Abstract
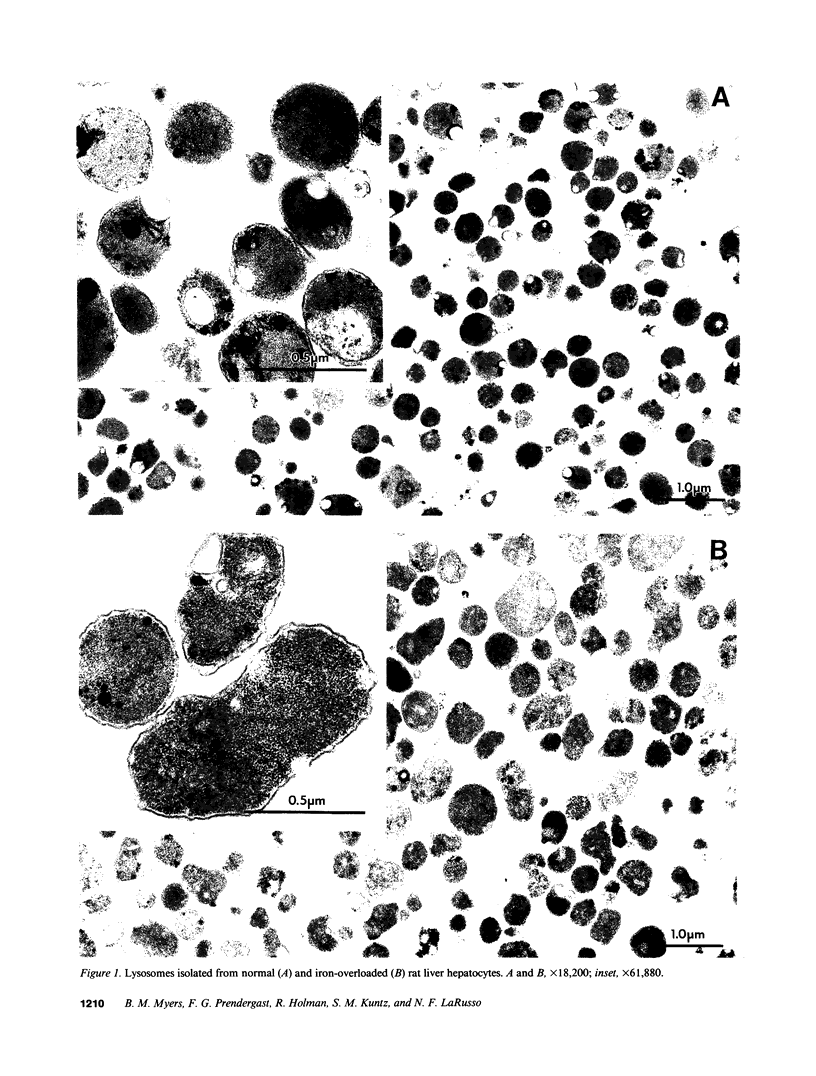
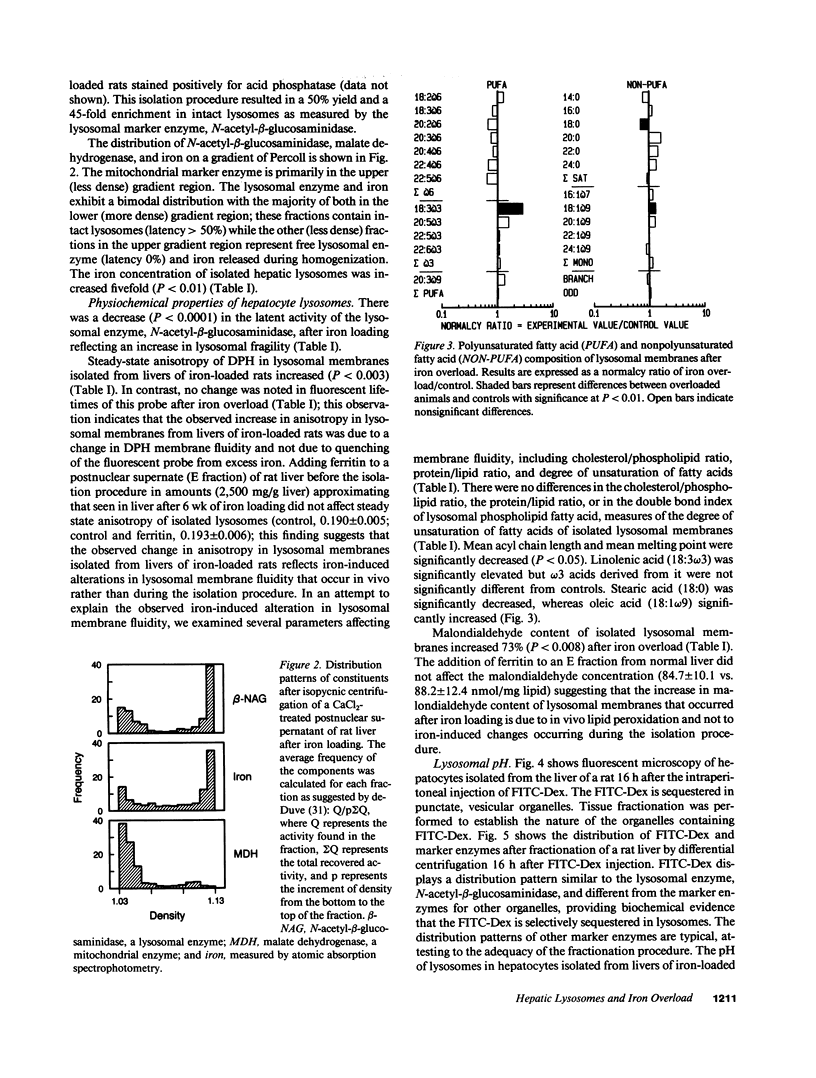
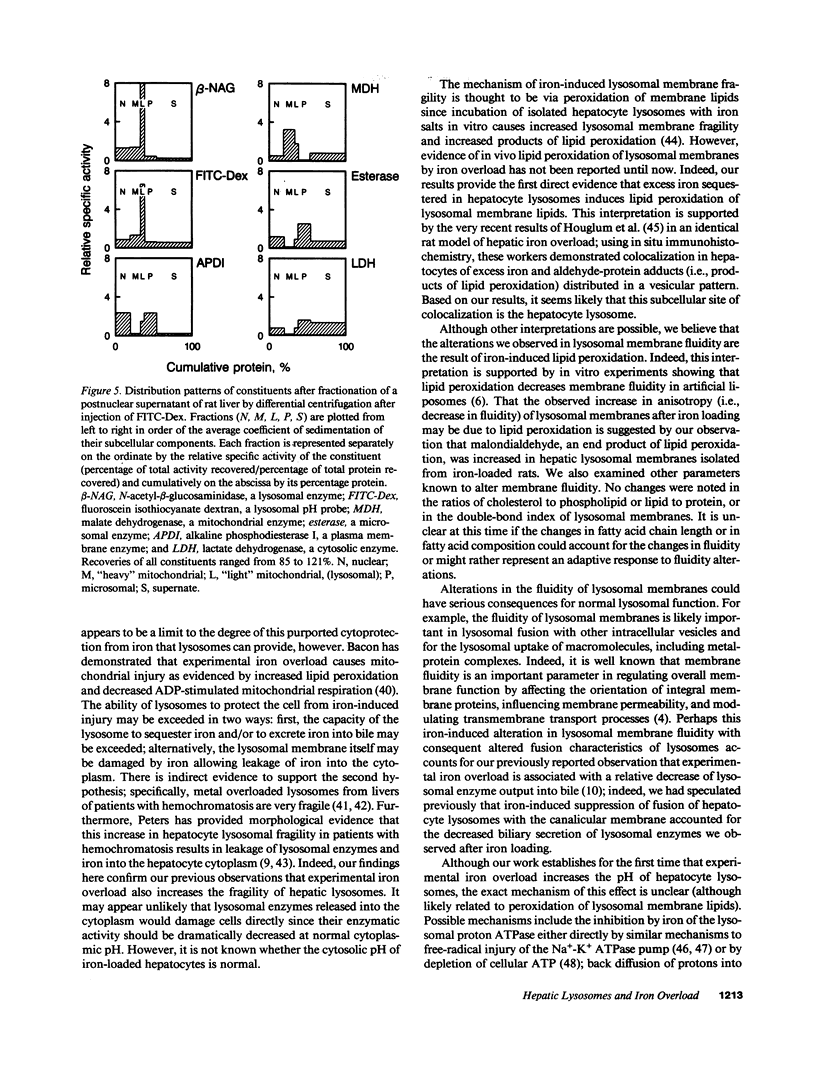
While hemochromatosis is characterized by sequestration of iron-protein complexes in hepatocyte lysosomes, little is known about the effects of excess iron on these organelles. Therefore, we studied the effects of experimental iron overload on hepatocyte lysosomal structure, physicochemical properties, and function in rats fed carbonyl iron. A sixfold increase (P less than 0.0001) in hepatic iron and a fivefold increase in lysosomal iron (P less than 0.01) was observed after iron loading; as a result, hepatocyte lysosomes became enlarged and misshapen. These lysosomes displayed increased (P less than 0.0001) fragility; moreover, the fluidity of lysosomal membranes isolated from livers of iron-loaded rats was decreased (P less than 0.0003) as measured by fluorescence polarization. Malondialdehyde, an end product of lipid peroxidation, was increased by 73% (P less than 0.008) in lysosomal membranes isolated from livers of iron-overloaded rats. While amounts of several individual fatty acids in isolated lysosomal membranes were altered after iron overload, cholesterol/phospholipid ratios, lipid/protein ratios, double-bond index, and total saturated and unsaturated fatty acids remained unchanged. The pH of lysosomes in hepatocytes isolated from livers of iron-loaded rats and measured by digitized video microscopy was increased (control, 4.70 +/- 0.05; iron overload, 5.21 +/- 0.10; P less than 0.01). Our results demonstrate that experimental iron overload causes marked alterations in hepatocyte lysosomal morphology, an increase in lysosomal membrane fragility, a decrease in lysosomal membrane fluidity, and an increase in intralysosomal pH. Iron-catalyzed lipid peroxidation is likely the mechanism of these structural, physicochemical, and functional disturbances.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon B. R., Park C. H., Brittenham G. M., O'Neill R., Tavill A. S. Hepatic mitochondrial oxidative metabolism in rats with chronic dietary iron overload. Hepatology. 1985 Sep-Oct;5(5):789–797. doi: 10.1002/hep.1840050514. [DOI] [PubMed] [Google Scholar]
