Abstract
Essential amino acids (EAA) stimulate skeletal muscle protein synthesis (MPS) in humans. Leucine may have a greater stimulatory effect on MPS than other EAA and/or decrease muscle protein breakdown (MPB). To determine the effect of 2 different leucine concentrations on muscle protein turnover and associated signaling, young men (n = 6) and women (n = 8) ingested 10 g EAA in 1 of 2 groups: composition typical of high quality proteins (CTRL; 1.8 g leucine) or increased leucine concentration (LEU; 3.5 g leucine). Participants were studied for 180 min postingestion. Fractional synthetic rate and leg phenylalanine and leucine kinetics were assessed on muscle biopsies using stable isotopic techniques. Signaling was determined by immunoblotting. Arterial leucine concentration and delivery to the leg increased in both groups and was significantly higher in LEU than in CTRL; however, transport into the muscle and intracellular availability did not differ between groups. MPS increased similarly in both groups 60 min postingestion. MPB decreased at 60 min only in LEU, but net muscle protein balance improved similarly. Components of mammalian target of rapamycin (mTOR) signaling were improved in LEU, but no changes were observed in ubiquitin-proteasome system signaling. Changes in light chain 3 and mTOR association with Unc-51-like kinase 1 indicate autophagy decreased more in LEU. We conclude that in 10 g of EAA, the leucine content typical of high quality proteins (~1.8 g) is sufficient to induce a maximal skeletal muscle protein anabolic response in young adults, but leucine may play a role in autophagy regulation.
Introduction
It is well established that essential amino acids (EAA)9 stimulate skeletal muscle protein synthesis (MPS) in animal and human models (1–4). Interestingly, the amino acid leucine stimulates MPS independently of all other amino acids in animal models and is a potent stimulator of the cell hypertrophy mammalian target of rapamycin complex 1 (mTORC1) pathway (1, 5–8). Additionally, leucine decreases muscle protein breakdown (MPB) and breakdown-associated cellular signaling and mRNA expression (9–12). These unique qualities have made leucine a target of interest in recent years as a potential intervention to promote MPS and decrease MPB, thereby facilitating the maintenance of skeletal muscle mass. However, the mechanisms involved in leucine regulation of muscle protein metabolism are largely unknown.
Increases in MPS following mixed EAA or leucine ingestion are associated with enhanced translation initiation via activation of mTORC1 and downstream targets ribosomal S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E binding protein 1 (4E-BP1) (7, 13, 14). Upstream of mTOR, Akt/protein kinase B can directly activate mTOR through phosphorylation (15) or indirectly by phosphorylating (and inhibiting) tuberous sclerosis complex 2 (16, 17), while further downstream, S6K1 signals to eukaryotic elongation factor 2 (eEF2) to enhance translation elongation (18). The mTORC1 pathway is a major contributor to the anabolic response following EAA or leucine ingestion, although multiple pathways are involved.
Similarly, regulation of MPB is a complex process controlled by multiple systems, including the ubiquitin-proteasome system (UPS), the autophagy-lysosome system, calpains, and caspases. Although mixed amino acids or EAA do not reduce MPB in humans (19, 20), evidence indicates infusion of branched-chain amino acids or leucine alone can decrease MPB (12, 21). In chick skeletal muscle, leucine decreased MPB in association with decreased ubiquitin and 20S proteasome C2 subunit mRNA expression (11). However, a study in rat skeletal muscle reported no change in proteasome mRNA expression or expression of E3 ubiquitin-ligases muscle atrophy F-box (MAFbx, also known as atrogin-1) and Muscle RING Finger 1 (MuRF1), but a decrease in autophagy marker microtubule-associated protein 1 light chain 3 (LC3) B-II expression (22). Overall, verification of the effects of leucine on MPB pathways with concurrent measures of muscle protein turnover in humans is lacking.
Despite strong evidence that leucine stimulates MPS in animal models, reports in humans vary, with several studies indicating no further improvement in MPS following leucine infusion (12), addition of leucine to an amino acid solution (23), or with increasing leucine concentrations in a mixture of EAA in healthy young adults (24). On the other hand, investigations conducted with similar study designs but differing amounts of EAA (6.7 vs. 15 g) and leucine content (1.72 vs. 2.79 g) indicate the higher amount of EAA, and thus higher leucine, does improve protein synthesis and overall net protein balance compared with a lower dose (25, 26). However, it is impossible to delineate whether this effect is due to the higher amount of leucine or simply a larger dose of all of the EAA. There are also conflicting results concerning the effects of leucine on MPB pathways in animal models and little information available in humans. Therefore, the purpose of this study was to determine the effects of different leucine concentrations in an isonitrogenous EAA mixture on muscle protein turnover (synthesis and breakdown) and associated cellular signaling. Specifically, we hypothesized that a concentration of leucine higher than that contained in a typical high-quality protein would elicit an increase in MPS and mTORC1 signaling and decrease MPB and UPS and/or autophagy-lysosomal signaling in young adults.
Materials and Methods
Participants.
