Abstract
Women of reproductive age living in resource-poor settings are at high risk of inadequate micronutrient intakes when diets lack diversity and are dominated by staple foods. Yet comparative information on diet quality is scarce and quantitative data on nutrient intakes is expensive and difficult to gather. We assessed the potential of simple indicators of dietary diversity, such as could be generated from large household surveys, to serve as proxy indicators of micronutrient adequacy for population-level assessment. We used 5 existing data sets (from Burkina Faso, Mali, Mozambique, Bangladesh, and the Philippines) with repeat 24-h recalls to construct 8 candidate food group diversity indicators (FGI) and to calculate the mean probability of adequacy (MPA) for 11 micronutrients. FGI varied in food group disaggregation and in minimum consumption required for a food group to count. There were large gaps between intakes and requirements across a range of micronutrients in each site. All 8 FGI were correlated with MPA in all sites; regression analysis confirmed that associations remained when controlling for energy intake. Assessment of dichotomous indicators through receiver-operating characteristic analysis showed moderate predictive strength for the best choice indicators, which varied by site. Simple FGI hold promise as proxy indicators of micronutrient adequacy.
Introduction
In resource-poor environments across the globe, low-quality, monotonous diets are the norm and the risk for a variety of micronutrient deficiencies is high. Infants and young children, and adolescent girls and women of reproductive age are among those most likely to suffer from deficiencies. The high nutrient demands of pregnancy and lactation put women in developing countries at high risk. HIV and other infections also play a role in elevating risk for some women. Very little information is available on women’s micronutrient status outside of developed countries. However, even with limited data, it is clear that micronutrient deficiencies among women are a global problem and are most severe for women in developing countries (1).
Comparable population-level information about diet patterns, dietary intakes, and micronutrient adequacy of diets for women across countries is also scarce. Because of the cost and complexity of quantitative dietary intake data collection, very few developing countries have nationally representative surveys providing information on micronutrient intakes. Available information on women’s intakes is very fragmented, usually from small studies representing either specific population subgroups or convenience samples (2). Further, most past studies of women’s nutrient intakes have used analytic methods that are now thought to provide incorrect estimates of the prevalence of nutrient adequacy; newer methods are based on the concepts of probability and risk and estimate prevalence taking into account both distributions of requirements and of estimated intakes (3).
Simple, valid proxy indicators are urgently needed to characterize micronutrient adequacy of the diet at population level in resource-poor countries, assess key diet quality problems (such as low intake of animal products, fruits, and vegetables), and identify population subgroups particularly at risk of consuming inadequate diets. Simple indicators are also needed to monitor and evaluate intervention programs (4). Without indicators to assess and monitor progress, development of interventions at all levels is constrained and not prioritized.
Dietary diversity, assessed as the number of foods consumed across and within food groups over a reference period, is widely recognized as being a key dimension of diet quality and is reflected in food-based dietary guidelines (5, 6). Accordingly, dietary diversity indicators have been proposed as potential proxy indicators for diet quality (7). There is ample evidence from developed countries showing that dietary diversity is indeed strongly associated with nutrient adequacy and is thus an essential element of diet quality (8–15). There is less evidence from developing countries where monotonous diets, relying mostly on a few plant-based staple foods, are typical. Even fewer studies from developing countries have aimed to confirm this association specifically among women of reproductive age. The available studies have generally supported the association between diversity and nutrient adequacy (16–19). Previous studies have been context specific and diversity has been operationalized differently in each study (7). Although this has made comparisons difficult, it has also suggested that the relationship is robust.
This study contributes to the development of simple indicators to serve as proxies in assessing the micronutrient adequacy of women’s diets. To our knowledge, ours is the first study to use a common protocol across multiple sites to construct a range of candidate indicators and analyze their relationship to micronutrient adequacy. Diet quality includes other dimensions, such as moderation (e.g. in intakes of energy, saturated/trans fat, cholesterol, sodium, and refined sugars) and balance, and these other dimensions are increasingly important as low-income countries enter into nutrition transitions (20). However, low-micronutrient intakes remain a problem even in countries undergoing transition and a dominant problem in many of the poorest regions. Therefore, this work focuses on proxy indicators for the micronutrient adequacy of diets. The study also provides descriptive information on diet patterns and is among the first to employ currently recommended methods for estimating prevalence of micronutrient adequacy for women of reproductive age in developing countries.
Methods
Selection of data sets
In 2005, the Food and Nutrition Technical Assistance Project initiated the Women’s Dietary Diversity Project (WDDP)17 by soliciting expressions of interest from a group of researchers with existing dietary data sets for women of reproductive age or plans to collect the same. The International Food Policy Research Institute responded and was selected to coordinate the WDDP. Other interested researchers were invited to participate if they had available dietary data consisting of 24-h recalls collected using well-documented methods, with a minimum sample size of 100 women of reproductive age (15–49 y) and a second recall for a subsample of at least 40 women. Investigators had to be willing to follow a common analytic protocol. Five data sets were selected; 4 (Bangladesh, Mali, Mozambique, and the Philippines) were originally collected for other purposes, and the fifth, Burkina Faso, had been planned with similar research objectives to those of the WDDP. Study sites, sampling, and dietary methods are fully described elsewhere (21–25).
Samples, ethical review, and exclusions
In general, each sample is representative of a study area; all sampling procedures involved either randomization or an invitation to all eligible households in the study area and none were convenience samples. In some sites, subsamples were used for this analysis (see below) based on judgments of the quality of dietary data. Sample designs varied by site based on the original study objectives (21–25). Study protocols for all sites were approved by institutional review boards.18
Some sites excluded women from analysis based on extreme energy intakes (<0.9 times or >3.0 times estimated basal metabolic rate). Recognizing that extreme single-day intakes are possible (26), decisions on exclusions were made differently across sites depending on the level of involvement of the researchers in the initial data collection and processing. In 3 sites, the proportion of exclusions based on extreme energy values was relatively low (Mali, 1%, implausible record; Mozambique, 6% for high intakes and 0.5% for implausible intakes; Bangladesh, 3% for low intakes and 7% for high intakes) (27). In Burkina Faso, 25% were excluded because of low intakes and 4% were excluded for high intakes, evaluated based on repeated recalls (26). In the Philippines data set, 45% of estimated energy intakes were low (27) and it was judged they could not be excluded without risk of substantial bias, so a lower cutoff was selected to determine exclusions (25).
Sources for nutrient data
Because some data sets were already processed (i.e. with nutrient intakes calculated), food composition tables (FCT) varied by study site. In each case, one FCT was primary but was supplemented with values from other FCT for foods not available in the following primary source: Bangladesh, International Minilist (28); Mali and Burkina Faso, Table de composition des aliments du Mali (29); Mozambique, USDA Release 19 (30); and the Philippines, Food and Nutrition Research Institute (31, 32). The Filipino FCT accounted for moisture yield, but retention factors could not be added due to the size of the database. In the other sites, nutrients were calculated for foods as eaten using USDA nutrient retention factors (33) for cooked foods or ingredients, as needed.
Operationalizing dietary diversity
Dietary diversity has been operationalized variously as the sum of individual foods and/or food groups consumed across varying time periods (7). More complex indicators incorporate portion size considerations. With an aim of examining candidate indicators that could potentially be gathered in large-scale surveys, we selected a set of 8 food group diversity indicators (FGI) that: 1) were based on food groups, not individual food items; 2) varied in level of aggregation of foods into groups; 3) varied in the minimum quantity of consumption required for a food group to “count” in the score (1 or 15 g); and 4) were based on recall of a single day. This set of FGI allowed us to explore the effect of aggregation and minimum quantities on indicator performance. Foods were aggregated into 6, 9, 13, or 21 food groups, with the 2 most aggregated reflecting groupings available from the Demographic and Health Surveys Phase 5 model questionnaire at the time the protocol was designed (34). The more disaggregated FGI separated subgroups rich in specific nutrients (e.g. vitamin C-rich fruits and vegetables from other fruits and vegetables) (Table 1). For vitamin A-rich and vitamin C-rich groups, foods were included if they were a “source” based on the Codex Alimentarius definition (35, 36); i.e. 60 Retinol Activity Equivalents per 100 g for vitamin A and 9 mg per 100 g for vitamin C.
TABLE 1.
