Abstract
In adults, maximal suppression of serum parathyroid hormone (PTH) has commonly been used to determine the sufficiency of serum 25-hydroxyvitamin D [25(OH)D]. In children and adolescents, the relationship between serum 25(OH)D and PTH is less clear and most studies reporting a relationship are derived from relatively small samples and homogeneous cohorts. Our objective was to determine the relationship between serum 25(OH)D and PTH in children and adolescents from a large and diverse U.S. cohort and to identify a point of inflection of serum 25(OH)D for maximal suppression of serum PTH. Data from 735 participants, ages 7–18 y, were pooled from 3 study sites located in Indiana, Texas, and Massachusetts. A two-phase linear spline was used to model the relationship between serum 25(OH)D and PTH. The value of serum 25(OH)D for maximal suppression of serum PTH was identified as the inflection point of the spline. Before adjustment for site, the inflection point of serum 25(OH)D for maximal suppression of serum PTH was 92.4 nmol/L (95% CI: 62.2, 130.7). After adjusting for site, the point of inflection was poorly defined and the relationship between serum 25(OH)D and PTH appeared to be linear. The lack of an inflection point of serum 25(OH)D for maximal suppression of PTH brings into question the value of using maximal suppression of serum PTH as a basis for determining optimal serum 25(OH)D for healthy children and adolescents.
Introduction
Dietary vitamin D requirements are ideally determined by identifying the optimal serum vitamin D status and corresponding dietary intake associated with 1 or more functional health outcomes. A principle function of vitamin D in bone health is to enhance calcium absorption. In adults, a positive linear relationship between 20–80 nmol/L serum 25-hydroxyvitamin D [25(OH)D], the major circulating vitamin D metabolite and indicator of vitamin D status, and calcium absorption (1, 2) has been described by some but not all investigators (3). However, in children and adolescents, either no relationship or a negative relationship between serum 25(OH)D and calcium absorption (4–6) has been reported. Vitamin D is important in the regulation of calcium and bone metabolism and is inversely related to serum parathyroid hormone (PTH) in adults, where low serum 25(OH)D results in elevated serum PTH, which leads to increased bone resorption and bone loss (7). Therefore, in adults, maximal suppression of serum PTH has been used to determine sufficiency for serum 25(OH)D. Maximal suppression of serum PTH in adults has been reported over a wide range of serum 25(OH)D from 8 to 44 μg/L (20–110 nmol/L) (8–16).
Several groups have recommended a cutoff for serum 25(OH)D of ~70–80 nmol/L for optimal vitamin D status in older adults based on skeletal and nonskeletal outcomes (16, 17). However, in children and adolescents, the relationship between serum 25(OH)D and skeletal outcomes is less clear. Furthermore, evidence that would be useful for setting an optimal level for serum 25(OH)D based on the relationship of this serum parameter and skeletal and nonskeletal outcomes in children and adolescents is scanty. A recent review of vitamin D status in children defined deficiency as <37.50 nmol/L and sufficiency as between 37.6 and 50 nmol/L (18). The American Academy of Pediatrics provided guidelines in 2008 (19) that recommended serum 25(OH)D ≥ 50 nmol/L for children. This was based partially on observations of increased serum PTH with low vitamin D status (20). However, it is unclear if serum PTH suppression is an appropriate method for determining optimal vitamin D status in children and adolescents.
Several studies have suggested that a negative relationship exists between serum 25(OH)D and PTH in children and adolescents (4, 6, 20–27). These reports have mostly been from studies with relatively small sample sizes of limited generalizability. In this study, we have pooled data from 737 children and adolescents aged 7–18 y, studied at 3 different sites representative of the northern, southern, and midwestern United States. Given the scarcity of data useful for setting optimal serum 25(OH)D in children and adolescents based on functional skeletal or nonskeletal outcomes, our study objectives were to determine the relationship between serum 25(OH)D and PTH and to identify a point of inflection of serum 25(OH)D for maximal suppression of serum PTH. A well-defined point of inflection of serum 25(OH)D for maximal suppression of serum PTH in children and adolescents may be useful in setting a cutpoint for vitamin D deficiency or sufficiency. But describing the relationship between serum 25(OH)D and PTH in a large, heterogeneous cohort of U.S. children and adolescents may have a broader application in understanding the relationship between these 2 calcium homeostatic hormones in this age group.