- Bacon B. R., Tavill A. S., Brittenham G. M., Park C. H., Recknagel R. O. Hepatic lipid peroxidation in vivo in rats with chronic iron overload. J Clin Invest. 1983 Mar;71(3):429–439. doi: 10.1172/JCI110787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett M. L., Halliday J. W., Powell L. W. Genetic hemochromatosis. Semin Liver Dis. 1984 Aug;4(3):217–227. doi: 10.1055/s-2008-1041772. [DOI] [PubMed] [Google Scholar]
- Beaufay H., Amar-Costesec A., Feytmans E., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol. 1974 Apr;61(1):188–200. doi: 10.1083/jcb.61.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradford W. D., Elchlepp J. G., Arstila A. U., Trump B. F., Kinney T. D. Iron metabolism and cell membranes. I. Relation between ferritin and hemosiderin in bile and biliary excretion of lysosome contents. Am J Pathol. 1969 Aug;56(2):201–228. [PMC free article] [PubMed] [Google Scholar]
- Bruch R. C., Thayer W. S. Differential effect of lipid peroxidation on membrane fluidity as determined by electron spin resonance probes. Biochim Biophys Acta. 1983 Sep 7;733(2):216–222. doi: 10.1016/0005-2736(83)90525-4. [DOI] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Gores G. J., Kost L. J., Miller L. J., LaRusso N. F. Processing of cholecystokinin by isolated liver cells. Am J Physiol. 1989 Aug;257(2 Pt 1):G242–G248. doi: 10.1152/ajpgi.1989.257.2.G242. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman R. T., Johnson S. B., Gerrard J. M., Mauer S. M., Kupcho-Sandberg S., Brown D. M. Arachidonic acid deficiency in streptozotocin-induced diabetes. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2375–2379. doi: 10.1073/pnas.80.8.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman R. T., Johnson S. B., Kokmen E. Deficiencies of polyunsaturated fatty acids and replacement by nonessential fatty acids in plasma lipids in multiple sclerosis. