Abstract
In addition to mental retardation, individuals with Down syndrome (DS) also develop the neuropathological changes typical of Alzheimer’s disease (AD) and the majority of these individuals become demented. The Ts65Dn mouse model of DS exhibits key features of these disorders, including early degeneration of cholinergic basal forebrain (CBF) neurons and impairments in functions dependent on the two CBF projection systems; namely, attention and explicit memory. Herein, we demonstrate that supplementing the maternal diet with excess choline during pregnancy and lactation dramatically improved attentional function of the adult trisomic offspring. Specifically, the adult offspring of choline-supplemented Ts65Dn dams performed significantly better than unsupplemented Ts65Dn mice on a series of five visual attention tasks, and in fact, on some tasks did not differ from the normosomic (2N) controls. A second area of dysfunction in the trisomic animals, heightened reactivity to committing an error, was partially normalized by the early choline supplementation. The 2N littermates also benefited from increased maternal choline intake on one attention task. These findings collectively suggest that perinatal choline supplementation might significantly lessen cognitive dysfunction in DS and reduce cognitive decline in related neurodegenerative disorders such as AD.
Keywords: Down syndrome, choline-early exposure delayed effects, Alzheimer’s Disease, Attention, Emotion regulation, Arousal
Introduction
Down syndrome (DS), one of the most common causes of mental retardation (MR), is caused by a trisomy of human chromosome 21 (HAS 21) as a result of non-disjunction during meiosis. Individuals with DS also develop the neuropathological changes typical of Alzheimer’s disease (AD) (Roizen and Patterson, 2003) and the majority of these individuals develop dementia (Wisniewski et al., 1985b; Wisniewski et al., 1985a; Mann, 1988; Lai and Williams, 1989; Visser et al., 1997). One of the most prominent neuropathological changes in both disorders is pronounced degeneration of cholinergic basal forebrain (CBF) neurons (Whitehouse et al., 1982; Sendera et al., 2000; Isacson et al., 2002) which innervate the entire cortical mantle and the hippocampus (Mesulam et al., 1983; Mufson et al., 2003). The precise mechanism(s) by which trisomy 21 leads to MR and early-onset of AD is unknown.
The availability of mouse models for DS provides an opportunity to elucidate the mechanisms which underlie the development of MR and AD-like symptomatology in this disorder and develop therapeutic interventions. A segmental trisomy mouse model of DS, termed the Ts65Dn mouse (Davisson et al., 1990) survivesto adulthood and exhibits a number of the morphological, biochemical, and transcriptional changes seen in the human disease (Davisson et al., 1990; Davisson et al., 1993; Reeves et al., 1995; Holtzman et al., 1996; Antonarakis et al., 2001; Capone, 2001). Ts65Dn mice possess a third copy of the distal region of mousechromosome 16 which contains approximately 94 genes orthologous to the DS critical region of HSA 21 (Davisson et al., 1990; Davisson et al., 1993; Gardiner et al., 2003). Notably, like humans with DS, these mice are born with intact CBF neurons (Holtzman et al., 1996) but exhibit atrophy of these neurons by 6 months of age, accompanied by astrocytic hypertrophy and microglial activation (Holtzman et al., 1992; Holtzman et al., 1996; Granholm et al., 2000). Consistent with these degenerative changes, the most pronounced cognitive deficits in humans with DS and this mouse model pertain to functions modulated by the two major CBF projection systems, namely: (a) explicit memory function (Crnic & Pennington, 2000; Hyde et al., 2001; Hyde and Crnic, 2001) subserved by projections from the medial septal nucleus to the hippocampus (Walsh et al., 1998) and (b) attention and working memory (Crnic, 2000; Driscoll et al., 2004) subserved by projections from the nucleus basalis to the frontal cortex (Robbins et al., 1989; Muir et al., 1992; Sarter et al., 2001; Risbrough et al., 2002). As in humans with DS, cognitive functioning in the Ts65Dn mouse declines during adulthood, coincident with the loss of the CBF neuronal phenotype (Granholm et al., 2000; Hyde and Crnic, 2001).
These findings collectively suggest that treatments which improve functioning of CBF neurons could prevent the pathological age-related cognitive decline in DS and probably also in related neurodegenerative disorders such as AD. One viable candidate is supplementation with choline during early development. This therapeutic approach was suggested by two lines of evidence namely, that the cognitive functions most impaired in humans with DS and the Ts65Dn mouse are subserved by CBF neurons and their projection systems (reviewed above), coupled with the effects of perinatal choline supplementation in normal rodents. Specifically, pre- and/or postnatal choline supplementation of normal rodents exerts organizational effects on CBF neuronal systems (Meck et al., 1989; Williams et al., 1998; Cermak et al., 1999; Montoya et al., 2000; Glenn et al., 2008; Napoli et al., 2008; Wong-Goodrich et al., 2008) and leads to lifelong enhancements of cognitive functions dependent on these systems (i.e., explicit memory and attention) (Meck et al., 1988; Schenk,1995; Meck and Williams, 1997; Mohler et al., 1998; Williams et al., 1998; Tees, 1999a, b; Tees and Mohammadi, 1999; Mohler et al., 2001; Meck et al., 2007). In addition, prenatal choline supplementation has been shown to provide neuroprotection against various neural insults (Meck and Williams, 1997; Blusztajn, 1998; Thomas et al., 2000; Yang et al., 2000; Guo-Ross et al., 2002; Meck and Williams, 2003; Wong-Goodrich et al., 2008) as well as lasting enhancement of neurotrophin function (Sandstrom et al., 2002; Glenn et al., 2007; Mellott et al., 2007; Nag et al., 2008; Wong-Goodrich et al., 2008). In light of these lasting effects, early choline supplementation may lessen the atrophy of CBF neurons in Ts65Dn mice, which appears to be caused by impaired retrograde transport of target-derived NGF (Cooper et al., 2001; Salehi et al., 2006), and which can be rescued by NGF administration (Cooper et al., 2001).
The present study was designed to test the putative cognitive benefit of perinatal choline supplementation in the Ts65Dn mouse model of DS, using a series of visual attention tasks which previously revealed dysfunction in this mouse model (Driscoll et al., 2004).
Methods
Subjects
Breeder pairs (Ts65Dn female & C57Bl/6J Eicher × C3H/HeSnJ F1 male) were purchased from Jackson Laboratories (Bar Harbor, ME) and mated at the University of Colorado Health Sciences Center. Upon verification of insemination, the pregnant dams were randomly allocated to one of two concentrations of choline chloride in the drinking water (0 or 25 mM), which was provided ad libitum. Preliminary experiments demonstrated that the addition of choline to the drinking water did not significantly affect water intake of either 2N or Ts65Dn mice. These two levels of maternal choline intake continued until the pups were weaned at postnatal day 21. The administration of choline was approved by the UCHSC Institutional Animal Care and Use Committee (IACUC). All dams were given ad libitum access to standard rodent chow (#2018; Harlan Tekland Global Diets).
With this regimen, the choline-supplemented dams consumed approximately 4.5 times the choline intake of the unsupplemented dams, within the range of dietary variation observed in the human population (Detopoulou et al., 2008). This choline supplementation regimen is the standard protocol used in prior research demonstrating that pre- and/or early postnatal choline supplementation produces lifelong improvement in memory function, neuroprotection, and organizational effects on CBF neuronal systems in normal rodents (reviewed in the Introduction). Although most of these prior studies pertain to rats rather than mice, this dosing regimen, administered prenatally, has also been shown to improve performance of adult mice in a signal detection task (Mohler et al., 2001) and a radial arm maze (Mohler et al., 1998) as well as alter hippocampal development of mice (Albright et al., 2003). Early postnatal choline administration, using this regimen, has also been shown to produce lasting improvement in motor function in a mouse model of Rett syndrome (Nag et al., 2008).
