Abstract
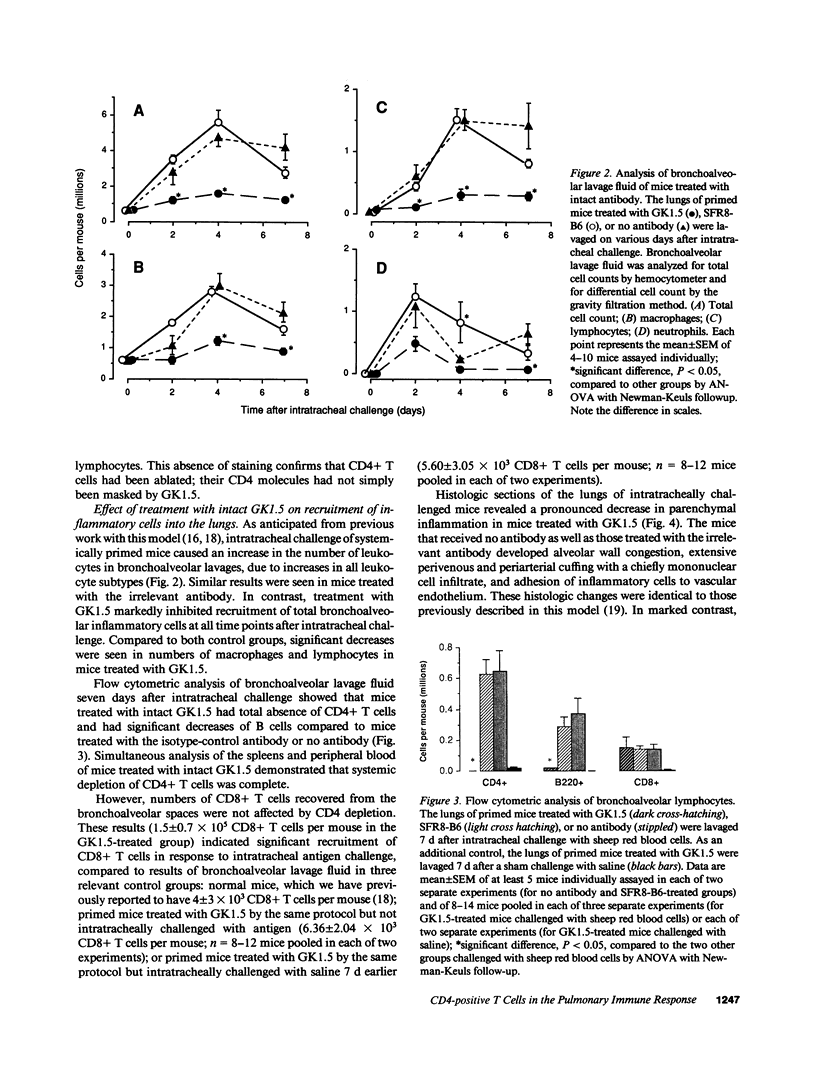
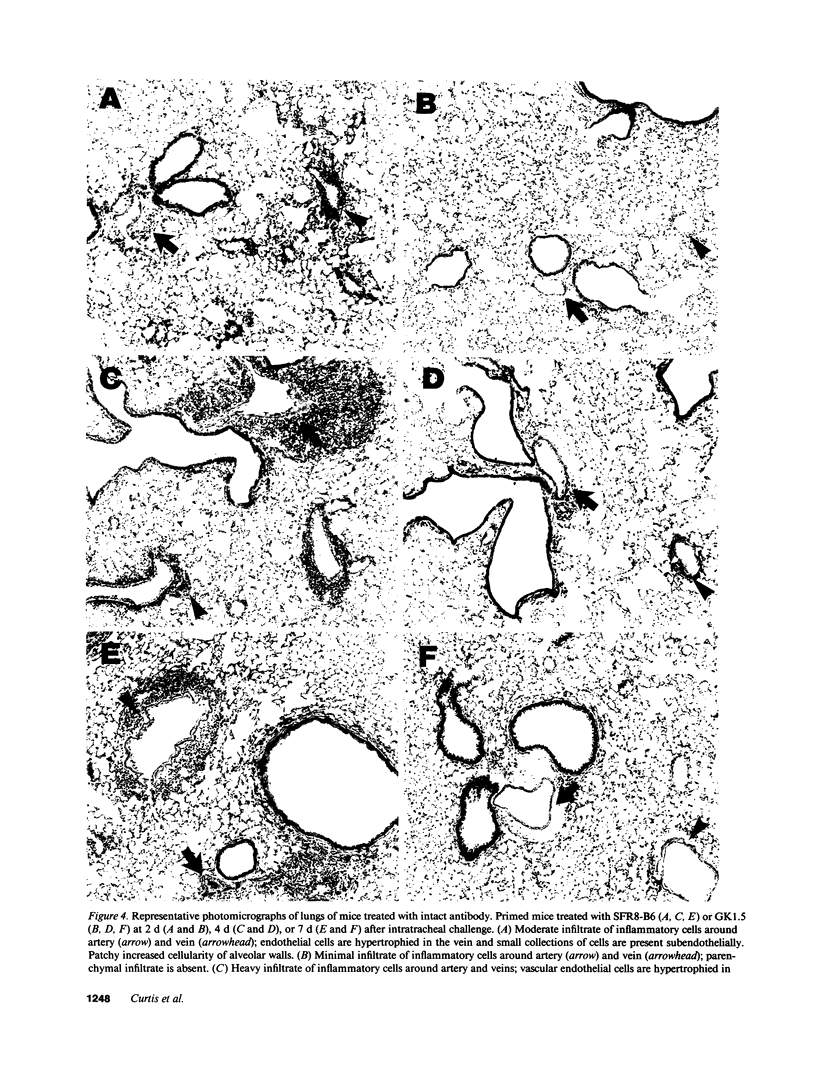
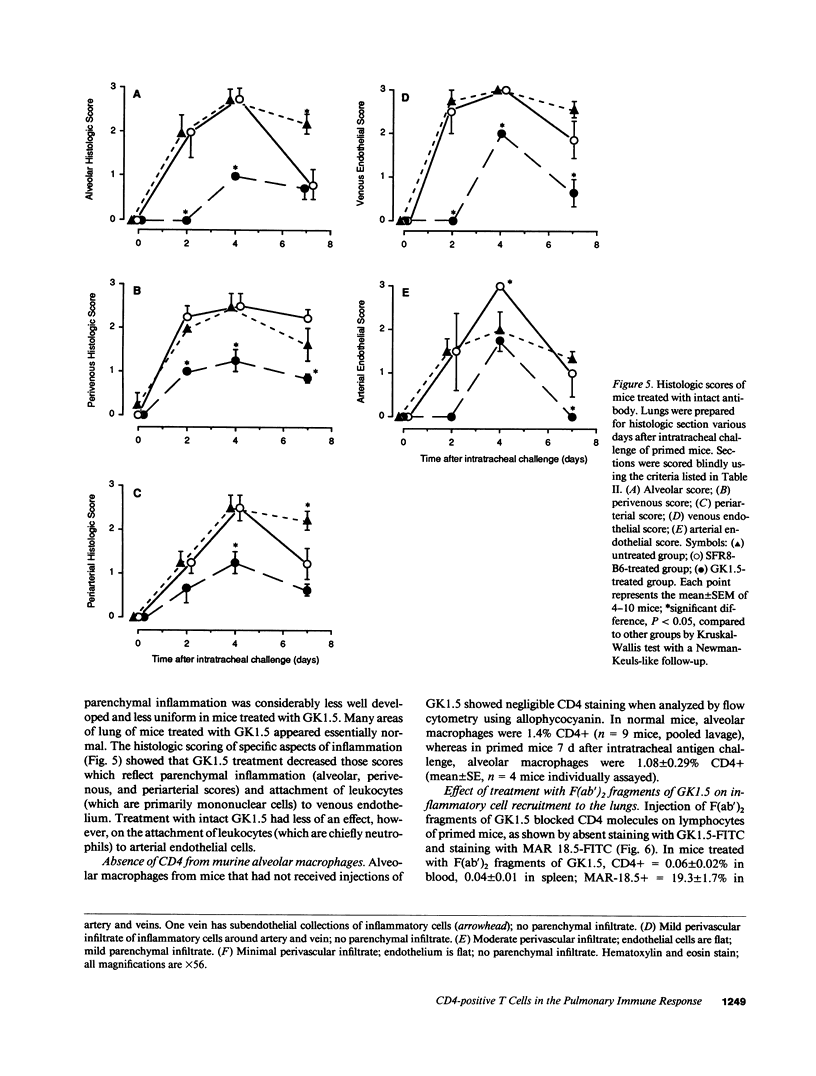
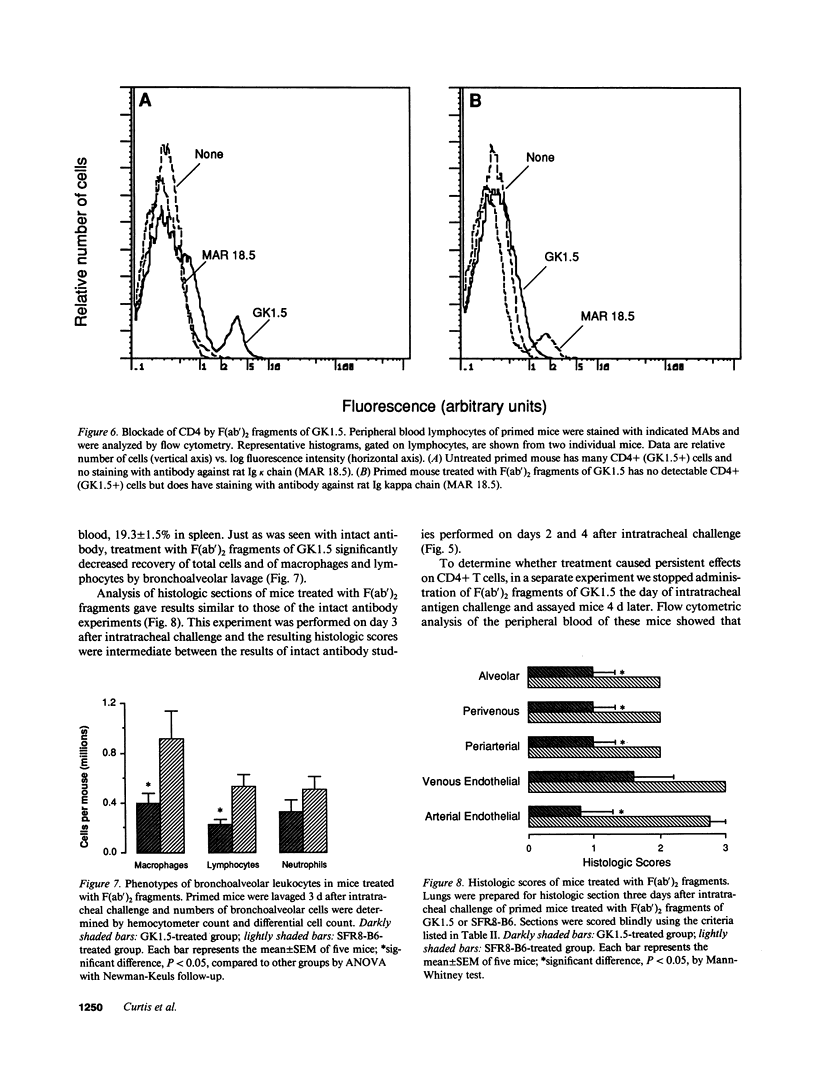
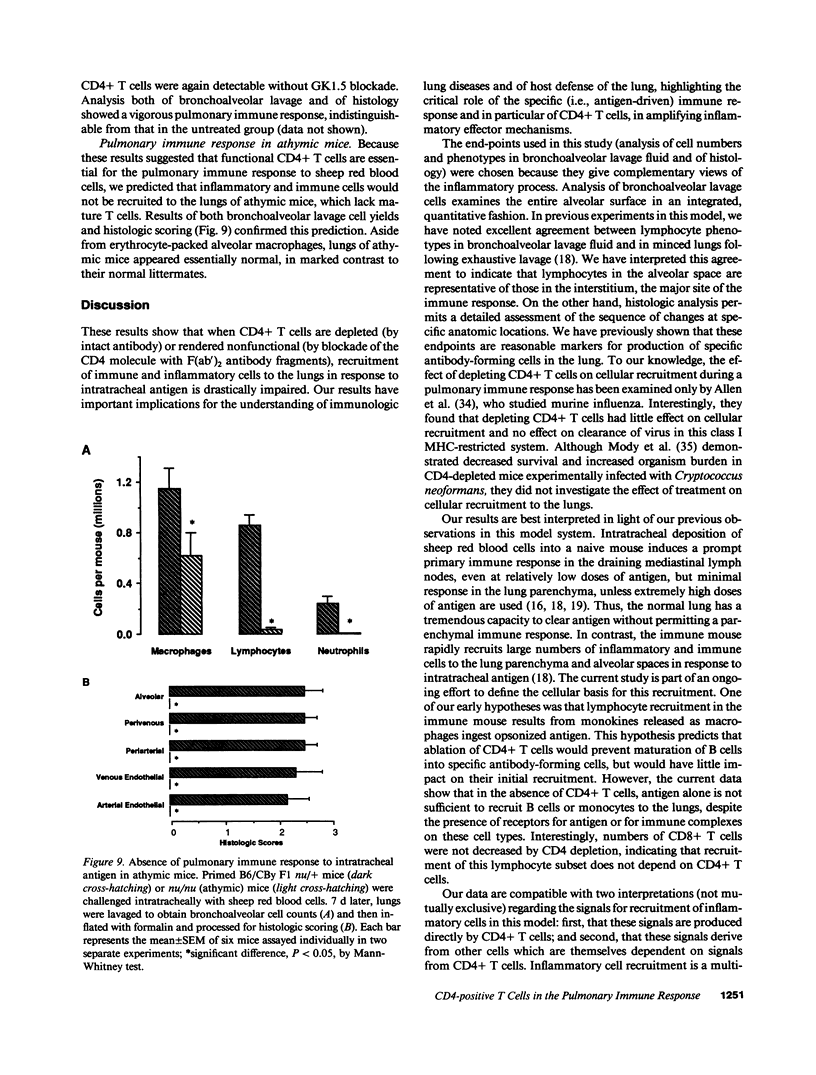
To determine whether CD4+ T cells participate in the recruitment of other lymphocyte subsets to the lungs, we examined pulmonary immune responses in C57BL/6 mice treated in vivo with the MAb GK1.5, either intact (which depletes CD4+ cells) or as F(ab')2 fragments (which block CD4 molecules). After intratracheal challenge with sheep erythrocytes, antigen-primed mice treated with intact GK1.5 had marked decreases in lymphocytes and macrophages in bronchoalveolar lavage fluid and minimal parenchymal inflammation, compared to primed mice treated with an isotype-matched irrelevant antibody or with no antibody. At 7 d after challenge, flow cytometric analysis showed that numbers of Thy 1.2+ and B220+ cells, but not of CD8+ cells, were markedly decreased in lavage fluid of CD4-depleted mice. Similar suppression of the pulmonary immune response to intratracheal challenge was found in primed mice injected repeatedly with F(ab')2 fragments of GK1.5, which did not deplete CD4+ T cells, and in athymic mice. These findings indicate that, in response to a single intratracheal antigen challenge, recruitment to the lungs of leukocytes other than CD8+ T cells depends largely on CD4+ T cells, possibly because of signals requiring T cell activation via interactions with antigen-presenting cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan W., Tabi Z., Cleary A., Doherty P. C. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990 May 15;144(10):3980–3986. [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank I., Chess L. Perturbation of the T4 molecule transmits a negative signal to T cells. J Exp Med. 1985 Oct 1;162(4):1294–1303. doi: 10.1084/jem.162.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. S., Beer D. J., Theodore A. C., Kornfeld H., Bernardo J., Center D. M. Lymphocyte recruitment to the lung. Am Rev Respir Dis. 1990 Jul;142(1):238–257. doi: 10.1164/ajrccm/142.1.238. