMed] [Google Scholar]
- Katz A., Sahlin K. Regulation of lactic acid production during exercise. J Appl Physiol (1985) 1988 Aug;65(2):509–518. doi: 10.1152/jappl.1988.65.2.509. [DOI] [PubMed] [Google Scholar]
- Kennaway N. G., Buist N. R., Darley-Usmar V. M., Papadimitriou A., Dimauro S., Kelley R. I., Capaldi R. A., Blank N. K., D'Agostino A. Lactic acidosis and mitochondrial myopathy associated with deficiency of several components of complex III of the respiratory chain. Pediatr Res. 1984 Oct;18(10):991–999. doi: 10.1203/00006450-198410000-00017. [DOI] [PubMed] [Google Scholar]
- LARSSON L. E., LINDERHOLM H., MUELLER R., RINGQVIST T., SOERNAES R. HEREDITARY METABOLIC MYOPATHY WITH PAROXYSMAL MYOGLOBINURIA DUE TO ABNORMAL GLYCOLYSIS. J Neurol Neurosurg Psychiatry. 1964 Oct;27:361–380. doi: 10.1136/jnnp.27.5.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Land J. M., Morgan-Hughes J. A., Clark J. B. Mitochondrial myopathy. Biochemical studies revealing a deficiency of NADH--cytochrome b reductase activity. J Neurol Sci. 1981 Apr;50(1):1–13. doi: 10.1016/0022-510x(81)90038-1. [DOI] [PubMed] [Google Scholar]
- Lee C. P., Martens M. E., Jankulovska L., Neymark M. A. Defective oxidative metabolism of myodystrophic skeletal muscle mitochondria. Muscle Nerve. 1979 Sep-Oct;2(5):340–348. doi: 10.1002/mus.880020504. [DOI] [PubMed] [Google Scholar]
- Lewis S. F., Haller R. G. Skeletal muscle disorders and associated factors that limit exercise performance. Exerc Sport Sci Rev. 1989;17:67–113. [PubMed] [Google Scholar]
- Lewis S. F., Haller R. G. The pathophysiology of McArdle's disease: clues to regulation in exercise and fatigue. J Appl Physiol (1985) 1986 Aug;61(2):391–401. doi: 10.1152/jappl.1986.61.2.391. [DOI] [PubMed] [Google Scholar]
- Liang C., Huckabee W. E. Mechanisms regulating the cardiac output response to cyanide infusion, a model of hypoxia. J Clin Invest. 1973 Dec;52(12):3115–3128. doi: 10.1172/JCI107511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderholm H., Essén-Gustavsson B., Thornell L. E. Low succinate dehydrogenase (SDH) activity in a patient with a hereditary myopathy with paroxysmal myoglobinuria. J Intern Med. 1990 Jul;228(1):43–52. doi: 10.1111/j.1365-2796.1990.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Linderholm H., Müller R., Ringqvist T., Sörnäs R. Hereditary abnormal muscle metabolism with hyperkinetic circulation during exercise. Acta Med Scand. 1969 Mar;185(3):153–166. doi: 10.1111/j.0954-6820.1969.tb07314.x. [DOI] [PubMed] [Google Scholar]
- MORRISON J. F. The activation of aconitase by ferrous ions and reducing agents. Biochem J. 1954 Dec;58(4):685–692. doi: 10.1042/bj0580685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J. J., Davies K. J., Dallman P. R., Packer L. Effects of dietary iron deficiency of iron-sulfur proteins and bioenergetic functions of skeletal muscle mitochondria. Biochim Biophys Acta. 1982 Feb 17;679(2):210–220. doi: 10.1016/0005-2728(82)90292-4. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Lee C. P. Biochemical studies of skeletal muscle mitochondria. I. Microanalysis of cytochrome content, oxidative and phosphorylative activities of mammalian skeletal muscle mitochondria. Arch Biochem Biophys. 1968 Jul;126(1):75–82. doi: 10.1016/0003-9861(68)90561-4. [DOI] [PubMed] [Google Scholar]
- Moreadith R. W., Batshaw M. L., Ohnishi T., Kerr D., Knox B., Jackson D., Hruban R., Olson J., Reynafarje B., Lehninger A. L. Deficiency of the iron-sulfur clusters of mitochondrial reduced nicotinamide-adenine dinucleotide-ubiquinone oxidoreductase (complex I) in an infant with congenital lactic acidosis. J Clin Invest. 1984 Sep;74(3):685–697. doi: 10.1172/JCI111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreadith R. W., Cleeter M. W., Ragan C. I., Batshaw M. L., Lehninger A. L. Congenital deficiency of two polypeptide subunits of the iron-protein fragment of mitochondrial complex I. J Clin Invest. 1987 Feb;79(2):463–467. doi: 10.1172/JCI112834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Hughes J. A., Darveniza P., Kahn S. N., Landon D. N., Sherratt R. M., Land J. M., Clark J. B. A mitochondrial myopathy characterized by a deficiency in reducible cytochrome b. Brain. 1977 Dec;100(4):617–640. doi: 10.1093/brain/100.4.617. [DOI] [PubMed] [Google Scholar]
