Abstract
Objectives
Although rapid childhood weight gain has been suggested to be a risk factor for lifetime obesity and other chronic diseases, few studies have been conducted in Asian populations. The aim of this study was, therefore, to ascertain whether rapid childhood weight gain is associated with indices of obesity in adulthood and, if so, which period in early development provides the greatest predictive value of future obesity in young Japanese women.
Methods
A total of 86 female university students aged 18–21 years old participated in this study. Current height, weight, body fat percentage (BFP) as evaluated by bioelectrical impedance analysis, and BMI were measured. Body weight at birth, 3 and 6 months, and 1.5 and 3 years of age was obtained from the maternity record book (Boshi-techo), and body weight at 6 years was obtained from school health records. We assessed infant weight gain by the weight change Z-score.
Results
Current BFP was found to be significantly correlated with weight change between 0 and 3 months (r = 0.26, P = 0.034) and between 3 and 6 months of age (r = −0.28, P = 0.031). However, none of the physical activity indices correlated with BMI and BFP.
Conclusions
Rapid weight gain in early infancy positively associates with BFP in young Japanese women.
Keywords: Body fat, Japanese, Infants, Rapid weight gain, Young women
Introduction
Rapid weight gain in childhood has been suggested to be a risk factor for lifetime obesity and other chronic diseases. A number of epidemiologic studies have shown that rapid weight gain between birth and 3 months of age correlates with body mass index (BMI) and other indices of obesity in adulthood [1]. While several such studies have been conducted on Caucasian populations, few studies have been conducted on Asian ones [2]. Because the prevalence of obesity and other chronic diseases are not uniform across ethnic groups, findings obtained from Europeans and North Americans may not be applicable to Asians [3]. The aim of this study was to ascertain whether rapid weight gain in childhood is associated with indices of obesity in adulthood and, if so, which period in early development provides the greatest predictive value of future obesity in young Japanese women.
Methods
Study design
This was a retrospective cohort study in which first-year female students at Niigata University of Health and Welfare were asked to participate. We initially contacted all 215 first-year female students in 2001. Of those contacted, 89 (41.4%) agreed to participate in this study. However, three students were excluded because they had diseases or conditions that may have affected metabolism (one had nephritic syndrome with long-term glucocorticoid treatment, one underwent growth-hormone replacement therapy, and one exceeded 3 standard deviations for body weight). Thus, 86 students were ultimately enrolled in the study. Written informed consent was obtained from all participants, and the study was approved by the Ethics Committee of Niigata University School of Medicine.
Data collection
Current height, weight, and body fat percentage (BFP) were measured in the afternoon using a segmental body composition analyzer (BC-118D; Tanita Corp, Tokyo, Japan). The BFP was determined by bioelectrical impedance analysis (BIA) with 8-point contact electrodes at a single-frequency (50 kHz). The accuracy of the BIA method has been experimentally verified in a comparison study with dual-energy X-ray absorptiometry (DXA) [4]. The BMI was calculated by weight (kg) divided by the square of height (m2). Information on current physical activity was obtained through interviews in which participants were asked to recall their physical activities during the past 7 days. Metabolic equivalent (MET) intensities were calculated according to the methodology of Sallis et al. [5]. Each MET reflects the ratio of the associated metabolic rate for a specific activity divided by the resting metabolic rate. Participants were also asked whether their activity levels in the past 7 days were lighter, similar, or more strenuous than their routine activity levels for a 3-month stretch.
Body weight at birth, 3 and 6 months, and 1.5 and 3 years of age were obtained from the maternity record books (Boshi-techo), which are provided to every pregnant woman in Japan by their local municipality. All events during pregnancy, delivery, and postpartum periods are recorded in the record book by the obstetrician or midwife. Body weight at 6 years of age was obtained from school health records. Postnatal weight gain is greatly affected by birth weight; however, a low-birth-weight (LBW) infant may gain the same numerical weight as a high-birth-weight (HBW) infant, but with drastically different implications for rate of change. For this reason, the simple recording of the numerical gain of kilograms insufficiently assesses an infant’s weight and metabolic change. Therefore, in this study, we used a change of weight Z-score to assess infant weight gain [Z-score = (individual weight − standardized weight)/standard deviation (SD)]. This score refers to the degree of deviation from an average growth chart [6].