Materials and Methods
The participants were 737 healthy 7- to 18-y-old children and adolescents. The distribution of sex and race in our cohort was 30% male, 36% white, 33% black, 16% Hispanic, 8% Asian, and 7% other races or multi-racial. They participated in study protocols, described in detail elsewhere (4, 20, 28–31), at the 3 study sites: Massachusetts (Boston, 42.36° N), Texas (Houston, 29.75° N), and Indiana (West Lafayette, 40.46° N). Study protocols were approved by the Committee on Clinical Investigation, Children’s Hospital Boston (Massachusetts site); Purdue University, Indiana University School of Medicine, and Clarian Institutional Review Boards (Indiana site); and the Institutional Review Board of Baylor College of Medicine and Affiliated Hospitals (Texas site). Annual mean daily solar radiation exposures are 325, 475, and 350 Gcal/cm2 (13.6, 19.9, 14.7 PJ/m2) for these sites, respectively (32). Calcium intake was assessed as habitual calcium intake by the Youth and Adolescent Questionnaire (33) in the Massachusetts participants and from diet records analyzed by Nutrition Data System for Research software (University of Minnesota, Minneapolis, MN) in the Texas participants. The Indiana participants participated in metabolic balance studies on controlled calcium intakes. The calcium intakes for the Indiana participants were determined from mineral analysis of meal composites as previously described (28–30). Fasting sera were analyzed for 25(OH)D by RIA (DiaSorin) in Indiana and Texas and by a competitive binding assay (Nichols Institute) in Boston, and for intact PTH by a 2-site immunoassay (Nichols Institute) in Indiana, an immunoradiometric assay (Diagnostic Systems Laboratories) in Texas, and by a 2-site chemiluminescence assay (Nichols Institute) in Boston. A cutoff for serum PTH was set at 4 ng/L, the midpoint between 0 and the detection limit of 8 ng/L. All serum PTH values < 4 ng/L (n = 13) were imputed as 4 ng/L. All samples in Indiana were collected during the summer and samples from Texas and Massachusetts were collected during various seasons throughout the year. Breast Tanner stage and genital Tanner stage (34) are reported for boys (n = 117) and girls (n = 291), respectively, for whom these data were available.
ANOVA and Tukey’s Studentized Range tests were used to determine differences between unadjusted means for age, serum 25(OH)D, and serum PTH among sites, racial groups, and between the sexes. ANCOVA with site as a covariate and Tukey’s Studentized Range tests were used to determine the differences between least squares means for serum 25(OH)D and serum PTH. Statistical significance was set at α = 0.05 and results are presented as means ± SD unless otherwise noted.
A two-phase linear regression (spline) approach (35) was used to model the relationship between serum PTH and serum 25(OH)D. The value of serum 25(OH)D for maximal suppression of serum PTH is the value corresponding to the inflection point (knot) of the spline. Because serum PTH was significantly higher for the Massachusetts site across the range of serum 25(OH)D [rather than if serum PTH and 25(OH)D from the Massachusetts participants were clustering at the high and low end of the ranges, respectively, as would have been anticipated due to geography], we adjusted serum PTH for site by subtracting the difference between the site mean and the grand mean. Our analyses were performed with and without this site adjustment. Bootstrap resampling (n = 5000) using the reflection method (36) was used to determine the 95% CI around the point estimate of serum 25(OH)D for maximal suppression of serum PTH. A series of subanalyses was performed to investigate potential differences in the point of inflection of serum 25(OH)D for maximal suppression of PTH among the subgroups and with the pooled analysis (main analysis). The subanalyses included: races separately, sexes separately, early (Tanner stage 1–2) and late (Tanner stage ≥ 3) pubertal participants separately, and sites separately. The relationship between serum PTH and age was also analyzed using a two-phase linear regression as suggested by smoothing plots of the data. The results are presented as the point estimate (95% CI). Simple linear regression was used where a two-phase regression was revealed to be inappropriate to model the data. All analyses were performed using SAS 9.2 (SAS Institute).
Results
A total of 735 participants were included in this analysis (Table 1). Two white female participants aged 18 and 15 y from the Massachusetts site were excluded due to hyperparathyroidism despite relatively high serum 25(OH)D (serum PTH = 68.7 and 69.7 ng/L and serum 25(OH)D = 173.1 and 147.7 nmol/L, respectively, with Studentized deleted residuals ≈ 2.5). There were 13 participants with serum PTH < 4 ng/L for whom 4 ng/L was imputed.