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bice D. E., Shopp G. M. Antibody responses after lung immunization. Exp Lung Res. 1988;14(2):133–155. doi: 10.3109/01902148809115121. [DOI] [PubMed] [Google Scholar]
- Bierer B. E., Sleckman B. P., Ratnofsky S. E., Burakoff S. J. The biologic roles of CD2, CD4, and CD8 in T-cell activation. Annu Rev Immunol. 1989;7:579–599. doi: 10.1146/annurev.iy.07.040189.003051. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Belin D., Vassalli J. D., de Kossodo S., Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986 Dec 1;164(6):2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Jefferies W. A., Clark S. J., Chung L. P., Gordon S. Species heterogeneity in macrophage expression of the CD4 antigen. J Exp Med. 1987 Aug 1;166(2):613–618. doi: 10.1084/jem.166.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank W. W., Berman J. S., Theodore A. C., Bernardo J., Center D. M. Lymphokine activation of T4+ T lymphocytes and monocytes. J Immunol. 1987 Jun 1;138(11):3817–3823. [PubMed] [Google Scholar]
- Curtis J. L., Kaltreider H. B. Characterization of bronchoalveolar lymphocytes during a specific antibody-forming cell response in the lungs of mice. Am Rev Respir Dis. 1989 Feb;139(2):393–400. doi: 10.1164/ajrccm/139.2.393. [DOI] [PubMed] [Google Scholar]
- Curtis J. L., Warnock M. L., Arraj S. M., Kaltreider H. B. Histologic analysis of an immune response in the lung parenchyma of mice. Angiopathy accompanies inflammatory cell influx. Am J Pathol. 1990 Sep;137(3):689–699. [PMC free article] [PubMed] [Google Scholar]
- Debs R. J., Fuchs H. J., Philip R., Montgomery A. B., Brunette E. N., Liggitt D., Patton J. S., Shellito J. E. Lung-specific delivery of cytokines induces sustained pulmonary and systemic immunomodulation in rats. J Immunol. 1988 May 15;140(10):3482–3488. [PubMed] [Google Scholar]