Statistical analyses
Data are expressed as means ± SD. Pearson’s product moment correlation coefficient (r) was calculated between the change in the infant weight Z-score and other variables. Multiple regression analysis was used to determine associations between predictor and outcome variables adjusted for physical activity levels (METs index). Variables of activity levels during the past 7 days (lighter, similar, or more strenuous) were treated as dummy variables. Data were analyzed utilizing SPSS for Windows ver. 17.0 (SPSS, Chicago, IL). A two-sided P value <0.05 was considered to be statistically significant.
Results and discussion
Demographic and physical characteristics of the participants are provided in Table 1. Some of the values for infant weight were missing.
Table 1.
Participant characteristics
| Demographic and physical characteristics | n | Mean ± SD | Range |
|---|---|---|---|
| Age (years) | 86 | 18.9 ± 0.6 | 18.0, 21.0 |
| Weight (kg) | 86 | 53.8 ± 6.9 | 38.6, 80.7 |
| Height (cm) | 86 | 158.7 ± 5.7 | 145.0, 175.0 |
| BMI (kg/m2) | 86 | 21.3 ± 2.3 | 16.7, 32.9 |
| Body fat (%) | 86 | 29.1 ± 4.9 | 19.0, 49.5 |
| METs index | 86 | 237 ± 11 | 223, 296 |
| Infant weight (kg) | |||
| Birth | 82 | 3.2 ± 0.5 | 2.0, 4.9 |
| 3 months | 72 | 6.4 ± 0.6 | 5.2, 8.6 |
| 6 months | 67 | 7.9 ± 0.8 | 5.5, 9.8 |
| 1.5 years | 71 | 10.5 ± 1.2 | 7.5, 13.6 |
| 3 years | 69 | 14.1 ± 1.7 | 9.4, 20.0 |
| 6 years | 82 | 21.6 ± 3.3 | 14.8, 34.0 |
| Weight change Z-score | |||
| 0–3 month | 69 | –0.03 ± 1.11 | −3.42, 3.06 |
| 3–6 month | 62 | 0.04 ± 0.75 | −1.77, 1.56 |
| 6 month–1.5 year | 63 | 0.12 ± 0.86 | −2.40, 2.84 |
| 1.5–3 year | 64 | −0.04 ± 0.67 | −2.23, 1.81 |
| 3–6 year | 66 | 0.05 ± 0.88 | −2.05, 2.62 |
BMI Body mass index, MET metabolic equivalent (ratio of working metabolic rate/resting metabolic rate), SD standard deviation
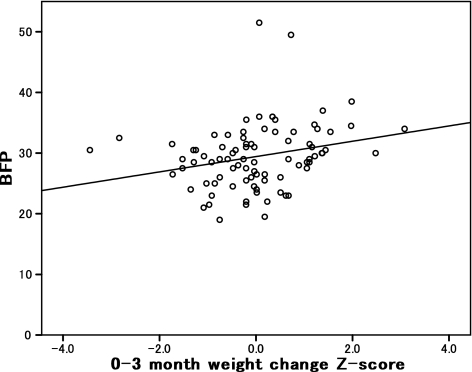
The results of the correlation analysis between the weight change Z-scores during early development and participants’ current BMI and BFP are shown in Table 2. Current BFP was statistically correlated with the Z-scores for weight change between 0 and 3 months (0–3 months) (r = 0.26, P = 0.034) and between 3 and 6 months (3–6 months) (r = −0.28, P = 0.031). However, the METs indices, suggestive of physical activity level, were not significantly correlated with current BMI (r = 0.17, P = 0.119) or BFP (r = 0.06, P = 0.571) (data not shown). Associations between 0–3 month weight change Z-scores and BFP [regression coefficient (β) = 1.338, standard error (SE) = 0.628, P = 0.037] and between 3–6 month weight change Z-scores and BFP (β = −2.347, SE = 0.907, P = 0.012) remained even after the METs index and activity levels were taken into account (data not shown). Figure 1 shows the relationship between current BFP and 0–3 month weight change Z-scores in young Japanese women.
