Abstract
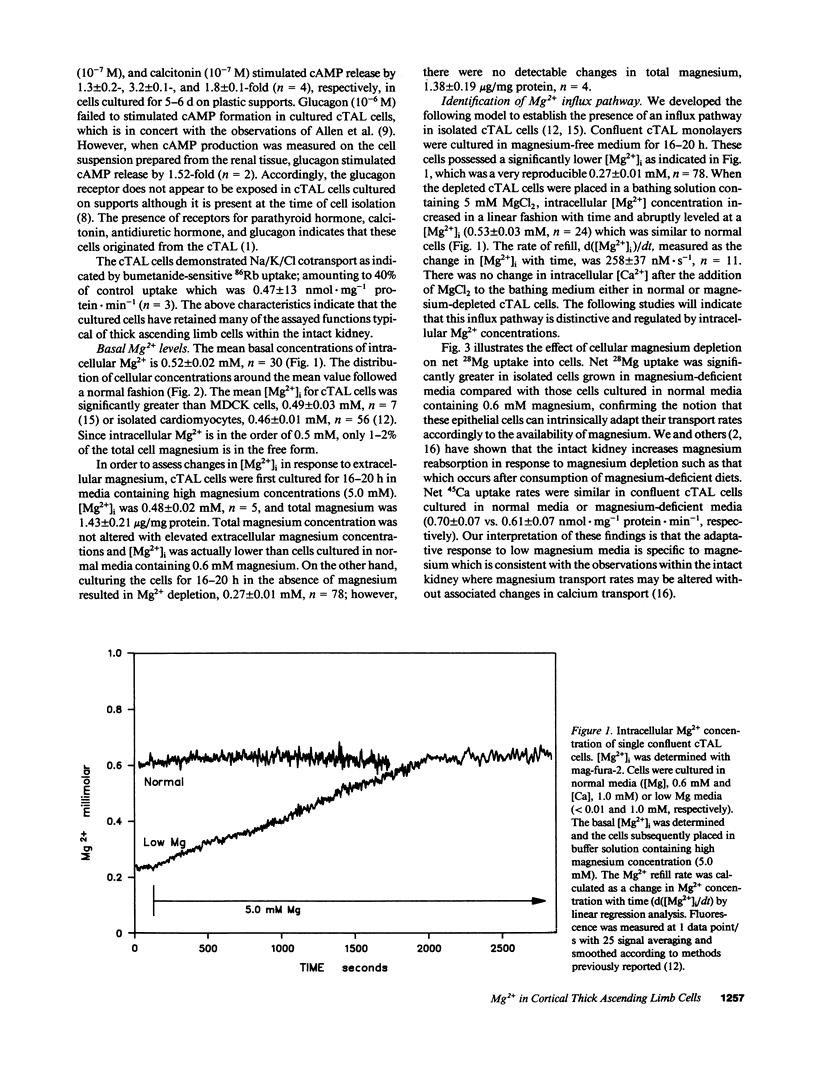
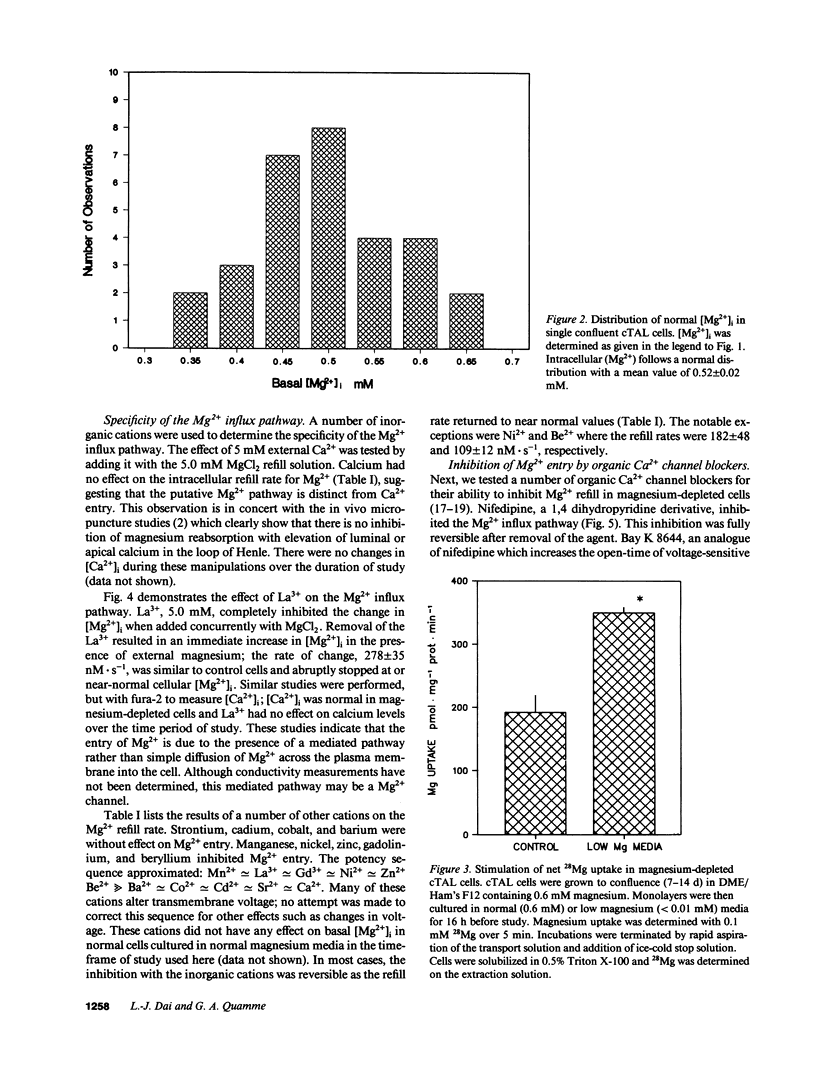
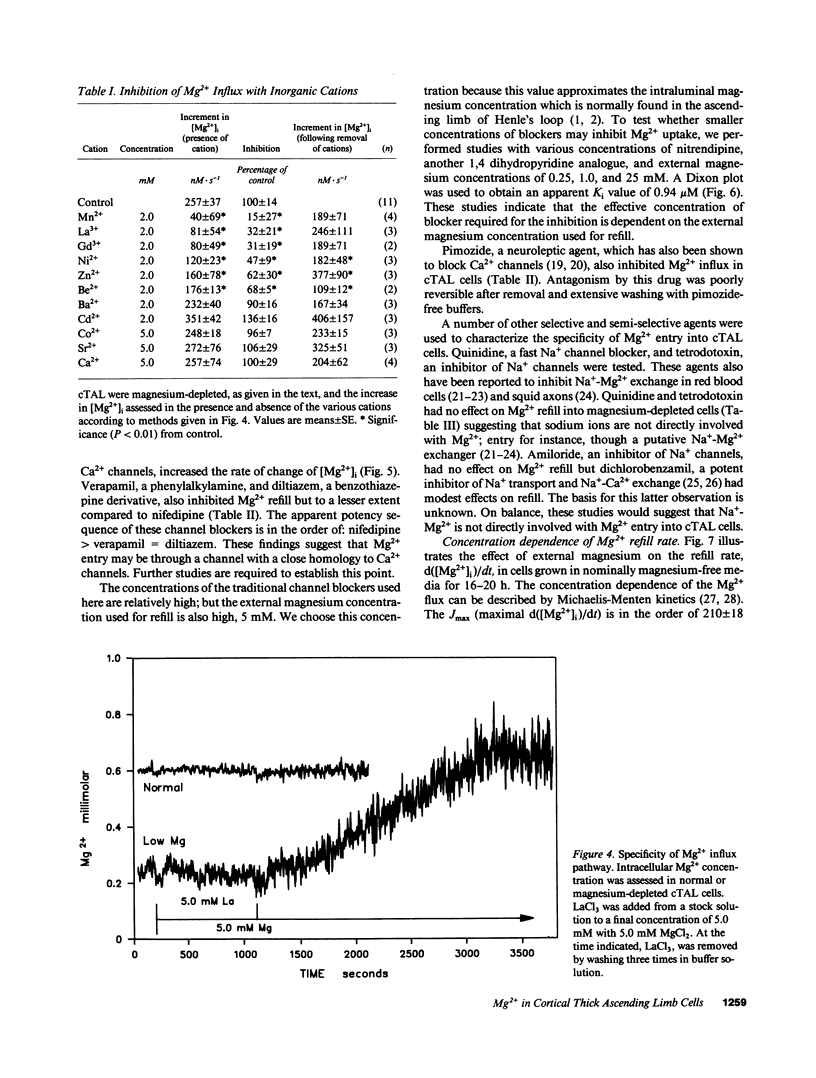
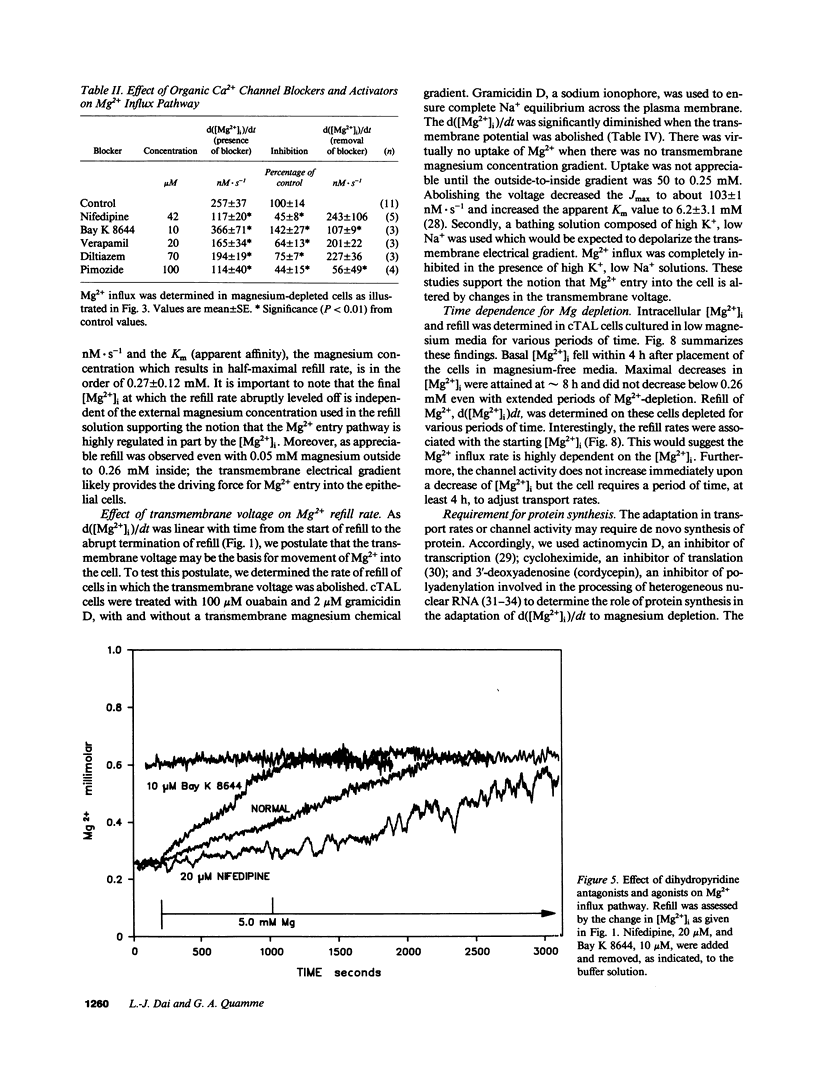
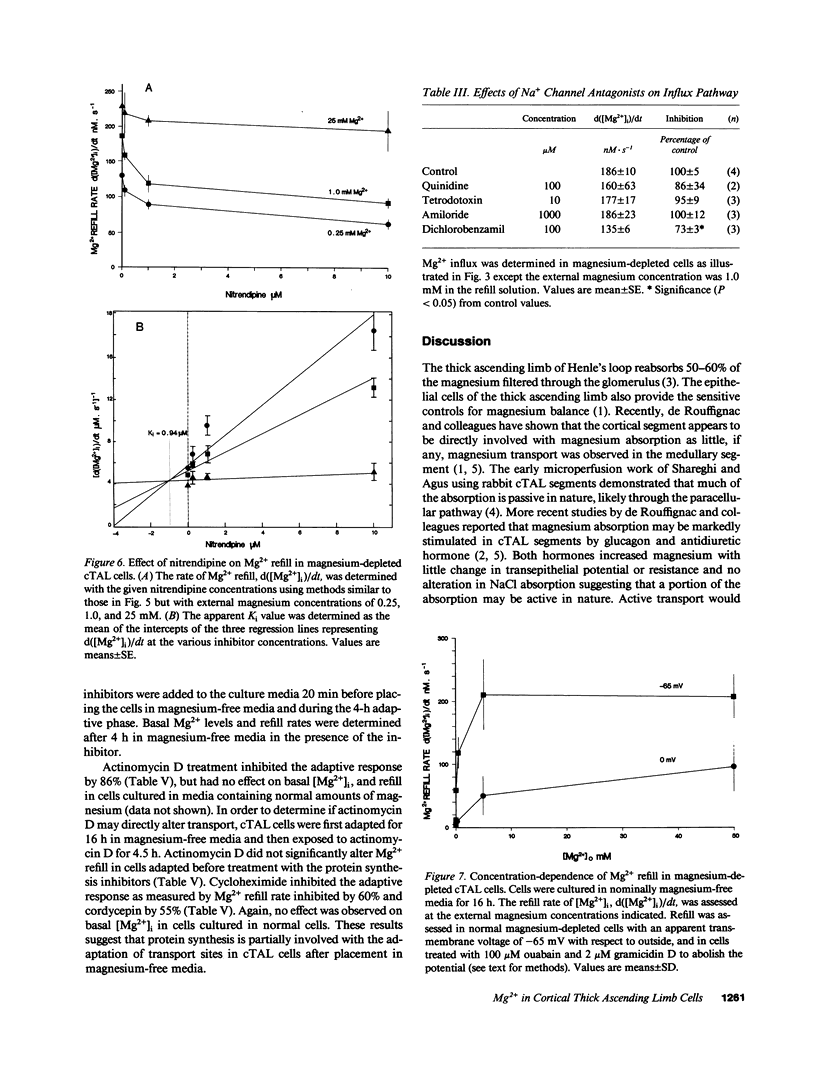
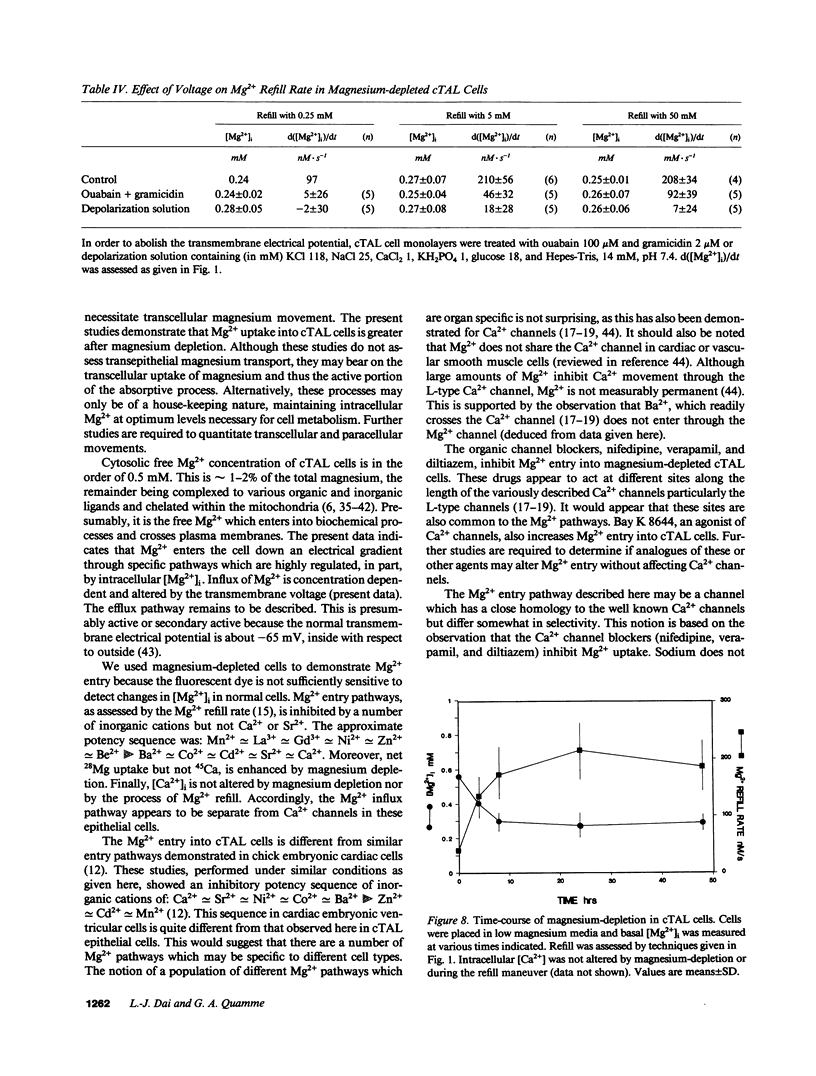
Magnesium reabsorption and regulation within the kidney occur principally within the cortical thick ascending limb (cTAL) cells of the loop of Henle. Fluorometry with the dye, mag-fura-2, was used to characterize intracellular Mg2+ concentration ([Mg2+]i) in single cTAL cells. Primary cell cultures were prepared from porcine kidneys using a double antibody technique (goat anti-human Tamm-Horsfall and rabbit anti-goat IgG antibodies). Basal [Mg2+]i was 0.52 +/- 0.02 mM, which was approximately 2% of the total cellular Mg. Cells cultured (16 h) in high magnesium media (5 mM) maintained basal [Mg2+]i, 0.48 +/- 0.02, in the normal range. However, cells cultured in nominally magnesium-free media possessed [Mg2+]i, 0.27 +/- 0.01 mM, which was associated with a significant increase in net Mg transport, (control, 0.19 +/- 0.03 and low Mg, 0.35 +/- 0.01 nmol.mg-1 protein.min-1) as assessed by 28Mg uptake. Mg(2+)-depleted cells were subsequently placed in high Mg solution (5 mM) and the Mg2+ refill rate was assessed by fluorescence. [Mg2+]i returned to normal basal levels, 0.53 +/- 0.03 mM, with a refill rate of 257 +/- 37 nM/s. Mg2+ entry was not changed by 5.0 mM Ca2+ or 2 mM Sr2+, Cd2+, Co2+, nor Ba2+ but was inhibited by Mn2+ approximately La3+ approximately Gd3+ approximately Zn2+ approximately Be2+ at 2 mM. Intracellular Ca2+ and 45Ca uptake was not