Abstract
Rapid ischemic tolerance, induced one hour following ischemic preconditioning, is mediated via the ubiq-uitin-proteasome system and the degradation of the pro-apoptotic bcl-2 family protein Bim. Previous studies implicate adenosine A1 receptors in mediating rapid ischemic tolerance. Since the A1 adenosine receptor antagonist DPCPX (10µM) blocked rapid ischemic tolerance in our model, we investigated whether adenosine-mediated preconditioning induces rapid ischemic tolerance via the proteasomal degradation of Bim. Cultured rat cortical neurons were incubated for 60 minutes with either adenosine (1µM) or (-)-N6-(2-Phenyl-isopropyl) adenosine (RPIA (1µM)), prior to a harmful dose of ischemia (120min oxygen and glucose deprivation). Preconditioned cells had significantly lower levels of cell death following harmful ischemia when compared to non-preconditioned cells. The proteasome inhibitor MG132 (0.1µM) blocked the protective effect of adenosine pre-conditioning. Immunoblot analysis revealed a decrease in Bim protein levels in adenosine and RPIA preconditioned neurons. Adenosine preconditioning induced neuroprotection and Bim degradation was blocked by the MEK inhibitor UO126 (10µM). Our data suggests that pharmacological preconditioning with adenosine results in proteasomal Bim degradation mediated by p42/44 MAPK. Therefore, pharmacological approaches may be able to induce rapid ischemic tolerance via similar molecular mechanisms as ischemic preconditioning.
Keywords: Adenosine, rapid ischemic tolerance, Bcl-2, proteasome, ubiquitin
Introduction
Ischemic tolerance is the phenomenon whereby prior exposure to a non-harmful ischemic challenge activates an endogenous protective mechanism, rendering the brain resilient to further normally harmful ischemia [1]. As such understanding of the molecular mechanisms which are critical for ischemic tolerance may help identify novel anti-stroke therapeutic strategies.
Two windows of neuroprotection have been reported following the preconditioning stimuli. The most commonly reported window is that of delayed (classic) ischemic tolerance, which occurs 24-72 hours following the preconditioning event [1]. Multiple preconditioning stimuli have been reported to induce delayed ischemic tolerance, including ischemia, hypoxia, anoxia, chemical ischemia inducing agents (i.e. 3-nitropropionic acid), seizures, hyperthermia (heat shock) and hypothermia [2-9], although, it is still unclear whether there are any common transducers of ischemic tolerance. Delayed ischemic tolerance is inhibited by the protein synthesis inhibitor cycloheximide, in both in vivo and in vitro models of ischemia [10, 11]. This suggests that new gene expression is required for the induction and acquisition of delayed ischemic tolerance. Consistent with this concept a number of studies have investigated the genomic signature of preconditioning and ischemic tolerance [2, 12]. Delayed ischemic tolerance is not considered further.
In contrast to delayed ischemic tolerance, rapid ischemic tolerance occurs 30-60 minutes following the preconditioning stimuli [13, 14] and has been reported using anoxia, ischemia, chemical ischemia, isoflurane and other chemical agents (diazoxide/ l-chromakalin and adenosine)as preconditioners [15-19]. Rapid ischemic tolerance is not blocked by the protein synthesis inhibitor cycloheximide, but is inhibited by protein kinase C inhibitors, MAPK inhibitors and KATP channel blockers [13, 16, 20, 21]. We have previously reported that the ubiquitinproteasome system plays a pivotal role in ischemic preconditioning [13, 21]. This suggests that rapidly acting intracellular biochemical signaling events confer tolerance to ischemia in the short time window following preconditioning.
The cells fate is determined by the relative balance between pro-survival and pro-cell death members of the Bcl-2 (B-cell lymphoma) families of proteins. Increased levels of the pro-survival Bcl-2 family proteins Bcl-2, Bcl-XL and Bcl-w increase the resistance of neurons to ischemia [22-24]. An increase in pro-survival Bcl -2 family expression may prevent mitochondrial dysfunction following subsequent ischemia. Pro-cell death Bcl-2 proteins either neutralize pro survival family members or directly promote the activation of Bax/Bak resulting in mitochondria instability and the release of apoptosis inducing proteins such as cytochrome C, SMAC/ Diablo and AIF. Bcl-2 interacting mediator of cell death (Bim) is an apical signaling protein, which has been shown to directly activate Bax promoting engagement of programmed cell death mechanisms [25]. We previously reported that neurons can degrade the potent cell death inducer Bim within one hour of an ischemic preconditioning stimulus, as an alternative strategy to prevent mitochondrial dysfunction following a subsequent ischemic insult [13].
