Abstract
Background and Aims
The gastric mucosa provides a stringent epithelial barrier and produces acid and enzymes that initiate digestion. In this regenerating tissue, progenitors differentiate continually into 4 principal specialized cell types, yet underlying mechanisms of differentiation are poorly understood. We identified stomach-restricted expression of the forkhead transcription factor FOXQ1.
Methods
We used a combination of genetic, histochemical, ultrastructural and molecular analysis to study gastric cell lineages with respect to FOXQ1.
Results
Within the developing and adult gastrointestinal tract, Foxq1 mRNA is restricted to the stomach and expressed predominantly in foveolar (pit) cells, the abundant mucin-producing cells that line the mucosal surface. Mice carrying Foxq1 coding mutations show virtual absence of mRNA and protein for the backbone of the major stomach mucin, MUC5AC. These observations correspond to a paucity of foveolar-cell secretory vesicles and notable loss of stomach but not intestinal mucus. Transcriptional profiling identified a surprisingly restricted set of genes with altered expression in Foxq1 mutant stomachs. MUC5AC is a highly tissue-restricted product that similarly depends on FOXQ1 in its other major site of expression, conjunctival goblet cells.
Conclusions
Taken together, these observations imply that promotion of gastric MUC5AC synthesis is a primary, cell-autonomous function of FOXQ1. This study is the first to implicate a transcription factor in terminal differentiation of foveolar cells and begins to define the requirements to assemble highly specialized organelles and cells in the gastric mucosa.
Keywords: mucin gene regulation, foveolar cell, stomach epithelial differentiation, forkhead, conjunctival goblet cell, surface mucous cell, MUC5AC, Satin, Foxq1
INTRODUCTION
The gastric mucosa contains four highly specialized cell types that differentiate from a common progenitor and help execute the stomach’s digestive functions. While much is known about the morphology and physiology of these cell types, few transcriptional regulators that govern their differentiation have been characterized.1–3 Surface mucous cells, also known as foveolar or pit cells, line the lumen of the glandular stomach in the corpus and antrum, including mucosal pits that vary in depth in different areas.4 Acid-secreting parietal cells dominate the corpus mucosa, which also houses zymogenic chief cells. These two cell lineages are excluded from the gastric antrum, where the proportion of foveolar cells is accordingly increased. Foveolar cells constitutively secrete a viscous mucus that protects the gastric epithelium from damage.
Secretory epithelial cells in different organs utilize distinct mucin polypeptides as the backbone for extensive glycosylation and mucus synthesis. Although more than one mucin may be present in a cell lineage, single mucin types tend to predominate and represent the major product of specialized secretory cells. Intestinal goblet cells produce only MUC2; in the stomach, mucous neck cells and basal cells in the antrum produce MUC6, whereas foveolar cells produce MUC5AC.5 While production of particular mucin peptides is a hallmark of gastric mucous cells, other markers distinguish these cell lineages further: pit cells express gastrokine1 and Trefoil factor (Tff) 1, whereas mucous neck cells produce Tff2.6, 7 MUC5AC is also produced by goblet cells in the conjunctiva, the stratified columnar epithelial lining of the eye.8 Regulation of individual mucin genes can thus provide important clues about lineage-specific cell differentiation in surface epithelia.
In a screen for transcription factor (TF) genes that are differentially expressed in developing mouse stomach and intestine,9 we observed that Foxq1 mRNA is excluded from intestine and expressed selectively in the stomach. Previous studies have noted Foxq1 expression in the stomach of various species but its exact function in this organ is unknown.10–13 To understand these functions, we studied Satin mice, a radiation-induced mutant strain that is homozygous for a null Foxq1 allele.14 We observed a specific and significant defect in gastric mucin production and secretory granule biogenesis in gastric foveolar cells, and traced these defects to virtually complete absence of MUC5AC and its glycosylated end-products. We confirmed the findings in independent strains of Foxq1 mutant mice and also found absence of MUC5AC in conjunctival goblet cells. A combination of studies in vitro and in animals indicated that the forkhead protein FOXQ1 has a limited but essential function in Muc5ac gene regulation. FOXQ1 is the first TF to be implicated in terminal differentiation of stomach foveolar cells.
MATERIALS AND METHODS
Mice
Mice were housed under pathogen-free conditions and handled according to protocols approved by an institutional Animal Care and Use Committee. Satin (SB/LeJ), Beige, 129/Sv and C57/BL6 mice were obtained from The Jackson Laboratories (Bar Harbor, ME), CD1 mice from Charles River Laboratories (Wilmington, MA), and mixed genetic backgrounds were generated by interbreeding. Foxq1ENU/+ mice were resurrected from cryopreserved sperm at the Mutant Mouse Regional Resource Center at the University of California, Davis. Genotyping for Foxq1 alleles was done by PCR as described previously.14
Details on expression analyses, histology, immunohistochemistry, electron microscopy, and transcriptional reporter assays are all included in the supplemental materials.
Microarray expression analysis
Stomachs from age-matched C57BL/6, Beige, and SB/LeJ mice were harvested immediately after euthanasia, washed, and the antrum isolated. RNA was extracted with Trizol reagent, labeled, and hybridized to 430A2.0 Mouse Expression Arrays (Affymetrix, Santa Clara, CA). Data were analyzed using dChip software15 and deposited in the GEO public database, with Accession number GSE8943.