altered by Mg depletion or Mg2+ refill, indicating that the entry is relatively specific to Mg2+. Mg2+ uptake was inhibited by nifedipine (117 +/- 20 nM/s), verapamil (165 +/- 34 nM/s), and diltiazem (194 +/- 19 nM/s) but enhanced by the dihydropyridine analogue, Bay K 8644 (366 +/- 71 nM/s). These antagonists and agonists were reversible with removal and [Mg2+]i subsequently returned to normal basal levels. Mg2+ entry rate was concentration and voltage dependent and maximally stimulated after 4 h in magnesium-free media. Cellular magnesium depletion results in increases in a Mg2+ refill rate which is dependent, in part, on de novo protein synthesis. These data provide evidence for novel Mg2+ entry pathways in cTAL cells which are specific for Mg2+ and highly regulated. These entry pathways are likely involved with renal Mg2+ homeostasis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. L., Nakao A., Sonnenburg W. K., Burnatowska-Hledin M., Spielman W. S., Smith W. L. Immunodissection of cortical and medullary thick ascending limb cells from rabbit kidney. Am J Physiol. 1988 Oct;255(4 Pt 2):F704–F710. doi: 10.1152/ajprenal.1988.255.4.F704. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Scarpa A., Tiffert T. The concentration of ionized magnesium in barnacle muscle fibres. J Physiol. 1977 Apr;266(3):545–565. doi: 10.1113/jphysiol.1977.sp011781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M., Burt C. T. 31P nuclear magnetic relaxation studies of phosphocreatine in intact muscle: determination of intracellular free magnesium. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4271–4275. doi: 10.1073/pnas.74.10.