TABLE 1.
Participant characteristics from 735 healthy children and adolescents1
| Site2 |
Sex |
Race3 |
||||||||
| IN | MA | TX | Boys | Girls | Asian | Black | Hispanic | White | Other | |
| n | 283 | 303 | 149 | 224 | 511 | 61 | 241 | 120 | 265 | 48 |
| Age, y | 13.3 ± 1.1b | 14.7 ± 2.0a | 10.8 ± 1.7c | 13.7 ± 1.6a | 13.2 ± 2.4b | 13.3 ± 1.6ab | 13.5 ± 2.4ab | 13.3 ± 2.7ab | 13.2 ± 1.9b | 14.0 ± 1.6a |
| Sex (M, F),4n | 69,214 | 107,196 | 48,101 | |||||||
| Race (A, B, H, W, O), n | 45,71,2,149,16 | 6,141,77,47,32 | 10,29,41,69,0 | 25,64,47,74,14 | 36,177,73,191,34 | |||||
| Ca intake, mg/d | 1214 ± 370a | 1165 ± 713a | 989 ± 293b | 1142 ± 556 | 1150 ± 520 | 1108 ± 351 | 1121 ± 603 | 1129 ± 651 | 1157 ± 406 | 1350 ± 601 |
| Serum 25(OH)D,5nmol/L | 68.0 ± 21.7b | 59.3 ± 26.9c | 76.9 ± 25.9a | 65.7 ± 21.9 | 66.2 ± 27.1 | 62.0 ± 18.4b | 55.0 ± 22.7c | 66.7 ± 24.4b | 77.9 ± 25.6a | 62.0 ± 23.6bc |
| Serum PTH, ng/L | 31.1 ± 13.8b | 46.3 ± 23.2a | 28.4 ± 17.2c | 38.8 ± 19.8a | 36.0 ± 20.7b | 41.5 ± 17.6a | 41.8 ± 23.4a | 36.0 ± 18.0ab | 30.8 ± 16.7b | 41.5 ± 24.5a |
| Adjusted serum PTH, ng/L | 37.9 ± 19.1 | 36.4 ± 18.7 | 46.1 ± 18.7a | 38.9 ± 21.1b | 32.9 ± 17.2b | 34.5 ± 15.6b | 37.1 ± 22.3b | |||
Values are means ± SD, except for categorical variables (sex, race), which are presented as per category. Differences among means for demographic groups were determined by ANOVA and Tukey's Studentized Range test for multiple comparisons. Means in each row without a common letter differ, P < 0.05.
MA, Massachusetts; IN, Indiana; and TX, Texas.
A, Asian; B, black; H, Hispanic; W, white; O, other race or multiracial.
M, Male, F, female.
ANOVA for the differences among groups for mean serum PTH and mean serum 25(OH)D was performed using the log values of these measures. The original units are reported in the table.
Unadjusted mean serum 25(OH)D was 66.2 ± 25.6 nmol/L and mean serum PTH was 36.8 ± 20.5 ng/L for all participants. There were no significant sex differences for mean serum 25(OH)D or PTH before or after adjustment for site. Racial differences in mean serum 25(OH)D and PTH before and after adjustment for site existed (Table 1). Whites had greater serum 25(OH)D than the other racial groups. Mean serum 25(OH)D was higher in Hispanics than in blacks. Whites had significantly higher mean serum PTH than the other racial groups prior to PTH adjustment by site. After site adjustment, mean serum PTH was different only for Asians compared with the other racial groups, where Asians had lower mean serum PTH.