We studied 14 young participants (6 men, 8 women) who reported not being currently engaged in any regular exercise training during the screening interview. Screenings of participants were performed with clinical history, physical exam, and laboratory tests, including complete blood count with differential, liver and kidney function tests, coagulation profile, fasting blood glucose and oral glucose tolerance tests, hepatitis B and C screening, HIV test, thyroid stimulating hormone, lipid profile, urinalysis, and drug screening. Volunteers were asked to refrain from performing vigorous physical activity for 48 h prior to participating in the study. All participants gave informed, written consent before participating in the study, which was approved by the Institutional Review Board of the University of Texas Medical Branch (which is in compliance with the Declaration of Helsinki as revised in 1983). A dual-energy X-ray absorptiometry scan (Hologic QDR 4500W) was performed to measure body composition and lean mass. The participants’ physical characteristics are summarized in Table 1.
TABLE 1.
Participants' characteristics1
| CTRL | LEU | |
| n (men, women) | 3, 4 | 3, 4 |
| Age, y | 32 ± 2 | 29 ± 2 |
| Height, cm | 170 ± 5 | 167 ± 6 |
| Weight, kg | 73 ± 3 | 71 ± 6 |
| BMI, −2 | 26 ± 1 | 25 ± 1 |
| Lean body mass, kg | 53 ± 4 | 50 ± 6 |
| Body fat, % | 25 ± 3 | 27 ± 4 |
Values are mean ± SEM.
Experimental design.
Details of the experimental design have been previously reported (27). Briefly, all participants were studied following an overnight fast under basal conditions and at the same time of day (between 0700 and 1600 h). After drawing a background blood sample, we started a primed continuous infusion of l-[13C6] phenylalanine (priming dose = 2 μmol · kg−1, infusion rate = 0.05 μmol · kg−1· min−1) and l-1-13C leucine (priming dose = 4.8 μmol · kg−1, infusion rate = 0.08 μmol · kg−1 · min−1) and maintained it at a constant rate until the end of the experiment (Isotec, Sigma-Aldrich).
At 2.5 h after starting the tracer infusion, and marking the beginning of the baseline period, the first muscle biopsy was obtained from the lateral portion of the vastus lateralis using sterile procedure and local anesthesia (1% lidocaine). After the first biopsy, infusion of indocyanine green (ICG; Akorn) was begun in the femoral artery (0.5 mg · min−1) and maintained for 10 min. Blood samples were obtained from the femoral vein and the arterialized hand vein to measure ICG concentration. Blood was also obtained from the femoral artery and vein and from the arterialized hand vein to measure amino acid enrichments, glucose, and insulin concentrations. The sequence was repeated for a total of 4 sets of blood draws per period, ∼15 min apart. Following the 4th set of blood draws, a second biopsy was obtained.
After the second baseline biopsy, participants ingested either the CTRL or LEU nutrient solution (composition below). Muscle biopsies were then sampled at 60, 120, and 180 min following ingestion of the nutrient solution. ICG infusion and blood sampling were performed in each of the periods as described previously.
Nutrient solution composition.
Both CTRL and LEU solutions contained 10 g EAA mixed in a noncaloric, noncaffeinated carbonated beverage (500 mL). The composition of the CTRL mixture was representative of high-quality protein and contained 18% leucine, whereas the LEU mixture contained 35% leucine (Sigma-Aldrich). To minimize changes in blood enrichments of the tracers, we added a small amount of l-[13C6] phenylalanine and l-1-13C leucine to the nutrient solutions (Isotec, Sigma-Aldrich; nutrient solution composition is reported in Table 2).
TABLE 2.
EAA content in beverage
| CTRL | LEU | |
| g | ||
| Histidine | 1.10 | 0.80 |
| Isoleucine | 1.00 | 0.80 |
| Leucine | 1.85 | 3.50 |
| Lysine | 1.55 | 1.20 |
| Methionine | 0.30 | 0.30 |
| Phenylalanine | 1.55 | 1.40 |
| Threonine | 1.45 | 1.00 |
| Valine | 1.20 | 1.00 |
| Total EAA | 10.0 | 10.0 |
| l-[13C6] phenylalanine | 0.10 | 0.09 |
| l-1-13C leucine | 0.10 | 0.19 |
Determination of blood flow, glucose, and insulin concentrations.
The serum ICG concentration to determine leg blood flow was measured spectrophotometrically (Beckman Coulter) at λ = 805 nm (28). The plasma glucose concentration was measured using an automated glucose and lactate analyzer (YSI). Plasma insulin concentrations were determined by ELISA (Millipore).
Amino acid enrichments and concentrations.
Enrichments of blood leucine and phenylalanine were determined on their tert-butyldimethylsilyl derivatives using l-leucine 5,5,5-d3 and l-[15N]phenylalanine, respectively, as internal standards and by GC-MS (6890 Plus GC, 5973N MSD/DS, 7683 autosampler, Agilent Technologies) to calculate amino acid concentrations as previously described (29). Additionally, we used the EZ:faast GC/MS Free (Physiological) Amino Acid Analysis kit (Phenomenex) to measure concentrations of 9 essential and 10 non-EAA in plasma from the femoral artery, according to the manufacturer’s instructions. Muscle tissue samples were ground and intracellular free amino acids and muscle proteins extracted as previously described (30). Intracellular free enrichments of phenylalanine were determined by GC-MS using l-[15N]phenylalanine as an internal standard, and protein-bound phenylalanine enrichments were measured by GC-MS (30).
Calculations.