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. L., Braverman R. The mechanism by which actinomycin D inhibits protein synthesis in animal cells. Nature. 1977 Oct 6;269(5628):527–529. doi: 10.1038/269527a0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Enyeart J. J., Biagi B. A., Day R. N., Sheu S. S., Maurer R. A. Blockade of low and high threshold Ca2+ channels by diphenylbutylpiperidine antipsychotics linked to inhibition of prolactin gene expression. J Biol Chem. 1990 Sep 25;265(27):16373–16379. [PubMed] [Google Scholar]
- Flatman P. W. Magnesium transport across cell membranes. J Membr Biol. 1984;80(1):1–14. doi: 10.1007/BF01868686. [DOI] [PubMed] [Google Scholar]
- Frenkel E. J., Graziani M., Schatzmann H. J. ATP requirement of the sodium-dependent magnesium extrusion from human red blood cells. J Physiol. 1989 Jul;414:385–397. doi: 10.1113/jphysiol.1989.sp017694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C. H. Measurement and control of intracellular magnesium ion concentration in guinea pig and ferret ventricular myocardium. Magnesium. 1986;5(5-6):306–316. [PubMed] [Google Scholar]
- Féray J. C., Garay R. A one-to-one Mg2+:Mn2+ exchange in rat erythrocytes. J Biol Chem. 1987 Apr 25;262(12):5763–5768. [PubMed] [Google Scholar]
- Geisbuhler T., Altschuld R. A., Trewyn R. W., Ansel A. Z., Lamka K., Brierley G. P. Adenine nucleotide metabolism and compartmentalization in isolated adult rat heart cells. Circ Res. 1984 May;54(5):536–546. doi: 10.1161/01.res.54.5.536. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Striessnig J. Molecular properties of calcium channels. Rev Physiol Biochem Pharmacol. 