- Duijvestijn A. M., Schreiber A. B., Butcher E. C. Interferon-gamma regulates an antigen specific for endothelial cells involved in lymphocyte traffic. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9114–9118. doi: 10.1073/pnas.83.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs H. J., McDowell J., Shellito J. E. Use of allophycocyanin allows quantitative description by flow cytometry of alveolar macrophage surface antigens present in low numbers of cells. Am Rev Respir Dis. 1988 Nov;138(5):1124–1128. doi: 10.1164/ajrccm/138.5.1124. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Goronzy J., Weyand C. M., Fathman C. G. Long-term humoral unresponsiveness in vivo, induced by treatment with monoclonal antibody against L3T4. J Exp Med. 1986 Sep 1;164(3):911–925. doi: 10.1084/jem.164.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Engers H. D., Louis J. A., Vassalli P., Lambert P. H. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986 Oct 1;137(7):2348–2354. [PubMed] [Google Scholar]
- Gutstein N. L., Seaman W. E., Scott J. H., Wofsy D. Induction of immune tolerance by administration of monoclonal antibody to L3T4. J Immunol. 1986 Aug 15;137(4):1127–1132. [PubMed] [Google Scholar]
- Gutstein N. L., Wofsy D. Administration of F(ab')2 fragments of monoclonal antibody to L3T4 inhibits humoral immunity in mice without depleting L3T4+ cells. J Immunol. 1986 Dec 1;137(11):3414–3419. [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad N., Helman T., Hoogenraad J. The effect of pre-injection of mice with pristane on ascites tumour formation and monoclonal antibody production. J Immunol Methods. 1983 Jul 29;61(3):317–320. doi: 10.1016/0022-1759(83)90225-9. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Bedell G. N., Zavala D. C., Monick M., Brady M. Role of interleukin-2 release by lung T-cells in active pulmonary sarcoidosis. Am Rev Respir Dis. 1983 Oct;128(4):634–638. doi: 10.1164/arrd.1983.128.4.634. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981 Aug 20;305(8):429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Glazier A. J., Monick M. M., Dinarello C. A. Interleukin-1 is a chemotactic factor for human T-lymphocytes. Am Rev Respir Dis. 1987 Jan;135(1):66–71. doi: 10.1164/arrd.1987.135.1.66. [DOI] [PubMed] [Google Scholar]