Before the mice were shipped to Cornell University for behavioral testing, they were typed for the presence of the extra chromosome by fluorescence in situ hybridization of blood smears using a bacterial artificial chromosome probe for the telomeric end of mouse chromosome 16 (Korenberg et al., 1999). DNA obtained from 1-mm tail clippings were typed by polymerase chain reaction amplification of the viral insert in the Pdeb6b gene that leads to retinal degeneration (Bowes et al., 1993). Mice homozygous for this mutation were excluded from the study. Whenever possible, one trisomic and one normosomic (2N) male pup were selected from each litter to participate in the behavioral testing.
Upon arrival at Cornell University, the mice (2–4 months of age) were housed individually in polycarbonate cages with food and water ad libitum. Single housing was implemented based on previous observations that male mice of this strain, caged in pairs, are prone to fighting when reunited after being removed for behavioral testing. The mice were maintained on a 12:12 reversed light dark cycle under temperature controlled conditions. All procedures were approved by the IACUC at Cornell University.
Food restriction schedule during behavioral testing
Prior to behavioral testing, the animals were placed on a food restriction regimen to ensure motivation for the food reward during each daily testing session. Target weights for the mice were calculated at approximately 85% of their ad libitum weight. The daily food allowance was gradually reduced and then maintained when the mouse reached this target weight. Feeding after each test session consisted of subtracting the number of calories obtained as reward in the testing chamber (Liquefied AIN-76A purified chow; Bio-Serv, Frenchtown, NJ) from the daily allowance of lab chow (ProLab 1000; Purina Mills, Richmond, IN).
Testing Apparatus
The mice were tested individually in one of six automated Plexiglas chambers, each controlled by a PC and enclosed in an insulated, sound attenuating chamber. The testing chambers were adapted from the nine hole operant chambers developed to assess attention in mice (Humby et al., 1999; Marston et al., 2001). The slightly curved rear wall contained five circular response ports,1 cm in diameter, located 2 cm above the floor and 5 mm apart. A nose-poke into any of these ports constituted a response (or choice). Infrared photodiodes, positioned inside each port 0.5 cm from the opening, monitored responses to the port. Green 4 mA LEDs, one embedded on the back surface of each port, provided the discriminative visual cues. On the chamber wall opposite the response ports was an alcove (15 mm wide, 2 cm above the floor) containing the dipper (ENV0302M, MED Associates, East Fairfield, VT) that dispensed the liquid reward. Access to the dipper alcove was controlled by a thin metal door, which was activated by a motor located outside of the testing chamber. As with the ports, nosepokes into the alcove were monitored by infrared photodiodes. A nosepoke into this alcove port was required to initiate each trial. All automated events in the chamber (door opening, dipper movement, responses, etc.) were timed, controlled, and recorded by custom programs written in QBASIC. Each chamber was fitted with an exhaust system which transported the air from each chamber directly to the room exhaust ventilator system at a rate of four complete air changes per minute.
Each chamber was equipped with a wide-angle infrared video camera so that the behaviors of the mice could be videotaped and later coded (described below). An infrared LED light source was attached to the ceiling directly over the center of each testing chamber. The camera allowed full view of the mouse at all times. Each camera was connected to a separate VHS videotape recorder. To facilitate identification of session events during videotape coding, an array of infrared LEDs was positioned outside the Plexiglas testing chamber but within viewing range of the camera. These LEDs signaled the initiation of the trial, omission responses, premature responses, correct responses, and time-outs.
Behavioral Testing
Daily behavioral testing began at 6 months of age and lasted for approximately 6 months. Each animal was randomly assigned to one of the six testing chambers, with the stipulation that each chamber was balanced for the four treatment conditions. All mice were tested 6 days a week; each daily session lasted for 30 minutes or 70 trials, whichever came first. All testing was conducted by individuals blind to the genotype and perinatal choline treatment condition of the animals. All testing equipment was thoroughly cleaned and dried following the testing of each mouse, using Odormute (R.C. Steele Co, Brockport, NY), a detergent containing an enzyme that removes olfactory cues (including pheromones).
Training
The training consisted of a series of four stages designed to familiarize the animals with the testing chamber and the sequence of responses necessary to complete a trial for the visual attention tasks. Briefly, during these four stages the mice learned that the door to the dipper alcove would be raised at the start of each trial and that a nosepoke into the dipper port, followed by a nosepoke into one of the five response ports, would produce the delivery of 0.01 ml of the liquid diet in the dipper alcove. During the final training stage, each mouse was required to respond for a fixed number of trials at each of the five response ports, to eliminate preferences or aversions to any of the ports. These training stages required a total of 10–15 sessions on average. For a more detailed description of these stages, see Driscoll et al., 2004.
Five-Choice Visual Discrimination Task
In this task, one of the five port LEDs was illuminated on each trial; the mouse was rewarded for making a nose-poke into the illuminated port. The location of the visual cue was pseudorandomized across trials, such that the number of cue presentations in each port was equal for each daily session. A 2-s delay separated trial initiation and cue onset; this delay (termed the “turn-around time”) allowed time for the mouse to turn around and orient toward the ports before cue illumination. The LED remained illuminated until the mouse made a response or until 32 s elapsed. Errors included responding to an incorrect response port after trial initiation (inaccurate response), failing to respond to any response port following trial initiation (omission error), and making a nose-poke into any response port prior to cue onset (premature response). A 5-s intertrial interval separated adjacent trials. All trials on which the mouse made an initiation poke into the dipper alcove (regardless of the outcome of the trial) were defined as response trials. Failures to initiate a trial within 60 s were termed nontrials; no cues were presented on these trials. A 5-s time-out period was imposed following a nontrial or an error. This time-out period was signaled by the illumination of a 3-W houselight on the ceiling of the chamber. Each mouse remained on this task until it reached a criterion of 80% correct for two out of three consecutive sessions.
Attention Tasks 1 and 2
The mice were subsequently tested on two variations of the initial visual discrimination task that were identical except for the duration of cue illumination, which was shortened to 2 s and 1 s, respectively, for the two tasks (see Table 1). These tasks tested attentional function and prepared the mice for the subsequent two attention tasks which were more attentionally-demanding. Attention tasks 1 and 2 were administered for 8 and 15 sessions, respectively.
Table 1.
Cue parameters and duration of testing for the six tasks.
| Task | Cue Duration | Pre-cue delay | Total # of Sessions |
|---|---|---|---|
| Five-Choice Visual Discrimination Task | 32 sa | no pre-cue delay | Criterion Taskb |
| Attention Task 1 | 2.0 sa | no pre-cue delay | 8 |
| Attention Task 2 | 1.0 sa | no pre-cue delay | 15 |
| Attention Task 3 | 1.0 sa | 0, 2, 4 sc | 20 |
| Attention Task 4 | 0.8, 1.0, 1.4 sc | 0, 2, 4 sc | 20 |
| Reward Omission Task d | 1.0 sa | 0, 2, 4 sc | 20 |
Constant across trials.
the criterion for this task was 80% correct for two out of three consecutive sessions
Variable across trials.
the reward was omitted on 20% of the correct responses
Attention task 3 (learning to wait for the cue)
This task was identical to the prior tasks except that the duration between trial initiation and cue onset varied pseudo-randomly across trials. The pre-cue delay varied between 0, 2, and 4 s (see Table 1). These variable pre-cue delays were added to the 2 s “turn-around time” (described above). Cue illumination duration was constant at 1 s. If a response was made prior to cue onset (a premature response), the trial was terminated and no cue was presented. As noted above, premature responses (as well as all types of errors) were followed by a 5-s time-out period, signaled by the illumination of a 3-W houselight on the ceiling of the chamber. The three pre-cue delays were presented pseudo-randomly, such that the number of presentations of each combination of pre-cue delay and response port (1–5) were balanced across each session. The mice were tested for 20 sessions on this task. In this task, the mice learned to wait for the cue as well as sustain attention until the cue was presented; thus the task tapped learning, inhibitory control, and sustained attention.