1990;114:1–105. doi: 10.1007/BFb0031018. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Serratos H., Rasgado-Flores H. Extracellular magnesium-dependent sodium efflux in squid giant axons. Am J Physiol. 1990 Oct;259(4 Pt 1):C541–C548. doi: 10.1152/ajpcell.1990.259.4.C541. [DOI] [PubMed] [Google Scholar]
- Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev. 1985 Jul;65(3):760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gupta R. K., Moore R. D. 31P NMR studies of intracellular free Mg2+ in intact frog skeletal muscle. J Biol Chem. 1980 May 10;255(9):3987–3993. [PubMed] [Google Scholar]
- Günther T., Vormann J., Höllriegl V. Characterization of Na(+)-dependent Mg2+ efflux from Mg2(+)-loaded rat erythrocytes. Biochim Biophys Acta. 1990 Apr 30;1023(3):455–461. doi: 10.1016/0005-2736(90)90139-f. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986 Sep;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Adesnik M., Salditt M., Sheiness D., Wall R., Molloy G., Philipson L., Darnell J. E. Further evidence on the nuclear origin and transfer to the cytoplasm of polyadenylic acid sequences in mammalian cell RNA. J Mol Biol. 1973 Apr 15;75(3):515–532. doi: 10.1016/0022-2836(73)90458-0. [DOI] [PubMed] [Google Scholar]
- Kaczorowski G. J., Slaughter R. S., King V. F., Garcia M. L. Inhibitors of sodium-calcium exchange: identification and development of probes of transport activity. Biochim Biophys Acta. 1989 May 9;988(2):287–302. doi: 10.1016/0304-4157(89)90022-1. [DOI] [PubMed] [Google Scholar]