Table 2.
Correlation coefficients of infant weight change Z-scores versus BMI and body fat percentage of young women
| Demographic and physical characteristics | n | BFP | BMI | ||
|---|---|---|---|---|---|
| r | P value | r | P value | ||
| Birth weight (kg) | 82 | 0.01 | 0.944 | 0.10 | 0.382 |
| Weight change Z-score | |||||
| 0–3 month | 69 | 0.26 | 0.034* | 0.18 | 0.132 |
| 3–6 month | 62 | −0.28 | 0.031* | −0.21 | 0.097 |
| 6 month–1.5 year | 63 | 0.14 | 0.267 | 0.11 | 0.397 |
| 1.5–3 year | 64 | 0.06 | 0.640 | 0.09 | 0.502 |
| 3–6 year | 66 | 0.00 | 0.987 | 0.02 | 0.877 |
BFP Body fat percentage
* Two-sided P < 0.05 was considered to be statistically significant according to Pearson’s product moment method
Pearson’s product moment correlation coefficient (r) was calculated between the infant weight change Z-scores and other variables. 0–3 and 3–6 month weight change Z-scores were significantly associated with current BFP in young Japanese women
Fig. 1.
Relationship between weight change Z-score (0–3 months) and body fat percentage (BFP) in young Japanese women (n = 69). Pearson’s product moment correlation coefficient (r) was calculated using Pearson’s product moment method. A significant correlation (r = 0.26, P = 0.034) was found. Two-sided P < 0.05 was considered to be statistically significant
We found a significant positive correlation between the BFP of young women and their 0–3 months weight gain Z-score, which is in line results from previous studies. Botton et al. [7] showed that weight gain velocity from birth to 3 months of age is associated with fat mass, overweight, and waist circumference in adolescence. Leunissen et al. [8] also showed that rapid weight gain during the first 3 months of life is associated with a higher BFP and several determinants of subsequent chronic diseases (i.e., cardiovascular diseases and type 2 diabetes). Ekelund et al. [9] revealed that increasing weight gain during infancy (0–6 months) was independently associated with fat mass in Swedish young adults.
Our findings may reflect the “early programming hypothesis” which occurs during the prenatal or early infant period [10]. Several studies have recently focused specifically on the immediate postnatal period. Stettler et al. [11] found that weight gain in the first week of life was associated with overweight status two to three decades later. These researchers pointed out that the importance of their findings was not so much to predict those infants at risk of becoming overweight adults, but more to highlight the importance of physiological programming during short early-life periods on the development of chronic disease over a lifetime.
We also found a significant negative correlation between weight change between 3 to 6 months of age and BFP. Leunissen et al. [8] also showed a negative correlation between weight gain between 3 and 6 months of life and adult BFP, although their correlation coefficient was not statistically significant. This may be considered a rebound effect, i.e., slowdown of weight gain for compensation.
The correlation between very early infant weight gain and later BFP may be particularly important for Asians. Since Asians have a higher BFP than Caucasians at the same BMI value, they are more likely to develop health problems at lower BMIs than Caucasians [12]. In line with this result, the World Health Organization Expert Consultation concluded that a substantial proportion of Asians are at high risk for type 2 diabetes and cardiovascular disease; Asians have a lower BMI cut-off compared to the existing WHO cut-off of 25 kg/m2 for being overweight [3].
The prevalence of obesity is not high in Japan. The prevalence of BMI ≥25 was 5.4% in 20- to 29-year-old Japanese women according to the National Nutrition Survey in Japan [13] and 4.7% in our study population (data not shown). Therefore, it is possible to generalize our results to the Japanese population. It should be noted, however, that we did not assess weight gain in early infancy in relation to obesity because of the limited sample size. Future studies will be needed to address this issue.