RESULTS
Stomach-restricted Foxq1 expression
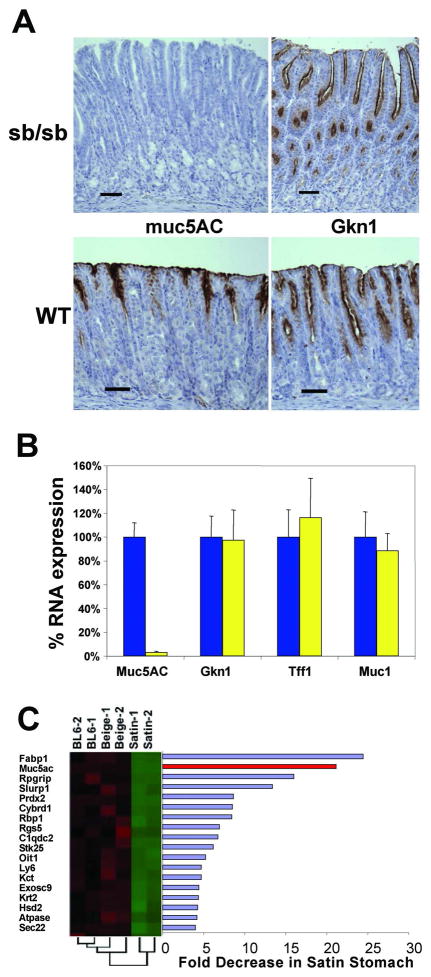
Transcriptional regulation of cell-specific gene expression in the gastrointestinal (GI) tract is not well understood. To take steps toward identifying relevant pathways, we recently surveyed the temporal and spatial expression of all known and predicted TF genes in developing mouse gut.9 Among mRNAs that are restricted to the stomach, we identified the forkhead TF Foxq1. Foxq1 transcripts first appear on embryonic day 13 and stomach-restricted expression is maintained throughout development (Fig. 1A). Similarly, Foxq1 transcripts are restricted to the adult stomach and absent from adult intestine (Fig. 1B). Further inspection of Foxq1 expression in the stomach corpus (where foveolar, parietal, and chief cells are evident by histochemical staining, Fig. 1C) by in situ hybridization indicated that Foxq1 transcripts in the adult stomach are restricted largely to surface mucous (pit or foveolar) cells (Fig. 1D), which are characterized by robust expression of the lineage marker Muc5AC (Fig. 1F). Foxq1 transcripts were also detected in pepsinogenic chief cells at the base of gastric gland units (Fig. 1D), although signals were considerably weaker than in surface mucous cells and may represent non-specific background staining. Control (sense) probes typically gave no staining (Fig. 1E). Previous studies using laser capture microdissection found foveolar cells enriched and chief cells lacking in Foxq1 transcripts, consistent with our results.3, 16
Figure 1. Restricted Foxq1 expression in fetal and adult stomach.
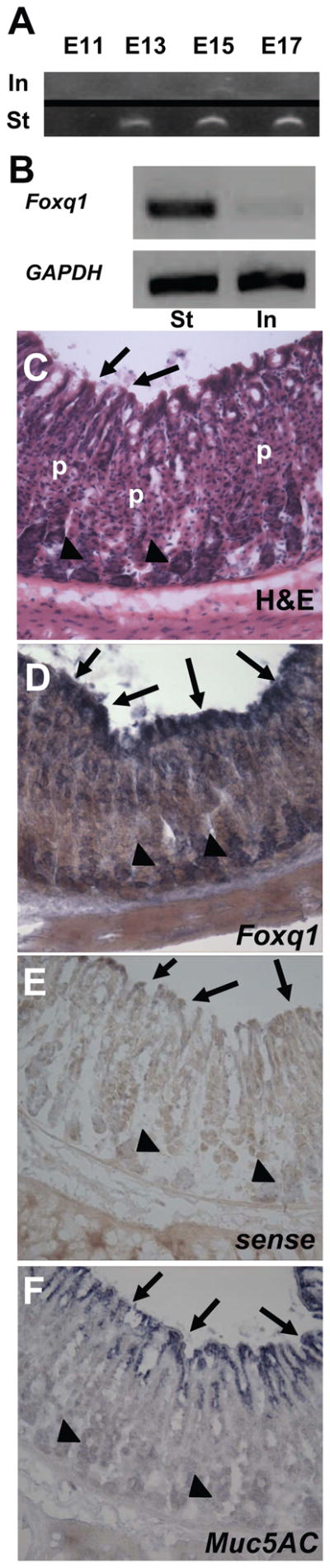
(A) RT-PCR results from the GIFT database 9. RNA from wild-type murine stomach (St) and intestine (In) at four different fetal stages (E11, E13, E15, E17) probed using Foxq1-specific primers. Foxq1 is expressed in the stomach with increasing abundance as the embryo approaches birth, but not detected in intestine. (B) RT-PCR in adult mouse stomach shows robust Foxq1 mRNA levels relative to the intestine; GAPDH was used as a loading control. (D–F) In situ hybridization on adult wild-type murine stomach using Foxq1 antisense probe (D), sense control (E) or Muc5AC antisense probe (F). The Foxq1 probe gave strong signal in surface epithelium (arrows) throughout the glandular stomach (images shown here are taken from the corpus, with an H&E-stained section as a reference (C)) and considerably weaker signal in chief cells (arrowheads); Muc5ac is specific to surface mucous cells (arrows in F). p, Parietal cells.
Delineation of Foxq1sa/sa stomach abnormalities
To study FOXQ1 functions, we took advantage of an existing recessive mutant mouse strain, Satin (Sa), which was generated by radiation mutagenesis17 and recognized originally by a shiny pelage resulting from disorganized hair-shaft medullae.14, 18 The genetic defect maps to a Foxq1 nonsense mutation that eliminates the C-terminal 112 amino acids of a 400-residue protein.14 Serial back-crosses isolated the mutation on a homogeneous genetic background with tight linkage to an additional mutation, beige; the resulting strain, SB/LeJ, is thus homozygous for both Foxq1sa and Lystbg alleles.17 Beige, a mutant allele of the Lyst lysosomal transport gene,19–23 increases susceptibility to infection owing to immune dysfunction but has no known role in stomach mucosa. The hair follicle defect in the Satin strain is well characterized14, 24 but the animals seem otherwise normal and stomach defects have not been investigated.
SB/LeJ mice (which we designate Foxq1sb/sb) show normal activity, feeding, growth, fertility and life span, and gross stomach morphology is intact, without mucosal ulceration or tumors. Histologic examination of Foxq1sb/sb stomachs revealed a normal mucosa (Fig. 2A), with differentiated cell types present in normal numbers and distribution, judging by the following immunohistochemical markers: H/K-ATPase for parietal cells, gastrin for antral G-cells, and pepsinogen and intrinsic factor for chief cells (Fig. 2I and data not shown). Alcian blue staining for acidic mucins also showed the typical weak signal in basal mucous cells in the antrum (data not shown). By contrast, periodic acid Schiff (PAS) staining, which identifies the neutral mucins secreted by foveolar cells, revealed a dramatic and completely penetrant defect (Fig. 2B–C versus Fig. 2F–G; N=10): some glands retained a faint rim of extracellular signal, but none displayed the prominent intracellular apical staining characteristic of control animals. The full scope of the defect can be appreciated in low-magnification photomicrographs (Suppl. Fig. 1A–D) and quantitation of stained gland units (Suppl. Fig. 2A). Defective mucin expression was confined to the stomach surface; PAS staining of intestinal goblet cells was unaffected (Fig. 2D,H).