The ubiquitination and proteasomal degradation of Bim following preconditioning requires a few key steps. Phosphorylation of the Ser69 residue (or Ser65 residue in the mouse and rat sequence) by p42/p44 mitogen activated protein kinase (p42/p44 MAPK, or Erk1/2) regulates Bim ubiquitination [26]. The poly-ubiquitination of Bim targets it for degradation by the 26S proteasome [26]. The rapid loss of Bim following preconditioning ischemia may account for the protection observed in rapid ischemic tolerance [13]. Indeed, a reduction in Bim protein levels protects neurons against ischemic-induced cell death, as well as other neurotoxic stimuli [13, 27, 28].
Rapid ischemic tolerance has been described following preconditioning with ischemia, anoxia and pharmacological agents (adenosine and KATP channel openers) [15, 18, 20]. Since the adenosine A1 receptor plays a key role in ischemic preconditioning both in vitro and in vivo [18, 29], we investigated whether adenosine receptor agonists may induce preconditioning via a molecular mechanism similar to those of ischemic preconditioning.
Materials and methods
Neuronal cultures were prepared from the cortex of one-day old Sprague Dawley rat pups, as previously described [21]. Experiments were preformed on 10-14 DIV (days in vitro) cultures. For immunoblot experiments, cells were plated onto 3.5cm dishes (3,500,000 cells/ dish) coated with poly-D-lysine. Cells were maintained in Neurobasal A media supplemented with B27 and 1% Glutamax (Invitrogen). Cell death experiments were performed on cells plated onto coverslips in 3.5cm dishes (350,000 cells/ coverslip). Oxygen glucose deprivation (OGD) experimental cultures were washed twice with phosphate buffered saline (0.5mM CaCl2, 1mM MgCl2; pH7.4) and placed in an anaerobic chamber (Forma Scientific, Marietta, OH) (85% N2, 5% H2, 10% CO2, 35°C) for either 30 or 120 minutes (preconditioning and harmful ischemia, respectively). After OGD, cultures were recovered in a solution of Neurobasal A media with 1% Glutamax in a normoxic incubator.
For adenosine preconditioning experiments, the cells were treated with 1µM Adenosine or 1µM (-)-N6-(2-Phenyl-isopropyl) adenosine (RPIA) for 60 minutes. Some cells were incubated with 10µM 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX), 10mM UO126, or 0.1µM MG132 following preconditioning with adenosine or 30 min OGD.
Cell death assay
Cell death in the ischemic-treated cultures was determined using propidium iodide (PI) exclusion assay, as previously described [13]. Briefly, cells were incubated with PI (1.5 µg/ ml) for 2 minutes, fixed in 4% PFA for 30 minutes and then permeablized with TritonX-100 (0.1%; 5min). Cells were DAPI stained and mounted for visualization with a Zeiss Axioimager fluorescence microscope fitted with an Axiocam. The coverslips were imaged for DAPI and PI using 365/395 and rhodamine filters, respectively (Zeiss). The number of DAPI and PI stained cells in a view were calculated using ImageJ and PI positive cells are expressed as a percentage of DAPI positive cells.
Immunoblotting
Tissue samples for immunoblotting were lysed in a buffer containing protease inhibitors (phenylmethylsulfonyl fluoride, leupeptin, pepstatin, aprotinin) and phosphatase inhibitors (NaVO4, NaF) and a phosphatase inhibitor cocktail (Sigma). The protein concentration was determined using Bradford Reagent (Sigma) spectrophotometrically at A595. Samples containing 25µg of protein were denatured in a loading buffer (10% SDS, 10% β-mercapto-ethanol) by boiling for 5 minutes, then loaded onto SDSpolyacrylimide gels (12%). Proteins were then transferred to polyvinylidene diflouride membranes and incubated with the following primary antibodies at 4°C overnight; Bim (#2933 or 2819), phospho p42/44 MAPK (#9101) (both Cell Signaling). Membranes were then incubated with anti-rabbit secondary antibody (Cell Signaling) conjugated to horseradish peroxidase for 1 hour at room temperature. After membranes were washed, the membranes were visualized using chemiluminescence (Visualizer™). Images were captured and quantified using a Kodak Imagestation 2000RT with Imagestation 3.6 software.