Food groups summed in diversity indicators
| 6-group indicators | 9-group indicators | 13-group indicators | 21-group indicators |
| All starchy staples | All starchy staples | All starchy staples | Grains and grain products |
| All other starchy staples | |||
| All legumes and nuts | All legumes and nuts | All legumes and nuts | Cooked dry beans and peas |
| Soybeans and soy products | |||
| Nuts and seeds | |||
| All dairy | All dairy | All dairy | Milk/yogurt |
| Cheese | |||
| Other animal source foods | Organ meat | Organ meat | Organ meat |
| Eggs | Eggs | Eggs | |
| Flesh foods and other miscellaneous small animal protein | Small fish eaten whole with bones | Small fish eaten whole with bones | |
| All other flesh foods and miscellaneous small animal protein | Large whole fish/dried fish/shellfish and other seafood | ||
| Beef, pork, veal, lamb, goat, game meat | |||
| Chicken, duck, turkey, pigeon, guinea hen, game birds | |||
| Insects, grubs, snakes, rodents, and other small animals | |||
| Vitamin A-rich fruits and vegetables | Vitamin A-rich dark green leafy vegetables | Vitamin A-rich dark green leafy vegetables | Vitamin A-rich dark green leafy vegetables |
| Other vitamin A-rich vegetables and fruits | Vitamin A-rich deep yellow/orange/red vegetables | Vitamin A-rich deep yellow/orange/red vegetables | |
| Vitamin A-rich fruits | Vitamin A-rich fruits | ||
| Other fruits and vegetables | Other fruits and vegetables | Vitamin C-rich vegetables | Vitamin C-rich vegetables |
| Vitamin C-rich fruits | Vitamin C-rich fruits | ||
| All other fruits and vegetables | All other vegetables |
Two minimum quantities were used in the FGI (1 and 15 g); indicators with the 1-g minimum were FGI-6, FGI-9, FGI-13, and FGI-21, depending on the number of food groups; those with the 15-g minimum consumption requirement were denoted as FGI-R (FGI-6R, FGI-9R, FGI-13R, and FGI-21R). With the objective of relevance across countries/regions and in large-scale surveys, it was not practical to explore quantity cutoffs based on serving sizes, which vary widely by food (within groups) and across regions with different diet patterns. Fifteen grams is approximately equivalent to 1 tablespoon, and it was considered potentially feasible to exclude trivial amounts of less than this in future surveys.
Selection of nutrients
We aimed to summarize micronutrient adequacy across a range of micronutrients with known public health relevance; most available information relates to potential effects on pregnancy outcomes (37) and breast milk content (38). We also considered the availability of nutrient data in the processed data sets and in available FCT. The following 11 micronutrients were selected: vitamin A, thiamin, riboflavin, niacin, vitamin B-6, folate, vitamin B-12, vitamin C, calcium, iron, and zinc.
Estimating probability of adequacy
We used the probability approach (3) to estimate adequacy for 10 of the 11 nutrients (all but calcium). We compared distributions of estimated usual intakes to the WHO/FAO requirement distributions for vitamins (39). Where EAR are not provided by the WHO/FAO, we back-calculated an EAR from Recommended Nutrient Intakes using the CV from the WHO/FAO (39) if available, or otherwise from the Institute of Medicine at the United States National Academy of Sciences (3). For iron requirements, which are known to be skewed for nonpregnant, nonlactating (NPNL) women, we used Institute of Medicine tables (40) but adjusted for absorption of 5% (Burkina Faso, Mozambique) or 10% (Bangladesh, Mali, the Philippines) for NPNL based on diet patterns, according to WHO/FAO guidance (39). For pregnant women, we lacked information on trimester, so assumed absorption of 23% (weighted mean of values for different trimesters) (40). For zinc, we used the International Zinc Nutrition Consultative Group EAR and CV (41), assuming low absorption (25%) in Burkina Faso and Mozambique and intermediate absorption (34%) in Bangladesh, Mali, and the Philippines. Finally, for calcium, we judged that the WHO/FAO EAR of 840 mg/d for calcium19 is high and not well justified. It is well above the United Kingdom EAR (525 mg/d) and is closer to the U.S. Adequate Intake of 1000 mg/d for adult women. We chose to evaluate probability of adequacy (PA) for calcium following the method used by Foote et al. (15).
We estimated usual intake distributions before calculating PA for each micronutrient. The mean PA for a group is equivalent to the prevalence of adequacy (3) for a particular micronutrient. We also averaged all 11 PA to form a summary variable for micronutrient adequacy, mean probability of adequacy (MPA). Like individual micronutrient PA, MPA has a possible range of 0 to 1. The distribution of MPA was also transformed if necessary to approximate normality for use in analyses.
Statistical analysis
Data were analyzed using Stata version 9 or 10 (42, 43), accounting for sample design characteristics as appropriate. Most statistical analyses were within sites. For cross-site comparisons of sample characteristics, energy contributions of food groups, FGI scores, and MPA, we used 1-way ANOVA with Bonferroni’s correction for multiple comparisons; each site was compared to all others, West African sites were compared to all others, and urban/peri-urban sites were compared to rural sites. Differences of P < 0.05 were considered significant for all tests. Results are presented separately for lactating women and NPNL women when subsample sizes allowed (at least 100 women). No site reported a sufficient number of pregnant women for separate analysis. Data are presented as means ± SD or medians (interquartile range). Except for PA and MPA, statistics reflect observations from a single day (the first of 2 days in most sites, but the second of 3 observation days in Burkina Faso20). We used Pearson correlations and simple linear regressions to describe relationships between FGI and MPA and controlled for energy using best linear unbiased predictors of estimated usual energy intake. Regression diagnostics included assessment of normality of residuals and heteroskedasticity tests. When diagnostics indicated violation of assumptions, regression results are not reported. Untransformed values of MPA are presented in descriptive tables and figures and the transformed variable was used in correlation and regression analyses.
We used receiver-operating characteristic (ROC) analysis to assess FGI prediction of MPA. The area under the ROC curve (AUC) summarizes the predictive power of each indicator across all possible FGI cutoffs. An AUC of 0.50 represents a null value (no predictive power). A significant AUC indicates predictive power, but AUC can be significant even when predictive power is weak. Because our sample sizes (and statistical power) varied across sites, we considered an AUC cutoff ≥0.70 as a rule-of-thumb criterion to indicate acceptable predictive power for FGI. We also assessed indicator characteristics (sensitivity, specificity, and misclassification) and compared estimates of prevalence yielded by indicator/cutoff combinations to estimates of prevalence based on observed MPA (above and below selected cutoffs for MPA).
Results
Description of samples
Mean age ranged from 29 to 35 y (Table 2). Mean height ranged from 150–151 cm in the 2 Asian sites to 163–166 cm in the urban West African sites. Mean BMI was similar in all 3 urban/peri-urban samples (Burkina Faso, Mali, and the Philippines). Higher mean BMI in the urban/peri-urban samples was accompanied by higher prevalence of overweight (BMI ≥ 25, 28–33% compared to 2–7% in the 2 rural sites, P < 0.001 for urban/rural comparison). Prevalence of low BMI (<18.5) ranged from 9 to 17% except in rural Bangladesh, where nearly one-half the NPNL women had low BMI. Energy intakes were similar across sites, with the exception of the Philippines (P < 0.001 for comparisons with each other site).
TABLE 2.
Selected characteristics of women of reproductive age in 5 study sites1
| Burkina Faso | Mali | Mozambique | Bangladesh | Philippines | |
| Location | Urban | Urban | Rural | Rural | Urban/peri-urban |
| Year (data collection) | 2006 | 2007 | 2006 | 1996 | 2005 |
| Sample size (1st/2nd recall)2 | 178/178 | 102/96 | 409/94 | 412/147 | 2045/2045 |
| Pregnant/lactating, %/% | 7/20 | 0/0 | 13/62 | 0/27 | 4/8 |
| Age, y | 31 ± 8 | 32 ± 10.5 | 29 ± 7 | 31 ± 9 | 35 ± 11.6 |
| Weight,3kg | 63.1 ± 11.7 | 65.0 ± 14.8 | 50.3 ± 7.1 | 42.7 ± 6.2 | 52.8 ± 11.0 |
| Height, cm | 163.1 ± 6.2 | 166.0 ± 5.9 | 153.6 ± 5.5 | 150.3 ± 5.1 | 151.0 ± 5.2 |
| BMI3 | 23.7 ± 4.2 | 23.6 ± 5.6 | 21.2 ± 2.5 | 18.9 ± 2.4 | 23.1 ± 4.5 |
| BMI < 18.5,3% | 9 | 17 | 12 | 47 | 16 |
| BMI ≥ 25,3% | 33 | 28 | 7 | 2 | 32 |
| Energy intake,34kcal/d | 2078 (1692–2791) | 2024 (1613–2513) | 2086 (1620–2547) | 2083 (1761–2445) | 1211 (875–1664) |
Values are means ± SD, median (25th, 75th percentiles), or percent. Statistics describe samples on the first recall day (second in Burkina Faso).