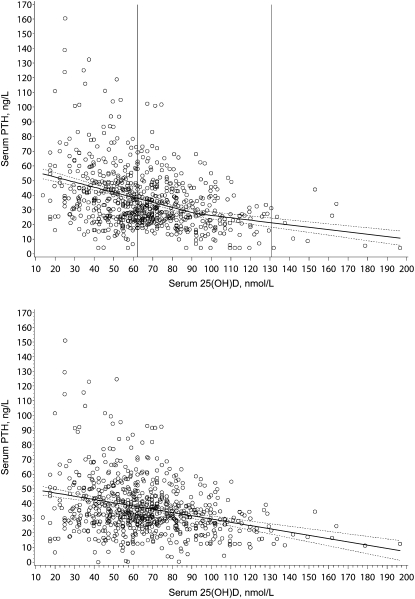
Without adjusting for site, 92.4 nmol/L (95% CI: 62.2, 130.7) was the value of serum 25(OH)D for maximal suppression of serum PTH (Fig. 1A). Due to the skewed distribution of serum PTH, the same analysis was performed using the natural log of serum PTH, which was successful in correcting the skewness. The point of inflection of serum 25(OH)D for maximal suppression of log serum PTH was similar to that in the analysis using the original units (data not shown).
FIGURE 1.
The relationship between serum 25(OH)D and PTH in healthy children and adolescents before (A) and after (B) adjustment of PTH for site. (A) Two-phase linear spline of serum 25(OH)D and serum PTH without adjustment for site. The equation for the line when serum 25(OH)D < 92.4 nmol/L is serum PTH (ng/L) = 59.14–0.35D, where D is serum 25(OH)D (nmol/L). The equation of the line when serum 25(OH)D ≥ 92.4 nmol/L is serum PTH (ng/L) = 42.43–0.16D. The solid line is the fitted two-phase linear spline and the surrounding dashed lines represent the 95% CI around the spline. The vertical lines represent the 95% CI around the inflection point for serum 25(OH)D. (B) Linear function of serum 25(OH)D and serum PTH after adjustment for site. Serum PTH was adjusted for site by subtracting the difference between the site mean and the grand mean. Therefore, serum PTH was adjusted for the Massachusetts site by subtracting 9.5 ng/L, for the Indiana site by adding 5.8 ng/L, and for the Texas site by adding 8.4 ng/L. For 1 observation, the site-adjusted serum PTH was <0 ng/L, so 0 was used for plotting purposes. The linear function is: serum PTH (ng/L) = 51.60–0.22D, where D is serum 25(OH)D, nmol/L. The solid line is the fitted linear function and the surrounding dashed lines represent the 95% CI around the linear function.
After adjusting for site, the location of the inflection point was very poorly defined; the 95% CI spread beyond the range of the data [serum 25(OH)D = 123.0 nmol/L (95% CI: −122.0, 252.1 nmol/L)]. Therefore, we used linear regression to describe the relationship between serum 25(OH)D and site-adjusted serum PTH (Fig. 1B). Serum 25(OH)D predicted 9% of the variation in adjusted serum PTH (R2 = 0.09; P < 0.0001) and race predicted an additional 2% of the variation (2 = 0.0; P = 0.0008).
In subanalyses of the separate sites, no inflection point of serum 25(OH)D for the maximal suppression of serum PTH was found for the Texas site, because the two-phase regression failed to converge. For the Massachusetts and Indiana sites, 84.4 nmol/L (95% CI: 52.2, 148.9) and 41.8 nmol/L (95% CI: −332.2, 102.3), respectively, were the values of serum 25(OH)D for maximal suppression of serum PTH, which were not significantly different from the unadjusted pooled analysis (above) as indicated by overlapping 95% CI. Linear regression revealed a negative relationship between serum PTH and serum 25(OH)D for all 3 sites: Indiana: serum PTH (ng/L) = 41.93–0.159D, R2 = 0.06, P < 0.0001; Massachusetts: serum PTH (ng/L) = 62.19–0.267D, R2 = 0.10, P < 0.0001; and Texas: serum PTH (ng/L) = 49.93 – 0.279D, R2 = 0.18, P < 0.0001. D is serum 25(OH)D in nmol/L in all equations.
Subanalyses of the races separately revealed overlapping 95% CI for the inflection point of serum 25(OH)D for maximal suppression of serum PTH among white, black, and Hispanic races: 112.6 nmol/L (95% CI: −104.7, 217.4), 91.0 nmol/L (95% CI: −71.2, 148.9), and 67.2 nmol/L (95% CI: −166.7, 148.7), respectively, which also overlapped the 95% CI for the pooled analysis (above). Linear regression revealed a negative linear relationship between serum PTH and serum 25(OH)D for whites, blacks, and Hispanics [serum PTH (ng/L) = 47.07 – 0.208D, R2 = 0.10, P < 0.0001; 64.74 – 0.417D, R2 = 0.16, P < 0.0001; and 49.06 – 0.195D, R2 = 0.07, P < 0.001, respectively]. The subanalyses for Asians revealed no point of inflection and a nonsignificant relationship between serum PTH and serum 25(OH)D [serum PTH (ng/L) = 56.2 – 0.23D, R2 = 0.06, P = 0.0535].