Attention Task 4
This task was identical to the prior task except that in addition to the variable pre-cue delay, cue duration also varied randomly across trials (see Table 1). The delays varied between 0, 2, and 4s (as in the prior task), and cue durations varied between 0.8, 1.0, and 1.4 s. Pre-cue delay and cue duration were balanced across each session. This task placed a greater demand on sustained attention because the cue duration was often very brief. The mice were tested on this task for 20 sessions.
Reward Omission (RO) Task
This task was identical to Attention Task 3 except that the reward was omitted on 20% of the correct responses (see Table 1). For these reward omission (RO) trials, the alcove door was not raised after the correct response, and no reward was provided. The mice were tested for 20 sessions on this task. Based on our prior findings that Ts65Dn mice exhibit a heightened emotional response to committing an error (Driscoll et al., 2004), it was hypothesized that they would also exhibit a heightened reaction to the omission of an expected reward.
Videotape Coding
All sessions of Attention Task 3 and the RO Task were videotaped. However, due to the labor intensiveness of the coding, only two sessions from each task were coded for analysis. Because it was not possible for the coders to simultaneously code all of the behaviors exhibited by the mice as well as their activity level, obtaining both types of information (behaviors exhibited and activity level) required scoring each videotape twice. For attention Task 3, both types of information were scored and analyzed, whereas for the RO task, only activity level was scored. The videotape coding program was written in Visual Basic and all coding was completed on a PC.
Behaviors coded (Attention Task 3)
The frequency and duration of three behaviors (wall-climbing, grooming, and jumping) were quantified, as well as the portion of the trial (before or after the response) in which the behavior was exhibited. As noted above, in our prior study (Driscoll et al., 2004), Ts65Dn mice were found to exhibit repetitive jumping specifically after an error, behavior not seen in the 2N mice.
Activity level (coded in Attention Task 3 and the RO Task)
To code activity level, the chamber was divided into four quadrants (upper left, lower left, upper right, and lower right). The movement of the animal was recorded and the number of crossings between quadrants, referred to as “square crossings”, was tallied.
Reliability
Both sessions of each task were coded by a single individual who was unaware of the animals’ genotype or treatment. Before coding these sessions, the coder practiced with other taped sessions until a high level of intra-rater reliability was obtained. Pearson correlation coefficients for all dependent measures were equal to or greater than .90 for the final practice sessions as well as for the sessions presented in this report.
Statistical Analyses
Automated performance measures
Statistical analyses were conducted using the Statistical Analysis System (Version 9.1; SAS institute, Cary, NC) on a Cornell University mainframe computer. Performance measures were analyzed using the PROC GLIMMIX program, a generalized linear mixed models procedure for conducting repeated measures analyses of both normal and non-normal data by specifying an appropriate link function and error distribution (Wolfinger et al., 1993). The dependent measures included: percent correct responses, percent inaccurate responses, percent premature responses, percent omission errors, and percent non-trials. These endpoints were assumed to have binomial errors and a logit link was used. In addition, alcove latency [the latency to make a nose-poke into the dipper alcove after it was raised (to initiate the next trial)] was analyzed as a function of the outcome of the prior trial, based on our prior findings (discussed above) that the Ts65Dn mice exhibit a heightened emotional response to committing an error (Driscoll et al., 2004). Because the data were not normally distributed, the dependent measure used to analyze the data was the percent of trials for which the alcove latency was greater than 5 s, which corresponds to the approximate 75th percentile of the alcove latency distribution. The models for these outcomes included the treatment group [Ts65Dn, WT (2N), Ts65Dn+Choline, 2N+Choline] plus (if appropriate) predictors describing the trial conditions present in the particular task [e.g., pre-stimulus delay, cue duration, trial-block (block of trials within each session), session-block and outcome of the previous trial (correct or incorrect]. Post hoc tests were conducted using Fisher’s least significance difference (LSD) procedure.
Videotape data
Behaviors (Attention Task 3)
The time spent grooming, wall climbing, and jumping were determined for each mouse on each trial. Mean time spent in each behavior per trial was calculated for each mouse by summing the total time across trials and dividing by the number of trials, as a function of whether the most recent response was correct or incorrect. In addition, for each mouse, the difference in the average behavior duration following an error and average behavior duration following a correct response was calculated because this last measure best captures the reactivity to an error (i.e., reaction to an error, correcting for the frequency/duration of the behavior after a correct response). Because the data were not normally distributed, they were analyzed using the nonparametric Wilcoxon Rank Sum test.
Activity level
Activity level in Attention Task 3 and the RO task was evaluated as a function of the most recent response. For Attention Task 3, the most recent response was designated as either correct or incorrect, and for the RO task, it was designated as either correct, incorrect, or reward omission (RO). The variables included in the analyses were treatment group, most recent response, and their interaction. The data were analyzed using the nonparametric Wilcoxon Rank Sum test.
Results
Final Sample Size
The final sample sizes for the four groups were: 10 Ts65Dn mice supplemented with choline, 9 Ts65Dn mice not supplemented with choline, 11 2N mice supplemented with choline, and 16 2N mice not supplemented with choline (referred to as “controls”). Nine were excluded during the study for the following reasons: four developed kidney stones (3 choline-supplemented 2N mice and 1 non-supplemented Ts65Dn mouse; one was found to have retinal degeneration (a choline-supplemented 2N mouse), and another four mice died from unknown causes (3 choline supplemented Ts65Dn and 1 unsupplemented 2N).
Although there may appear to be a greater incidence of deaths or kidney stones in the supplemented mice, the most parsimonious interpretation is that this pattern is spurious, based on the fact that the many studies which have been conducted on pre- and/or early postnatal choline supplementation in rodents over the past 20 years have not indicated an increased incidence of either of these endpoints (e.g., Cheng et al., 2008, Glenn et al., 2008; Meck et al., 1989; 1998; 2007; Mohler et al., 2001, Nag et al., 2008). However, additional studies are needed to confirm the safely of this particular early choline regimen as most of these prior studies supplemented the maternal diet for a shorter period of time, most commonly limited to either the prenatal or postnatal period (but see Holmes et al., 2002).
Body Weight
The four treatment groups did not differ significantly in body weight [F(3, 42) = 2.24, p = 0.10].
Non-trials
The percentage of non-trials (trials on which the mouse did not initiate a trial by making a nose-poke into the dipper alcove at trial onset) did not differentiate the four groups for any of the 6 tasks (all p > 0.10), suggesting that appetitive motivation was comparable for all groups.
Initial Visual Discrimination (no pre-cue delay, cue duration: 32 s)
The four groups of animals did not differ significantly in the number of trials to criterion [F(3, 135) = 0.40, p = 0.74] or errors to criterion [F(3, 46) = 1.06, p = 0.37; see Table 2]. These findings indicate that basic associative learning ability was not impaired in the trisomic mice, nor was it modified by perinatal choline supplementation.