Burkina Faso (22) selected the second of 3 recalls as primary ( = 178); n = 181 for first and n = 173 for 3rd.
Among the NPNL subsample: of 130 in Burkina Faso; 103 in Mozambique; 299 in Bangladesh; 1798 in Philippines. In Mali, all women were NPNL; anthropometry was available for a subsample of 64 and energy intake data for all 102.
1 kcal = 4.184 kJ.
Diet patterns
Diet patterns and nutrient intakes were similar among NPNL and lactating women (results not shown). Consumption of food groups varied by site and the impact of imposing the 15-g minimum varied both across food groups and across sites (Table 3). The 15-g minimum made the least difference to the assessment of food group consumption in Mozambique; i.e. in general, when a food group was consumed it was consumed in nontrivial amounts (≥15 g). Contributions of major food groups to energy intake also varied by site (Table 4). In the 2 urban West African sites, the proportion of total energy intake from starchy staples was lower than in the 2 rural sites, whereas energy contributions from fats, oils, and sweets were higher (P < 0.001 for all comparisons). In the Philippines, energy intake from animal-source foods was high and reflects the inclusion of commonly eaten very fatty meats in this food group. In Mozambique, the proportion of energy intake from fruits and vegetables was higher than in other sites (P < 0.001 for all comparisons) due to high intake of mango, which provided 12% of energy intakes. Total carbohydrate as a percentage of energy ranged from 57 to 66% in the urban sites and was 82% in the rural sites. Conversely, total fat intakes were very low at 6–7% of energy in rural sites and ranged up to 32% in urban Mali.
TABLE 3.
Percentage of all women who consumed 9 food groups on a single recall day, by study site
| Burkina Faso | Mali | Mozambique | Bangladesh | Philippines | |
| n | 178 | 102 | 409 | 412 | 2,045 |
| Starchy staples | |||||
| ≥1 g, % | 100 | 100 | 100 | 100 | 100 |
| ≥15 g, % | 100 | 100 | 100 | 100 | 100 |
| Legumes and nuts | |||||
| ≥1 g, % | 85 | 73 | 58 | 35 | 41 |
| ≥15 g, % | 61 | 39 | 56 | 33 | 26 |
| Dairy | |||||
| ≥1 g, % | 18 | 48 | 0 | 19 | 26 |
| ≥15 g, % | 18 | 47 | 0 | 18 | 13 |
| Organ meat | |||||
| ≥1 g, % | 0 | 0 | 0 | 0 | 11 |
| ≥15 g, % | 0 | 0 | 0 | 0 | 6 |
| Eggs | |||||
| ≥1 g, % | 1 | 8 | 6 | 7 | 26 |
| ≥15 g, % | 1 | 7 | 6 | 3 | 16 |
| Fish, meat, poultry1 | |||||
| ≥1 g, % | 93 | 98 | 46 | 72 | 99 |
| ≥15 g, % | 71 | 95 | 41 | 57 | 93 |
| Vitamin A-rich dark green leafy vegetables | |||||
| ≥1 g, % | 78 | 41 | 34 | 51 | 30 |
| ≥15 g, % | 55 | 28 | 34 | 49 | 23 |
| Other vitamin A-rich vegetables and fruits | |||||
| ≥1 g, % | 72 | 86 | 77 | 64 | 22 |
| ≥15 g, % | 32 | 25 | 77 | 16 | 9 |
| Other fruits and vegetables | |||||
| ≥1 g, % | 96 | 100 | 63 | 100 | 63 |
| ≥15 g, % | 93 | 100 | 53 | 82 | 46 |
Also includes other miscellaneous small protein, such as insects, grubs, and snakes.
TABLE 4.
Percentage of energy from major food groups and from macronutrients, all women, by study site
| Burkina Faso | Mali | Mozambique | Bangladesh | Philippines | |
| n | 178 | 102 | 409 | 412 | 2,045 |
| Starchy staples, % | 56 | 46 | 68 | 86 | 56 |
| Legumes and nuts, % | 10 | 11 | 11 | 2 | 2 |
| All animal source foods, % | 7 | 12 | 4 | 4 | 301 |
| All fruits and vegetables, % | 7 | 6 | 15 | 4 | 2 |
| Fats, oils, sweets, alcohol,2% | 20 | 25 | 2 | 4 | 101 |
| Carbohydrate, % | 66 | 57 | 82 | 82 | 65 |
| Protein, % | 11 | 11 | 11 | 10 | 16 |
| Fat, % | 22 | 32 | 7 | 6 | 19 |
In the Philippines, the animal source food group included very fatty meats, which were commonly consumed and difficult to classify.
Across all sites, very few women reported alcohol consumption.
FGI scores
The highest mean FGI scores were observed in the 2 urban samples from West Africa (Table 5). This was true across all levels of food group disaggregation (P < 0.01 for all comparisons except for FGI-21, where Mali did not differ from Bangladesh). Mozambique scores were lowest (5th) on all 1-g FGI (P < 0.01) but ranked 3rd or 4th for all FGI-R. In Mozambique, it was rare for women to report trivial intakes (<15 g; Table 3); fewer foods were eaten, but in nontrivial quantities. Women in the Philippines site ranked last in all 4 FGI-R.
TABLE 5.
FGI scores for all women, by study site1
| Burkina Faso, n = 178 |
Mali, n = 102 |
Mozambique, n = 409 |
Bangladesh, n = 412 |
Philippines, n = 2045 |
||||||
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| FGI-6 | 4.8 ± 0.7 | 2–6 | 5.1 ± 0.7 | 3–6 | 3.5 ± 0.9 | 2–5 | 4.1 ± 0.9 | 2–6 | 3.8 ± 1.2 | 2–6 |
| FGI-6R | 4.2 ± 0.9 | 2–6 | 4.3 ± 0.9 | 2–6 | 3.4 ± 0.8 | 1–5 | 3.5 ± 1.0 | 1–6 | 3.1 ± 1.0 | 1–6 |
| FGI-9 | 5.4 ± 1.0 | 2–7 | 5.5 ± 1.0 | 3–8 | 3.8 ± 0.9 | 2–7 | 4.5 ± 1.1 | 2–7 | 4.2 ± 1.5 | 2–9 |
| FGI-9R | 4.3 ± 1.1 | 2–7 | 4.4 ± 1.1 | 2–7 | 3.7 ± 0.8 | 1–7 | 3.6 ± 1.1 | 1–7 | 3.3 ± 1.1 | 1–7 |
| FGI-13 | 6.6 ± 1.6 | 2–10 | 6.4 ± 1.3 | 3–10 | 4.2 ± 1.2 | 2–8 | 5.7 ± 1.3 | 2–10 | 4.6 ± 1.8 | 2–11 |
| FGI-13R | 4.6 ± 1.2 | 2–8 | 4.9 ± 1.3 | 2–9 | 3.9 ± 1.0 | 1–7 | 3.7 ± 1.3 | 1–8 | 3.5 ± 1.3 | 1–9 |
| FGI-21 | 7.3 ± 1.8 | 2–11 | 7.1 ± 1.5 | 3–11 | 4.7 ± 1.6 | 2–9 | 6.5 ± 1.6 | 2–11 | 5.7 ± 2.4 | 2–15 |
| FGI-21R | 4.9 ± 1.4 | 2–9 | 5.6 ± 1.6 | 2–10 | 4.4 ± 1.3 | 2–9 | 4.4 ± 1.5 | 1–9 | 4.1 ± 1.6 | 1–11 |
Values are mean ± SD or range of values.
Ranges were wider for more disaggregated FGI, but not in proportion to the added number of food groups. For FGI-6, across sites, scores ranged from 2 to 6, whereas for FGI-21 the range was 2 to 15. Scores for FGI-6R ranged from 1 to 6 and those for FGI-21R ranged from 1 to 11.