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4720–4724. doi: 10.1073/pnas.86.12.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houglum K., Filip M., Witztum J. L., Chojkier M. Malondialdehyde and 4-hydroxynonenal protein adducts in plasma and liver of rats with iron overload. J Clin Invest. 1990 Dec;86(6):1991–1998. doi: 10.1172/JCI114934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultcrantz R., Angelin B., Björn-Rasmussen E., Ewerth S., Einarsson K. Biliary excretion of iron and ferritin in idiopathic hemochromatosis. Gastroenterology. 1989 Jun;96(6):1539–1545. doi: 10.1016/0016-5085(89)90524-6. [DOI] [PubMed] [Google Scholar]
- Kapitulnik J., Tshershedsky M., Barenholz Y. Fluidity of the rat liver microsomal membrane: increase at birth. Science. 1979 Nov 16;206(4420):843–844. doi: 10.1126/science.493984. [DOI] [PubMed] [Google Scholar]
- Keefee E. B., Scharschmidt B. F., Blankenship N. M., Ockner R. K. Studies of relationship among bile flow, liver plasma membrane NaK-ATPase, and membrane microviscosity in the rat. J Clin Invest. 1979 Dec;64(6):1590–1598. doi: 10.1172/JCI109620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. S., Akera T. O2 free radicals: cause of ischemia-reperfusion injury to cardiac Na+-K+-ATPase. Am J Physiol. 1987 Feb;252(2 Pt 2):H252–H257. doi: 10.1152/ajpheart.1987.252.2.H252. [DOI] [PubMed] [Google Scholar]
- Kornbrust D. J., Mavis R. D. Microsomal lipid peroxidation. I. Characterization of the role of iron and NADPH. Mol Pharmacol. 1980 May;17(3):400–407. [PubMed] [Google Scholar]
- LaRusso N. F., Fowler S. Coordinate secretion of acid hydrolases in rat bile. J Clin Invest. 1979 Oct;64(4):948–954. doi: 10.1172/JCI109561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRusso N. F., Kost L. J., Carter J. A., Barham S. S. Triton WR-1339, a lysosomotropic compound, is excreted into bile and alters the biliary excretion of lysosomal enzymes and lipids. Hepatology. 1982 Mar-Apr;2(2):209–215. doi: 10.1002/hep.1840020204. [DOI] [PubMed] [Google Scholar]