Kinetics were calculated using both the 2-pool and 3-pool models of leg muscle amino acid kinetics and have been described in detail elsewhere (31–33). Kinetics calculated for leucine included: 1) delivery to the leg (Fin = BF · CA); 2) transport into the muscle (Fm,a = {[CV · (EM – EV)/(EA – EM)] + CA}· BF); and 3) intracellular availability {Fm.o + Fm,a, where Fm,o = Fm,a · [(EA · EM) – 1]}. Phenylalanine kinetics were calculated for: 1) net balance [NB = BF · (CA – CV)]; 2) rate of appearance and disappearance of phenylalanine (whole body Ra = BF · CA · EA/EV; leg Ra = total Ra − Fin; and leg Rd = leg Ra + NB); 3) release from proteolysis (Fm.o); and 4) utilization for protein synthesis (Fo,m = Fm,o + NB). CA and CV are plasma concentrations in the femoral artery and vein, respectively. EA, EV, and EM are free tracer enrichments (tracer:tracee ratio) in the femoral artery and vein and in muscle, respectively. BF is leg blood flow. The fractional synthetic rate (FSR) of mixed muscle proteins was calculated from phenylalanine as (ΔEp/t) / [(EM(1) + EM(2))/2] · 60 · 100, where ΔEp is the incremental change in enrichment of protein-bound phenylalanine between the 2 biopsies, t is the time between biopsies, and EM(1) and EM(2) are the phenylalanine enrichments in the free intracellular pool in the 2 biopsies. Data are expressed as %/h.
Immunoblot analysis.
Details of the homogenization and immunoblotting techniques can be found elsewhere (34). Briefly, equal amounts of total protein (determined spectrophotometrically) were loaded into each lane and the samples separated by electrophoresis (Criterion, BioRad). Proteins were then transferred to a polyvinylidene difluoride membrane (BioRad) and incubated in primary and secondary antibodies. Chemiluminescent solution (ECL plus, Amersham BioSciences) was applied to each blot. Optical density measurements (ChemiDoc, BioRad) were taken and densitometric analysis performed using Quantity One 4.5.2 software (BioRad). Membranes containing phospho-detected proteins were stripped of primary and secondary antibodies using Restore Western blot Stripping Buffer (Pierce Biotechnology) then reprobed for total protein. Total protein for each phospho-protein was determined for each blot and did not change over the course of the experiment from baseline. Data for phospho-proteins are presented as phosphorylation status relative to a normalization control and as fold of baseline. Data for LC3B-II, MuRF-1, and MAFbx are presented as total protein expression relative to the normalization control and as fold of baseline.
To quantify the mTOR:UNC-51-like kinase (ULK1) complex, mTOR protein was isolated in whole muscle homogenates using an immunoprecipitation technique previously characterized by Williamson et al. (35). Buffer compositions have been previously reported (36). Approximately 30 mg of tissue was homogenized in CHAPS buffer (1:9 wt:v, pH 7.5). Samples were centrifuged at 2500 × g for 5 min at 4°C and total protein content determined spectrophotometrically. Samples (700 μg protein) were combined with 2 μL total mTOR antibody and rocked overnight at 4°C. Samples were combined with 500 μL of BioMag goat anti-rabbit IgG beads (QIAGEN) suspended in CHAPS buffer and 1% nonfat dry milk for 1 h at 4°C and isolated using a magnetic tube rack. Following 2 washes in CHAPS buffer, samples were washed with a second buffer (HEPES, NaCl, pH 7.5). Sixty microliters of 2× sample buffer was added to each sample, boiled for 5 min at 100°C, and separated from beads using the magnetic tube rack. Samples were loaded onto gels as described above and probed with ULK1 followed by total mTOR after stripping. Values of ULK1 are expressed relative to total mTOR in the precipitates.
Antibodies.
The primary phospho- antibodies were purchased from Cell Signaling (Beverley, MA): Akt (Ser473), mTOR (Ser2448), 4E-BP1 (Thr37/46) at 1:1000; S6K1 (Thr389) at 1:500; and eEF2 (Thr56) at 1:2000. Total antibodies purchased from Cell Signaling were Akt, mTOR, S6K1, 4E-BP1, and LC3B at 1:1000; eEF2 at 1:5000; ULK1 at 1:500. MuRF1 and MAFbx (1:1000) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody was purchased from Amersham Bioscience (1:2000; Piscataway, NJ)
Statistical methods.
Values are expressed as mean ± SEM. Comparisons were performed using 2-way repeated-measures ANOVA (SigmaPlot v. 11.0; Systat Software), with the effects of group (CTRL, LEU) and time (baseline and 60, 120, and 180 min postnutrient ingestion; baseline and 30, 60, 120, and 180 min postnutrient ingestion for insulin; or 16 time point intervals for leucine kinetics). Post hoc testing was performed using the Bonferroni’s t test for multiple comparisons compared with baseline and CTRL. If a test of normality or equal variance failed, simple transformations were performed. An independent 2 sample Student’s t test was used to assess differences in calculated area under the curve (AUC) for FSR and phenylalanine kinetics. For all analyses, significance was set at P ≤ 0.05.
Results
Blood flow, glucose, and insulin.