- Nuutinen E. M., Nishiki K., Erecińska M., Wilson D. F. Role of mitochondrial oxidative phosphorylation in regulation of coronary blood flow. Am J Physiol. 1982 Aug;243(2):H159–H169. doi: 10.1152/ajpheart.1982.243.2.H159. [DOI] [PubMed] [Google Scholar]
- Petty R. K., Harding A. E., Morgan-Hughes J. A. The clinical features of mitochondrial myopathy. Brain. 1986 Oct;109(Pt 5):915–938. doi: 10.1093/brain/109.5.915. [DOI] [PubMed] [Google Scholar]
- Plaut G. W., Beach R. L., Aogaichi T. Substrate activity of structural analogs of isocitrate for isocitrate dehydrogenases from bovine heart. Biochemistry. 1975 Jun 17;14(12):2581–2588. doi: 10.1021/bi00683a004. [DOI] [PubMed] [Google Scholar]
- Riggs J. E., Schochet S. S., Jr, Fakadej A. V., Papadimitriou A., DiMauro S., Crosby T. W., Gutmann L., Moxley R. T. Mitochondrial encephalomyopathy with decreased succinate-cytochrome c reductase activity. Neurology. 1984 Jan;34(1):48–53. doi: 10.1212/wnl.34.1.48. [DOI] [PubMed] [Google Scholar]
- Rivner M. H., Shamsnia M., Swift T. R., Trefz J., Roesel R. A., Carter A. L., Yanamura W., Hommes F. A. Kearns-Sayre syndrome and complex II deficiency. Neurology. 1989 May;39(5):693–696. doi: 10.1212/wnl.39.5.693. [DOI] [PubMed] [Google Scholar]
- Rose I. A., O'Connell E. L. Mechanism of aconitase action. I. The hydrogen transfer reaction. J Biol Chem. 1967 Apr 25;242(8):1870–1879. [PubMed] [Google Scholar]
- Rosenbaum L. C., Nilaver G., Hagman H. M., Neuwelt E. A. Detection of low-molecular-weight polypeptides on nitrocellulose with monoclonal antibodies. Anal Biochem. 1989 Dec;183(2):250–257. doi: 10.1016/0003-2697(89)90475-2. [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Morgan-Hughes J. A., Landon D. N., Clark J. B. Mitochondrial myopathy with a defect of mitochondrial-protein transport. N Engl J Med. 1990 Jul 5;323(1):37–42. doi: 10.1056/NEJM199007053230107. [DOI] [PubMed] [Google Scholar]
- Sengers R. C., Fischer J. C., Trijbels J. M., Ruitenbeek W., Stadhouders A. M., ter Laak H. J., Jaspar H. H. A mitochondrial myopathy with a defective respiratory chain and carnitine deficiency. Eur J Pediatr. 1983 Sep;140(4):332–337. doi: 10.1007/BF00442676. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperl W., Ruitenbeek W., Trijbels J. M., Sengers R. C., Stadhouders A. M., Guggenbichler J. P. Mitochondrial myopathy with lactic acidaemia, Fanconi-De Toni-Debré syndrome and a disturbed succinate: cytochrome c oxidoreductase activity. Eur J Pediatr. 1988 May;147(4):418–421. doi: 10.1007/BF00496424. [DOI] [PubMed] [Google Scholar]
- Triebwasser J. H., Johnson R. L., Burpo R. P., Campbell J. C., Reardon W. C., Blomqvist C. G. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med. 1977 Mar;48(3):203–209. [PubMed] [Google Scholar]
- Wahren J., Linderholm H., Felig P. Amino acid metabolism in patients with a hereditary myopathy and paroxysmal myoglobinuria. Acta Med Scand. 1979;206(4):309–314. doi: 10.1111/j.0954-6820.1979.tb13516.x. [DOI] [PubMed] [Google Scholar]
- Wilcock A. R., Goldberg D. M. Kinetic determination of malate dehydrogenase activity eliminating problems due to spontaneous conversion of oxaloacetate to pyruvate. Biochem Med. 1972 Apr;6(2):116–126. doi: 10.1016/0006-2944(72)90029-4. [DOI] [PubMed] [Google Scholar]
- Willis W. T., Brooks G. A., Henderson S. A., Dallman P. R. Effects of iron deficiency and training on mitochondrial enzymes in skeletal muscle. J Appl Physiol (1985) 1987 Jun;62(6):2442–2446. doi: 10.1152/jappl.1987.62.6.2442. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Bonilla E., DeVivo D. C., DiMauro S. Mitochondrial diseases. Neurol Clin. 1989 Feb;7(1):123–156. [PubMed] [Google Scholar]