- Kim H. D., Tsai Y. S., Franklin C. C., Turner J. T. Characterization of Na+/K+/Cl- cotransport in cultured HT29 human colonic adenocarcinoma cells. Biochim Biophys Acta. 1988 Dec 22;946(2):397–404. doi: 10.1016/0005-2736(88)90415-4. [DOI] [PubMed] [Google Scholar]
- Lansman J. B., Hess P., Tsien R. W. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J Gen Physiol. 1986 Sep;88(3):321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel F., Roinel N., Le Grimellec C. Electron probe analysis of tubular fluid composition. Nephron. 1969;6(3):350–364. doi: 10.1159/000179738. [DOI] [PubMed] [Google Scholar]
- Murphy E., Steenbergen C., Levy L. A., Raju B., London R. E. Cytosolic free magnesium levels in ischemic rat heart. J Biol Chem. 1989 Apr 5;264(10):5622–5627. [PubMed] [Google Scholar]
- Nakao A., Allen M. L., Sonnenburg W. K., Smith W. L. Regulation of cAMP metabolism by PGE2 in cortical and medullary thick ascending limb of Henle's loop. Am J Physiol. 1989 Mar;256(3 Pt 1):C652–C657. doi: 10.1152/ajpcell.1989.256.3.C652. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. J Physiol. 1988 Jan;395:695–729. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K., Kawasaki F., Kita H. Mn and Mg influxes through Ca channels of motor nerve terminals are prevented by verapamil in frogs. Brain Res. 1990 Mar 5;510(2):289–295. doi: 10.1016/0006-8993(90)91379-u. [DOI] [PubMed] [Google Scholar]
- Pope A. J., Jennings I. R., Sanders D., Leigh R. A. Characterization of Cl- transport in vacuolar membrane vesicles using a Cl(-)-sensitive fluorescent probe: reaction kinetic models for voltage- and concentration-dependence of Cl- flux. J Membr Biol. 1990 Jun;116(2):129–137. doi: 10.1007/BF01868671. [DOI] [PubMed] [Google Scholar]