Blood flow did not differ across time or between groups. The arterial glucose concentration decreased at 120 min postnutrient ingestion in the LEU group compared with baseline (4.8 ± 0.1 vs. 5.0 ± 0.1 mmol·L−1, respectively; P < 0.05) and at 180 min postingestion in both groups compared with baseline (CTRL = 5.0 ± 0.1 vs. 5.1 ± 0.1 mmol·L−1; LEU = 4.8 ± 0.1 vs. 5.0 ± 0.1 mmol·L−1, respectively; P < 0.05) with no group differences. Plasma insulin concentrations were elevated in both groups at 30 min following nutrient ingestion over baseline (CTRL = 103 ± 18 vs. 30 ± 5 pmol·L−1; LEU = 92 ± 12 vs. 33 ± 6 pmol·L−1; P < 0.05) and remained elevated at 60 min only in the LEU group (56 ± 8 pmol·L−1; P < 0.05 vs. baseline).
Leucine kinetics.
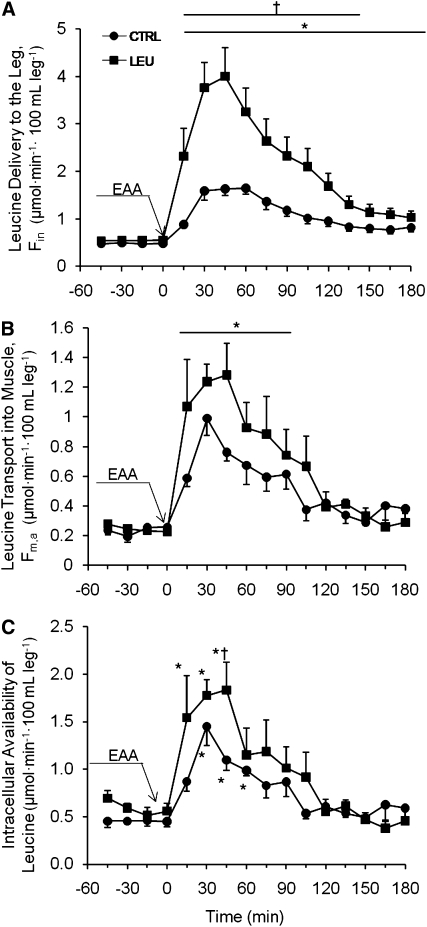
The arterial leucine concentration (Supplemental Table 1) and leucine delivery to the leg (Fin; Fig. 1A) increased in both groups at all time points following beverage ingestion (P < 0.05 vs. baseline) and were higher in the LEU group than in the CTRL group for 135 min following ingestion (P < 0.05). Leucine transport into the muscle (Fm,a) increased at 90 min postbeverage ingestion (P < 0.05; Fig. 1B) with no group differences. Intracellular availability of leucine increased in both groups during the first 60 min following beverage ingestion and was significantly elevated in the LEU group compared with the CTRL group 45 min following ingestion (P < 0.05; Fig. 1C).
FIGURE 1.
Time course of leucine delivery to the leg (A), transport into the muscle (B), and intracellular availability (C) following ingestion of 10 g EAA in young men and women. Values are mean ± SEM, n = 7. *Different from t = −45 (baseline), P < 0.05; †different from CTRL at that time, P < 0.05.
Amino acid concentrations.
The groups did not differ in most of the amino acids following nutrient ingestion (Supplemental Table 1). The groups differed in isoleucine, methionine, and phenylalanine at single time points; isoleucine was lower in the LEU group at 180 min postingestion compared with the CTRL group; methionine was higher in LEU at 15 min postingestion; and phenylalanine was higher in the LEU group at 45 min postingestion (P < 0.05).
Muscle protein turnover.
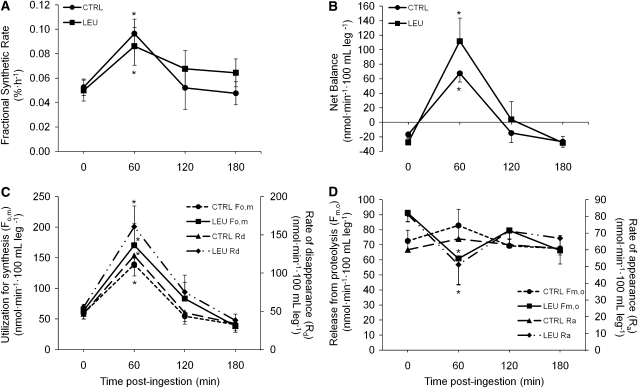
Mixed muscle FSR was elevated in both groups 60 min following nutrient ingestion (P < 0.05; Fig. 2A). FSR calculated over the 180-min period following beverage ingestion was elevated above baseline in the LEU group (0.050%·h−1 vs. 0.076%· h−1; P = 0.05) but not in EAA (0.053%·h−1 vs. 0.061%·h−1; P = 0.52). The AUC for FSR and the 16-point phenylalanine kinetic measures of Fo,m and Rd did not differ between groups (data not shown). However, similar to the FSR results, hourly means of MPS (Fo,m and Rd) were elevated in both groups 60 min postingestion (P < 0.05) with no group differences (Fig. 2C). Net balance increased 60 min following nutrient ingestion in both groups (P < 0.05) with no group differences (Fig. 2B). MPB (Fm,o and Ra) decreased 60 min following nutrient ingestion only in the LEU group (P < 0.05 vs. baseline; Fig. 2D).