Table 2.
Errors to Criterion and Trials to Criterion (mean ± SEM) in Initial Visual Discrimination
| Group | Errors to Criterion (Mean ± SEM) | Trials to Criterion (Mean ± SEM) |
|---|---|---|
| 2N Choline | 273 ± 33.08 | 658.64 ± 63.04 |
| Ts65Dn Choline | 282.5 ± 34.70 | 638.2 ± 66.12 |
| 2N | 272.19 ± 27.44 | 637.69 ± 52.27 |
| Ts65Dn | 272.11 ± 36.57 | 608.11 ± 69.69 |
Alcove Latency (AL) analysis did not reveal a significant main effect of Group or a significant interaction of Group and previous trial outcome (correct or incorrect).
The Attention Tasks
Across the five attention tasks, consistent and significant effects were seen for several task variables (pre-cue delay, cue duration, prior trial outcome), for all treatment groups. To streamline the presentation of results, these effects are presented first, followed by a description of the group differences within each of the five tasks.
In all tasks, percent correct decreased as the duration of the pre-cue delay increased (p’s < 0.0001), reflecting the increasing demands on both inhibitory control and focused attention with increasing pre-cue delay. In addition, in all tasks, a significant effect of previous trial outcome (correct or incorrect) was observed (p’s < 0.0001), reflecting the fact that performance was significantly better on trials that followed a correct response than on trials following an error, indicative of the disruptive effect of committing an error. Finally, in Attention Task 4, the only task in which cue duration varied, a significant effect of this parameter was seen [F(2, 1418) = 133.04, p < 0.0001], reflecting the fact that performance declined with decreasing cue duration due to the increasing demands on attention for trials with briefer cues.
Although each of the different error types was analyzed in addition to overall percent correct, only the results for this measure are presented to streamline the presentation of results. The impairment of the trisomic mice and the benefit of perinatal choline supplementation were most pronounced for the percent correct measure, due to the fact that both omission errors and inaccurate responses were increased in the trisomic mice (relative to controls) and decreased by early choline supplementation (relative to the control diet). Percent correct provided an index which enabled the effects of genotype and diet on these two error types to summate.
Attention Task 1 (no pre-cue delay, cue duration: 2 s)
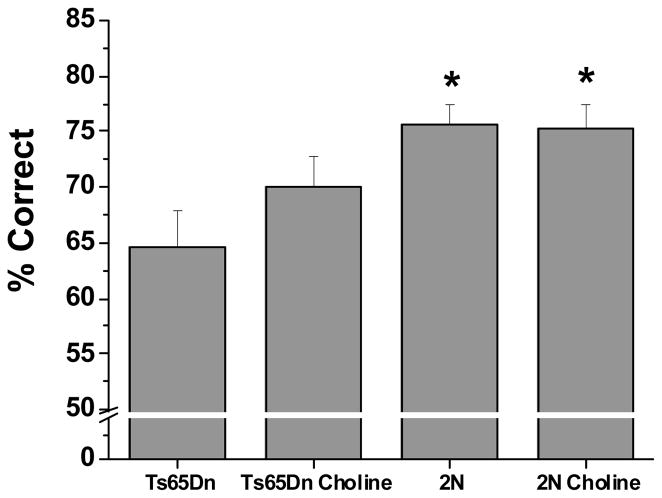
In Attention Task 1, a significant group effect was observed for percent correct [F(3, 42.2) = 2.90, p = 0.05]. The unsupplemented Ts65Dn mice performed significantly more poorly than both the unsupplemented (p < 0.0007) and supplemented (p < 0.005) 2N mice (see Figure 1). The performance of the choline-supplemented trisomic mice did not differ from either group of 2N mice, but also did not differ significantly from the unsupplemented trisomic mice.
Figure 1. Mean (±SE) percentage of correct responses in Attention task 1 (no pre-cue delay, 2 s cue duration), collapsed across all sessions.
The unsupplemented Ts65Dn mice performed significantly worse than both groups of wild-type mice (2N). The performance of the trisomic mice supplemented with choline early in life did not differ from the 2N mice but also did not differ significantly from their unsupplemented counterparts.
* p < 0.05, relative to the unsupplemented Ts65Dn mice.
Analysis of alcove latency did not reveal a main effect of Group nor a significant interaction of Group and previous trial outcome (correct or incorrect).
Attention Task 2 (no pre-cue delay, cue duration: 1 s)
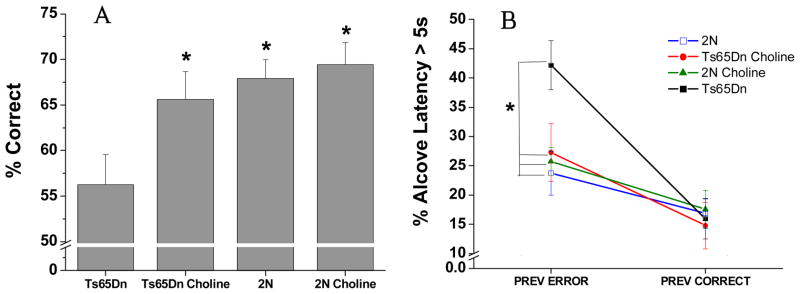
The analysis of percentage correct revealed a significant effect of treatment group [F (3, 38.8) = 4.24, p = 0.05]. As seen in Figure 2A, the unsupplemented Ts65Dn mice performed significantly worse than both groups of 2N mice (p < 0.004). In contrast, the choline-supplemented Ts65Dn mice did not differ significantly from either group of 2N mice (p > 0.3), and performed significantly better than their unsupplemented counterparts (p = 0.05). Early choline supplementation did not affect performance of the 2N mice in this task.
Figure 2. Attention task 2 (no pre-cue delay, 1s cue duration).
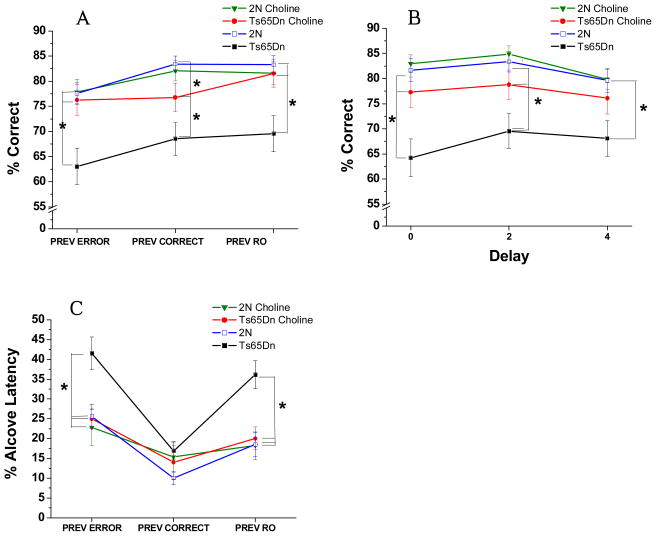
(A) Mean (± SE) percentage of correct responses, collapsed across all sessions. The Ts65Dn mice performed significantly more poorly than both groups of 2N mice. In contrast, the choline-supplemented Ts65Dn mice did not differ significantly from either group of 2N mice, and performed significantly better than their unsupplemented counterparts. (B) Mean (± SE) percentage of a long (> 5 s) Alcove Latency, collapsed across all sessions: There were no treatment differences for trials that followed a correct response whereas for the trials that followed an error, the incidence of long ALs was significantly greater for the unsupplemented Ts65Dn mice than for either group of 2N mice, as well as their choline-supplemented counterparts. The supplemented trisomic mice did not differ from either group of 2N mice.