Subanalyses of the sexes separately revealed overlapping 95% CI for the inflection point of serum 25(OH)D for maximal suppression of serum PTH. The value of the inflection point was 57.5 nmol/L (95% CI: −17.2, 110.0) for boys and 110.0 nmol/L (95% CI: 77.8, 137.0) for girls. These values were not significantly different from the pooled analyses as indicated by overlapping 95% CI. Linear regression of the relationship between serum PTH and serum 25(OH)D showed a negative relationship between these 2 variables for both boys [serum PTH (ng/L) = 55.27–0.290D, R2 = 0.12, P < 0.0001] and girls [serum PTH (ng/L) = 59.73–0.317D, R2 = 0.15, P < 0.0001].
In subanalyses of the relationship between serum PTH and serum 25(OH)D for early pubertal (Tanner stage ≤ 2; n = 145) and peri/late pubertal adolescents (Tanner stage > 2; n = 263), the inflection point of serum 25(OH)D for maximal suppression of serum PTH was not evident for either group. A negative linear relationship was observed in the early pubertal stage [serum PTH (ng/L) = 44.9–0.234D, nmol/L, R2 = 0.15, P < 0.0001] and the peri/late pubertal stage [serum PTH (ng/L) = 45.2–0.190D, R2 = 0.08, P < 0.0001].
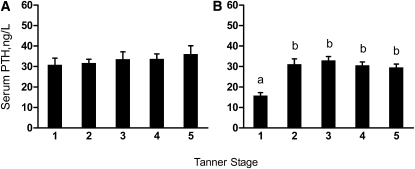
The age at which serum PTH peaked was 11.7 y (95% CI: 10.9, 12.4). Serum PTH was greater in Tanner stages 2–5 than in Tanner stage 1 in girls but did not differ among Tanner stages in boys (Fig. 2).
FIGURE 2.
Serum PTH concentrations in boys (A) and girls (B) at different Tanner stages. Values are means ± SE, n = 9–81. Means without a common letter differ, P < 0.05.
Discussion
This study was undertaken to clarify the relationship between serum 25(OH)D and PTH in children and adolescents. A major strength of this study was pooling data from a large number of diverse children and adolescents from a broad range of latitudes in the US into a relatively large dataset. However, site differences were observed that may have been due to factors other than latitude such as differences in assays, season of sample collection, race and sex distribution, age differences among sites, and differences in calcium intake among participants. Thus, we performed our analyses with and without adjustment for site, which helped to correct for these potential confounders. Additionally, our subanalysis of the relationship between serum 25(OH)D and PTH in each site separately revealed either a lack of or similar point of inflection of serum 25(OH)D compared with our pooled analysis. This finding suggests that unknown site differences or inter-assay variation did not confound our results and conclusions regarding the point of inflection of serum 25(OH)D for maximal suppression of PTH from our pooled analysis.
Higher serum PTH in boys compared with girls has been reported (24), but this has not been a consistent finding (4, 20). We found no difference between boys and girls for serum 25(OH)D or PTH; therefore, we conclude that difference in sex distribution among the sites was not a confounder in this study. Racial distribution varied among the sites and could be a potential confounder considering our observation of differences in mean serum 25(OH)D and PTH among races. However, adjusting for race did not remove our observed differences in serum 25(OH)D and PTH between sites. One would not expect the racial distributions in these 3 different regions of the country to be identical, and the contribution of racial variety from each of the sites can be considered an overall strength of this study, adding to the generalizability to the entire U.S. In addition, in our subanalysis of the relationship between serum 25(OH)D and PTH in the races separately, the point of inflection of serum 25(OH)D for maximal suppression of PTH did not differ among the races or compared with our pooled analyses.