FIGURE 2.
FSR (A), net protein balance across the leg (B), protein synthesis (C), and protein breakdown (D) following ingestion of 10 g EAA in young men and women. Values are mean ± SEM, n = 7. *Different from t = 0 (baseline), P < 0.05; †different from CTRL, P < 0.05.
Cellular signaling.
Phosphorylation of Akt (Ser473) was not different at any time point or between groups (Table 3). Phosphorylation of mTOR (Ser2448) increased from baseline at 60 min in the LEU group and at 120 and 180 min in both groups (P < 0.05). S6K1 phosphorylation (Thr389) increased at all time points following beverage ingestion in the LEU group and was elevated at 60 and 180 min in the CTRL group (P < 0.05). Phospho-4E-BP1 (Thr37/46) increased only in the LEU group (P < 0.05 vs. baseline at 60 and 120 min; P < 0.05 vs. CTRL at 60 min). Phosphorylation of eEF2 (Thr56) decreased in both groups at 60 min (P < 0.05) and remained decreased at 120 min only in the LEU group (P < 0.05 vs. baseline). Total ULK1 complexed with mTOR increased at 180 min in the LEU group (P < 0.05 vs. baseline and CTRL). Conversion of LC3B-I to LC3B-II did not differ between groups but tended to decrease 120 min following nutrient ingestion in the LEU group (P = 0.1) and had decreased at 180 min (P < 0.05). It tended to decrease at 180 min in the CTRL group (P = 0.09). There were no changes over time or differences between groups for total protein MuRF1 or MAFbx.
TABLE 3.
Western-blot analyses of synthesis- and breakdown-associated signaling proteins following ingestion of 10 g EAA in young men and women1
| 60 min |
120 min |
180 min |
||||
| Time postnutrient ingestion | CTRL | LEU | CTRL | LEU | CTRL | LEU |
| Fold of baseline | ||||||
| Akt (Ser473) | 0.95 ± 0.14 | 1.38 ± 0.13 | 1.21 ± 0.27 | 1.17 ± 0.23 | 0.79 ± 0.13 | 0.87 ± 0.15 |
| mTOR (Ser2448) | 1.48 ± 0.16 | 2.79 ± 0.72* | 2.12 ± 0.44* | 2.48 ± 0.5* | 2.04 ± 0.41* | 2.64 ± 0.58* |
| S6K1 (Thr389) | 12.5 ± 3.38* | 39.5 ± 12.1* | 2.04 ± 0.35 | 4.69 ± 1.31* | 6.19 ± 1.26* | 9.62 ± 3.72* |
| 4E-BP1 (Thr37/46) | 1.02 ± 0.10 | 1.86 ± 0.34* | 1.39 ± 0.18 | 2.01 ± 0.48* | 1.26 ± 0.27 | 1.52 ± 0.42 |
| eEF2 (Thr56) | 0.66 ± 0.13 | 0.66 ± 0.06* | 1.20 ± 0.14 | 0.83 ± 0.18* | 1.03 ± 0.15 | 0.96 ± 0.24 |
| mTOR:ULK1 | 1.07 ± 0.21 | 2.14 ± 0.43 | 1.28 ± 0.41 | 2.12 ± 0.71 | 0.91 ± 0.2 | 2.31 ± 0.49*† |
| LC3B-I | 1.02 ± 0.15 | 1.21 ± 0.26 | 1.04 ± 0.18 | 1.29 ± 0.35 | 1.03 ± 0.16 | 1.24 ± 0.48 |
| LC3B-II | 0.72 ± 0.15 | 0.66 ± 0.08 | 0.70 ± 0.19 | 0.45 ± 0.09 | 0.60 ± 0.11 | 0.41 ± 0.15* |
| MuRF1 | 1.09 ± 0.07 | 1.06 ± 0.07 | 1.35 ± 0.28 | 1.21 ± 0.18 | 1.27 ± 0.22 | 1.16 ± 0.15 |
| MAFbx | 1.42 ± 0.26 | 0.96 ± 0.06 | 1.71 ± 0.48 | 1.00 ± 0.16 | 1.69 ± 0.45 | 0.97 ± 0.18 |
Values are mean ± SEM, = 7. *Different from baseline, P < 0.05; †different from CTRL at that time, P < 0.05.
Discussion
Despite numerous studies in animal models concerning the positive effects of leucine infusion or ingestion on muscle protein turnover, conflicting results from investigations in humans make reaching a consensus on the effects of leucine in human skeletal muscle difficult. It appears from numerous investigations that protein intake with a sufficient amount of EAA and/or leucine maximally stimulates MPS such that further increases in protein content will not result in additional increases in protein synthesis or net protein balance (37–39). Still, the optimal amount of EAA and/or leucine to maximize the protein anabolic response in young men and women is not clear. We sought to determine the effects of 2 different concentrations of leucine in an isonitrogenous EAA mixture on muscle protein turnover and associated cellular signaling. Specifically, we were interested in whether a higher concentration of leucine would enhance MPS and mTORC1 signaling, and/or decrease MPB and activity of the autophagy-lysosome or UPS. Our results indicate that in 10 g of EAA, a leucine content typical of high-quality proteins (CTRL, 1.8 g) elicits a similar muscle protein anabolic response in young adults compared with higher leucine concentrations (LEU). However, a higher leucine concentration than that typical of high-quality proteins transiently decreased MPB and tended to enhance mTORC1 signaling. Nonetheless, small changes in mTORC1 signaling and MPB did not translate into significant improvements in MPS or overall muscle protein net balance.