This study has several limitations. First, the sample size was relatively small, possibly resulting in a limited detection of other possible correlations. A total of 67 subjects is required to detect a correlation coefficient of 0.3 with a statistical power of 80% [14]. Second, we did not adjust for possible confounding factors, such as current energy level or nutritional intake. Third, participants were students of a “Health and Welfare” university, so generalizations based on results of our study to the entire Japanese population should be made with caution. According to the 2004 National Health and Nutrition Survey in Japan [13], the average BMI of 18- to 21-year-old women was 20.7 at the time of the survey. The average BMI in our study population was 21.3, which is slightly higher than the national average. This difference should be taken into consideration when generalizing the results. Fourth, we assessed current physical activity levels, but we did not assess those during the early growth phase. The latter should be addressed in future studies. Lastly, given that this study statistically compared many variables, our significant findings might belong to type 1 errors.
Despite these limitations, our study is strengthened by the power of available epidemiological data in the Japanese population. Namely, we could obtain accurate infant weight data using the maternity record book (Boshi-techo) and school health records. Boshi-techo also records the pregnancy weight of mothers. Although we did not use these data, it would be interesting to study changes in weight during pregnancy in relation to indices of obesity in adulthood in the future.
In conclusion, our data suggest that rapid weight gain in the early post-natal period and infancy positively correlates to adult body fat mass in young Japanese women. This finding should be confirmed in larger epidemiologic studies.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research (©) No.16500460 from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
References
- 1.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka T, Matsuzaki A, Kuromaru R, Kinukawa N, Nose Y, Matsumoto T, et al. Association between birth weight and body mass index at 3 years of age. Pediatr Int. 2001;43:641–646. doi: 10.1046/j.1442-200X.2001.01468.x. [DOI] [PubMed] [Google Scholar]
- 3.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 4.Sato S, Demura S, Kitabayashi T, Noguchi T. Segmental body composition assessment for obese Japanese adults by single-frequency bioelectrical impedance analysis with 8-point contact electrodes. J Physiol Anthropol. 2007;26:533–540. doi: 10.2114/jpa2.26.533. [DOI] [PubMed] [Google Scholar]
- 5.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 6.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. Br Med J. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botton J, Heude B, Maccario J, Ducimetière P, Charles MA, FLVS Study Group Postnatal weight and height growth velocities at different ages between birth and 5 y and body composition in adolescent boys and girls. Am J Clin Nutr. 2008;87:1760–1768. doi: 10.1093/ajcn/87.6.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009;301:2234–2242. doi: 10.1001/jama.2009.761. [DOI] [PubMed] [Google Scholar]
- 9.Ekelund U, Ong K, Linné Y, Neovius M, Brage S, Dunger DB, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES) Am J Clin Nutr. 2006;83:324–330. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 10.Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007;66:423–434. doi: 10.1017/S0029665107005691. [DOI] [PubMed] [Google Scholar]
- 11.Stettler N, Stalling VA, Troxel AB, Zhao J, Schinnar R, Nelson SE, et al. Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Circulation. 2005;111:1897–1903. doi: 10.1161/01.CIR.0000161797.67671.A7. [DOI] [PubMed] [Google Scholar]
- 12.Kagawa M, Uenishi K, Kuroiwa C, Mori M, Binns CW. Is the BMI cut-off level for Japanese females for obesity set too high? A consideration from a body composition perspective. Asia Pac J Clin Nutr. 2006;15:502–507. [PubMed] [Google Scholar]
- 13.The National Health and Nutrition Survey in Japan, 2004. Tokyo: Daiichi Shuppan; 2006. [Google Scholar]
- 14.Browner WS, Black D, Newman TB, Hulley SB. Estimating sample size and power. In: Hulley SB, Cummings SR, editors. Designing clinical research. Baltimore: Williams & Wilkins; 1988. pp. 139–150. [Google Scholar]