Abstract
Primary tumours release soluble factors, including VEGF-A, TGFβ and TNFα, which induce expression of the chemokines S100A8 and S100A9 in the myeloid and endothelial cells within the lung before tumour metastasis. These chemokine-activated premetastatic niches support adhesion and invasion of disseminating malignant cells, thereby establishing a fertile habitat for metastatic tumours.
It has long been recognized that the preferential colonization of specific tissues by certain tumour types is, in part, determined by the unique nature of the microenvironment within distant target organs1. Emerging evidence suggests that certain primary tumour cells release soluble factors that induce a specific population of non-malignant haematopoietic cells to mobilize and engraft distant organ tissues, thereby establishing a ‘premetastatic niche’ that lay a foundation for incoming circulating cancer cells2,3. However, the identity of these soluble factors secreted by the tumour cells is not fully known.
On page 1369 in this issue, Hiratsuka et al.4 demonstrate that primary tumour cells release VEGF-A, TGFβ and TNFα that, in turn, induce the expression of the chemoattractants, S100A8 (also known as MRP8, Calgranulin A)5 and S100A9 (also known as MRP14, Calgranulin B)6 by lung endothelium and myeloid cells, thereby facilitating the homing of tumour cells to the premetastatic sites within the lung parenchyma (Fig, 1). S100A8 and S100A9 also increase the motility of circulating cancer cells by p38-mediated activation of pseudopodia for invasion (invadopodia), which accelerates the assembly of the metastatic focus. These findings support the concept that specific soluble factors released by the primary tumour induce the expression of S100-chemokines, thereby preparing the premetastatic niche for the engraftment of tumour cells.
Figure1.
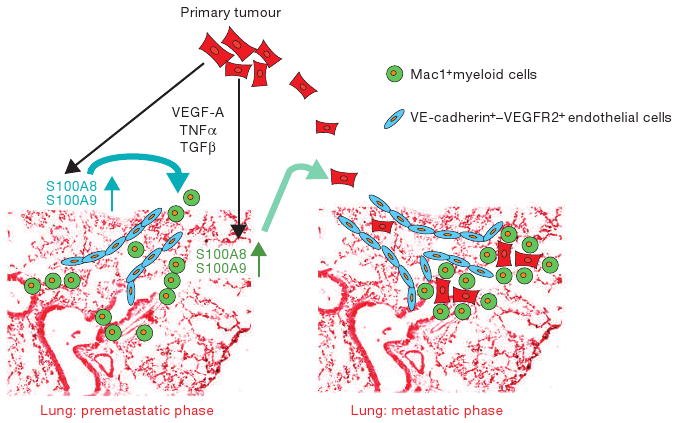
Establishment of a premetastatic niche by tumour cells. Primary tumour cells secrete pro-inflammatory factors such as VEGF-A, TGFβ and TNFα, which induce selective expression of chemoattractants S100A8 and S100A9 by lung VE-cadherin+-endothelial cells (blue) and Mac1+-myeloid cells (green). S100A8 and S100A9 expressed by the endothelial cells recruit Mac1+-myeloid cells, which set the stage for the arrival and docking of S100-responsive tumour cells (red) to the permissive lung tissue.
Metastasis of primary tumours to common distant target-organ site(s), such as lung and liver, requires activation of a complex set of proteases, adhesion molecules, inflammatory factors and chemokines1,7,8. Certain tumours, such as melanomas, metastasize to many organs, whereas prostate cancer primarily settles within the marrow microenvironment. It has been suggested that tumour cells randomly metastasize through either venous or lymphatic channels. Furthermore, these models speculate that localization of metastatic cancers within a particular organ may be random — disseminated cancer cells, or clusters of cancer cells associated with thrombotic cells, may be non-specifically trapped within the narrowing vessels1.
However, it was recently demonstrated that primary tumour cells release soluble factors that induce a specific subset of bone marrow-derived cells (BMDCs), which express vascular endothelial growth factor receptor-1 (VEGFR1, also known as Flt-1), to colonize the target organ before the arrival of tumour cells2,9. Recruitment of VEGFR1+VLA4+ haematopoietic cells is associated with a simultaneous increase in the deposition of fibronectin, resulting in formation of a highly fertile habitat for adhesion and docking of circulating tumour cells. In experiments with mice that were implanted with metastatic lung cancers or melanoma cells, BMDCs arrived at the pre-metastatic sites before the invasion of cancer cells. Interference with mobilization of VEGFR1+ cells from the bone marrow, and their incorporation into the premetastatic niche, resulted in a significant decrease in subsequent tumour metastasis. Moreover, depleting VEGFR1+ cells, or inhibiting the function of VEGFR1 itself, was also sufficient to block neo-angiogenesis10,11 and retard tumour metastasis. Notably, tumour types determined the pattern of organ localization of BMDCs — most likely by introducing specific soluble factors into the plasma. For example, melanoma cells, which have the capacity to metastasize to virtually every organ, secreted factors that directed incorporation of VEGFR1+ BMDCs to all of the organs that are known to be the common sites for melanoma metastasis. However, the identity of factors that directed the recruitment of marrow-derived cells to a particular premetastatic niche has not been fully elucidated.