Large doses of leucine cause a small and transient increase in circulating insulin levels in animal models (6, 7, 40) and we report similar findings of small increases in plasma insulin compared with baseline values in both groups 30 min following nutrient ingestion. This increase in insulin was sustained only in the LEU group at 60 min. Because insulin decreases MPB (19, 41, 42), the prolonged insulin response in the LEU group may have contributed to the observed decreases in MPB at 60 min postingestion. However, despite the small increases in insulin levels in both groups, we did not detect any changes in Akt phosphorylation. This result is not completely unexpected, because leucine has been reported to decrease insulin-stimulated phosphatidylinositol 3-kinase activity in rat skeletal muscle (43). Additionally, Akt is an upstream inhibitor of the FOXO (Forkhead box) family of transcription factors (44), which control MuRF1 and MAFbx expression; similar to Akt, no changes were observed in the total protein content of either E3 ubiquitin ligase.
In contrast to the UPS, there were significant changes in components of the autophagy-lysosome system. ULK1 is essential for autophagy (45) and is part of a complex (with autophagy factor Atg13 and focal adhesion kinase family-interacting protein of 200kDa) that is bound to, phosphorylated, and inhibited by mTORC1 in nutrient-rich conditions, preventing autophagy (46). ULK1 complexed to mTOR (i.e. inhibition of autophagy) was significantly elevated at 180 min in the LEU group, consistent with greater decreases in MPB in this group. Similarly, the autophagy marker LC3B-II decreased in both groups over the 180-min period but more so in LEU. Overall, these results indicate subtle differences in insulin action and markers of autophagy occurred in conjunction with a small but significant decrease in measures of MPB with a higher leucine concentration.
Similar to a previous report that found no changes in intracellular concentrations of amino acids despite their higher circulating levels (47), this study found that higher arterial concentrations of leucine in the LEU group did not translate to an increased rate of transport into the muscle compared with the CTRL group at any time point. Also, despite the leucine concentration and delivery to the leg being elevated for the entire 180-min period following nutrient ingestion, transport and intracellular availability were increased for shorter durations. Previous reports have hypothesized and/or concluded that human MPS is modulated by extracellular amino acid availability (48, 49), which would suggest higher MPS in the LEU group due to higher extracellular leucine concentrations. However, no group differences were observed for any measure of MPS despite significantly higher extracellular concentrations of leucine. These data support the idea that MPS may not be regulated solely by extracellular amino acid availability but is also influenced by amino acid transport into the cell and intracellular availability (50, 51).
It is well established that a decrease in blood amino acid availability inhibits MPS (52–54). Here, we report 19 amino acid concentrations across the 180-min time course following nutrient beverage ingestion (Supplemental Table 1). The majority of amino acids remained elevated or stable for at least the first 120 min following beverage ingestion. Therefore, because the majority of blood amino acids were maintained but MPS increased only during the first 60 min following ingestion (i.e. when intracellular amino acid availability increased), further considerations about the role of extracellular compared with intracellular amino acid availability in regulating MPS are warranted. The fact that higher arterial leucine concentrations did not result in increased leucine transport or intracellular availability suggests a rate-limiting step at the level of leucine transport from blood into the cell, perhaps by lack of available amino acid transporters such as the l-glutamine transporter SNAT2 (a System A-type sodium-coupled neutral amino acid transporter) (27, 55). Alternatively, we acknowledge the possibility that small decreases in some of the amino acids may be responsible for the inability of MPS to remain elevated for longer periods of time and/or be rate limiting for membrane transport. For example, leucine was elevated in the LEU group due to the higher leucine content of the nutrient beverage, whereas the discrepancy in isoleucine concentration between groups by 180 min may be attributable to the lowered isoleucine content of the LEU beverage. Because of the increased leucine and decreased isoleucine concentrations, the leucine:isoleucine ratio was increased in the LEU group at several time points. Therefore, the decline in isoleucine concentration may have been the limiting factor for leucine transport into the cell and future investigations are required to examine this potential explanation.
Although there were no significant group differences in any measure of MPS (FSR, Fo,m, Rd) or mTORC1 signaling for the most part, the common theme throughout was a prolonged response with the higher leucine concentration. The AUC did not differ between groups for any measure of synthesis, but FSR calculated over the entire 180-min postprandial period (biopsy 2 to biopsy 5) was significantly elevated compared with baseline only in the LEU group. In addition, the net balance remained positive at 120 min in the LEU group but returned to baseline values in the CTRL group, similar to results reported by Katsanos et al. (24) in which net balance remained elevated for a longer time with a higher leucine concentration in young participants. These results correspond to earlier and/or greater increases of mTOR and 4E-BP1 phosphorylation and a prolonged dephosphorylation of eEF2 (indicative of enhanced translation elongation) in the LEU group. Although the findings support the idea that mTORC1 signaling is acutely responsive to differing concentrations of insulin and amino acids (specifically leucine) (56), the question of what level of changes in mTORC1 signaling translate to significant increases in MPS remains unresolved. Further studies are required to determine whether these small improvements in MPS and mTORC1 signaling can generate significant increases in net muscle protein accretion over time, while considering potential adverse effects that may occur with long-term consumption of high quantities of leucine (8).