Figure 2. Foxq1sb/sb stomachs are deficient in surface mucin.
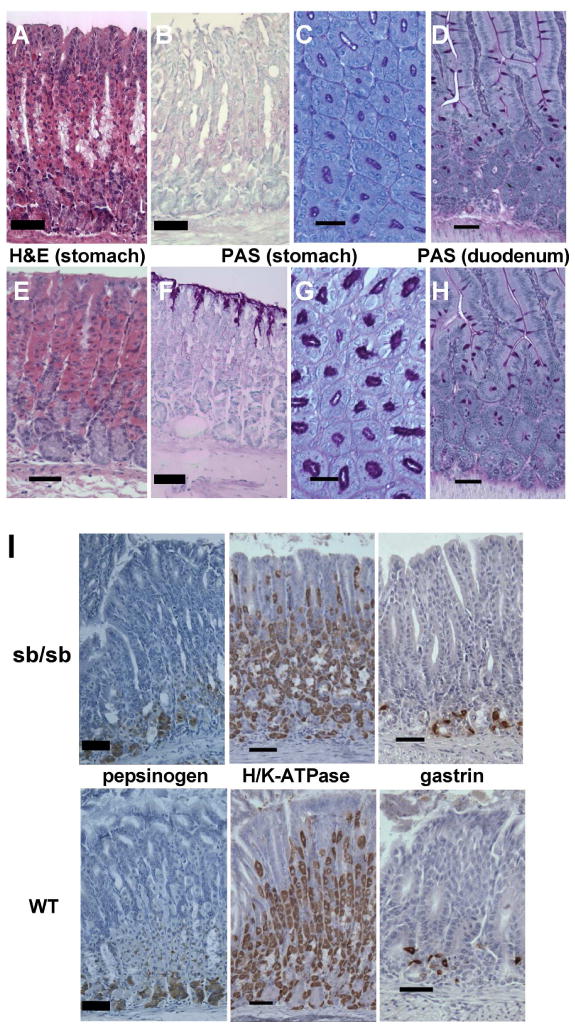
(A–H) Adult mouse stomach or duodenal tissue (Foxq1sb/sb in top 4 panels and controls in lower 4) stained with hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS), as indicated. H&E staining reveals normal character of gastric glandular mucosa; images are taken from the gastric corpus. PAS stain reveals marked attenuation of surface epithelial mucous staining in Foxq1sb/sb stomach (B,C) but normal duodenal goblet-cell staining (D). (I) Immunohistochemistry of adult murine gastric mucosa (Foxq1sb/sb in top 3 panels and wild type in lower 3), showing normal distribution of markers for chief (pepsinogen) and parietal (H/K-ATPase) cells in the corpus and enteroendocrine G (gastrin) cells in the antrum. Scale bars: 60 μm for PAS-stained cross-sections (C,G), 120 μm for all others. Low-power photomicrographs are shown in Suppl. Fig. 1A–D.
High-resolution light microscopy revealed absence of the apical zone of PAS staining in Foxq1sb/sb pit cells (Fig. 3A,B), and we used transmission electron microscopy to characterize the defect further. We observed normal size, shape and polarity of Foxq1sb/sb foveolar cells (Fig. 3E), but apical mucous granule numbers were markedly reduced compared to controls (Fig. 3C,D). In mutant animals a few of these granules showed the typical morphology and electron density, but most were reduced in both size and density. The prominent reduction in PAS staining can thus be attributed to a significant defect in the organelles that store and release neutral gastric mucin.
Figure 3. Foveolar-cell apical granule deficiency in Foxq1sb/sb stomach.
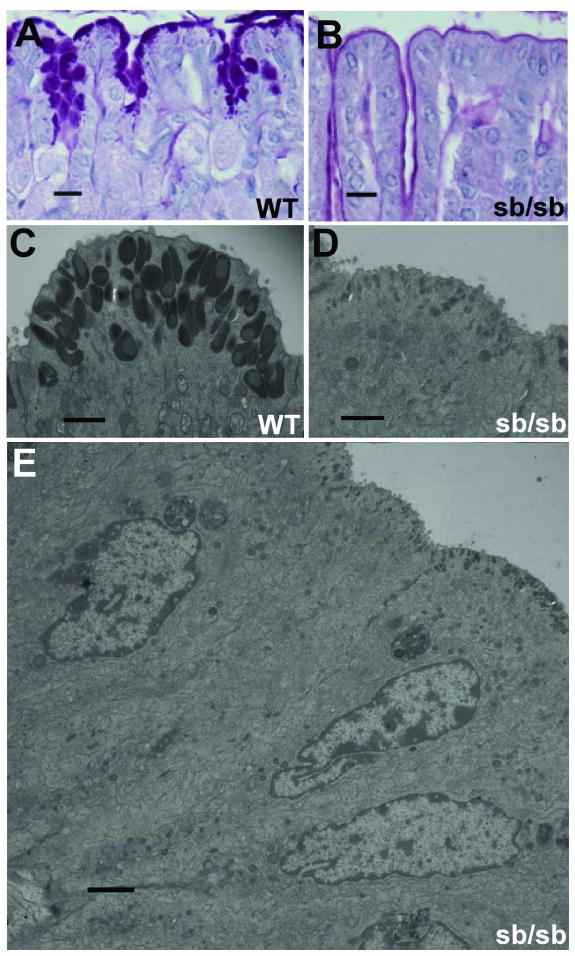
(A–B) High-magnification view of PAS-stained gastric foveolar cells from adult wild-type (A) and Foxq1sb/sb (B) mice. (C–D) Transmission electron microscopy of adult gastric foveolar cells, emphasizing the apical granular zone in wild-type (C) and Foxq1sb/sb (D) cells. (E) Full ultrastructural profile of Foxq1sa/sa gastric foveolar cells, indicating intact architecture except for apical granule deficiency. Scale bars: A–B, 60 μm; C–D, 1 μm; E, 2 μm.
Selective loss of MUC5AC in the absence of Foxq1 function
To distinguish whether paucity of apical granules in Foxq1sb/sb pit cells reflects absence of MUC5AC synthesis or a failure to glycosylate and store the protein, we used a specific antibody. MUC5AC protein was completely absent from Foxq1sa/sa stomach samples (Fig. 4A, N=5), indicating that FOXQ1 is required to produce the polypeptide backbone for neutral stomach mucin. Judging by other stains, including Alcian blue, synthesis of other mucins, Muc2 and Muc6, is intact in the absence of FOXQ1 function (data not shown). Gastrokine-1, another secreted and granule-bound pit-cell product of unknown function,7 is also expressed normally in Foxq1sb/sb surface mucous cells (Fig. 4A). Finally, quantitative RT-PCR analysis of Foxq1sb/sb stomach revealed normal gastrokine-1, Mucin 1, and stomach trefoil-family factor TFF1 mRNA levels, whereas Muc5ac transcripts were reduced to <3% of levels observed in control samples (Fig. 4B).