Our data can be used to evaluate children and adolescents 7–18 y of age. This is a relatively large age range and includes prepubertal children to late pubertal adolescents. Depending on age and pubertal stage, serum PTH may be higher or lower to correspond with the rate of bone turnover for skeletal modeling. In our cohort, serum PTH increased with age until it peaked at ~11.7 y of age. This age corresponds approximately to the years of peak height velocity observed for boys and girls (12–14 y and 11–13 y, respectively) (37). We found that serum PTH was higher in girls in Tanner stages 2 through 5 compared with Tanner stage 1, but the mean serum PTH did not differ among Tanner stages in boys. In contrast, greater variation in serum PTH was observed for both sexes during the middle and later Tanner stages compared with Tanner stage 1, suggesting that different hormonal statuses and rates of growth during puberty may be contributing to variation in serum PTH. This may partially explain the relatively small relationship (R2 = 0.09) between serum 25(OH)D and serum PTH in our study. Indeed, we have found that sexual maturity and pubertal hormones contribute more to the variability in calcium retention in adolescents than do vitamin D metabolites or PTH (30, 38). Sexual maturation and pubertal status may account for some of the unexplained variation in serum PTH. Our subanalysis of early pubertal (Tanner stages 1 and 2) and peri/late pubertal (Tanner stages 3–5) revealed that the relationship between serum 25(OH)D and PTH in was linear for both groups, indicating that our conclusion from our main analysis [that an inflection point of serum 25(OH)D for maximal suppression of PTH in children and adolescents is not evident] was not confounded by the wide age range studied.
Race explained only an additional 2% of the variation in serum PTH. In a smaller previous study of the relationship between serum 25(OH)D, PTH, and calcium absorption in black and white adolescent girls from 1 site (6), we found a significant race effect where there was the anticipated negative relationship between serum 25(OH)D and PTH for white girls but no relationship between these 2 variables for black girls. In this larger analysis, there was no difference in the relationship between serum 25(OH)D and PTH, possibly due to the increased number of participants or from the increased heterogeneity provided by inclusion of participants from the other 2 sites. The Massachusetts site contributed data from 141 black adolescents, making up almost 60% of black participants in our pooled dataset. These participants provided a broader range in both serum PTH and 25(OH)D compared with our smaller study of black and white girls, which improved our ability to assess the relationship between serum PTH and 25(OH)D.
Our unadjusted (for site) analysis indicates that the inflection point of serum 25(OH)D for maximal suppression of serum PTH in children and adolescents is 92.4 nmol/L, which is similar to the inflection point reported in a study of Ohio boys and girls (89.6 nmol/L) (23) but higher than the inflection point reported for girls in Northern Ireland (59.8 nmol/L) (24). Whereas no CI were reported for the inflection points in these previous studies, we report a relatively large 95% CI around the inflection point, which spans from 62.3 to 130.0 nmol/L in serum 25(OH)D. Note the lower bound of our 95% CI is similar to the inflection point reported in the Northern Ireland study (24). Furthermore, when we adjusted for site, we were unable to identify an inflection point of serum 25(OH)D for maximal suppression of serum PTH. These results suggest that an inflection point of serum 25(OH)D for maximal suppression of serum PTH is elusive. Without a clearly defined inflection point, we turn to the question: Is maximal suppression of serum PTH a reasonable goal in children and adolescents?
In adults, higher serum PTH is associated with increased bone remodeling, which in adulthood generally favors bone resorption. High serum PTH values in children and adolescents, however, should be evaluated with caution, because the normal range for serum PTH in this age group is not well established. Serum PTH is elevated during growth to support higher bone turnover for skeletal modeling and growth (39). Perhaps the lack of a positive relationship between serum 25(OH)D and calcium absorption or retention in adolescents is a result of suppression of serum PTH and bone modeling with increasing serum 25(OH)D, which may have a detrimental effect on the skeleton during this time of growth when high bone turnover is needed. Because both positive (4) and no relationships (6) between serum PTH and calcium absorption have been observed in this age group, this hypothesis requires further investigation.
Above deficient levels (>25 nmol/L), serum 25(OH)D has not been associated with improved calcium absorption or retention in children and adolescents (4–6) and serum PTH may be positively associated with calcium absorption in children and adolescents (4). Therefore, we infer that our inability to clearly identify an inflection point of serum 25(OH)D for maximal suppression of serum PTH supports that maximal suppression of serum PTH is inappropriate as a basis for determining optimal serum 25(OH)D for healthy children and adolescents.