In summary, a higher concentration of leucine in 10 g of EAA slightly prolongs the insulin response, which may contribute to a decrease in MPB. Despite higher circulating leucine concentrations in the LEU group, leucine transport and intracellular availability did not differ between groups, suggesting both a rate-limiting step at the level of membrane transport and that MPS is more dependent on intracellular compared with extracellular amino acid availability. Although mTORC1 signaling was slightly enhanced and autophagy-lysosome system markers decreased with higher leucine concentration, effects on measures of muscle protein turnover were small and most likely negligible. Future studies are required to determine whether the seemingly small improvements in net muscle protein balance with higher leucine translate into clinically meaningful changes in skeletal muscle mass over time and whether nutrients (EAAs and/or leucine) play a regulatory role in the control of human skeletal muscle autophagy. However, taken in the context of the current literature regarding the amount of leucine able to elicit a maximal protein anabolic response, our results are in agreement with others that indicate ~1.8 g in an amino acid mixture is sufficient.
Supplementary Material
Acknowledgments
We thank Ming-Qian Zheng and Shelley Medina for technical assistance and Dr. Sarah Toombs-Smith for editing the manuscript. B.B.R. and E.L.G. developed the research proposal; B.B.R. and E.V. developed the research design; E.L.G. and B.B.R. wrote the manuscript; E.L.G., C.S.F., M.J.D., E.V., and B.B.R. analyzed the data; E.L.G., C.S.F., M.J.D., K.L.T., and S.D. collected data and reviewed the manuscript. All authors read and approved the final draft of the manuscript.
Footnotes
Supported by NIH/NIAMS grant AR049877 (B.B.R.) and was conducted at the Institute for Translational Sciences Clinical Research Center at the University of Texas Medical Branch and supported in part by grant 1UL1RR029876-01 from the NIH/National Center for Research Resources and NIH T32-HD07539.
Supplemental Table 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AUC, area under curve; CTRL, control group; EAA, essential amino acid; 4E-BP1, eukaryotic initiation factor 4E binding protein 1; eEF2, eukaryotic elongation factor 2; FSR, fractional synthetic rate; ICG, indocyanine green; LC3, microtubule-associated protein 1 light chain 3; LEU, increased leucine concentration group; MAFbx, muscle atrophy F-box (Atrogin-1); MPB, muscle protein breakdown; MPS, muscle protein synthesis; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; MuRF1, muscle RING Finger 1; S6K1, ribosomal S6 kinase 1; ULK1, Unc-51-like kinase 1; UPS, ubiquitin-proteasome system.
Literature Cited
- 1.Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-[1–13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1–13C]valine. Am J Physiol. 1992;262:E372–6 [DOI] [PubMed] [Google Scholar]
- 2.Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol. 1998;275:E73–8 [DOI] [PubMed] [Google Scholar]
- 3.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garlick PJ, Grant I. Amino-acid infusion increases the sensitivity of muscle protein-synthesis in vivo to insulin: effect of branched-chain amino-acids. Biochem J. 1988;254:579–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:E612–21 [DOI] [PubMed] [Google Scholar]
- 6.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–45 [DOI] [PubMed] [Google Scholar]
- 7.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–9 [DOI] [PubMed] [Google Scholar]
- 8.Garlick PJ. The role of leucine in the regulation of protein metabolism. J Nutr. 2005;135:S1553–6 [DOI] [PubMed] [Google Scholar]
- 9.Mordier S, Deval C, Bechet D, Tassa A, Ferrara M. Leucine limitation induces autophagy and activation of lysosome-dependent proteolysis in C2C12 myotubes through a mammalian target of rapamycin-independent signaling pathway. J Biol Chem. 2000;275:29900–6 [DOI] [PubMed] [Google Scholar]
- 10.Nagasawa T, Kido T, Yoshizawa F, Ito Y, Nishizawa N. Rapid suppression of protein degradation in skeletal muscle after oral feeding of leucine in rats. J Nutr Biochem. 2002;13:121–7 [DOI] [PubMed] [Google Scholar]
- 11.Nakashima K, Ishida A, Yamazaki M, Abe H. Leucine suppresses myofibrillar proteolysis by down-regulating ubiquitin-proteasome pathway in chick skeletal muscles. Biochem Biophys Res Commun. 2005;336:660–6 [DOI] [PubMed] [Google Scholar]
- 12.Nair KS, Schwartz RG, Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol. 1992;263:E928–34 [DOI] [PubMed] [Google Scholar]
- 13.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007;582:813–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–4 [DOI] [PubMed] [Google Scholar]
- 15.Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344:427–31 [PMC free article] [PubMed] [Google Scholar]
- 16.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57 [DOI] [PubMed] [Google Scholar]
- 17.Manning BD, Tee AR, Logsdon N, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Mol Cell. 2002;10:151–62 [DOI] [PubMed] [Google Scholar]
- 18.Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–8 [DOI] [PubMed] [Google Scholar]
- 19.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–9 [DOI] [PubMed] [Google Scholar]