Figure 4. Foxq1sb/sb fail to produce MUC5AC protein or mRNA.
(A) Immunohistochemistry of adult Foxq1sa/sa (top) and wild-type (bottom) mouse gastric mucosa for MUC5AC (left) and gastrokine-1 (GKN1, right), indicating absence of MUC5AC but preserved GKN1 expression in Foxq1sb/sb stomach; scale bars, 120 μm. (B) qRT-PCR analysis of selected mucins and secreted foveolar-cell products: Muc5AC, Gkn1, trefoil factor 1 (Tff1), and Muc1. mRNA expression was first normalized against Gapdh and the levels in Foxq1sb/sb stomach (yellow bars) are expressed as a percentage of wild-type levels (blue bars, 100%). The results reveal marked attenuation of Muc5ac transcripts, with intact expression of all other tested markers. (C) Graphic depiction of relative mRNA levels revealed in expression microarray analysis of stomach antra from age-matched wild-type C57BL/6 (BL6), Beige, and Foxq1sb/sb (Satin) adult mice. Most transcripts showed nearly identical expression in the 3 strains; data for all mRNAs with >4-fold decrease are shown in expression-heat maps (red: high expression; green, low expression) and as a bar graph. Muc5ac was the second most differentially expressed transcript (red bar). The dendrogram below the heat map depicts results of hierarchical clustering analysis of the samples for all probes and indicates greatest similarity first between duplicate samples and secondly between BL6 and Beige; Foxq1sb/sb (Satin) samples cluster separately.
These results reveal virtual absence of Muc5ac mRNA and hint at selective loss of MUC5AC among apical granule contents. To determine the potentially broader scope of Foxq1 function in foveolar cells, we used oligonucleotide microarrays to profile mRNA expression in the gastric antrum. The epithelium of the antrum, the most distal portion of the stomach, carries no zymogenic or parietal cells and a correspondingly high fraction of pit cells;4 accordingly, differences in gene expression related to absence of Foxq1 function should be most evident in this region. Because Foxq1sb/sb mice carry tightly linked mutations in the Foxq1 and Lyst genes, we also used expression profiling to compare Foxq1sb/sb stomach with that from the Beige strain, which carries a mutation only in the Lyst gene and is congenic with the C57BL/6 line.25 Analysis of antral RNA from Foxq1sb/sb, Beige and C57BL/6 control mice disclosed fewer than 20 genes with >4-fold reduction in Foxq1sb/sb samples, and Muc5ac was one of only 4 transcripts reduced >10-fold in Foxq1sb/sb antrum (Fig. 4C). RT-PCR on independent stomach samples validated the change in Fabp1 mRNA recorded in expression profiling (Suppl. Fig. 2B). These results together implicate stomach Foxq1 function in a restricted range of activities; Foxq1sb/sb surface mucous cells seem intact in most respects but deficient in apical granules and their principal protein product, MUC5AC, likely reflecting substantially reduced Muc5ac gene transcription.
Validation of the role of Foxq1 in foveolar cell function
As the parental strain on which the Foxq1sb/sb mutations appeared is no longer available, in the foregoing histochemical analyses we used a panel of laboratory mouse strains (CD1, C57BL/6, and 129/Sv-C57BL/6 hybrids) as controls. In contrast to Foxq1sb/sb stomach, we observed abundant apical mucus staining in every control (Fig. 5A and data not shown), suggesting that strain background is unlikely to account for the Foxq1sb/sb phenotype. The only other mutation in this strain maps to the closely linked Lyst gene,19–21, 25 which regulates biogenesis and transport of membranous organelles and enables lysosome-mediated plasma membrane repair.26, 27 In hierarchical analysis of mRNA expression profiles, Beige and C57BL/6 antra clustered together, whereas Satin samples clustered separately (Fig. 4C; the cluster dendrogram reflects the complete array dataset). To further exclude the possibility that pit-cell defects in Foxq1sb/sb mice might reflect Lyst gene inactivity, we examined bg/bg mice more closely. PAS staining and MUC5AC immunohistochemistry of adult bg/bg stomach were similar to congenic C57BL/6 controls, with abundant signal in surface mucous cells (Fig. 5A and Suppl. Fig. 3). Thus, LYST deficiency alone cannot account for the foveolar-cell defect, which likely results from the Foxq1sa mutation rather than the Lystbg allele.
Figure 5. Foxq1sb/sb stomach defects specifically reflect Foxq1 gene mutations.
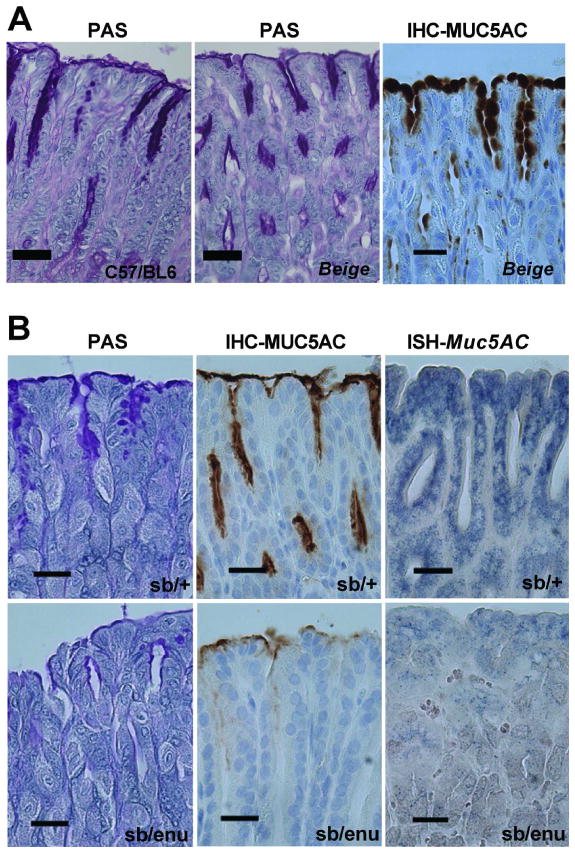
(A) Staining of gastric mucosa from C57BL/6 (left) and Beige (right 2 panels) mice for PAS (left 2 panels) and MUC5AC immunohistochemistry (IHC, far right), showing normal expression of neutral stomach mucins and MUC5AC. Images are taken from the gastric corpus and additional data are shown in Suppl. Fig. 3. (B) Complementation analysis of the Foxq1 gene: examination of Foxq1sb/+ (top row, control) and Foxq1sb/enu (bottom row) stomach corpus for PAS stain (left), and stomach antrum for MUC5AC immunostain (middle), and Muc5ac in situ hybridization (right). The Foxq1sb/enu strain effectively phenocopies Satin (Foxq1sb/sb) mice, and the results hint that the Foxq1enu allele may be hypomorphic. Additional data are shown in Suppl. Fig. 4. All scale bars: 60 μm.