- LeSage G. D., Kost L. J., Barham S. S., LaRusso N. F. Biliary excretion of iron from hepatocyte lysosomes in the rat. A major excretory pathway in experimental iron overload. J Clin Invest. 1986 Jan;77(1):90–97. doi: 10.1172/JCI112307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak I. T., Weglicki W. B. Characterization of iron-mediated peroxidative injury in isolated hepatic lysosomes. J Clin Invest. 1985 Jan;75(1):58–63. doi: 10.1172/JCI111697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Moriyama Y., Takano T. Identification and characterization of a proton pump on lysosomes by fluorescein-isothiocyanate-dextran fluorescence. Proc Natl Acad Sci U S A. 1982 May;79(9):2758–2762. doi: 10.1073/pnas.79.9.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
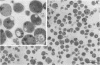
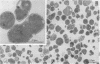
- Park C. H., Bacon B. R., Brittenham G. M., Tavill A. S. Pathology of dietary carbonyl iron overload in rats. Lab Invest. 1987 Nov;57(5):555–563. [PubMed] [Google Scholar]
- Peters T. J., Seymour C. A. Acid hydrolase activities and lysosomal integrity in liver biopsies from patients with iron overload. Clin Sci Mol Med. 1976 Jan;50(1):75–78. doi: 10.1042/cs0500075. [DOI] [PubMed] [Google Scholar]
- Quinn P. J. The fluidity of cell membranes and its regulation. Prog Biophys Mol Biol. 1981;38(1):1–104. doi: 10.1016/0079-6107(81)90011-0. [DOI] [PubMed] [Google Scholar]
- Reijngoud D. J., Oud P. S., Tager J. M. Effect of ionophores on intralysosomal pH. Biochim Biophys Acta. 1976 Oct 5;448(2):303–313. doi: 10.1016/0005-2736(76)90244-3. [DOI] [PubMed] [Google Scholar]
- Roeschlau P., Bernt E., Gruber W. Enzymatic determination of total cholesterol in serum. Z Klin Chem Klin Biochem. 1974 May;12(5):226–226. [PubMed] [Google Scholar]
- Schachter D. Fluidity and function of hepatocyte plasma membranes. Hepatology. 1984 Jan-Feb;4(1):140–151. doi: 10.1002/hep.1840040124. [DOI] [PubMed] [Google Scholar]