Acknowledgments
C.M.W., S.A.A., and C.M.G. planned the study; C.M.W., S.A.A., C.M.G, and K.M.H. conducted the research; K.M.H., G.P.M., and L.D.M. performed the data analysis; K.M.H., C.M.W., and G.P.M. wrote the paper; and K.M.H. was responsible for final content. All authors read and approved the final manuscript.
Footnotes
Supported by the NIH (grant nos. AR-40553, HD-36609, DK-066108, M01-RR-2172, RR-00188, AR-43740, and HD-K24-01288), USDA/Agricultural Research Service Cooperative Agreement 58-6250-6-001), Project 5-T71-MC-000-10-S1-R0 from the Maternal and Child Health Bureau, the Charles H. Hood Foundation, Boston, and the McCarthy Family Foundation, New York.
Literature Cited
- 1.Hansen KE, Jones AN, Lindstrom MJ, Davis LA, Engelke JA, Shafer MM. Vitamin D insufficiency: disease or no disease? J Bone Miner Res. 2008;23:1052–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–6 [DOI] [PubMed] [Google Scholar]
- 3.Need AG, Nordin BE. Misconceptions: vitamin D insufficiency causes malabsorption of calcium. Bone. 2008;42:1021–4 [DOI] [PubMed] [Google Scholar]
- 4.Abrams SA, Griffin IJ, Hawthorne KM, Gunn SK, Gundberg CM, Carpenter TO. Relationships among vitamin D levels, parathyroid hormone, and calcium absorption in young adolescents. J Clin Endocrinol Metab. 2005;90:5576–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee WT, Cheng JCY, Jiang J, Hu P, Hu X, Roberts DC. Calcium absorption measured by stable calcium isotopes (42Ca & 44Ca) among Northern Chinese adolescents with low vitamin D status. J Orthop Surg (Hong Kong). 2002;10:61–6 [DOI] [PubMed] [Google Scholar]
- 6.Weaver CM, McCabe LD, McCabe GP, Braun M, Martin BR, Dimeglio LA, Peacock M. Vitamin D status and calcium metabolism in adolescent black and white girls on a range of controlled calcium intakes. J Clin Endocrinol Metab. 2008;93:3907–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94:1244–50 [DOI] [PubMed] [Google Scholar]
- 8.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–43 [DOI] [PubMed] [Google Scholar]
- 9.Dawson-Hughes B, Harris SS, Dallal GE. Plasma calcidiol, season, and serum parathyroid hormone concentrations in healthy elderly men and women. Am J Clin Nutr. 1997;65:67–71 [DOI] [PubMed] [Google Scholar]
- 10.Krall EA, Sahyoun N, Tannenbaum S, Dallal GE, Dawson-Hughes B. Effect of vitamin D intake on seasonal variations in parathyroid hormone secretion in postmenopausal women. N Engl J Med. 1989;321:1777–83 [DOI] [PubMed] [Google Scholar]
- 11.Lips P, Wiersinga A, van Ginkel FC, Jongen MJ, Netelenbos JC, Hackeng WH, Delmas PD, van der Vijgh WJ. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67:644–50 [DOI] [PubMed] [Google Scholar]
- 12.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6 [DOI] [PubMed] [Google Scholar]
- 13.McKenna MJ. Differences in vitamin D status between countries in young adults and the elderly. Am J Med. 1992;93:69–77 [DOI] [PubMed] [Google Scholar]
- 14.Peacock M. Effects of calcium and vitamin D insufficiency on the skeleton. Osteoporos Int. 1998;8Suppl 2:S45–51 [DOI] [PubMed] [Google Scholar]
- 15.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83 [DOI] [PubMed] [Google Scholar]
- 16.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28 [DOI] [PubMed] [Google Scholar]
- 17.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–6 [DOI] [PubMed] [Google Scholar]
- 18.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417 [DOI] [PubMed] [Google Scholar]
- 19.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52 [DOI] [PubMed] [Google Scholar]
- 20.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531–7 [DOI] [PubMed] [Google Scholar]