These results do not, however, exclude the formal possibility that combined mutation of the Foxq1 and Lyst genes is required to produce the stomach pit-cell phenotype. As tight linkage of the satin and beige loci prohibits their separation, we asked if another Foxq1 mutant strain, generated independently by ethylnitrosourea (ENU) mutagenesis,14 carries the same foveolar cell defect. In a previous study, Foxq1enu mice phenocopied the hair defect when crossed to Satin mice but Foxq1enu/sb stomachs were not examined.12 As Foxq1enu/enu mice were not viable at weaning in our crosses (p=0.008), we generated Foxq1enu/sb mice. Unlike Foxq1enu/+ or Foxq1sb/+ littermates, compound heterozygous Foxq1enu/sb mice showed loss of PAS staining (Fig. 5B and Suppl. Fig. 4, N=3), similar to SB/LeJ, and hence provide genetic proof that Foxq1 is responsible for the phenotype. Very weak MUC5AC expression in Foxq1enu/sb stomachs, which we detected by in situ hybridization and immunohistochemistry (Fig. 5B and Suppl. Fig. 4), implies that the Foxq1enu allele is hypomorphic for stomach function. Such a hypomorphic phenotype may result from the relatively subtle missense mutation in the Foxq1enu allele (Ile128Ser in the winged helix domain) compared to the nonsense mutation in the SB/LeJ strain.14 These results collectively indicate that Muc5AC production in gastric pit cells depends on Foxq1.
The role of FOXQ1 in Muc5ac gene regulation
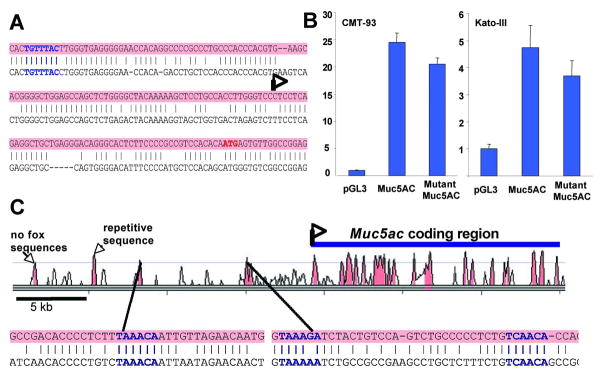
As FOXQ1 is a forkhead protein with a likely role in transcriptional regulation and Muc5ac mRNA levels are low in its absence, we asked if FOXQ1 may regulate the Muc5ac gene directly. Previous study of the mouse Muc5ac promoter found that it could be activated by transforming growth factor-β signaling, along with Smad- and Sp1-family TFs.28 Whereas FOXQ1 is reported to bind an AT-rich sequence in the telokin promoter, its consensus DNA recognition sequence is unknown.13 However, most forkhead TFs recognize the sequence RYMAAYA,29 and the binding preference for FOXF2, which is closely related to FOXQ1,30 has been determined empirically.31 We identified an evolutionarily conserved forkhead consensus binding sequence in the mouse Muc5ac promoter, 100 bp upstream of the transcriptional start site (Fig. 6A). To assess the function of this site, we cloned the Muc5ac promoter sequence from −199 to +3 upstream of the firefly luciferase gene and tested its ability to activate reporter gene expression. Compared to a promoterless reporter construct, this Muc5ac promoter fragment induced robust expression of the reporter gene in CMT-93 colonic epithelial cells, a cell line chosen on the basis of demonstrated Muc5ac promoter activity,27 as well as in the human gastric cancer cell line Kato-III (Fig. 6B). However, deletion of two core nucleotides in the putative forkhead element (TGTTTAC → TG--TAC) had little effect on promoter activity (Fig. 6B), and co-transfection of a Foxq1 expression plasmid did not increase it (data not shown).
Figure 6. Conserved regions in the mouse Foxq1 locus contain consensus forkhead binding sites but these may not activate Muc5ac gene transcription directly.
(A) Comparison of Muc5ac promoter sequences from mouse (top, shaded) and human (bottom) reveals a conserved Foxf2 consensus element (bold type) within a region previously shown to be essential for Muc5ac promoter activity.28 (B) A firefly luciferase reporter under control of the native or FOX site-mutant murine Muc5ac promoter was transfected into CMT-93 or Kato-III cells and luciferase activity was compared to pGL3 promoterless control. Both constructs activated the reporter gene equally, indicating lack of a requirement for the putative FOX-binding activity in reporter assays on this promoter. (C) Sequence conservation profile of the murine Foxq1 locus, adapted from Vista Browser (http://pipeline.lbl.gov/cgi-bin/gateway2). Conservation was highest in the coding region, but narrow peaks (>70% identity, pink shading) were also identified in the promoter and 4 distant regions, one that contains repetitive elements and 2 that carry consensus forkhead-binding elements, T(A/c)AA(c/t)A. Sequence from these 2 regions is shown below, with alignment of mouse (top, shaded) and human (bottom) sequences and bold highlighting of putative forkhead-binding elements.