Reagents, drugs, and chemicals
All cell lysis protease and phosphatase inhibitors, as well as adenosine agonists were purchased from Sigma. The DPCPX and RPIA were purchased from Tocris. All antibodies were purchased from Cell Signaling Technologies. Cell culture media were purchased from Invitrogen.
Results
Adenosine preconditioning reduces ischemia-induced cell death in vitro
We have previously established a model of rapid ischemic tolerance, whereby cortical neuronal cultures are preconditioned with 30 minutes of oxygen and glucose deprivation (OGD) one hour prior to prolonged 120 minutes of OGD [13]. Following exposure of the cultures to 120 minutes OGD we observe 40-50% cell death in the culture as determined by propidium iodide exclusion assay. Preconditioning the cells with 30 minutes exposure to OGD, one hour prior to prolonged OGD reduced cell death in the culture by 60% (Figure 1a), consistent with our previous findings [13, 21].
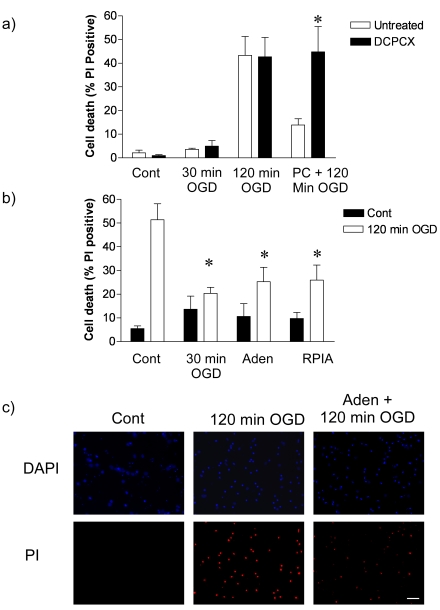
Figure 1.
Adenosine receptor 1 antagonist block and adenosine receptor agonists mimic rapid ischemic tolerance. a) Cortical neuronal cultures were subjected to 30 min OGD preconditioning prior to 120min OGD. Cell death was determined 24h later by propidium iodide exclusion assay. Some cells were incubated with 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX: 10 µM) for one hour following preconditioning and prior to harmful ischemia. Control cells received antagonist only, 30 min OGD then antagonist or one hour antagonist followed by 120min OGD. Data shown are mean ± s.e.m. n=4. * denotes P<0.05 vs. PC + 120min OGD group (one-way ANOVA followed by post-hoc Bonferroni's test). b) Cortical neuronal cultures were preconditioned with either 30min OGD, adenosine (Aden: 1µM), or the adenosine agonist RPIA (1µM) for one hour. Cells were subjected to 120 min OGD and cell death determined by PI assay. Data shown are mean ± s.e.m, n=4/5 * denotes P<0.01 vs. control 120 min OGD (one-way ANOVA with post hoc Dunnet's test for multiple comparison to control 120 min OGD group). c) Sample DAPI and PI stained images of control, 120 min OGD treated and adenosine preconditioned cells subjected to 120 min OGD. Scale bar represents 50µm.
Previous studies show adenosine A1 receptors regulate ischemic tolerance [18, 29]. To test the role of the adenosine A1 receptor in rapid ischemic tolerance, the A1 receptor antagonist DPCPX (10µM) was added during the one hour recovery period, immediately following 30 minutes OGD preconditioning. DPCPX had no affect on control, 30 minutes or 120 minutes OGD-induced cell death (Figure 1a). However, the neuroprotection observed following preconditioning was significantly attenuated in cells treated with DPCPX (Figure 1a). This suggests a potential role of adenosine receptors in our model of rapid ischemic tolerance.
To determine the role that adenosine itself may play in preconditioning we investigated the effect of adenosine (1µM) and an adenosine agonist (-)-N6-(2-Phenyl-isopropyl)adenosine (RPIA: 1µM) in our rapid ischemic tolerance paradigm (Figure 1b). Neither adenosine, nor RPIA significantly increased cell death under control conditions. Cell death following 120 minutes OGD was reduced by prior incubation of the cultures with either adenosine or RPIA (Figure 1b). These data suggest that adenosine, or an adenosine receptor agonist, can induce tolerance in ischemia modeled in vitro.
Inhibition of the proteasome blocks adenosine-mediated Bim degradation and rapid ischemic tolerance
Our previous study identified the Bcl-2 family protein Bim as playing a key role in rapid ischemic tolerance [13]. This led us to question whether pharmacological preconditioning with adenosine also affects Bim protein levels. Following 1 hour incubation with either adenosine and RPIA, there was almost a 40% reduction of Bim, when compared to non-preconditioned control cells (Figure 2a). Therefore two pharma-cologically-related preconditioning agents, adenosine and RPIA, both induce a loss of Bim protein at the time point when tolerance is observed. Accordingly, we decided to focus our investigation on the molecular effects of adenosine.