Micronutrient adequacy
Unlike diet patterns and nutrient intakes, PA varied widely by physiological group due to higher nutrient requirements during lactation (Table 6). For NPNL women, considering all micronutrients and all sites, the estimated prevalence of adequacy was below 50% for more than one-half (34 of 55 cells in Table 5). Considering results by site, prevalence of adequacy was below 50% for 5 of 11 micronutrients in Mali, 6 in Mozambique, 7 in Burkina Faso and Bangladesh, and 9 in the Philippines. Considering results by micronutrient, prevalence of adequacy was below 50% in at least 4 of 5 sites for riboflavin, niacin, folate, vitamin B-12, calcium, and iron. For lactating women (data available in 3 sites) prevalence of adequacy was below 50% for nearly all micronutrients in all sites (28 of 33 cells in Table 5).
TABLE 6.
Estimated prevalence of adequacy and MPA across 11 micronutrients, by study site and physiological status
| Country | n | Thiamin | Riboflavin | Niacin | Vitamin B-6 | Folate | Vitamin B-12 | Vitamin C | Vitamin A | Calcium | Iron | Zinc | MPA ± SD |
| Burkina Faso | % | ||||||||||||
| NPNL | 130 | 49 | 16 | 19 | 70 | 15 | 6 | 70 | 73 | 30 | 15 | 70 | 0.39 ± 0.20 |
| Mali | |||||||||||||
| NPNL | 102 | 59 | 28 | 31 | 67 | 0 | 17 | 88 | 50 | 27 | 54 | 96 | 0.47 ± 0.18 |
| Mozambique | |||||||||||||
| Lactating | 306 | 35 | 6 | 23 | 47 | 12 | 20 | 78 | 67 | 17 | 7 | 65 | 0.34 ± 0.21 |
| NPNL | 103 | 68 | 45 | 49 | 90 | 45 | 26 | 90 | 86 | 18 | 1 | 76 | 0.54 ± 0.17 |
| Bangladesh | |||||||||||||
| Lactating | 113 | 0 | 2 | 21 | 28 | 0 | 18 | 23 | 38 | 26 | 26 | 94 | 0.25 ± 0.13 |
| NPNL | 299 | 9 | 15 | 30 | 82 | 2 | 20 | 52 | 53 | 21 | 10 | 92 | 0.35 ± 0.17 |
| Philippines | |||||||||||||
| Lactating | 247 | 3 | 3 | 39 | 13 | 29 | 71 | 7 | 12 | 17 | 28 | 38 | 0.24 ± 0.19 |
| NPNL | 1798 | 12 | 11 | 60 | 45 | 47 | 78 | 13 | 38 | 15 | 12 | 48 | 0.34 ± 0.23 |
When PA were averaged across all 11 micronutrients, the resulting MPA for NPNL women ranged from 0.34–0.35 in the 2 Asian sites up to 0.54 in Mozambique. For lactating women, MPA ranged from 0.24–0.25 (Asian sites) to 0.34 in Mozambique.
Although MPA was highest in Mozambique for both NPNL and lactating women (P < 0.01 for all comparisons), it would likely be lower outside of mango season. Mango provided one-half the vitamin A, one-half the vitamin C, and over 10% of the energy, thiamin, riboflavin, niacin, vitamin B-6, folate, and calcium consumed by all women in Mozambique (and a higher proportion of each among consumers). For the 95 women with repeated 24-h recalls, MPA varied greatly with the number of days mango was consumed: median MPA was 0.18 if mango was not consumed, 0.35 if consumed 1 day, and 0.45 if consumed both days.
Associations between FGI and MPA
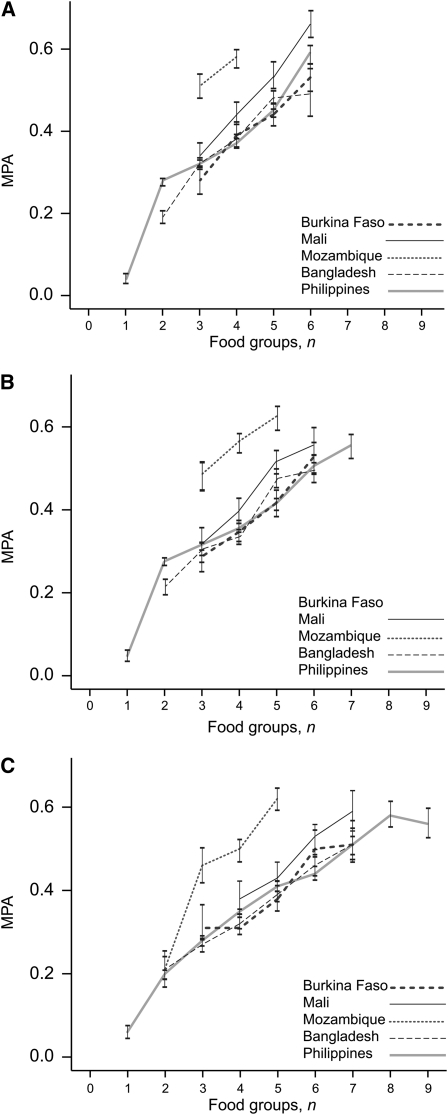
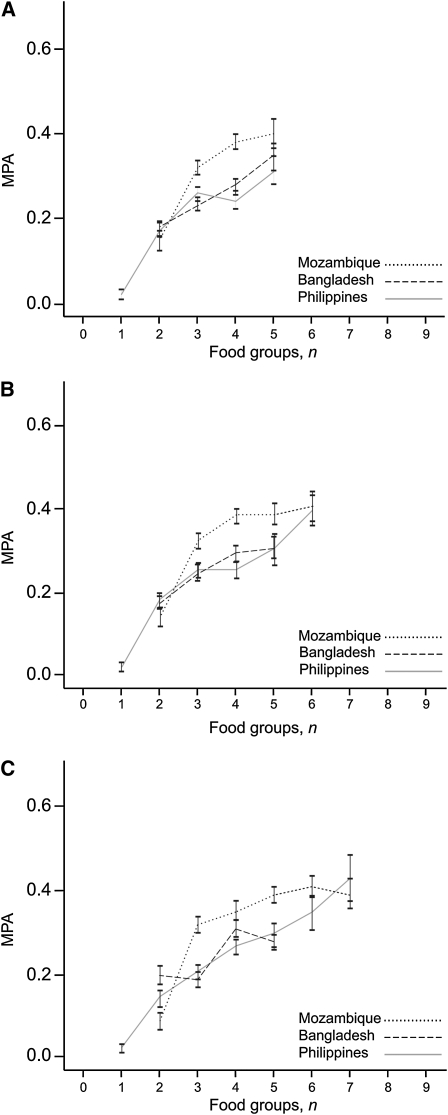
Higher FGI scores were associated with higher MPA and the pattern was consistent across sites, as shown for 3 FGI-R for NPNL women (Fig. 1) and for lactating women (Fig. 2). Among NPNL women, MPA was higher in Mozambique than in other sites when 3, 4, or 5 food groups were consumed (P < 0.001 for all comparisons); MPA also tended to be higher for lactating women. This is consistent with results presented previously, which suggest a pattern of few food groups eaten in substantial amounts and the influence of mango intake.
FIGURE 1.
MPA by FGI score, NPNL women, for FGI-9R (A), FGI-13R (B), and FGI-21R (C). Values are means ± SEM. Data points representing fewer than 10 observations are not included. Burkina Faso, n = 130; Mali, n = 102; Mozambique, n = 103; Bangladesh, n = 299; Philippines, n = 1798.
FIGURE 2.
MPA by FGI score, lactating women, for FGI-9R (A), FGI-13R (B), and FGI-21R (C). Values are means ± SEM. Data points representing fewer than 10 observations are not included. Mozambique, n = 252; Bangladesh, n = 111; Philippines, n = 167.
For NPNL women, correlations between all 8 FGI and MPA were significant in all sites (Table 7). When energy was controlled for, correlations were attenuated, but all remained significant; the attenuation was most marked in Mozambique. When energy intake was not controlled for, results for Mozambique were similar to or stronger than those in other sites. The size of the correlations ranged from 0.21 to 0.53 and 0.12 to 0.46 when energy intake was controlled for. Correlations tended to be lower for lactating women.
TABLE 7.