*, p < 0.05, relative to the unsupplemented Ts65Dn mice
The analysis of AL (percent of trials with AL > 5 s) did not reveal a significant effect of Group [F(3, 41) = 1.69, p = 0.19], but a significant main effect of Prior trial outcome (correct or incorrect) was seen [F(1, 41) = 45.25, p < 0.0001) as well as a significant interaction of Group and Previous trial outcome [F(3, 41) = 5.71, p = 0.002; see Fig. 2B]. Contrasts revealed that no treatment differences were seen for trials that followed a correct response. However, the increased incidence of long ALs following an error, relative to after a correct response, was significantly greater for the unsupplemented trisomic mice than for the other three groups (all p < 0.045).. As a result, for trials following an error, the incidence of long ALs was significantly greater for the unsupplemented trisomic mice than for either group of 2N mice (p’s < 0.002) as well as the supplemented trisomic mice (p = 0.03). The supplemented trisomic mice did not differ from either group of 2N mice.
Attention Task 3 (Variable pre cue delay: 0, 2, 4 s; constant 1 s cue duration)
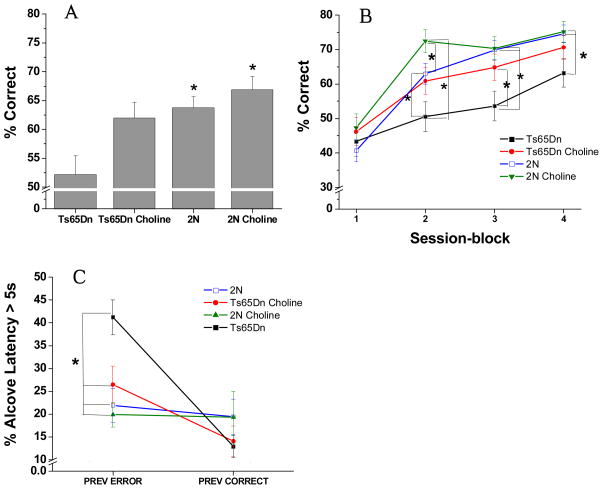
The analysis of percentage correct revealed a nonsignificant trend for the main effect of Treatment Group [F(3, 42.3) = 2.63, p = 0.06; See Figure 3A], and a significant interaction between Group and Session block [F(9, 925) = 8.02, p < 0.0001; see Figure 3B]. All groups performed very poorly during the first session-block (Sessions 1–5) and did not differ significantly during this stage. In these early sessions on the task, the mice were learning that the cue would be presented after a delay on some trials, and that they must wait to respond. During the learning of this new rule, they committed a high percentage of premature responses. Group differences were seen, however, during the final 15 sessions (session blocks 2, 3, and 4) on the task. The unsupplemented Ts65Dn mice performed significantly more poorly than both groups of 2N mice throughout these three blocks of sessions (p < 0.05). A significant beneficial effect of the early choline supplementation was seen for the Ts65Dn mice: They did not differ from the unsupplemented 2N mice for any session-block. In addition, the supplemented trisomic animals performed significantly better than their unsupplemented counterparts mice for session block 3 (p = 0.04); a similar nonsignificant trend was seen for session-block 2 (p = 0.07). Notably, for this task, the early choline supplementation also benefited the 2N mice: They performed significantly better than their unsupplemented counterparts during session-block 2 (p = 0.01), indicating more rapid mastery of the new task demands; i.e., learning to wait for the cue.
Figure 3. Attention task 3 (Variable pre-cue delay: 0, 2, 4 s; constant 1 s cue duration).
(A) Mean (± SE) percentage of correct responses (averaged across the 20 sessions). The unsupplemented Ts65Dn mice performed significantly worse than both groups of 2N mice. The choline-supplemented Ts65Dn mice did not differ significantly from either group of 2N mice, and tended to perform better than their unsupplemented counterparts. (B) Mean (± SE) percentage of correct responses, as a function of the four session-blocks (5 sessions/block). Although the four groups did not differ in the first session block, differences emerged during the last three session blocks. During these latter 15 sessions, the unsupplemented Ts65Dn mice performed significantly more poorly than both groups of 2N mice. In contrast, the choline-supplemented Ts65Dn mice did not differ from the unsupplemented 2N mice for any session-block, and performed better than their unsupplemented counterparts mice for session blocks 2 and 3. The choline-supplemented 2N mice performed significantly better than their unsupplemented counterparts during session-block 2. (C) Mean (± SE) percentage of a long (> 5s) Alcove Latency: Whereas no treatment differences were seen for trials that followed a correct response, the incidence of long ALs following an error was significantly greater for the unsupplemented trisomic mice than for ether group of 2N mice as well as the supplemented trisomic mice.
*, p < 0.05
The analysis of AL (percent of trials with AL > 5 s) showed a similar pattern as in Attention Task 2. A Group effect was not seen [F(3, 41) = 1.60, p = 0.20], but a significant main effect of Prior trial outcome (correct or incorrect) was found [F(1, 41) = 21.84, p < 0.0001) as well as a significant interaction of Group and Previous trial outcome [F(3, 41) = 7.94, p = 0.0003; see Figure 3C]. Contrasts revealed that no treatment differences were seen for trials that followed a correct response. However, the increase in long ALs following an error, relative to after a correct response, was significantly greater for the unsupplemented trisomic mice than for the 2N controls (p’s < 0.0003) or the supplemented trisomic mice (p = 0.005). As a result, for trials following an error, the incidence of long ALs was significantly greater for the unsupplemented trisomic mice than for ether group of 2N mice (p’s < 0.0008) as well as the supplemented trisomic mice (p = 0.01). The supplemented trisomic mice did not differ from either group of 2N mice.
Attention Task 4 (Variable pre-cue delay: 0, 2, 4 s; variable cue duration: 0.8, 1.0, or 1.4 s)
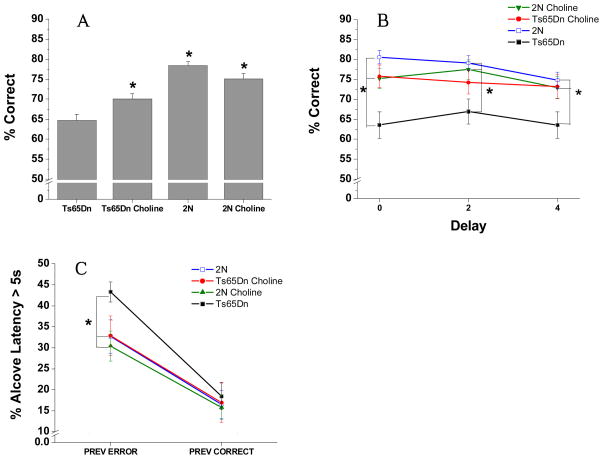
The analysis of percentage correct for the 20 sessions of Attention Task 4 revealed a significant main effect of Group [F(3, 39.7) = 5.90, p = 0.005; See figure 4A], reflecting the fact that overall, the unsupplemented Ts65Dn mice performed significantly worse than the two 2N control groups (p’s < 0.03). The Ts65Dn mice supplemented with choline early in life performed significantly better than the unsupplemented Ts65Dn mice (p = 0.05) and did not differ from the 2N mice.
Figure 4. Attention task 4 (Variable pre-cue delay: 0, 2, 4 s; variable cue duration: 0.8, 1.0, or 1.4 s).