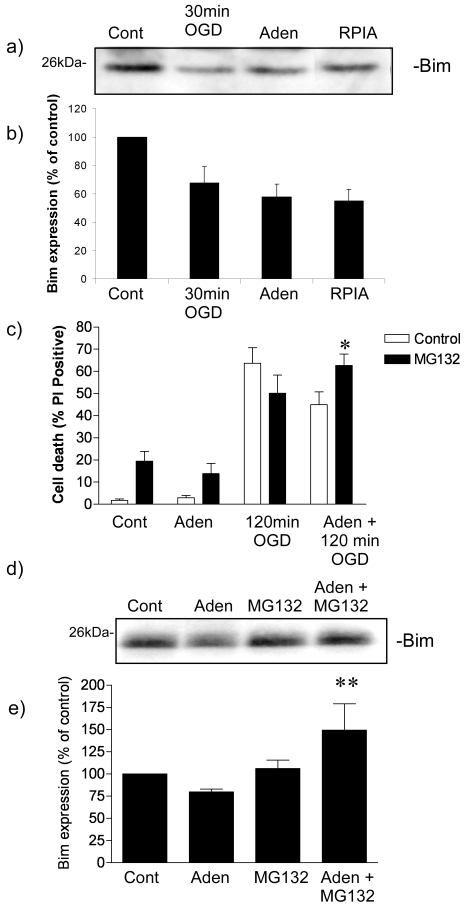
Figure 2.
Adenosine promotes proteasome mediated degradation of Bim. a) Representative immmunoblot showing loss of Bim protein one hour following ischemic preconditioning, adenosine (Aden: 1µM) preconditioning and RPIA (1µM) preconditioning. b) Quantification of optical density of immunoblots. Data shown are mean ± s.e.m. n=4. c) Adenosine preconditioning is reduced by the proteasome inhibitor MG132. Cells were preconditioned with adenosine (Aden: 1µM) for 1 hour prior to 120 min OGD. Some cells were incubated with MG132 (0.1µM) in the presence of adenosine. As a control cells received MG132 only, one hour adenosine plus MG132, or one hour MG132 followed by 120min OGD. Data shown are mean ± s.e.m. n=7, * denotes P < 0.01 vs. Aden + 120min OGD. d) Representative immunoblot of cells incubated with adenosine (Aden: 1µM), MG132 (0.1µM) or adenosine + MG132 for one hour. E) Quantification of optical density. Data shown are mean ± s.e.m. of n=4 experiments ** denotes P<0.01 vs. Aden (one-way ANOVA followed by post-hoc Bonferroni's test).
In order to investigate the role of the protea-some in regulating Bim protein levels following adenosine preconditioning, we incubated cells with adenosine in the presence of MG132 (0.1µM), a concentration which inhibits rapid ischemic tolerance [13]. MG132 caused an increase in cell death in control and adenosine treated cells (P<0.01) (Figure 2c). The protective effect of adenosine was significantly reduced in MG132 treated cells (Figure 2c), suggesting that the protective effect of adenosine preconditioning in our model is reduced by proteasomal inhibition.
We then determined the role of the proteasome in regulating Bim protein levels following incubation with adenosine. Cells were incubated with adenosine in the presence or absence of MG132 (0.1µM), the concentration which blocks rapid tolerance to ischemia induced by adenosine preconditioning. As can be seen in Figure 2d, the loss of Bim protein levels observed following adenosine preconditioning is blocked by MG132. Interestingly, the levels of Bim protein in cells treated with both adenosine and MG132 were higher than control levels, the significance of this observation is not clear. These data suggest that adenosine induces proteasomal Bim degradation resulting in rapid ischemic tolerance.
Adenosine mediates rapid ischemic tolerance via the activation of p42/44 MAPK
The p42/p44 mitogen activated protein kinase (p42/p44 MAPK, also known as Erk1/2) regulates proteasomal degradation of Bim, via phosphorylation [30]. First we examined the phosphorylation of p42/p44 MAPK on Ser202/Thr204 in adenosine preconditioned samples, as a marker of p42/p44 MAPK activation. Immunoblot revealed a increase in phosphorylated levels of p42/44 MAPK in both adenosine and ischemia preconditioned cells (Figure 3a). This finding is consistent with our previous observation that preconditioning with ischemia induces phosphorylation of p42/p44 MAPK [13]. To determine that the effect of adenosine was due to MEK-mediated phosphorylation of p42/p44 MAPK, we incubated cells with U0126 and adenosine. As can be seen in Figure 3b, U0126 decreased the phosphorylation of p42/p44 MAPK in the presence of adenosine.