- Scharschmidt B. F., Van Dyke R. W. Proton transport by hepatocyte organelles and isolated membrane vesicles. Annu Rev Physiol. 1987;49:69–85. doi: 10.1146/annurev.ph.49.030187.000441. [DOI] [PubMed] [Google Scholar]
- Selden C., Owen M., Hopkins J. M., Peters T. J. Studies on the concentration and intracellular localization of iron proteins in liver biopsy specimens from patients with iron overload with special reference to their role in lysosomal disruption. Br J Haematol. 1980 Apr;44(4):593–603. doi: 10.1111/j.1365-2141.1980.tb08714.x. [DOI] [PubMed] [Google Scholar]
- Seymour C. A., Peters T. J. Organelle pathology in primary and secondary haemochromatosis with special reference to lysosomal changes. Br J Haematol. 1978 Oct;40(2):239–253. doi: 10.1111/j.1365-2141.1978.tb03661.x. [DOI] [PubMed] [Google Scholar]
- Smith M. T., Thor H., Hartizell P., Orrenius S. The measurement of lipid peroxidation in isolated hepatocytes. Biochem Pharmacol. 1982 Jan 1;31(1):19–26. doi: 10.1016/0006-2952(82)90230-1. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stubbs C. D., Smith A. D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984 Jan 27;779(1):89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Takayama M., Itoh S., Nagasaki T., Tanimizu I. A new enzymatic method for determination of serum choline-containing phospholipids. Clin Chim Acta. 1977 Aug 15;79(1):93–98. doi: 10.1016/0009-8981(77)90465-x. [DOI] [PubMed] [Google Scholar]
- Tribble D. L., Aw T. Y., Jones D. P. The pathophysiological significance of lipid peroxidation in oxidative cell injury. Hepatology. 1987 Mar-Apr;7(2):377–386. doi: 10.1002/hep.1840070227. [DOI] [PubMed] [Google Scholar]
- Wills E. D. Effects of iron overload on lipid peroxide formation and oxidative demethylation by the liver endoplasmic reticulum. Biochem Pharmacol. 1972 Jan 15;21(2):239–247. doi: 10.1016/0006-2952(72)90274-2. [DOI] [PubMed] [Google Scholar]
- Yamada H., Hayashi H., Natori Y. A simple procedure for the isolation of highly purified lysosomes from normal rat liver. J Biochem. 1984 Apr;95(4):1155–1160. doi: 10.1093/oxfordjournals.jbchem.a134704. [DOI] [PubMed] [Google Scholar]