(A) Mean (± SE) percentage of correct responses (averaged across all other conditions): The unsupplemented Ts65Dn mice performed significantly worse than the two groups of 2N mice. The Ts65Dn mice supplemented with choline early in life performed significantly better than the unsupplemented Ts65Dn mice and did not differ from the 2N mice. (B) Mean (± SE) percentage of correct responses as a function of the pre-cue delay: The unsupplemented Ts65Dn mice performed significantly worse than the unsupplemented 2N controls at all delays. In contrast, the choline-supplemented Ts65Dn mice did not differ significantly from either group of 2N mice for trials with either a 0s or 4s pre-cue delay, while still not differing from the supplemented 2N mice. In addition, the supplemented trisomic mice performed significantly better than their unsupplemented counterparts for trials with a 0 s or 4s pre-cue delay. (C) Mean (± SE) percentage of trials with a long Alcove Latency (> 5s), as a function of the outcome of the previous trial (correct or incorrect): No group differences were seen for trials that followed a correct response. However, for trials that followed an error, the incidence of trials with a long AL was significantly greater for the unsupplemented trisomic mice than for the two groups of 2N mice and the supplemented trisomic mice.
*, p < 0.05, compared with the unsupplemented Ts65Dn mice
A significant interaction between Group and Pre-cue Delay was also found [F(6, 878) = 3.10, p = 0.007; see figure 4B]. Contrasts revealed that the unsupplemented Ts65Dn mice performed significantly worse than the unsupplemented 2N controls at all delays (all p’s < 0.002). In contrast, the choline-supplemented Ts65Dn mice did not differ significantly from either group of 2N mice for trials with either a 0s or 4s pre-cue delay. In addition, the supplemented trisomic mice performed better than the unsupplemented trisomic mice for trials with a 0 s (p = 0.002) or 4s (p = 0.02) pre-cue delay.
The analysis of AL (percent of trials with AL > 5 s) showed the same pattern as the prior two attention tasks. A Group effect was not seen [F(3, 41) = 1.61, p = 0.20], but a significant main effect of Prior trial outcome (correct or incorrect) was seen [F(1, 41) = 83.15, p < 0.0001) as well as a nonsignificant trend for the interaction of Group and Previous trial outcome [F(3, 41) = 2.69, p = 0.06]. Contrasts revealed that no treatment differences were seen for trials that followed a correct response. However, the increase in long ALs for trials following an error, relative to after a correct response, was significantly greater for the unsupplemented trisomic mice than for the other three groups (see Figure 4C). As a result, for trials following an error, the incidence of long ALs was significantly greater for the unsupplemented trisomic mice than for ether group of 2N mice (p’s < 0.03); a trend also seen for the comparison between the unsupplemented and supplemented trisomic mice (p = 0.06). The supplemented trisomic mice did not differ from either group of 2N mice.
Reward Omission Task
The analysis of percent correct responses for the 20 sessions on the RO Task revealed a main effect of Group [F(3, 39.8) = 6.99, p = 0.0007], as well significant interactions between Group and Previous Trial Outcome (correct, incorrect, or reward omission) [F(6, 334) = 2.27; p = 0.04], and Group and Delay [F(6, 434) = 2.30, p = 0.03]. As seen in Figure 5A, the interaction of Group and Previous Trial Outcome reflected the fact that, whereas the unsupplemented trisomic mice were impaired relative to both groups of 2N mice regardless of prior trial outcome (all p’s < 0.05), the benefit provided by the perinatal choline supplementation varied somewhat by prior trial outcome. The supplemented trisomic mice performed significantly better than the unsupplemented trisomic mice regardless of prior trial outcome (p’s < 0.05). However, the extent to which the early choline supplementation normalized the performance of the Ts65Dn mice (relative to controls) varied by prior trial outcome: The choline-supplemented trisomic mice did not differ from the 2N mice for trials following an error or RO, whereas they performed significantly less well than the unsupplemented 2N mice for trials following a correct response (p = 0.04). They did not differ, however, from the supplemented 2N group regardless of prior trial outcome.
Figure 5. Reward Omission Task (Variable pre-cue delay: 0, 2, 4 s; constant 1 s cue duration; no reward on 20% of the correct responses).
(A) Mean (± SE) percentage of correct responses, as a function of the outcome of the previous trial: The unsupplemented trisomic mice performed more poorly than both groups of 2N mice regardless of prior trial outcome. The perinatal choline supplementation was effective: the supplemented trisomic mice performed significantly better than their unsupplemented counterparts, regardless of prior trial outcome. The Ts65Dn mice supplemented with choline did not differ from the 2N mice for trials following an error or RO, whereas they performed significantly less well than the unsupplemented 2N mice for trials following a correct response. They did not differ, however, from the supplemented 2N group regardless of prior trial outcome. (B) Mean (± SE) percentage of correct responses as a function of the pre-cue delay: The impairment of the unsupplemented trisomic mice relative to the 2N controls was most pronounced on trials with a 0 s delay prior to cue presentation. The perinatal choline supplementation was very effective in alleviating this attentional dysfunction of the trisomic mice, as they did not differ from either group of 2N at any pre-cue delay, and they were superior to the unsupplemented trisomic mice at the 0 s and 2 s delays. (C) Mean (± SE) percentage of a long Alcove Latency (> 5s)as a function of the outcome of the previous trial [correct, incorrect, or reward omission (RO)): The percentage of trials with an AL was significantly greater for the unsupplemented trisomic mice than for either group of 2N mice for trials following an error or a RO. For trials following an error or RO, the incidence of long ALs of the supplemented trisomic mice did not differ from that of controls, and was significantly lower than that of their unsupplemented counterparts.
*, p < 0.05
The interaction of Group and Delay [F(6, 254) = 2.61, p = 0.01] reflected the fact that the impairment of the unsupplemented trisomic mice (relative to the 2N controls), although seen at all pre-cue delays (p’s < 0.008), was most pronounced on trials with a 0 s delay prior to cue presentation (see Figure 5B). This pattern indicates that the unsupplemented trisomic mice had particular difficulty engaging and focusing attention immediately at trial onset. The perinatal choline supplementation was very effective in alleviating this attentional dysfunction of the trisomic mice, as they did not differ from either group of 2N at any pre-cue delay, and they were superior to the unsupplemented trisomic mice at the 0 s (p = 0.009) and 2 s (p = 0.05) delays, a trend also seen at the 4 s delay (p = 0.09).
Analysis of percent long AL (> 5s) revealed significant group differences for trials following an error or RO but not for trials following a correct response (see Fig. 5C). The percentage of trials with a long AL (>5 s) was significantly greater for the unsupplemented trisomic mice than for either group of 2N mice for trials following an error (p’s < 0.003) or a RO (p’s < 0.0006). Perinatal choline supplementation was effective in normalizing the incidence of long AL’s following an error or RO: For both conditions, the incidence of long ALs of the supplemented trisomic mice did not differ from that of controls, and was significantly lower than that of their unsupplemented counterparts. (p = 0.001 for post-error AL; p = 0.0008 for post-RO AL).
Videotape Findings
Attention Task 3
Sessions 6 and 7 were selected for coding, based on the finding that all four treatment groups differed in performance (percent correct) during these two sessions.
Behaviors
As noted above, the time spent in each behavior was evaluated separately for each trial outcome (correct or incorrect), and in addition, a difference score was analyzed (i.e., the difference in the average behavior duration following an error and average behavior duration following a correct response). Because this last measure best captures the reactivity to an error (i.e., reaction to an error, correcting for the frequency/duration of the behavior after a correct response, the presented results focus on this index. However, the conclusions drawn from these analyses did not differ from those drawn from the analyses conducted separately for each prior trial outcome.