Correlation between FGI and MPA, by study site and physiological status1
| Controlling for energy |
||||||||||
| Burkina Faso, n = 130/02 |
Mali, n = 102/02 |
Mozambique, n = 103/2522 |
Bangladesh, n = 299/1112 |
Philippines, n = 1798/1672 |
||||||
| No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | |
| NPNL women | ||||||||||
| FGI-6 | 0.30*** | 0.23** | 0.32** | 0.25* | 0.30** | 0.20* | 0.39*** | 0.32*** | 0.21** | 0.12*** |
| FGI-6R | 0.38*** | 0.35*** | 0.50*** | 0.48*** | 0.38*** | 0.22* | 0.50*** | 0.44*** | 0.27*** | 0.21*** |
| FGI-9 | 0.33*** | 0.24** | 0.36*** | 0.33*** | 0.34*** | 0.24* | 0.48*** | 0.42*** | 0.26*** | 0.15** |
| FGI-9R | 0.42*** | 0.38*** | 0.45*** | 0.48*** | 0.43*** | 0.27** | 0.52*** | 0.46*** | 0.34*** | 0.25*** |
| FGI-13 | 0.27** | 0.20* | 0.30** | 0.27** | 0.38*** | 0.30** | 0.46*** | 0.39*** | 0.26*** | 0.15*** |
| FGI-13R | 0.43*** | 0.37*** | 0.42*** | 0.38*** | 0.42*** | 0.32*** | 0.51*** | 0.44*** | 0.33*** | 0.24*** |
| FGI-21 | 0.33*** | 0.28** | 0.34*** | 0.32** | 0.48*** | 0.33*** | 0.47*** | 0.38*** | 0.32*** | 0.16** |
| FGI-21R | 0.47*** | 0.39*** | 0.41*** | 0.41*** | 0.53*** | 0.37*** | 0.50*** | 0.42*** | 0.45*** | 0.29*** |
| Lactating women3 | ||||||||||
| FGI-6 | 0.19** | 0.12 | 0.28** | 0.15 | 0.19* | 0.12 | ||||
| FGI-6R | 0.28*** | 0.21*** | 0.38** | 0.33*** | 0.23** | 0.22** | ||||
| FGI-9 | 0.30*** | 0.26*** | 0.35** | 0.26** | 0.23** | 0.15* | ||||
| FGI-9R | 0.38*** | 0.36*** | 0.41*** | 0.35*** | 0.32*** | 0.28*** | ||||
| FGI-13 | 0.27*** | 0.22*** | 0.39*** | 0.30** | 0.20** | 0.13 | ||||
| FGI-13R | 0.36*** | 0.33*** | 0.41** | 0.34*** | 0.30** | 0.26*** | ||||
| FGI-21 | 0.27*** | 0.15* | 0.29** | 0.16 | 0.28*** | 0.09 | ||||
| FGI-21R | 0.34*** | 0.23** | 0.37*** | 0.27** | 0.39*** | 0.6*** | ||||
Values are Pearson correlations. * < 0.05; **P < 0.01; ***P < 0.001.
NPNL women/Lactating women.
There were too few lactating women for separate analysis in Burkina Faso and none in Mali.
Within each site, correlations tended to be consistently higher when the 15-g minimum was imposed. There also appeared to be a tendency toward higher correlations with higher levels of disaggregation, but this pattern was not entirely consistent. Whether or not energy intake was controlled for, correlations were lowest for FGI-6 in almost all sites and for both NPNL and lactating women. For NPNL women, correlations were highest for FGI-21R in 3 sites (Burkina Faso, Mozambique, and the Philippines) but were highest for FGI-6R in Mali and FGI-9R in Bangladesh. For lactating women, correlations for FGI-9R were highest in Mozambique and Bangladesh and were also highest in the Philippines when energy was controlled for.
Results for simple linear regressions confirmed that FGI remained significant in models controlling for age and height (Supplemental Table 1). When energy intake was included in the models, coefficients were attenuated, but most remained significant for NPNL women (exceptions were FGI-6 and FGI-21 in Mali and FGI-6 and FGI-9 in Mozambique). For lactating women, coefficients remained significant for 6 of 8 FGI in Mozambique and 4 of 8 (the 4 FGI-R) in the Philippines.21 The decrease in coefficients highlights that part of the positive relationship between FGI and MPA can be attributed to the increase in energy intake (i.e. quantity of foods consumed) that accompanies higher FGI (results not shown).
For NPNL women, coefficients ranged from 0.03 to 0.13 when energy was not in the models and from 0.02 to 0.10 when energy was controlled for. In the 2 West African sites, MPA was sufficiently normally distributed and no transformation was necessary, which facilitates interpretation of coefficients. In these sites, for FGI with the 15-g minimum, coefficients ranged from 0.06 to 0.12 when energy was not in the models. This would indicate increases of 6–12 percentage points in MPA for each 1-point increase in the FGI.
Indicator performance
For NPNL women, ROC analysis for MPA cutoffs of 0.50, 0.60, and 0.70 showed that most FGI significantly predicted MPA above each cutoff but with weak to moderate strength (Table 8). There were too few NPNL women above higher MPA cutoffs and too few lactating women above any cutoff 0.50 or higher to allow analysis. Results are consistent with those for correlations and regressions, with AUC tending to be higher for FGI-R (results for FGI with 1-g minimum not shown). At an MPA cutoff of 0.50, all AUC were significant. At this cutoff, AUC for FGI-9R, FGI-13R, and FGI-21R exceeded 0.70 in 4 of the sites, whereas only FGI-21R met this criterion for the Philippines. At MPA cutoffs of 0.60 and 0.70, results were less consistent. At an MPA cutoff of 0.60, AUC for several FGI (1-g minimum) were not significant in Mali and Mozambique. Although all AUC for FGI-R were significant, fewer exceeded 0.70 than was the case at the lower MPA cutoff. At an MPA cutoff of 0.70, in 2 of the 5 sites, most AUC were not significant.
TABLE 8.
Area under the ROC curve for FGI predicting MPA above selected cutoffs and percent above cutoffs, for NPNL by study site1
| Burkina Faso | Mali | Mozambique | Bangladesh | Philippines | |
| n | 130 | 102 | 103 | 299 | 1798 |
| MPA > 0.50 | |||||
| Above cutoff, % | 28 | 46 | 61 | 20 | 27 |
| FGI-6R | 0.68*** | 0.75*** | 0.69** | 0.72*** | 0.63*** |
| FGI-9R | 0.72*** | 0.75*** | 0.70** | 0.74*** | 0.66*** |
| FGI-13R | 0.74*** | 0.74*** | 0.70** | 0.75*** | 0.65*** |
| FGI-21R | 0.76*** | 0.74*** | 0.77*** | 0.72*** | 0.71*** |
| MPA > 0.60 | |||||
| Above cutoff, % | 15 | 25 | 46 | 7 | 16 |
| FGI-6R | 0.69** | 0.71** | 0.63* | 0.78*** | 0.64*** |
| FGI-9R | 0.68** | 0.70** | 0.65* | 0.82*** | 0.67*** |
| FGI-13R | 0.74*** | 0.68** | 0.67** | 0.84*** | 0.67*** |
| FGI-21R | 0.79*** | 0.68** | 0.74*** | 0.80*** | 0.73*** |
| MPA > 0.70 | |||||
| Above cutoff, % | 9 | 11 | 24 | 4 | 8 |
| FGI-6R | 0.63* | 0.78** | 0.56 | 0.78** | 0.68*** |
| FGI-9R | 0.63 | 0.75** | 0.60 | 0.81*** | 0.71*** |
| FGI-13R | 0.73*** | 0.66 | 0.63 | 0.83*** | 0.71*** |
| FGI-21R | 0.80*** | 0.68 | 0.70** | 0.81*** | 0.75*** |
Values are AUC or percent. * < 0.05; **P < 0.01; ***P < 0.001.
Dichotomous indicators are often preferred for advocacy and communication purposes. This requires both definition of an MPA cutoff, as above, and also a cutoff for number of food groups for any given FGI. We assessed indicator characteristics (sensitivity, specificity, and total misclassification) of all FGI for predicting the 3 MPA cutoffs. We also compared the proportion of women above various food group cutoffs to the proportion of women above the 3 MPA cutoffs. These analyses also indicated that no FGI and no specific cutoff for number of food groups could be identified for universal use (results not shown). There was substantial (≥20%) misclassification for all indicators (Table 9).
TABLE 9.