- Porzig H. Pharmacological modulation of voltage-dependent calcium channels in intact cells. Rev Physiol Biochem Pharmacol. 1990;114:209–262. doi: 10.1007/BFb0031020. [DOI] [PubMed] [Google Scholar]
- Preston R. R. A magnesium current in Paramecium. Science. 1990 Oct 12;250(4978):285–288. doi: 10.1126/science.2218533. [DOI] [PubMed] [Google Scholar]
- Quamme G. A. Control of magnesium transport in the thick ascending limb. Am J Physiol. 1989 Feb;256(2 Pt 2):F197–F210. doi: 10.1152/ajprenal.1989.256.2.F197. [DOI] [PubMed] [Google Scholar]
- Quamme G. A., Dai L. J. Presence of a novel influx pathway for Mg2+ in MDCK cells. Am J Physiol. 1990 Sep;259(3 Pt 1):C521–C525. doi: 10.1152/ajpcell.1990.259.3.C521. [DOI] [PubMed] [Google Scholar]
- Quamme G. A., Rabkin S. W. Cytosolic free magnesium in cardiac myocytes: identification of a Mg2+ influx pathway. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1406–1412. doi: 10.1016/0006-291x(90)90679-h. [DOI] [PubMed] [Google Scholar]
- Raju B., Murphy E., Levy L. A., Hall R. D., London R. E. A fluorescent indicator for measuring cytosolic free magnesium. Am J Physiol. 1989 Mar;256(3 Pt 1):C540–C548. doi: 10.1152/ajpcell.1989.256.3.C540. [DOI] [PubMed] [Google Scholar]
- Rossow P., Radeos M. S., Amos H. Metabolic effects of glucose starvation in animal cell cultures. Arch Biochem Biophys. 1975 Jun;168(2):520–524. doi: 10.1016/0003-9861(75)90282-9. [DOI] [PubMed] [Google Scholar]