- 21.Louard RJ, Barrett EJ, Gelfand RA. Effect of infused branched-chain amino-acids on muscle and whole-body amino-acid-metabolism in man. Clin Sci (Lond). 1990;79:457–66 [DOI] [PubMed] [Google Scholar]
- 22.Sugawara T, Ito Y, Nishizawa N, Nagasawa T. Regulation of muscle protein degradation, not synthesis, by dietary leucine in rats fed a protein-deficient diet. Amino Acids. 2009;37:609–16 [DOI] [PubMed] [Google Scholar]
- 23.Koopman R, Verdijk LB, Beelen M, Gorselink M, Kruseman AN, Wagenmakers AJM, Kuipers H, van Loon LJC. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br J Nutr. 2008;99:571–80 [DOI] [PubMed] [Google Scholar]
- 24.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–7 [DOI] [PubMed] [Google Scholar]
- 25.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–73 [DOI] [PubMed] [Google Scholar]
- 26.Paddon-Jones D, Sheffield-Moore M, Zhang X-J, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–8 [DOI] [PubMed] [Google Scholar]
- 27.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–73 [DOI] [PubMed] [Google Scholar]
- 29.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research principles and practice of kinetic analysis. 2nd ed Hoboken (NJ): Wiley-Liss; 2005 [Google Scholar]
- 30.Wolfe RR. Radioactive and stable isotope tracers in biomedicine. Principles and practice of kinetic analysis. New York: Wiley-Liss; 1992 [Google Scholar]
- 31.Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Am J Physiol Endocrinol Metab. 2007;292:E77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell JA, Fujita S, Volpi E, Cadenas JG, Rasmussen BB. Short-term insulin and nutritional energy provision do not stimulate muscle protein synthesis if blood amino acid availability decreases. Am J Physiol Endocrinol Metab. 2005;289:E999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol. 1995;268:E75–84 [DOI] [PubMed] [Google Scholar]
- 34.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E–BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williamson DL, Bolster DR, Kimball SR, Jefferson LS. Time course changes in signaling pathways and protein synthesis in C2C12 myotubes following AMPK activation by AICAR. Am J Physiol Endocrinol Metab. 2006;291:E80–9 [DOI] [PubMed] [Google Scholar]
- 36.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, Ritz P, Antoine JM, Prugnaud J, et al. Protein feeding pattern does not affect protein retention in young women. J Nutr. 2000;130:1700–4 [DOI] [PubMed] [Google Scholar]
- 38.Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, Ballevre O, Beaufrere B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001;280:E340–8 [DOI] [PubMed] [Google Scholar]
- 39.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89:161–8 [DOI] [PubMed] [Google Scholar]
- 40.Rocha DM, Unger RH, Faloona GR. Glucagon-stimulating activity of 20 amino-acids in dogs. J Clin Invest. 1972;51:2346–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gelfand RA, Barrett EJ. Effect of physiological hyperinsulinemia on skeletal-muscle protein-synthesis and breakdown in man. J Clin Invest. 1987;80:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Louard RJ, Fryburg DA, Gelfand RA, Barrett EJ. Insulin sensitivity of protein and glucose-metabolism in human forearm skeletal-muscle. J Clin Invest. 1992;90:2348–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baum JI, O'Connor JC, Seyler JE, Anthony TG, Freund GG, Layman DK. Leucine reduces the duration of insulin-induced PI 3-kinase activity in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E86–91 [DOI] [PubMed] [Google Scholar]
- 44.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68 [DOI] [PubMed] [Google Scholar]
- 45.Chan EYW, Kir S, Tooze SA. SiRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J Biol Chem. 2007;282:25464–74 [DOI] [PubMed] [Google Scholar]
- 46.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13–FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borsheim E, Kobayashi H, Traber DL, Wolfe RR. Compartmental distribution of amino acids during hemodialysis-induced hypoaminoacidemia. Am J Physiol Endocrinol Metab. 2006;290:E643–52 [DOI] [PubMed] [Google Scholar]
- 49.Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol. 2003;552:315–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab. 2009;296:E603–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995;268:E514–20 [DOI] [PubMed] [Google Scholar]
- 52.Wolfe RR. Regulation of muscle protein by amino acids. J Nutr. 2002;132:S3219–24 [DOI] [PubMed] [Google Scholar]
- 53.Nissen S, Haymond MW. Changes in leucine kinetics during meal absorption: effects of dietary leucine availability. Am J Physiol. 1986;250:E695–701 [DOI] [PubMed] [Google Scholar]
- 54.Giordano M, Castellino P, DeFronzo RA. Differential responsiveness of protein synthesis and degradation to amino acid availability in humans. Diabetes. 1996;45:393–9 [DOI] [PubMed] [Google Scholar]
- 55.Evans K, Nasim Z, Brown J, Butler H, Kauser S, Varoqui H, Erickson JD, Herbert TP, Bevington A. Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signalling to protein synthesis in L6 muscle cells. J Am Soc Nephrol. 2007;18:1426–36 [DOI] [PubMed] [Google Scholar]
- 56.Anthony TG, McDaniel BJ, Knoll P, Bunpo P, Paul GL, McNurlan MA. Feeding meals containing soy or whey protein after exercise stimulates protein synthesis and translation initiation in the skeletal muscle of male rats. J Nutr. 2007;137:357–62 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.