Summary of indicator characteristics relative to predicting MPA > 0.50, for NPNL women across all sites
| Food group cutoffs | Sensitivity1 | Specificity | False positives, % | False negatives, % | Total misclassified, % | Cutoff yielding closest estimate of % of women with MPA > 0.50 (percentage point difference)2 |
| FGI-9R | ||||||
| ≥3 | 86–100 | 4–32 | 33–69 | 0–4 | 33–69 | |
| ≥4 | 57–98 | 30–65 | 16–51 | 1–18 | 34–54 | Mozambique (−3), Philippines (+14) |
| ≥5 | 14–67 | 66–100 | 0–25 | 9–52 | 23–52 | Burkina Faso (+15), Mali (−4), Bangladesh (+1), Philippines (−12) |
| ≥6 | 2–36 | 94–100 | 0–5 | 18–60 | 21–60 | Burkina Faso (−13) |
| FGI-13R | ||||||
| ≥3 | 86–100 | 2–32 | 33–69 | 0–4 | 34–69 | |
| ≥4 | 59–98 | 18–62 | 18–53 | 1–15 | 33–56 | Mozambique (+4) |
| ≥5 | 30–79 | 59–93 | 3–30 | 6–43 | 23–46 | Mali (+12), Bangladesh (+8), Philippines (−5) |
| ≥6 | 10–50 | 84–100 | 0–9 | 14–55 | 22–55 | Burkina Faso (−6), Mali (−16), Bangladesh (−12) |
| ≥7 | 2–19 | 95–100 | 0–3 | 19–60 | 21–60 | |
| FGI-21R | ||||||
| ≥4 | 83–98 | 13–46 | 21–58 | 1–9 | 30–61 | Mozambique (+13) |
| ≥5 | 59–87 | 36–83 | 7–34 | 5–24 | 29–40 | Mozambique (−17), Philippines (+9) |
| ≥6 | 22–75 | 71–100 | 0–16 | 7–48 | 23–48 | Burkina Faso (+9), Mali (+3), Bangladesh (+2) |
| ≥7 | 11–40 | 89–100 | 0–6 | 16–54 | 21–54 | |
Sensitivity assesses the proportion of all those who have MPA > 0.50 who are identified by the indicator.
The difference is the site-specific prevalence for MPA > 0.50, less the prevalence estimated by the FGI at a given cutoff. When 2 cutoffs yielded estimates that were similar (within 5 percentage points) in distance from “true” prevalence, both are listed.
In each site, it was possible to identify indicator/food group cutoff combinations that yielded acceptable estimates of the proportion of women above selected MPA cutoffs, as shown for 3 FGI-R for the MPA cutoff of 0.50 (Table 9). There was some consistency in the range of “best” cutoffs at 4–6 food groups, across FGI and across sites.
Discussion
Our results show a consistent and moderately strong relationship between very simple indicators of food group diversity and micronutrient adequacy of the diet for women of reproductive age in 5 resource-poor settings (Table 7; Figs. 1 and 2). Relationships were stronger for NPNL women and for indicators with a 15-g minimum consumption required in order for a food group to count in the score.
We also documented large gaps between intakes and requirements across a range of micronutrients. It is notable that prevalence of adequacy was low for numerous micronutrients even in the urban/peri-urban sites, where macronutrient balance was acceptable (Table 4) (44). For NPNL women, gaps were most consistently problematic for 6 micronutrients: riboflavin, niacin, folate, vitamin B-12, calcium, and iron. For lactating women, prevalence of adequacy was low for all micronutrients and in all sites (Table 6).
These findings underscore the need to improve diets and micronutrient intakes for women of reproductive age. The findings further motivate the search for new tools, such as those described here, for assessment and tracking progress. Our results extend knowledge about the performance, potential usefulness, and limitations of FGI. Our results also speak to several issues around operationalizing such indicators.
Given the low distributions of MPA in our 5 sites, we could not explore whether FGI could predict high MPA (e.g. above 0.80, above 0.90). In our data sets, performance was most consistent for the lowest MPA cutoff of 0.50. Analysis of data sets with higher MPA distributions could shed light on the utility of these indicators for predicting MPA at or above higher cutoffs.
The moderate strength of associations between FGI and MPA was reflected in indicator performance (AUC, sensitivity, specificity, and misclassification). The AUC provide an overall assessment of each indicator, across all possible food group cutoffs. We suggest that at the population level, these indicators are meaningful, as reflected by AUC exceeding 0.70 (Table 8) and the bivariate relationships illustrated in Figures 1 and 2. Potential uses for “quasi-continuous” FGI scores include comparisons across time (so long as seasonality is accounted for); such uses have been proposed, e.g., by FAO (45).
When dichotomous indicators are required, e.g. to present prevalence estimates rather than a mean FGI score, indicator characteristics should be considered. Interpretation of these characteristics is a function of the desired uses of the indicator (46). A certain amount of misclassification may be acceptable in indicators for population-level assessment and for tracking progress at population level. Good estimates of prevalence may be obtained even in the presence of moderate to substantial misclassification. Such population-level indicators can highlight problems, motivate interventions, and allow accountability for progress. Simple FGI may be best suited for these purposes rather than for tasks that demand lower misclassification (e.g. decision-making for allocation of scarce resources, individual-level screening or diagnosis).
Thus, in evaluating the FGI, we examined the balance between sensitivity and specificity but also compared estimates of prevalence yielded by the indicators to the proportion of women above MPA cutoffs. Indicators and cutoffs can be identified that provide reasonable estimates of the proportion of women above selected MPA cutoffs (Table 9); these indicators and cutoffs vary by study site and no universal dichotomous indicator and cutoff could be identified.
The studies summarized here all employed 24-h recall methodologies and both the FGI and the MPA were generated from the same data sets. If simple FGI are used in the future, data collection would be through simpler methodologies, such as those employed in the Demographic and Health Surveys. Because our results are based on 24-h recalls, they inform methodological issues only in a few specific ways. Our results do suggest that it is desirable to avoid counting very trivial amounts of food in FGI scoring; further research is needed regarding how to operationalize this (47). Second, our results suggest that relationships between food group diversity and micronutrient adequacy may vary by season, as evidenced by the strong impact of mango season in the Mozambique site, which led to higher MPA at each FGI score as compared to other sites. This should be considered if FGI are used to compare across time or between regions with different agricultural cycles. Third, there was some evidence that increasing disaggregation of food groups yielded better indicator performance. The 6-group indicators did not perform as well as others; in several countries, FGI-21R performed best. However, the more disaggregated indicators are also more difficult to operationalize, because more distinctions and decisions on food groupings must be correctly made when designing data collection instruments. These practical considerations must be balanced against possible improvements in performance.
Our study had several limitations. Most of our data sets had relatively small sample sizes, as is typical of quantitative 24-h recalls in developing countries, and this restricted statistical power. The 2 larger data sets showed evidence of underreporting (Philippines) and, to a lesser extent, possible overreporting (Bangladesh).21 This may reflect a trade-off between quantity and quality of data.
Further, as noted, the MPA and the FGI were generated from the same data sets; measurement errors may be correlated and this could have biased the magnitude of correlations. Conversely, the random errors inherent in all dietary data are known to attenuate measures of association, such as correlation and regression coefficients. Specifically, high intra-individual variation in intakes across days can have this effect. In our analyses, we correlated single-day diversity measures with estimates of usual nutrient intakes with inherent variability; precise individual-level estimates of nutrient intakes require many repeated recalls. This problem has been characterized and addressed analytically, primarily in the context of assessing diet-health associations (48–50). Further research could address the effects of correlated errors, high random error, or both on correlations between FGI and measures of micronutrient adequacy (51).
Although these limitations could affect estimated coefficients in either direction, we judge that they would be very unlikely to change our main conclusions from this work. Micronutrient intakes for women of reproductive age are far from adequate, and FGI may provide a very simple proxy tool for population-level assessment and tracking progress over time. Although our results do not suggest a single indicator or a single cutoff for global use, they provide evidence for selection of site-specific indicators in our study areas and, arguably, similar areas in the study regions. Our results are consistent with other studies that have documented associations between dietary diversity indicators and micronutrient intakes or adequacy in developing countries (16–19). Taken together, these studies suggest the relationship is robust and FGI can be meaningfully operationalized in various ways. This is relevant, because as countries develop and adopt food-based dietary guidelines, they can also adopt simple, meaningful FGI that reflect those guidelines.