To identify potential Muc5ac enhancers that may fall under FOXQ1 control, we searched for conserved intergenic sequences. Only two conserved regions (75% and 72% homology between mouse and human) contained forkhead consensus sequences (Fig. 6C); no other regions are conserved in either direction until the next structural genes. We cloned these regions upstream of the Muc5ac promoter-reporter and tested FOXQ1-dependent transcriptional activation, but observed no enhancement over promoter activity alone (data not shown). Thus, despite identification of putative FOXQ1 cis-elements, we gathered no conclusive evidence for direct Muc5ac gene regulation by FOXQ1. It is, however, important to note that Fox proteins may remodel chromatin, a function not accurately reflected in plasmid-based reporter assays.32, 33
Muc5ac expression defects in Satin mice are not restricted to the stomach
More than half of Foxq1sb/sb mice over 9 months old developed ocular surface abnormalities and accumulated surface debris, which impaired opening of one or both eyes (Fig. 7A). Other animals maintained in the same colony never showed the same pathology, and we noted that Muc5ac is also present in conjunctival goblet cells.8 Most conjunctival epithelial cells produce the membrane-spanning mucins Muc1 and Muc4 8; goblet cells occupy the conjunctival fornix, resemble intestinal goblet cells in morphology and, like mucus in the GI tract, secrete products that function to lubricate the surface, clear debris, and provide anti-microbial defense.34 Initial inspection revealed the typical frequency and appearance of goblet cells in Foxq1sb/sb conjunctivae, and mutant goblet cells stained with both PAS and Alcian blue, similar to controls (Fig. 7B–G); these findings differ from the dramatic loss of PAS signal in Foxq1sb/sb stomach (Fig. 2A). However, conjunctival goblet cells differ from gastric pit cells, which express only MUC5AC, in that they express multiple mucin glycoproteins.34 Indeed, immunostaining revealed striking absence of MUC5AC in Foxq1sb/sb conjunctival goblet cells (Fig. 7H). Muc5ac gene expression thus appears to depend on Foxq1 function in more than one tissue, and its absence from the conjunctiva is sufficient to produce an overt ocular phenotype.
Figure 7. Absence of MUC5AC expression in Foxq1sb/sb conjunctiva.
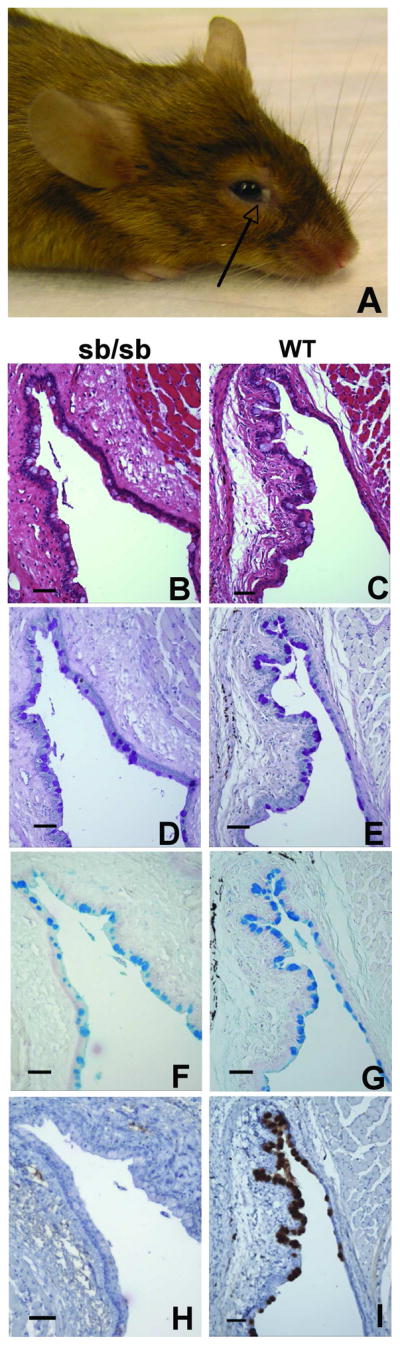
(A) Ocular phenotype of >50% of aged (>6 months) Foxq1sb/sb mice: accumulation of debris (arrow) around the eye. (B–I) Histochemical analysis of serial tissue sections of adult murine conjunctiva from Foxq1sb/sb (B, D, F, H) and age-matched wild-type (C, E, G, I) mice. B,C: hematoxylin & eosin; D,E: PAS; F,G: Alcian Blue; H,I: MUC5AC immunostain. All scale bars represent 60 μm.
DISCUSSION
Surface GI epithelia engage in continuous self-renewal and differentiation of highly specialized cells. Few genetic studies have identified TFs that are responsible for particular cell features, especially in the stomach.1–3 Here we report the characterization of a TF required for normal gastric foveolar cell differentiation. We show that Foxq1 mutations in mice severely limit foveolar cells’ ability to synthesize MUC5AC and to fill the secretory granules that characterize this unique cell lineage. Besides MUC5AC, other foveolar cell products such as TFF1 and Gkn1, which are also stored in mucous granules,7, 35–37 are unaffected by loss of Foxq1 function, and expression profiling revealed a narrow spectrum of dysregulated genes. In this light, we expected to find normal numbers and appearance of pit-cell mucous granules, but Foxq1sb/sb stomach ultrastructure disclosed fewer and smaller granules. This suggests that absence of MUC5AC, the backbone for the major content of these granules (neutral stomach-specific mucin), may preclude normal mucous granule formation or stability, similar to the effect of MUC2 loss on intestinal goblet cells.38 On the other hand, mutant pit cells are not devoid of granules and gastrokine-1 immunostaining localizes correctly to the cell apex. The sum of these findings is explained most conservatively by failure of granule filling when cellular MUC5AC levels are limiting, which suggests that Foxq1 may have evolved to fulfill a limited but essential function in pit cells. RNA microarray analysis revealed a handful of additional genes that are dysregulated in Foxq1 mutant stomach, including Rpgrip, Sec22 and Stk25, which have known roles in assembly and function of membranous organelles.39–41 These data raise the particular possibility that FOXQ1 makes additional contributions toward assembly, stability or the structure of pit-cell apical granules.
Although our data establish that Foxq1 is required for Muc5ac expression in diverse tissues, the underlying mechanisms remain unresolved. Our analysis of a limited promoter region and putative enhancers do not support the elementary possibility that FOXQ1 directly activates Muc5ac gene transcription. On the one hand, cell transfection assays may limit the ability to determine FOXQ1 functions if, for example, an essential co-factor is missing or the epigenetic state is non-permissive. On the other hand, FOXQ1 may regulate Muc5ac transcription through a distant enhancer that eluded our detection or via intermediary effectors; FOXQ1 may also be just one of several TFs that regulates Muc5ac. These possibilities will require specific antibodies and other tools to resolve definitively. One group previously suggested that FOXQ1 may repress transcription,13 in which case its effects on Muc5ac gene expression could be complex.