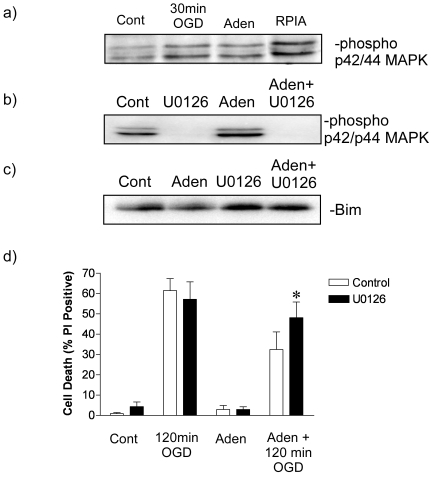
Figure 3.
Adenosine preconditioning and Bim degradation is mediated by p42/p44 MAPK. a) Representative immunoblot showing increase in p42/p44 MAPK phosphorylation following incubation of cortical cells with adenosine (Aden 1µM) or (-)-N6-(2-Phenyl-isopropyl) adenosine (RPIA: 1µM). As a comparison 30min OGD also increase p42/p44 MAPK phosphorylation. Data shown is a representative blot of 3 similar experiments. b) Representative blot of the effect of blocking adenosine (Aden: 1µM) mediated p42/p44 MAPK activation with the MEK inhibitor U0126 (10 µM). Note the dephosphorylation of p42/p44 by the upstream MEK inhibitor U0126. c) Representive blot of the adenosine-mediated Bim degradation was reduced by U0126. NB note the different loading order between 3band 3c. d) Cells were incubated with Adenosine (Aden: 1µM) in the presence or absence of U0126 (10µM) for 1 hour and then subjected to 120min OGD. Cell death was determined 24 h later by PI assay. Data shown are mean ± s.e.m. n=6. ** denotes P<0.05 vs. Aden + 120 min OGD (one-way ANOVA followed by post-hoc Bonferroni's test).
We investigated the role of p42/p44 MAPK in regulating the effect of adenosine on rapid ischemic tolerance and Bim protein loss using the p42/p44 MAPK selective inhibitor U0126 (10µM). Phosphorylation of Bim by p42/p44 is deemed a pre-requisite for Bim ubiquitination and degradation. Our attempts to measure phosphorylated Bim protein levels with a phos-pho-Bim specific antibody (Cell Signaling phos-pho-Bim (Ser65) specific antibody) were unsuccessful (data not shown). Therefore, we determined the effect of the MEK inhibitor U0126 on Bim protein loss following incubation with adenosine. As can be seen in Figure 3b loss of Bim protein following incubation with adenosine is blocked by U0126.
Finally, if p42/p44 MAPK-mediated Bim degradation is a key regulator of adenosine-induced ischemic tolerance, then blocking p42/p44 MAPK activation would be expected to block adenosine induced rapid ischemic tolerance. Cells preconditioned with adenosine had U1026 added for the duration of the preconditioning (1 hour). U0126 had no effect of cell death in control, adenosine-treated or 120 min OGD treated cells. U0126 blocked the protection observed in adenosine preconditioned cells subject to an additional 120 minutes OGD (Figure 3c). Taken together these data suggest that adenosine-mediated Bim degradation and rapid ischemic tolerance is mediated via the activation of p42/p44 MAPK.
Discussion
Here we show adenosine increases the proteasomal degradation of the pro-cell death protein Bim resulting in rapid tolerance to ischemia. In our model system, rapid ischemic tolerance following ischemic preconditioning was blocked by the A1 adenosine receptor antagonist DPCPX, implying that adenosine signaling plays a role. Adenosine has been shown to regulate neuroprotection to ischemia and other neurotoxic insults. Adenosine antagonists have been shown to block both rapid and delayed ischemic tolerance following preconditioning with anoxia and ischemia, in both in vitro and in vivo models. Isoflurane, adenosine and chemical ischemia ,induced by iodoacetate, have been shown to induce rapid ischemic tolerance [18] and the effect of these agents are blocked by the adenosine receptor antagonist DPCPX in both in vitro and in vivo models of ischemic tolerance [29]. The preconditioning effect of adenosine and adenosine receptor agonists (RPIA) are blocked by glibenclamide and tolbutamide, blockers of potassium-sensitive ATP channels (KATP), suggesting that opening of KATP channels is an important step for the induction of rapid ischemic tolerance [16, 20, 31]. Indeed, KATP opening compounds, such as L-cromakalin, diazoxide and pinacidil have been shown to induce tolerance to ischemia [15], although, heat shock-induced ischemic tolerance is not sensitive to KATP channel blockers. Taken together, previous studies strongly support a role of adenosine in regulating rapid ischemic tolerance following ischemic preconditioning.