- 21.Cheng S, Tylavsky F, Kroger H, Karkkainen M, Lyytikainen A, Koistinen A, Mahonen A, Alen M, Halleen J, et al. Association of low 25-hydroxyvitamin D concentrations with elevated parathyroid hormone concentrations and low cortical bone density in early pubertal and prepubertal Finnish girls. Am J Clin Nutr. 2003;78:485–92 [DOI] [PubMed] [Google Scholar]
- 22.Guillemant J, Cabrol S, Allemandou A, Peres G, Guillemant S. Vitamin D-dependent seasonal variation of PTH in growing male adolescents. Bone. 1995;17:513–6 [DOI] [PubMed] [Google Scholar]
- 23.Harkness L, Cromer B. Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos Int. 2005;16:109–13 [DOI] [PubMed] [Google Scholar]
- 24.Hill TR, Cotter AA, Mitchell S, Boreham CA, Dubitzky W, Murray L, Strain JJ, Flynn A, Robson PJ, et al. Vitamin D status and parathyroid hormone relationship in adolescents and its association with bone health parameters: analysis of the Northern Ireland Young Heart's Project. Osteoporos Int. 2010;21:695–700 [DOI] [PubMed] [Google Scholar]
- 25.Outila TA, Karkkainen MU, Lamberg-Allardt CJ. Vitamin D status affects serum parathyroid hormone concentrations during winter in female adolescents: associations with forearm bone mineral density. Am J Clin Nutr. 2001;74:206–10 [DOI] [PubMed] [Google Scholar]
- 26.Pettifor JM. Rickets and vitamin D deficiency in children and adolescents. Endocrinol Metab Clin North Am. 2005;34:537–53 [DOI] [PubMed] [Google Scholar]
- 27.Rajakumar K, Fernstrom JD, Janosky JE, Greenspan SL. Vitamin D insufficiency in preadolescent African-American children. Clin Pediatr (Phila). 2005;44:683–92 [DOI] [PubMed] [Google Scholar]
- 28.Braun M, Martin BR, Kern M, McCabe GP, Peacock M, Jiang Z, Weaver CM. Calcium retention in adolescent boys on a range of controlled calcium intakes. Am J Clin Nutr. 2006;84:414–8 [DOI] [PubMed] [Google Scholar]
- 29.Braun M, Palacios C, Wigertz K, Jackman LA, Bryant RJ, McCabe LD, Martin BR, McCabe GP, Peacock M, et al. Racial differences in skeletal calcium retention in adolescent girls with varied controlled calcium intakes. Am J Clin Nutr. 2007;85:1657–63 [DOI] [PubMed] [Google Scholar]
- 30.Jackman LA, Millane SS, Martin BR, Wood OB, McCabe GP, Peacock M, Weaver CM. Calcium retention in relation to calcium intake and postmenarcheal age in adolescent females. Am J Clin Nutr. 1997;66:327–33 [DOI] [PubMed] [Google Scholar]
- 31.Singh R, Martin BR, Hickey Y, Teegarden D, Campbell WW, Craig BA, Schoeller DA, Kerr DA, Weaver CM. Comparison of self-reported, measured, metabolizable energy intake with total energy expenditure in overweight teens. Am J Clin Nutr. 2009;89:1744–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227–31 [DOI] [PubMed] [Google Scholar]
- 33.Rockett HR, Breitenbach M, Frazier AL, Witschi J, Wolf AM, Field AE, Colditz GA. Validation of a youth/adolescent food frequency questionnaire. Prev Med. 1997;26:808–16 [DOI] [PubMed] [Google Scholar]
- 34.Tanner J. Growth at adolescence. Oxford: Blackwell; 1962 [Google Scholar]
- 35.Hinkley DV. Inference in two-phase regression. J Am Stat Assoc. 1971;66:736–43 [Google Scholar]
- 36.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, FL: CRC Press LLC; 1998 [Google Scholar]
- 37.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the University of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14:1672–9 [DOI] [PubMed] [Google Scholar]
- 38.Hill KM, Braun M, Kern M, Martin BR, Navalta JW, Sedlock DA, McCabe L, McCabe GP, Peacock M, et al. Predictors of calcium retention in adolescent boys. J Clin Endocrinol Metab. 2008;93:4743–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krabbe S, Transbol I, Christiansen C. Bone mineral homeostasis, bone growth, and mineralisation during years of pubertal growth: a unifying concept. Arch Dis Child. 1982;57:359–63 [DOI] [PMC free article] [PubMed] [Google Scholar]