Jumping
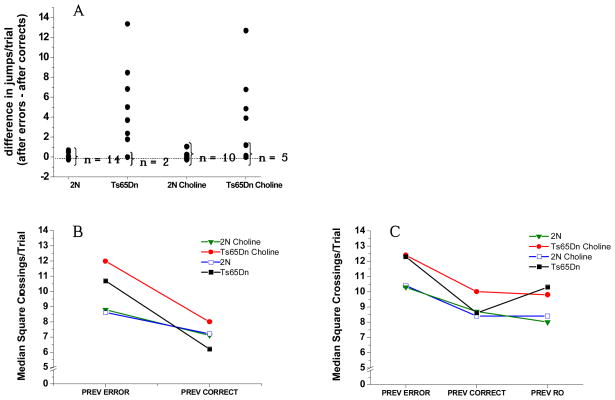
Corroborating the results of our prior study (Driscoll et al., 2004), the unsupplemented trisomic mice exhibited pronounced jumping specifically after an error, contrary to the controls who jumped relatively little after either an error or a correct response. The difference score (mean frequency of jumping per trial after an error minus mean frequency of jumping per trial after a correct response) was significantly higher for the unsupplemented trisomic mice than for either group of 2N mice (supplemented or not) (p’s < 0.008; see Figure 6A). Note that the 2N and Ts65Dn mice did not differ in jumping after a correct response (p = .40); thus, this difference is due to the increased jumping of the trisomic mice specifically after an error. The perinatal choline supplementation was not effective in normalizing this heightened reactivity to committing an error (p = 0.60; unsupplemented vs. supplemented Ts65Dn). Consistent with this finding, the difference score for the supplemented Ts65Dn mice was marginally larger than for either group of 2N mice (p’s < 0.05), indicating excessive error-induced jumping in these mice as well. Finally, the perinatal choline supplementation did not affect this measure for the 2N mice (p = 0.50).
Figure 6. Videotape results.
(A) Difference between the average number of jumps/trial following an error and following a correct response (in Attention Task 3). Each dot represents one mouse. The unsupplemented trisomic mice exhibited pronounced jumping specifically after an error, contrary to the 2N mice who jumped relatively little after either an error or a correct response. The perinatal choline supplementation was not effective in normalizing this heightened reactivity to committing an error (p = 0.60; unsupplemented vs. supplemented trisomic). The perinatal choline supplementation did not affect this measure for the 2N mice (p =0 .50). (B) Activity level (square crossings/trial) in Attention Task 3, as a function of the outcome of the previous trial: The four groups did not differ significantly in square crossings per trial following a correct response (treatment group, p = 0.72). In contrast, the increase in activity level following an error (relative to after a correct response) was significantly greater for the trisomic mice than for the 2N controls (p = 0.003); this effect was not modified by perinatal choline supplementation (p = 0.21; unsupplemented vs supplemented trisomic mice). Perinatal choline supplementation did not affect activity level of the 2N mice for any condition. (C) Activity level (square crossings/trial) on RO Task, as a function of previous trial outcome: The increase in activity level seen following an error or a RO, relative to a correct response was significantly greater for the unsupplemented trisomic mice than for the 2N controls (respectively, p = 0.02 and 0.007). In contrast, the trisomic mice supplemented with choline early in life did not differ from the 2N controls for the difference score relating post-error activity to that seen after a correct response (p < 0.36), or post-RO activity relative to after a correct response (p > 0.33). Correspondingly, the post-error increase in activity (relative to after a correct response) was significantly greater for the unsupplemented trisomic mice than for their supplemented counterparts (p < 0.03).
Grooming
No group differences were seen for this behavior regardless of prior trial outcome.
Wall-climbing
No group differences were seen for this behavior regardless of prior trial outcome.
Activity level (square crossings/trial)
The four groups did not differ significantly in square crossings per trial following a correct response (treatment group, p = 0.72). In contrast, the increase in activity level following an error (relative to after a correct response) was significantly greater for the trisomic mice than for the 2N controls (p = 0.003; see Figure 6B); this effect was not modified by perinatal choline supplementation (p = 0.21; unsupplemented vs supplemented trisomic mice). Thus, the increase in activity level seen following an error (relative to after a correct response) was also significantly greater for the supplemented trisomic mice than for the 2N controls (p’s < 0.004). Perinatal choline supplementation also did not affect activity level of the 2N mice for any condition.
RO Task
Sessions 1 and 2 were selected for coding because it was hypothesized that the emotional reaction engendered by the omission of an expected reward would be most pronounced at the beginning of the task. As noted above, only activity level (square-crossings/trial) was coded for this task.
Activity level (square crossings/trial)
The increase in activity level seen following an error or a RO, relative to a correct response (i.e., the difference score), was significantly greater for the unsupplemented trisomic mice than for the 2N controls (p’s for the two conditions, respectively, 0.02 and 0.007; see Figure 6C). In contrast, the trisomic mice supplemented with choline early in life did not differ from the 2N controls for the difference score relating post-error activity to that seen after a correct response (p = 0.36), or post-RO activity relative to after a correct response (p = 0.33). Correspondingly, the post-error increase in activity (relative to after a correct response) was significantly greater for the unsupplemented trisomic mice than for their supplemented counterparts (p < 0.03), a trend also seen following a RO (p = .12), again relative to activity following a correct response (i.e., difference scores). Thus, contrary to post-error activity in Attention Task 3, these data suggest some normalization of arousal-induced hyperactivity in the trisomic mice by perinatal choline supplementation.
Discussion
The results of this study replicate our prior findings that Ts65Dn mice exhibit profound attentional dysfunction as well as heightened reactivity to committing an error (Driscoll et al., 2004). The present study also revealed heightened reactivity of the trisomic mice to omission of an expected reward. Moreover, these findings demonstrate that increased maternal choline intake during pregnancy and lactation significantly ameliorated attentional functioning of the trisomic offspring, and provided some benefit, although less pervasive, to their 2N littermates. The effect of increased maternal choline intake on the heightened reactivity to errors and reward omission seen in the trisomic mice was more equivocal, but the pattern of findings indicates some benefit for this area of dysfunction as well.
Attentional Dysfunction in unsupplemented Ts65Dn mice
The pattern of findings indicates that the impaired performance of the unsupplemented Ts65Dn mice was due to attentional dysfunction, consistent with our prior study (Driscoll et al., 2004). They performed significantly more poorly than 2N littermate controls on all five attentionally-demanding tasks, but did not differ from these littermates in the initial visual discrimination task. The absence of group differences on this latter task indicates that they did not differ from controls in basic associative ability, motivation, visual acuity, motor functioning, or understanding of the basic task rules. The inference that the inferior performance of the unsupplemented trisomic mice reflects attentional dysfunction was also informed by the results of the analyses of the various types of errors in the five attention tasks (data not shown). These trisomic mice did not differ from their 2N littermates in the percentage of premature responses in any of the tasks, arguing against impulsivity or impaired inhibitory control as a cause of their impaired performance. Rather the trisomic mice committed a higher percentage of omission errors and inaccurate responses than the 2N mice, particularly on trials with the briefest cues and the longest pre-cue delays, consistent with our prior findings (Driscoll et al., 2004). Overall, this pattern of group differences implicates attentional dysfunction in the unsupplemented trisomic mice.