We found that adenosine, and the synthetic agonist RPIA, induced a decrease in Bim protein levels and provided neuroprotection. The effect of adenosine on the protein Bim was investigated further and shown to be blocked by both U0126 and MG132. This is consistent with our previous study, which showed that Bim degradation following preconditioning ischemia was blocked by both the MEK inhibitor U0126 and the proteasome inhibitor MG132 [13]. In addition, adenosine-mediated ischemic tolerance was blocked by U0126 and MG132. While we did not measure Bim ubiquitination in this study, these data are consistent with the known biology of Bim ubiquitination, which has been shown to be regulated by its phosphorylation by p42/p44 MAPK, but not other members of the MAPK family of protein kinases [30, 32]. Taken together, these data would suggest that ischemia induces adenosine release, which activates the A1 receptor resulting in MAPK activation and Bim degradation.
Following preconditioning ischemia there is an increase in protein ubiquitination and rapid ischemic tolerance is blocked by multiple proteasome inhibitors, including MG132, MG115 and β-clasto-lactacystin [21]. The ubiquitination and degradation of Bim could be a key strategy in regulating cell death for multiple therapeutic applications. Currently a number of therapeutic strategies are being devised to regulate cell death in tumors, via the manipulation of bcl-2 family protein signaling, for example ABT 737 [33]. Pro-survival bcl-2 proteins have been shown to be increased following preconditioning ischemia, but in the context of delayed ischemic tolerance [22, 23]. Rapid ischemic tolerance appears to involve the rapid degradation of Bim [13], which would reduce the potential for proapoptotic signaling following a subsequent ischemic insult. Hence, both rapid and delayed ischemic tolerance use different mechanisms to tip the balance of bcl-2 protein signaling in favor of cell survival. Manipulation of the Bim-ubiquitination system may be a good target for anti-stroke therapeutic strategies, especially since we show here that pharmacological manipulation of neurons can enhance Bim degradation resulting in neuroprotection.
The molecular mechanisms whereby adenosine imparts its protection are far from clear. Previous studies show a role of KATP channels [16, 20, 31], and here we show a role of p42/p44 MAPK and protein degradation via the protea-some. However, in our studies we did not observe a blockade of tolerance by glibenclamide (a KATP channel blocker) nor was there a consistent affect of diazoxide on Bim protein levels (data not shown). These data may suggest that the molecular signaling effects of adenosine diverge, requiring both KATP channel activation and Bim degradation to confer rapid ischemic tolerance to neurons. Whether adenosine and diazoxide also result in synaptic reorganization, which we have recently shown to be critical for rapid ischemic tolerance, is currently under investigation [21].
The molecular mechanisms by which all preconditioning stimuli result in tolerance to ischemia are far from clear. There can be two logical mechanisms by which preconditioning stimuli work, either a common biological signature or disparate molecular programs. The consequence of each has implications for how we would translate preconditioning into clinical use. If a common mechanism mediates preconditioning, then tolerance could be induced clinically with the most benign preconditioning stimuli. Our experiments suggest that some preconditioning agents may induce rapid ischemic tolerance via similar molecular mechanisms, which leads us to suggest the pharmacological activation of tolerance may have the potential for therapeutic application.
Summary
Here we show that pharmacological preconditioning promotes the degradation of the pro-cell death protein Bim. If other pharmacological agents can be identified that promote Bim degradation this pro-apoptotic target may have utility as an anti-stroke strategy or for prophylactic therapy in patients undergoing procedures where ischemic conditions are likely, such as cardio-pulmonary bypass.
Acknowledgments
This work was funded by NIH/ NINDS grants to R Meller (NS59588) and RP Simon (NS24728).