The gastric phenotype of Foxq1sb/sb and Foxq1sb/enu mice notably resembles that reported in mice with Slp2a gene mutations,42 specifically in the reduced number of pit-cell mucous granules. Slp2a is a synaptotagmin-like protein that interacts selectively with Rab27, a small-GTPase known to regulate intracellular vesicle transport in diverse cells,43 and Slp2a-Rab interaction seems to be essential for mucous granule formation and exocytosis.42 qRT-PCR analysis of Foxq1sb/sb stomach did not uncover altered RNA levels of Slp- or Rab-family genes (data not shown). Stable synthesis of foveolar cell mucous granules thus requires at least two independent processes: intact Slp2a-Rab function and Foxq1-dependent synthesis of MUC5AC and a small number of other gene products.
Elaboration of mucus is an essential function of certain epithelia, and properties of the mucus secreted in different tissues are dictated in part by the mucin polypeptide. MUC5AC is by far the predominant mucin produced in gastric pit cells; secretion of the mature, glycosylated product protects the epithelium from acid, enzymatic or physical damage. Foxq1 is selectively expressed in surface mucous cells and our data argue for its requirement in Muc5ac gene regulation. Nearly complete absence of MUC5AC does not produce overt disease in the stomach, including ulcers, inflammation or tumors, which may reflect the artificial environment of animals maintained in the laboratory; by contrast, aging Foxq1sb/sb mice develop ocular symptoms that can be attributed in principle to chronic MUC5AC deficiency. In some human dry-eye (keratitis sicca) conditions such as Sjögren’s syndrome, reduced Muc5ac mRNA and protein levels correlate with disease severity.44 Future studies might thus apply Foxq1 mutant mice to investigate pathophysiology of gastric and ocular mucus deficiency.
Supplementary Material
Acknowledgments
Statement of funding sources: Grant R01DK61139 (RAS) and fellowship training grants T32DK007533 (AHK) and T32DK07477 (MPV) from the National Institutes of Health, and a Crohn’s and Colitis Foundation Fellowship (MPV).
We thank Luanne Peters, Qiang Liu, and members of our laboratory for critical review of the manuscript; Karin Oien for gastrokine-1 antiserum; David Alpers for antibody against gastric intrinsic factor; and Adam Bass and Dan Podolsky for gifts of gastric cancer cell lines.
Abbreviations
- ENU
ethylnitrosourea
- GI
gastrointestinal
- PAS
Periodic Acid-Schiff
- TF
transcription factor
Footnotes
No conflicts of interest exist
Transcript profiling accession number: GSE8943
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jacobsen CM, Narita N, Bielinska M, Syder AJ, Gordon JI, Wilson DB. Genetic mosaic analysis reveals that GATA-4 is required for proper differentiation of mouse gastric epithelium. Dev Biol. 2002;241:34–46. doi: 10.1006/dbio.2001.0424. [DOI] [PubMed] [Google Scholar]
- 2.Lee CS, Perreault N, Brestelli JE, Kaestner KH. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–97. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–22. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 4.Karam SM. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci. 1999;4:D286–98. doi: 10.2741/karam. [DOI] [PubMed] [Google Scholar]
- 5.Lee HS, Lee HK, Kim HS, Yang HK, Kim YI, Kim WH. MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric carcinomas: their roles as prognostic indicators. Cancer. 2001;92:1427–34. doi: 10.1002/1097-0142(20010915)92:6<1427::aid-cncr1466>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Longman RJ, Douthwaite J, Sylvester PA, Poulsom R, Corfield AP, Thomas MG, Wright NA. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut. 2000;47:792–800. doi: 10.1136/gut.47.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oien KA, McGregor F, Butler S, Ferrier RK, Downie I, Bryce S, Burns S, Keith WN. Gastrokine 1 is abundantly and specifically expressed in superficial gastric epithelium, down-regulated in gastric carcinoma, and shows high evolutionary conservation. J Pathol. 2004;203:789–97. doi: 10.1002/path.1583. [DOI] [PubMed] [Google Scholar]
- 8.Gipson IK, Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1–49. doi: 10.1016/s0074-7696(03)31001-0. [DOI] [PubMed] [Google Scholar]
- 9.Choi MY, Romer AI, Hu M, Lepourcelet M, Mechoor A, Yesilaltay A, Krieger M, Gray PA, Shivdasani RA. A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development. Development. 2006;133:4119–29. doi: 10.1242/dev.02537. [DOI] [PubMed] [Google Scholar]
- 10.Bieller A, Pasche B, Frank S, Glaser B, Kunz J, Witt K, Zoll B. Isolation and characterization of the human forkhead gene FOXQ1. DNA Cell Biol. 2001;20:555–61. doi: 10.1089/104454901317094963. [DOI] [PubMed] [Google Scholar]
- 11.Choi VM, Harland RM, Khokha MK. Developmental expression of FoxJ1.2, FoxJ2, and FoxQ1 in Xenopus tropicalis. Gene Expr Patterns. 2006;6:443–7. doi: 10.1016/j.modgep.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Frank S, Zoll B. Mouse HNF-3/fork head homolog-1-like gene: structure, chromosomal location, and expression in adult and embryonic kidney. DNA Cell Biol. 1998;17:679–88. doi: 10.1089/dna.1998.17.679. [DOI] [PubMed] [Google Scholar]
- 13.Hoggatt AM, Kriegel AM, Smith AF, Herring BP. Hepatocyte nuclear factor-3 homologue 1 (HFH-1) represses transcription of smooth muscle-specific genes. J Biol Chem. 2000;275:31162–70. doi: 10.1074/jbc.M005595200. [DOI] [PubMed] [Google Scholar]
- 14.Hong HK, Noveroske JK, Headon DJ, Liu T, Sy MS, Justice MJ, Chakravarti A. The winged helix/forkhead transcription factor Foxq1 regulates differentiation of hair in satin mice. Genesis. 2001;29:163–71. doi: 10.1002/gene.1020. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Hung Wong W. Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol. 2001;2:RESEARCH0032. doi: 10.1186/gb-2001-2-8-research0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller A, Merrell DS, Grimm J, Falkow S. Profiling of microdissected gastric epithelial cells reveals a cell type-specific response to Helicobacter pylori infection. Gastroenterology. 2004;127:1446–62. doi: 10.1053/j.gastro.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 17.Lane PW, Murphy ED. Susceptibility to spontaneous pneumonitis in an inbred strain of beige and satin mice. Genetics. 1972;72:451–60. doi: 10.1093/genetics/72.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundberg JP, Hogan ME. Handbook of mouse mutations with skin and hair abnormalities. CRC Press, Inc; 1994. [Google Scholar]