- Itescu S., Brancato L. J., Buxbaum J., Gregersen P. K., Rizk C. C., Croxson T. S., Solomon G. E., Winchester R. A diffuse infiltrative CD8 lymphocytosis syndrome in human immunodeficiency virus (HIV) infection: a host immune response associated with HLA-DR5. Ann Intern Med. 1990 Jan 1;112(1):3–10. doi: 10.7326/0003-4819-112-1-3. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Rojo J., Saizawa K., Dianzani U., Portoles P., Tite J., Haque S., Jones B. The co-receptor function of murine CD4. Immunol Rev. 1989 Jun;109:77–92. doi: 10.1111/j.1600-065x.1989.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltreider H. B., Byrd P. K., Curtis J. L. Expression of Ia by murine alveolar macrophages is upregulated during the evolution of a specific immune response in pulmonary parenchyma. Am Rev Respir Dis. 1988 Jun;137(6):1411–1416. doi: 10.1164/ajrccm/137.6.1411. [DOI] [PubMed] [Google Scholar]
- Kaltreider H. B., Caldwell J. L., Byrd P. K. The capacity of normal murine alveolar macrophages to function as antigen-presenting cells for the initiation of primary antibody-forming cell responses to sheep erythrocytes in vitro. Am Rev Respir Dis. 1986 Jun;133(6):1097–1104. doi: 10.1164/arrd.1986.133.6.1097. [DOI] [PubMed] [Google Scholar]
- Kaltreider H. B., Curtis J. L., Arraj S. M. The mechanism of appearance of specific antibody-forming cells in lungs of inbred mice after immunization with sheep erythrocytes intratracheally. II. Dose-dependence and kinetics of appearance of antibody-forming cells in hilar lymph nodes and lungs of unprimed and primed mice. Am Rev Respir Dis. 1987 Jan;135(1):87–92. doi: 10.1164/arrd.1987.135.1.87. [DOI] [PubMed] [Google Scholar]