Hiratsuka et al.4 showed that when normal lung tissue is cultured with serum from mice harbouring Lewis Lung Carcinoma or B16 melanoma, the expression of S100A8 and S100A9 chemokines was induced in VE-cadherin+ endothelial cells and Mac1+ myeloid cells derived from the lungs, and this activity was destroyed following heat inactivation. They also showed that S100A8 induced phosphorylation of p38, thereby leading to migration of the tumour cells. Neutralizing monoclonal antibodies against S100A8 and S100A9 reduced the colonization by tumours cells in the lungs dramatically. This indicated that blocking S100A8 and S100A9 expression and infiltration of Mac1+-myeloid cells into the lung at the premetastatic stage could inhibit the migration of disseminating cancer cells.
Remarkably, soluble factors released by the primary tumours induced the expression of S100A8 and S100A9, primarily in the lungs, but only minimally in liver or kidneys. As such, inhibition of S100A8 and S100A9 was more effective in abrogating metastasis to the lung than to other organs. These data suggested that selective upregulation of S100A8 and S100A9 in the lungs set the stage for the metastasis specifically to this site.
These findings also suggest that factors released by primary tumours induce expression of specific chemokines within a given metastatic target. Using microarray analysis and quantitative real-time PCR, Hiratsuka et al. demonstrated that the production of the chemokine MIP1-α in premetastatic lungs was stimulated by primary lung tumours, but not by B16 melanoma4. Indeed, inhibition of MIP1-α in tumour-bearing lungs partially blocked colonization within the Lewis Lung carcinoma cells.
These new data set forth the novel concept that tumour metastatic potential may not only be dependent on the oncogenicity of the cancer cells, but also on the existence of ‘hot spots’ within each specific organ that are receptive to metastatic cells. It is conceivable that the number and capacity of these premetastatic hot spots that permit attachment of tumour cells may be determined by the genetic makeup of any given individual. This may explain, for example, why subsets of patients with early-stage cancer are more prone to lung and liver metastasis, whereas others with an identical stage of cancer and oncogenic repertoire are cured of their disease with timely surgery and adjuvant chemotherapy.
Despite the conceptual novelty of these findings, there are several issues that demand further investigation: First, it remains to be determined whether localization of the marrow-derived cells to organ-specific premetastatic niches is random, or whether there are specific sites within each target organ that are receptive for colonization by the S100-activated marrow-derived cells. For example, it is unclear whether S100A8 or S100A9 are selectively upregulated in specific niches within the lungs or randomly upregulated throughout the pulmonary vascular tree. Second, it is unclear how S100A8 and S100A9 are selectively upregulated within each specific organ. Hiratsuka et al. show that induction of S100A8 and S100A9 was stronger in the lungs than in the liver and kidneys4. This suggests that organ specificity of the tumour metastasis may be dependent on selective induction of organ-specific chemoattractants, suggesting that other, as yet unrecognized, organ-specific inducible chemokines may support the recruitment of metastatic tumour cells. Third, the precise molecular and cellular phenotype of Mac1+ cells is unknown. Mac1 is expressed on a wide variety of myeloid cells, including progenitor cells, suggesting that, similarly to VEGFR1+ cells2,12, Mac1+ cells may have the potential to undergo further cell expansion once recruited to the premetastatic niches. Whether this unique feature of Mac1+ cells, which may contain a subset of VEGFR1+ cells, contributes to acceleration of metastasis is unknown and will require further investigation. Finally, Mac1+ cells, by releasing S100 chemokines, encourage tumour-cell entry into lung tissue. However, Mac1+ cells are known to express metalloproteinases, such as MMP-9 (ref. 3), which may also support tumour invasion. Further studies are needed to determine how S100-activated lung-endothelial cells and Mac1+ cells allow docking of subsets of metastatic tumour cells.
Despite these unresolved issues, the proposition that primary tumours prepare specific sites within target organs for tumour metastasis has important clinical ramifications. Conventional diagnostic techniques, such as light microscopy, may fail to detect very small micrometastatic tumours in target organs or lymph nodes positioned in the immediate vicinity of the primary tumour. The presence of Mac1+- or VEGFR1+-cell clusters may be a useful prognostic indicator of impending metastatic disease, detectable even before micrometastases. The presence or absence of these clusters could be used in clinical-risk stratification to identify more clearly which patients would benefit from less or more intensive adjuvant or neo-adjuvant chemotherapy. Whether metastasis to a particular organ is predetermined by a specific set of soluble factors and a defined set of organ-specific chemokines is an important proposition and will open up new avenues of research to elucidate the complex molecular and cellular pathways involved in selective organ metastasis. This may lead to development of new treat-ments to block tumour metastasis or increase the efficacy of life-saving clinical therapies.
Contributor Information
Shahin Rafii, Email: srafii@med.cornell.edu.
David Lyden, Email: DCL2001@med.cornell.edu.
References
- 1.Steeg PS. Nature Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan RN, et al. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiratsuka S, et al. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 4.Hiratsuka S, et al. Nature Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 5.Roth J, Vogl T, Sorg C, Sunderkotter Trends Immunol. 2003;24:155–158. doi: 10.1016/s1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 6.Vogl T, et al. Blood. 2004;104:4260–4268. doi: 10.1182/blood-2004-02-0446. [DOI] [PubMed] [Google Scholar]
- 7.Minn AJ, et al. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steeg PS. Nature. 2005;438:750–751. doi: 10.1038/438750b. [DOI] [PubMed] [Google Scholar]
- 10.Lyden D, et al. Nature Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 11.Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Nature Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 12.Jin DK, et al. Nature Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]