Acknowledgments
We thank Elaine Ferguson for many contributions made during discussions of methodology and of preliminary results; we thank Eunyong Chung, Kathleen Kurz, Suzanne Murphy, Marie Ruel, and Anne Swindale for very helpful comments on a previous draft. M.A. and L.E.T. designed the research; A.C. and M.J. developed statistical methods; all authors collaborated in refining methodologies; M.A., E.B., M.D., N.F., M.J., G.K., Y.M-P., and D.W. analyzed data; all authors contributed substantially to the development of the manuscript; M.A. wrote the paper and had responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
This paper was submitted as part of the supplement, “Developing Simple Measures of Women's Diet Quality in Developing Countries: Methods and Findings”. The paper underwent the standard JN peer review process.
Published in a supplement to The Journal of Nutrition. Findings of research carried out under the USAID funded Food and Nutrition Technical Assistance Project’s (FANTA) Women’s Dietary Diversity Project (WDDP). The supplement coordinators for this supplement were Megan Deitchler, AED, and Marie T. Ruel, International Food Policy Research Institute. Supplement Coordinator disclosures: Megan Deitchler is an employee of AED. Marie T. Ruel declares no conflict of interest. The supplement is the responsibility of the Guest Editor to whom the Editor of The Journal of Nutrition has delegated supervision of both technical conformity to the published regulations of The Journal of Nutrition and general oversight of the scientific merit of each article. The Guest Editor for this supplement was Jennifer Nettleton. Guest Editor disclosure: no conflicts of interest. This publication is made possible by the generous support of the American people through the support of the Office of Health, Infectious Disease, and Nutrition, Bureau for Global Health, United States Agency for International Development (USAID), under terms of Cooperative Agreement No. GHN-A-00-08-00001-00, through the Food and Nutritional Technical Assistance II Project (FANTA-2), managed by AED. The opinions expressed herein are those of the authors and do not necessarily reflect the views of USAID or the United States Government. Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of The Journal of Nutrition.
Supported by the German Federal Ministry for Economic Cooperation and Development, the Norwegian Research Council, the International Food Policy Research Institute, and Iowa State University. Funding for data collection and data processing for individual study sites was received from the Danish International Development Assistance; United States Agency for International Development (USAID); the Institute of Research for Development; the European Union through the FONIO Project (EU/INCO No 0015403); HarvestPlus Project; and the NIH, Fogarty International Center (grant no. RO1TW05596). Funding for protocol development, data analysis, and project coordination was made possible by the support of the Office of Health, Infectious Disease, and Nutrition, Bureau for Global Health, USAID, through both the Food and Nutrition Technical Assistance II Project (FANTA-2) under the terms of Cooperative Agreement No. GHNA-00-08-00001-00, and the FANTA Project (1998-2008) under the terms of Cooperative Agreement No. HRNA-00-98-00046-00, managed AED.
Supplemental Table 1 is available with the online posting of this paper at jn.nutrition.org.
The abstract has been previously published (FASEB J. 2009;23:917.3). Some results have been presented in posters (Experimental Biology, April 2009; International Conference on Diet and Activity Methods, June 2009) and orally (19th International Congress of Nutrition, October 2009). In addition, most results will be available in a report to be published on the Food and Nutrition Technical Assistance II Project Web site (http://www.fanta-2.org/): Dietary Diversity as a Measure of the Micronutrient Adequacy of Women’s Diets in Resource-Poor Areas: Summary of Results from Five Sites. Washington, DC: Food and Nutrition Technical Assistance II Project, AED, forthcoming.
The views expressed in this publication are those of the author(s) and do not necessarily reflect the views of FAO.
Abbreviations used: AUC, area under the receiver-operating characteristic curve; EAR, estimated average requirement; FCT, food composition table; FGI, food group diversity indicator; FGI-R, food group diversity indicator with a 15-g minimum consumption requirement; MPA, mean probability of adequacy; NPNL, nonpregnant, nonlactating; PA, probability of adequacy; ROC, receiver-operating characteristic; WDDP, Women’s Dietary Diversity Project.
Approvals were from the ethical review committees or Institutional Review Boards of: Bangladesh Medical Research Council; Burkina Faso: Ministry of Health; Mali: National Institute of Public Health Research; Mozambique: Ministry of Health, and International Food Policy Research Institute; Philippines: University of North Carolina at Chapel Hill.
840 mg/day is the WHO/FAO EAR for NPNL women, and is the same for lactating women (39). The EAR is 940 mg/day for pregnant women.
In Burkina Faso, the first 24-h recall was also a validation exercise and enumerators were in the home weighing foods and ingredients on the day before the recall. As this can cause changes in behavior, the second recall day was selected as more similar to recall days in the other studies.
Results are not presented for lactating women in Bangladesh because MPA could not be transformed to approximate normal and regression residuals were non-normally distributed for a majority of models.
Literature Cited
- 1.Kennedy E, Meyers L. Dietary reference intakes: development and uses for assessment of micronutrient status of women: a global perspective. Am J Clin Nutr. 2005;81S:1194–7 [DOI] [PubMed] [Google Scholar]
- 2.Torheim LE, Ferguson E, Penrose K, Arimond M. Women in resource-poor settings are at risk of inadequate intakes of multiple micronutrients. J Nutr. 2010;140:2051–8 [DOI] [PubMed] [Google Scholar]
- 3.Dietary Reference Intakes IOM Applications in dietary assessment. Washington, DC: National Academies Press; 2000 [PubMed] [Google Scholar]
- 4.Bryce J, Coitinho D, Darton-Hill I, Pinstrup-Anderson P. Maternal and child undernutrition: effective action at national level. Lancet. 2008;371:510–26 [DOI] [PubMed] [Google Scholar]
- 5.WHO Preparation and use of food-based dietary guidelines. WHO Technical Report Series No. 880. Geneva: WHO/FAO; 1998 [PubMed] [Google Scholar]
- 6.FAO. [Internet]. Rome; FAO; c2010 [updated 2009 Mar 25; cited 2009 Dec 29]. Food-based dietary guidelines. Available from: http://www.fao.org/ag/humannutrition/nutritioneducation/fbdg/en/
- 7.Ruel MT. Operationalizing dietary diversity: a review of measurement issues and research priorities. J Nutr. 2003;133S:3911–26 [DOI] [PubMed] [Google Scholar]
- 8.Randall E, Nichaman MZ, Contant CF. Diet diversity and nutrient intake. J Am Diet Assoc. 1985;85:830–6 [PubMed] [Google Scholar]
- 9.Krebs-Smith SM, Smiciklas-Wright H, Guthrie HA, Krebs-Smith J. The effects of variety in food choices on dietary quality. J Am Diet Assoc. 1987;87:897–903 [PubMed] [Google Scholar]
- 10.Kant AK. Indexes of overall diet quality: a review. J Am Diet Assoc. 1996;96:785–91 [DOI] [PubMed] [Google Scholar]
- 11.Drewnowski A, Henderson SA, Driscoll A, Rolls BJ. The dietary variety score: assessing diet quality in healthy young and older adults. J Am Diet Assoc. 1997;97:266–71 [DOI] [PubMed] [Google Scholar]
- 12.Cox DR, Skinner JD, Carruth BR, Moran J III, Houck KS. A Food Variety Index for Toddlers (VIT): development and application. J Am Diet Assoc. 1997;97:1382–6 [DOI] [PubMed] [Google Scholar]
- 13.Lowik MR, Hulshof KF, Brussaard JH. Food-based dietary guidelines: some assumptions tested for The Netherlands. Br J Nutr. 1999;81:S143–9 [DOI] [PubMed] [Google Scholar]
- 14.Bernstein MA, Tucker KL, Ryan ND, O’Neil EF, Clements KM, Nelson ME, Evans WJ, Fiatarone Singh MA. Higher dietary variety is associated with better nutritional status in frail elderly people. J Am Diet Assoc. 2002;102:1096–104 [DOI] [PubMed] [Google Scholar]
- 15.Foote JA, Murphy SP, Wilkens LR, Basiotis PP, Carlson A. Dietary variety increases the probability of nutrient adequacy among adults. J Nutr. 2004;134:1779–85 [DOI] [PubMed] [Google Scholar]
- 16.Ogle BM, Hung PH, Tuyet HT. Significance of wild vegetables in micronutrient intakes of women in Vietnam: an analysis of food variety. Asia Pac J Clin Nutr. 2001;10:21–30 [DOI] [PubMed] [Google Scholar]
- 17.Torheim LE, Barikmo I, Parr CL, Hatloy A, Ouattara F, Oshaug A. Validation of food variety as an indicator of diet quality assessed with a food frequency questionnaire for western Mali. Eur J Clin Nutr. 2003;57:1283–91 [DOI] [PubMed] [Google Scholar]
- 18.Torheim LE, Ouattara F, Diarra MM, Thiam FD, Barikmo I, Hatloy A, Oshaug A. Nutrient adequacy and dietary diversity in rural Mali: association and determinants. Eur J Clin Nutr. 2004;58:594–604 [DOI] [PubMed] [Google Scholar]
- 19.Roche ML, Creed-Kanashiro HM, Tuesta I, Kuhnlein HV. Traditional food diversity predicts dietary quality for the Awajun in the Peruvian Amazon. Public Health Nutr. 2008;11:457–65 [DOI] [PubMed] [Google Scholar]
- 20.Popkin BM. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with non communicable diseases. Am J Clin Nutr. 2006;84:289–98 [DOI] [PubMed] [Google Scholar]
- 21.Arimond M, Torheim LE, Wiesmann D, Joseph M, Carriquiry A. dietary diversity as a measure of the micronutrient adequacy of women’s diets: results from rural Bangladesh Site [Internet]. Washington, DC: Food and Nutrition Technical Assistance II Project, Academy for Educational Development; 2009 [cited 2009 Dec 29]. Available from: http://www.fantaproject.org/downloads/pdfs/WDDP_Bangladesh_Dec09.pdf.