- Kornfeld H., Berman J. S., Beer D. J., Center D. M. Induction of human T lymphocyte motility by interleukin 2. J Immunol. 1985 Jun;134(6):3887–3890. [PubMed] [Google Scholar]
- Kradin R. L., Divertie M. B., Colvin R. B., Ramirez J., Ryu J., Carpenter H. A., Bhan A. K. Usual interstitial pneumonitis is a T-cell alveolitis. Clin Immunol Immunopathol. 1986 Aug;40(2):224–235. doi: 10.1016/0090-1229(86)90025-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Wortis H. H. Role of antigen-specific B cells in the induction of SRBC-specific T cell proliferation. J Immunol. 1984 May;132(5):2253–2258. [PubMed] [Google Scholar]
- McLeod E., Caldwell J. L., Kaltreider H. B. Pulmonary immune responses of inbred mice. Appearance of antibody-forming cells in C57BL/6 mice after intrapulmonary or systemic immunization with sheep erythrocytes. Am Rev Respir Dis. 1978 Sep;118(3):561–571. doi: 10.1164/arrd.1978.118.3.561. [DOI] [PubMed] [Google Scholar]
- Ming W. J., Bersani L., Mantovani A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol. 1987 Mar 1;138(5):1469–1474. [PubMed] [Google Scholar]
- Mody C. H., Lipscomb M. F., Street N. E., Toews G. B. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J Immunol. 1990 Feb 15;144(4):1472–1477. [PubMed] [Google Scholar]
- Parks D. R., Herzenberg L. A. Fluorescence-activated cell sorting: theory, experimental optimization, and applications in lymphoid cell biology. Methods Enzymol. 1984;108:197–241. doi: 10.1016/s0076-6879(84)08086-1. [DOI] [PubMed] [Google Scholar]
- Pober J. S. Warner-Lambert/Parke-Davis award lecture. Cytokine-mediated activation of vascular endothelium. Physiology and pathology. Am J Pathol. 1988 Dec;133(3):426–433. [PMC free article] [PubMed] [Google Scholar]
- Ranges G. E., Sriram S., Cooper S. M. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985 Sep 1;162(3):1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Shigekane V. M., Fisher W. L., Locksley R. M. Cellular and humoral immunity to Leishmania major in genetically susceptible mice after in vivo depletion of L3T4+ T cells. J Immunol. 1987 Aug 15;139(4):1303–1309. [PubMed] [Google Scholar]