- Shafik I. M., Quamme G. A. Early adaptation of renal magnesium reabsorption in response to magnesium restriction. Am J Physiol. 1989 Dec;257(6 Pt 2):F974–F977. doi: 10.1152/ajprenal.1989.257.6.F974. [DOI] [PubMed] [Google Scholar]
- Shareghi G. R., Agus Z. S. Magnesium transport in the cortical thick ascending limb of Henle's loop of the rabbit. J Clin Invest. 1982 Apr;69(4):759–769. doi: 10.1172/JCI110514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siev M., Weinberg R., Penman S. The selective interruption of nucleolar RNA synthesis in HeLa cells by cordycepin. J Cell Biol. 1969 May;41(2):510–520. doi: 10.1083/jcb.41.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L., Foy M. A., Cragoe E. J., Jr A role for Na+/Ca2+ exchange in the generation of superoxide radicals by human neutrophils. J Biol Chem. 1990 Aug 15;265(23):13449–13456. [PubMed] [Google Scholar]
- Smith W. L., Garcia-Perez A. Immunodissection: use of monoclonal antibodies to isolate specific types of renal cells. Am J Physiol. 1985 Jan;248(1 Pt 2):F1–F7. doi: 10.1152/ajprenal.1985.248.1.F1. [DOI] [PubMed] [Google Scholar]
- Wittner M., di Stefano A., Wangemann P., Nitschke R., Greger R., Bailly C., Amiel C., Roinel N., de Rouffignac C. Differential effects of ADH on sodium, chloride, potassium, calcium and magnesium transport in cortical and medullary thick ascending limbs of mouse nephron. Pflugers Arch. 1988 Oct;412(5):516–523. doi: 10.1007/BF00582541. [DOI] [PubMed] [Google Scholar]
- de Rouffignac C., Elalouf J. M., Roinel N. Physiological control of the urinary concentrating mechanism by peptide hormones. Kidney Int. 1987 Feb;31(2):611–620. doi: 10.1038/ki.1987.42. [DOI] [PubMed] [Google Scholar]