References
- 1.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 2.Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- 3.Kuroiwa T, Yamada I, Endo S, Hakamata Y, Ito U. 3-Nitropropionic acid preconditioning ameliorates delayed neurological deterioration and infarction after transient focal cerebral ischemia in gerbils. Neuroscience Letters. 2000;283:145–148. doi: 10.1016/s0304-3940(00)00937-x. [DOI] [PubMed] [Google Scholar]
- 4.Weih M, Bergk A, Isaev NK, Ruscher K, Megow D, Riepe M, Meisel A, Victorov IV, Dirnagl U. Induction of ischemic tolerance in rat cortical neurons by 3-nitropropionic acid: chemical preconditioning. Neuroscience Letters. 1999;272:207–210. doi: 10.1016/s0304-3940(99)00594-7. [DOI] [PubMed] [Google Scholar]
- 5.Loftus LT, Gala R, Yang T, Jessick VJ, Ashley MD, Ordonez AN, Thompson SJ, Simon RP, Meller R. Sumo-2/3-ylation following in vitro modeled ischemia is reduced in delayed ischemic tolerance. Brain Res. 2009;1272:71–80. doi: 10.1016/j.brainres.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emerson MR, Nelson SR, Samson FE, Pazdernik TL. A global hypoxia preconditioning model: neuroprotection against seizure-induced specific gravity changes (edema) and brain damage in rats. Brain Res Brain Res Protoc. 1999;4:360–366. doi: 10.1016/s1385-299x(99)00041-0. [DOI] [PubMed] [Google Scholar]
- 7.Ota A, Ikeda T, Xia XY, Xia YX, Ikenoue T. Hypoxic-ischemic tolerance induced by hyperthermic pretreatment in newborn rats. J Soc Gynecol Investig. 2000;7:102–105. [PubMed] [Google Scholar]
- 8.Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K, et al. ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 1990;528:21–24. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- 9.Towfighi J, Housman C, Mauger D, Vannucci RC. Effect of seizures on cerebral hypoxic-ischemic lesions in immature rats. Brain Res Dev Brain Res. 1999;113:83–95. doi: 10.1016/s0165-3806(99)00004-8. [DOI] [PubMed] [Google Scholar]
- 10.Barone FC, White RF, Spera PA, Ellison J, Currie RW, Wang X, Feuerstein GZ. Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29:1937–1950. doi: 10.1161/01.str.29.9.1937. discussion 1950-1931. [DOI] [PubMed] [Google Scholar]
- 11.Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan JQ, Henshall DC, Simon RP. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:234–246. doi: 10.1038/sj.jcbfm.9600024. [DOI] [PubMed] [Google Scholar]
- 12.Dhodda VK, Sailor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of precondi-tioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J Neurochem. 2004;89:73–89. doi: 10.1111/j.1471-4159.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- 13.Meller R, Cameron JA, Torrey DJ, Clayton CE, Ordonez AN, Henshall DC, Minami M, Schindler CK, Saugstad JA, Simon RP. Rapid degradation of Bim by the ubiquitin-proteasome pathway mediates short-term ischemic tolerance in cultured neurons. J Biol Chem. 2006;281:7429–7436. doi: 10.1074/jbc.M512138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Pinzon MA, Xu GP, Mumford PL, Dietrich WD, Rosenthal M, Sick TJ. Rapid ischemic preconditioning protects rats from cerebral an-oxia/ischemia. Adv Exp Med Biol. 1997;428:155–161. doi: 10.1007/978-1-4615-5399-1_22. [DOI] [PubMed] [Google Scholar]
- 15.Reshef A, Sperling O, Zoref-Shani E. Opening of ATP-sensitive potassium channels by cromakalim confers tolerance against chemical ischemia in rat neuronal cultures. Neurosci Lett. 1998;250:111–114. doi: 10.1016/s0304-3940(98)00458-3. [DOI] [PubMed] [Google Scholar]
- 16.Reshef A, Sperling O, Zoref-Shani E. Role of K(ATP) channels in the induction of ischemic tolerance by the ‘adenosine mechanism’ in neuronal cultures. Adv Exp Med Biol. 2000;486:217–221. [PubMed] [Google Scholar]