- Saltini C., Hemler M. E., Crystal R. G. T lymphocytes compartmentalized on the epithelial surface of the lower respiratory tract express the very late activation antigen complex VLA-1. Clin Immunol Immunopathol. 1988 Feb;46(2):221–233. doi: 10.1016/0090-1229(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Saltini C., Spurzem J. R., Lee J. J., Pinkston P., Crystal R. G. Spontaneous release of interleukin 2 by lung T lymphocytes in active pulmonary sarcoidosis is primarily from the Leu3+DR+ T cell subset. J Clin Invest. 1986 Jun;77(6):1962–1970. doi: 10.1172/JCI112525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman W. E., Wofsy D. Selective manipulation of the immune response in vivo by monoclonal antibodies. Annu Rev Med. 1988;39:231–241. doi: 10.1146/annurev.me.39.020188.001311. [DOI] [PubMed] [Google Scholar]
- Sertl K., Takemura T., Tschachler E., Ferrans V. J., Kaliner M. A., Shevach E. M. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J Exp Med. 1986 Feb 1;163(2):436–451. doi: 10.1084/jem.163.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellito J., Suzara V. V., Blumenfeld W., Beck J. M., Steger H. J., Ermak T. H. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J Clin Invest. 1990 May;85(5):1686–1693. doi: 10.1172/JCI114621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S. J., Fujimoto J., Levy R. Human T lymphocytes and monocytes bear the same Leu-3(T4) antigen. J Immunol. 1986 May 15;136(10):3773–3778. [PubMed] [Google Scholar]
- Titus R. G., Ceredig R., Cerottini J. C., Louis J. A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically-susceptible BALB/c mice. J Immunol. 1985 Sep;135(3):2108–2114. [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Waldmann H. Manipulation of T-cell responses with monoclonal antibodies. Annu Rev Immunol. 1989;7:407–444. doi: 10.1146/annurev.iy.07.040189.002203. [DOI] [PubMed] [Google Scholar]