- 19.Nagle DL, Karim MA, Woolf EA, Holmgren L, Bork P, Misumi DJ, McGrail SH, Dussault BJ, Jr, Perou CM, Boissy RE, Duyk GM, Spritz RA, Moore KJ. Identification and mutation analysis of the complete gene for Chediak-Higashi syndrome. Nat Genet. 1996;14:307–11. doi: 10.1038/ng1196-307. [DOI] [PubMed] [Google Scholar]
- 20.Perou CM, Moore KJ, Nagle DL, Misumi DJ, Woolf EA, McGrail SH, Holmgren L, Brody TH, Dussault BJ, Jr, Monroe CA, Duyk GM, Pryor RJ, Li L, Justice MJ, Kaplan J. Identification of the murine beige gene by YAC complementation and positional cloning. Nat Genet. 1996;13:303–8. doi: 10.1038/ng0796-303. [DOI] [PubMed] [Google Scholar]
- 21.Barbosa MD, Nguyen QA, Tchernev VT, Ashley JA, Detter JC, Blaydes SM, Brandt SJ, Chotai D, Hodgman C, Solari RC, Lovett M, Kingsmore SF. Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature. 1996;382:262–5. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsay M. Protein trafficking violations. Nat Genet. 1996;14:242–5. doi: 10.1038/ng1196-242. [DOI] [PubMed] [Google Scholar]
- 23.Spritz RA. Genetic defects in Chediak-Higashi syndrome and the beige mouse. J Clin Immunol. 1998;18:97–105. doi: 10.1023/a:1023247215374. [DOI] [PubMed] [Google Scholar]
- 24.Potter CS, Peterson RL, Barth JL, Pruett ND, Jacobs DF, Kern MJ, Argraves WS, Sundberg JP, Awgulewitsch A. Evidence That the Satin Hair Mutant Gene Foxq1 Is among Multiple and Functionally Diverse Regulatory Targets for Hoxc13 during Hair Follicle Differentiation. J Biol Chem. 2006;281:29245–55. doi: 10.1074/jbc.M603646200. [DOI] [PubMed] [Google Scholar]
- 25.Perou CM, Pryor RJ, Naas TP, Kaplan J. The bg allele mutation is due to a LINE1 element retrotransposition. Genomics. 1997;42:366–8. doi: 10.1006/geno.1997.4740. [DOI] [PubMed] [Google Scholar]
- 26.Harris E, Wang N, Wu WL, Weatherford A, De Lozanne A, Cardelli J. Dictyostelium LvsB mutants model the lysosomal defects associated with Chediak-Higashi syndrome. Mol Biol Cell. 2002;13:656–69. doi: 10.1091/mbc.01-09-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huynh C, Roth D, Ward DM, Kaplan J, Andrews NW. Defective lysosomal exocytosis and plasma membrane repair in Chediak-Higashi/beige cells. Proc Natl Acad Sci U S A. 2004;101:16795–800. doi: 10.1073/pnas.0405905101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonckheere N, Van Der Sluis M, Velghe A, Buisine MP, Sutmuller M, Ducourouble MP, Pigny P, Buller HA, Aubert JP, Einerhand AW, Van Seuningen I. Transcriptional activation of the murine Muc5ac mucin gene in epithelial cancer cells by TGF-beta/Smad4 signalling pathway is potentiated by Sp1. Biochem J. 2004;377:797–808. doi: 10.1042/BJ20030948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- 30.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–6. [PubMed] [Google Scholar]
- 31.Pierrou S, Enerback S, Carlsson P. Selection of high-affinity binding sites for sequence-specific, DNA binding proteins from random sequence oligonucleotides. Anal Biochem. 1995;229:99–105. doi: 10.1006/abio.1995.1384. [DOI] [PubMed] [Google Scholar]
- 32.Cirillo LA, Zaret KS. An early developmental transcription factor complex that is more stable on nucleosome core particles than on free DNA. Mol Cell. 1999;4:961–9. doi: 10.1016/s1097-2765(00)80225-7. [DOI] [PubMed] [Google Scholar]
- 33.Friedman JR, Kaestner KH. The Foxa family of transcription factors in development and metabolism. Cell Mol Life Sci. 2006;63:2317–28. doi: 10.1007/s00018-006-6095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dartt DA. Control of mucin production by ocular surface epithelial cells. Exp Eye Res. 2004;78:173–85. doi: 10.1016/j.exer.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Beck S, Sommer P, dos Santos Silva E, Blin N, Gott P. Hepatocyte nuclear factor 3 (winged helix domain) activates trefoil factor gene TFF1 through a binding motif adjacent to the TATAA box. DNA Cell Biol. 1999;18:157–64. doi: 10.1089/104454999315547. [DOI] [PubMed] [Google Scholar]
- 36.Hanby AM, Poulsom R, Singh S, Elia G, Jeffery RE, Wright NA. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993;105:1110–6. doi: 10.1016/0016-5085(93)90956-d. [DOI] [PubMed] [Google Scholar]
- 37.Karam SM, Tomasetto C, Rio MC. Trefoil factor 1 is required for the commitment programme of mouse oxyntic epithelial progenitors. Gut. 2004;53:1408–15. doi: 10.1136/gut.2003.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–9. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 39.Kaiser CA, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–33. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Y, Hong DH, Pawlyk B, Yue G, Adamian M, Grynberg M, Godzik A, Li T. The retinitis pigmentosa GTPase regulator (RPGR)- interacting protein: subserving RPGR function and participating in disk morphogenesis. Proc Natl Acad Sci U S A. 2003;100:3965–70. doi: 10.1073/pnas.0637349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preisinger C, Short B, De Corte V, Bruyneel E, Haas A, Kopajtich R, Gettemans J, Barr FA. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3zeta. J Cell Biol. 2004;164:1009–20. doi: 10.1083/jcb.200310061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saegusa C, Tanaka T, Tani S, Itohara S, Mikoshiba K, Fukuda M. Decreased basal mucus secretion by Slp2-a-deficient gastric surface mucous cells. Genes Cells. 2006;11:623–31. doi: 10.1111/j.1365-2443.2006.00964.x. [DOI] [PubMed] [Google Scholar]
- 43.Fukuda M. Rab27 and its effectors in secretory granule exocytosis: a novel docking machinery composed of a Rab27. effector complex. Biochem Soc Trans. 2006;34:691–695. doi: 10.1042/BST0340691. [DOI] [PubMed] [Google Scholar]
- 44.Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–11. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.