- 17.Reshef A, Sperling O, Zoref-Shani E. Preconditioning of primary rat neuronal cultures against ischemic injury: characterization of the “time window of protection’. Brain Res. 1996;741:252–257. doi: 10.1016/s0006-8993(96)00939-0. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Pinzon MA, Mumford PL, Rosenthal M, Sick TJ. Anoxic preconditioning in hippocampal slices: role of adenosine. Neuroscience. 1996;75:687–694. doi: 10.1016/0306-4522(96)00311-9. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Xiong L, Chen S, Wang Q. Isoflurane tolerance against focal cerebral ischemia is attenuated by adenosine A1 receptor antagonists. Can J Anaesth. 2006;53:194–201. doi: 10.1007/BF03021827. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Pinzon MA, Born JG. Rapid preconditioning neuroprotection following anoxia in hippocampal slices: role of the K+ ATP channel and protein kinase C. Neuroscience. 1999;89:453–459. doi: 10.1016/s0306-4522(98)00560-0. [DOI] [PubMed] [Google Scholar]
- 21.Meller R, Thompson SJ, Lusardi TA, Ordonez AN, Ashley MD, Jessick V, Wang W, Torrey DJ, Henshall DC, Gafken PR, Saugstad JA, Xiong ZG, Simon RP. Ubiquitin proteasome-mediated synaptic reorganization: a novel mechanism underlying rapid ischemic tolerance. J Neurosci. 2008;28:50–59. doi: 10.1523/JNEUROSCI.3474-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bossenmeyer-Pourie C, Daval JL. Prevention from hypoxia-induced apoptosis by preconditioning: a mechanistic approach in cultured neurons from fetal rat forebrain. Brain Res Mol Brain Res. 1998;58:237–239. doi: 10.1016/s0169-328x(98)00123-5. [DOI] [PubMed] [Google Scholar]
- 23.Minami M, Jin KL, Li W, Nagayama T, Henshall DC, Simon RP. Bcl-w expression is increased in brain regions affected by focal cerebral ischemia in the rat. Neurosci Lett. 2000;279:193–195. doi: 10.1016/s0304-3940(99)00987-8. [DOI] [PubMed] [Google Scholar]
- 24.Kato K, Shimazaki K, Kamiya T, Amemiya S, Inaba T, Oguro K, Katayama Y. Differential effects of sublethal ischemia and chemical preconditioning with 3-nitropropionic acid on protein expression in gerbil hippocampus. Life Sci. 2005;77:2867–2878. doi: 10.1016/j.lfs.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 25.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 Signaling Pathway Promotes Phosphorylation and Protea-some-dependent Degradation of the BH3-only Protein, Bim. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 27.Putcha G, Le S, Frank S, Besirli CG, Clark K, Chu B, Alix S, Youle RJ, LaMarche A, Maroney AC, Johnson EM. JNK-Mediated BIM Phosphorylation Potentiates BAX-Dependent Apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 28.Shinoda S, Schindler CK, Meller R, So NK, Araki T, Yamamoto A, Lan JQ, Taki W, Simon RP, Henshall DC. Bim regulation may determine hippocampal vulnerability after injurious seizures and in temporal lobe epilepsy. J Clin Invest. 2004;113:1059–1068. doi: 10.1172/JCI19971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura M, Nakakimura K, Matsumoto M, Sakabe T. Rapid tolerance to focal cerebral ischemia in rats is attenuated by adenosine A1 receptor antagonist. J Cereb Blood Flow Metab. 2002;22:161–170. doi: 10.1097/00004647-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Ley R, Ewings KE, Hadfield K, Howes E, Balmanno K, Cook SJ. Extracellular signal-regulated kinases 1/2 are serum-stimulated “Bim(EL) kinases” that bind to the BH3-only protein Bim(EL) causing its phosphorylation and turnover. J Biol Chem. 2004;279:8837–8847. doi: 10.1074/jbc.M311578200. [DOI] [PubMed] [Google Scholar]
- 31.Reshef A, Sperling O, Zoref-Shani E. Opening of K(ATP) channels is mandatory for acquisition of ischemic tolerance by adenosine. Neuroreport. 2000;11:463–465. doi: 10.1097/00001756-200002280-00007. [DOI] [PubMed] [Google Scholar]
- 32.Ley R, Hadfield K, Howes E, Cook SJ. Identification of a DEF-type docking domain for extracellular signal-regulated kinases 1/2 that directs phosphorylation and turnover of the BH3-only protein BimEL. J Biol Chem. 2005;280:17657–17663. doi: 10.1074/jbc.M412342200. [DOI] [PubMed] [Google Scholar]
- 33.Kline MP, Rajkumar SV, Timm MM, Kimlinger TK, Haug JL, Lust JA, Greipp PR, Kumar S. ABT-737, an inhibitor of Bcl-2 family proteins, is a potent inducer of apoptosis in multiple myeloma cells. Leukemia. 2007;21:1549–1560. doi: 10.1038/sj.leu.2404719. [DOI] [PubMed] [Google Scholar]