- 22.Becquey E, Capon G, Martin-Prével Y. Dietary diversity as a measure of the micronutrient adequacy of women’s diets: results from Ouagadougou, Burkina Faso Site [Internet]. Washington, DC: Food and Nutrition Technical Assistance II Project, Academy for Educational Development; 2009 [cited 2009 Dec 29]. Available from: http://www.fantaproject.org/downloads/pdfs/WDDP_BurkinaFaso_Dec09.pdf.
- 23.Kennedy G, Fanou N, Seghieri C, Brouwer ID. Dietary diversity as a measure of the micronutrient adequacy of women’s diets: results from Bamako, Mali Site [Internet]. Washington, DC: Food and Nutrition Technical Assistance II Project, Academy for Educational Development; 2009 [cited 2009 Dec 29]. Available from: http://www.fantaproject.org/downloads/pdfs/WDDP_Mali_Dec09.pdf.
- 24.Wiesmann D, Arimond M, Loechl C. Dietary diversity as a measure of the micronutrient adequacy of women’s diets: results from rural Mozambique Site [Internet]. Washington, DC: Food and Nutrition Technical Assistance II Project, Academy for Educational Development; 2009 [cited 2009 Dec 29]. Available from: http://www.fantaproject.org/downloads/pdfs/WDDP_Mozambique_Dec09.pdf.
- 25.Daniels MC. Dietary diversity as a measure of the micronutrient adequacy of women’s diets: results from metropolitan Cebu, Philippines Site [Internet]. Washington, DC: Food and Nutrition Technical Assistance II Project, Academy for Educational Development; 2009 [cited 2009 Dec 29]. Available from: http://www.fantaproject.org/downloads/pdfs/WDDP_Philippines_Dec09.pdf.
- 26.Black AE. Critical evaluation of energy intake using the Goldberg cutoff for energy intake: basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes Relat Metab Disord. 2000;24:1119–30 [DOI] [PubMed] [Google Scholar]
- 27.Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, Prentice AM. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. derivation of cutoff limits to identify under-recording. Eur J Clin Nutr. 1991;45:569–81 [PubMed] [Google Scholar]
- 28.International Network of Food Data Systems. Rome: FAO [updated 1997 Jul 23; cited 2009 Dec 29]. WorldFood Dietary Assessment System, version 2, International Minilist. Available from: http://www.fao.org/infoods/software_worldfood_en.stm.
- 29.Barikmo I, Ouattara F, Oshaug A. Table de composition des aliments du Mali. Oslo: Akershus University College; 2004 [Google Scholar]
- 30.USDA Agricultural Research Service [Internet]. Washington, DC: USDA [updated 2009 October 2; cited 2009 Dec 29]. USDA National Nutrient Database for Standard Reference, Release 19. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=15973 [Google Scholar]
- 31.Food and Nutrition Research Institute Food composition tables. Manila (Philippines): Department of Science and Technology; 1980 [Google Scholar]
- 32.Food and Nutrition Research Institute Food composition table and menu eval. Manila (Philippines): Department of Science and Technology; 2000 [Google Scholar]
- 33.USDA Agricultural Research Service Washington, DC: USDA [updated 2009 Aug 14; cited 2009 Dec 29]. USDA Table of Nutrient Retention Factors, Release 6. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=9448 [Google Scholar]
- 34. MEASURE DHS [Internet]. Calverton (MD): ICF Macro [cited 2009 Dec 29]. Demographic and Health Surveys: DHS model questionnaires. Available from: http://www.measuredhs.com/aboutsurveys/dhs/questionnaires.cfm.
- 35. Codex Alimentarius Commission [Internet]. Rome/Geneva: FAO/WHO [cited 2009 Dec 29]. Guidelines on nutrition labelling. CAC/GL 2–1985, revision 1, 1993. Available from: http://www.codexalimentarius.net/web/more_info.jsp?id_sta=34.
- 36. Codex Alimentarius Commission [Internet]. Rome/Geneva: FAO/WHO [cited 2009 Dec 29]. Guidelines for use of nutrition and health claims. CAC/GL 23–1997, Revision 1, 2004. Available from: http://www.codexalimentarius.net/web/more_info.jsp?id_sta=351.
- 37.Allen LH. Multiple micronutrients in pregnancy and lactation: an overview. Am J Clin Nutr. 2005;81S:1206–12 [DOI] [PubMed] [Google Scholar]
- 38.Bartley KA, Underwood BA, Deckelbaum RJ. A life cycle micronutrient perspective for women’s health. Am J Clin Nutr. 2005;81:S1188–93 [DOI] [PubMed] [Google Scholar]
- 39.WHO/FAO Vitamin and mineral requirements in human nutrition. 2nd ed Geneva: WHO; 2004 [Google Scholar]
- 40.IOM Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academies Press; 2000 [PubMed] [Google Scholar]
- 41.IZiNCG Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004;25:S99–203 [PubMed] [Google Scholar]
- 42.StataCorp Stata statistical software release 9.0. College Station (TX): Stata Corp; 2005 [Google Scholar]
- 43.StataCorp Stata statistical software release 10.0. 2007. College Station (TX): Stata Corp; 2007 [Google Scholar]
- 44.WHO Nutrition and the prevention of chronic diseases: report of a Joint WHO/FAO Expert Consultation. Technical Report Series 916. Geneva: WHO; 2003 [Google Scholar]
- 45.FAO Guidelines for measuring household and individual dietary diversity. Version 2. Rome: FAO; June2007 [Google Scholar]
- 46.Habicht JP, Meyers L, Brownie C. Indicators for identifying and counting the improperly nourished. Am J Clin Nutr. 1982;35:1241–54 [DOI] [PubMed] [Google Scholar]
- 47.Martin-Prével Y, Becquey E, Arimond M. Food group diversity indicators derived from qualitative list-based questionnaire misreported some foods compared to same indicators derived from quantitative 24-hour recall in Urban Burkina Faso J Nutr. 2010;140:2086–93 [DOI] [PubMed] [Google Scholar]
- 48.Launer LJ, Kardjati S, Kusin JA, Reed GF. Patterns of variability in the nutrient intake of nutritionally vulnerable pregnant women. Eur J Clin Nutr. 1991;45:131–8 [PubMed] [Google Scholar]
- 49.Berti PR, Leonard WR. Demographic and socioeconomic determinants of variation in food and nutrient intake in an Andean community. Am J Phys Anthropol. 1998;105:407–17 [DOI] [PubMed] [Google Scholar]
- 50.Ferrari P, Day NE, Boshuizen HC, Roddam A, Hoffmann K, Thiébaut A, Pera G, Overvad K, Lund E, et al. The evaluation of the diet/disease relation in the EPIC study: considerations for the calibration and the disease models. Int J Epidemiol. 2008;37:368–78 [DOI] [PubMed] [Google Scholar]
- 51.Joseph ML, Carriquiry A. A measurement error approach to assess the association between dietary diversity, nutrient intake and mean probability of adequacy. J Nutr. 2010;140:2094–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]