- Walker C., Herzog C., Rieber P., Riethmüller G., Müller W., Pichler W. J. Anti-CD4 antibody treatment of patients with rheumatoid arthritis: II. Effect of in vivo treatment on in vitro proliferative response of CD4 cells. J Autoimmun. 1989 Oct;2(5):643–649. doi: 10.1016/s0896-8411(89)80003-4. [DOI] [PubMed] [Google Scholar]
- Warren J. S., Yabroff K. R., Remick D. G., Kunkel S. L., Chensue S. W., Kunkel R. G., Johnson K. J., Ward P. A. Tumor necrosis factor participates in the pathogenesis of acute immune complex alveolitis in the rat. J Clin Invest. 1989 Dec;84(6):1873–1882. doi: 10.1172/JCI114374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]
- Wofsy D. Administration of monoclonal anti-T cell antibodies retards murine lupus in BXSB mice. J Immunol. 1986 Jun 15;136(12):4554–4560. [PubMed] [Google Scholar]
- Wofsy D., Mayes D. C., Woodcock J., Seaman W. E. Inhibition of humoral immunity in vivo by monoclonal antibody to L3T4: studies with soluble antigens in intact mice. J Immunol. 1985 Sep;135(3):1698–1701. [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Reversal of advanced murine lupus in NZB/NZW F1 mice by treatment with monoclonal antibody to L3T4. J Immunol. 1987 May 15;138(10):3247–3253. [PubMed] [Google Scholar]
- Wood G. S., Warner N. L., Warnke R. A. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]
- Woodcock J., Wofsy D., Eriksson E., Scott J. H., Seaman W. E. Rejection of skin grafts and generation of cytotoxic T cells by mice depleted of L3T4+ cells. Transplantation. 1986 Dec;42(6):636–642. doi: 10.1097/00007890-198612000-00012. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Rosen S. D. Lymphocyte homing. Adv Immunol. 1989;44:313–378. doi: 10.1016/s0065-2776(08)60645-8. [DOI] [PubMed] [Google Scholar]
- Yu C. L., Haskard D. O., Cavender D., Johnson A. R., Ziff M. Human gamma interferon increases the binding of T lymphocytes to endothelial cells. Clin Exp Immunol. 1985 Dec;62(3):554–560. [PMC free article] [PubMed] [Google Scholar]