Perinatal Choline Supplementation Ameliorates the Attentional Dysfunction of the trisomic mice
Increased maternal choline intake during gestation and lactation produced a significant amelioration of the attentional functioning of the trisomic offspring, when tested during adulthood. The degree of benefit provided by the early choline supplementation varied somewhat across the five attention tasks and across various conditions within each task, but in general, the performance of the trisomic mice supplemented with choline early in life was significantly better than that of their unsupplemented trisomic counterparts and did not differ significantly from controls. It should be noted, however, that attentional function of the supplemented trisomic mice was not completely normalized. In some tasks, the supplemented trisomic mice committed a higher rate of omission errors than controls, even in instances where the overall percent correct did not reveal significant differences between these groups. Nonetheless, there is no question that the increased maternal choline intake significantly improved the attentional abilities of the trisomic mice, even if complete normalization was not achieved.
Effect of choline supplementation on heightened emotionality of the trisomic mice
Several findings indicated that the unsupplemented Ts65Dn mice exhibited an increased emotional response to committing an error or not receiving an expected reward. First, the analysis of the coded videotapes from Attention Task 3 revealed that the unsupplemented Ts65Dn mice exhibited repetitive jumping specifically after committing an error, behavior not seen in the 2N littermate controls, replicating our previous results (Driscoll et al., 2004). Consistent with this finding, in all five attention tasks, the unsupplemented trisomic mice exhibited a significantly greater incidence of long alcove latencies following an error or reward omission than the controls, indicating a greater hesitancy to respond following these trial outcomes. Finally, analysis of videotapes from Attention Task 3 and the Reward Omission task revealed that the increase in activity level produced by an error or reward omission (relative to after a correct response) was significantly greater for the unsupplemented trisomic mice than for the controls. These findings collectively indicate that, for the unsupplemented trisomic mice, the emotional reaction to committing an error or not receiving an expected reward was more excessive or less well regulated than for the 2N mice.
The effect of perinatal choline supplementation on this area of dysfunction was somewhat equivocal. The incidence of long ALs (> 5 s) following an error or reward omission was not different for the supplemented trisomic mice than for the 2N controls. However, heightened activity and jumping following an error were seen to a comparable degree in both groups of trisomic mice in Attention Task 3; i.e., a benefit of early choline supplementation was not seen. Nonetheless, in the Reward Omission Task, the choline supplementation partially normalized the heightened activity level produced by either an error or reward omission (relative to after a correct response). This latter finding is somewhat difficult to interpret, however, because the activity level of the supplemented trisomic mice did not differ from their unsupplemented counterparts for trials following either an error or reward omission; the inference that the early choline supplementation was beneficial is based on the relative increase in activity for these conditions; i.e., compared to that seen after a correct response.
The most parsimonious explanation for these findings, collectively, is that the greater incidence of trials with a long AL following an error or reward omission seen for the unsupplemented trisomic mice reflects not only increased activity and jumping but also some hesitancy to respond, and that this latter effect was normalized by the early choline supplementation, resulting in a normalization of AL despite the fact that the increased post-error or post- reward omission activity and jumping were not normalized (Attention Task 3) or only partially normalized (the Reward Omission Task). In sum, the overall pattern of findings suggests partial amelioration of this area of dysfunction by perinatal choline supplementation, consistent with a recent report suggesting improved emotion regulation in rats supplemented with choline prenatally (Cheng et al., 2008). This type of effect may be mediated by lasting changes in the functioning of the amygdala and/or orbito-frontal cortex, structures subserving emotion or affect that have strong cholinergic innervation.
Effect of choline supplementation in the wild-type (2N) mice
Providing excess choline during early development also significantly improved the performance of the 2N mice but only during session block 2 (sessions 6–10) of Attention Task 3. The pattern of results suggests that the supplemented 2N mice learned the new rule (i.e., that the cue would be presented after a delay on some trials) faster than their unsupplemented counterparts. Future studies are needed to ascertain whether this benefit is specific to the learning process involved in this type of task or whether it extends to associative function in general.
Although the perinatal choline supplementation facilitated learning of Attention Task 3 by the 2N mice, this task series did not reveal an effect on the attentional function of these mice, as indicated by asymptotic performance on these tasks after the basic rules had been mastered. However, it seems likely that such an effect would have been seen had the tasks been more attentionally-demanding due to briefer cues and/or longer pre-cue delays, This suggestion is based on the findings of Mohler and colleagues (2001), in which prenatal choline supplementation improved performance of normal mice in a signal detection task, but only when very brief cues were used. This improvement of attentional function as a result of early choline supplementation is consistent with the evidence suggesting that this early nutritional supplementation exerts lasting effects on cholinergic basal forebrain neurons and their projections to frontal cortex and hippocampus (cited in the Introduction), coupled with the evidence that cholinergic projections from the nucleus basalis to frontal cortex play an important role in this aspect of attention (e.g., Sarter et al., 2001).
The demonstration of this benefit for the 2N mice in the present study adds to the substantial evidence that perinatal choline supplementation produces lifelong improvements in cognitive functioning in normal rats and mice. Most of these prior findings, however, have involved rats and most have shown improved performance in spatial mazes and tests of timing ability (Meck et al., 1988; Meck et al., 1989; Meck et al., 1997; Tees et al., 1999; Tees et al., 1999; Williams et al., 1998; Cheng et al., 2008). There are, however, two prior studies that have demonstrated that prenatal choline supplementation improves specific aspects of attentional function, one (discussed above) showing improved performance of mice in a signal detection task (Mohler et al., 2001) and one showing an enhanced ability of rats to simultaneously time auditory and visual signals (Meck and Williams, 1997). Thus the present study extends the type of task and aspect of cognitive functioning influenced by this early nutritional manipulation in normal rodents, and adds to the evidence that such effects are seen in mice as well as rats.
Putative neural mechanisms
The neural mechanism(s) underlying the lasting cognitive and affective benefit of perinatal choline supplementation for the Ts65Dn mice remains to be elucidated but prior studies provide a plausible hypothesis; namely, that the early nutritional manipulation reduced the adult-onset atrophy of CBF neurons, possibly via a normalization of neurotrophin function. This hypothesis is based on several lines of converging evidence. First, the attentional dysfunction of Ts65Dn mice is likely caused by the atrophy of CBF neurons projecting to frontal cortex, which in turn has been shown to result from impaired retrograde transport of target-derived NGF (Cooper et al., 2001; Salehi et al., 2006), due, in part, to triplication of App (Cataldo et al., 2003; Salehi et al., 2006). Findings from AD patients and animal models (including Ts65Dn) indicate that CBF neurons atrophy and lose characteristic phenotypic markers (e.g., choline acetyltransferase and the neurotrophin receptors, p75NTR and TrkA), but survive rather than die outright (Sofroniew et al., 1990, Gilmor et al., 1999) and can be rescued by neurotrophin treatment (Cooper et al., 2001; Tuszynski et al., 2005). Second, perinatal choline supplementation has been shown to produce lasting effects on cholinergic neurons (Williams et al., 1998), cholinergic neurotransmission (Blusztajn et al., 1998) and various neurotrophins (Sandstrom et al., 2002, Glenn et al., 2007; Wong-Goodrich et al., 2008) in normal rats. Notably, recent findings point to alterations in several neural growth factors as a proximate mechanism underlying the neuroprotective effects of prenatal choline supplementation (Wong-Goodrich et al., 2006Wong-Goodrich et al., 2008; Napoli et al., 2008). Finally, NGF administration lessens learning and memory deficits in aged rats, coupled with a partial reversal of CBF neuron atrophy (Fischer et al., 1991, Kaisho et al., 1999).
As choline is the major dietary source of methyl groups, it is possible that many of these observed biological and behavioral effects of pre- and/or early postnatal choline supplementation may be mediated by alterations in DNA methylation status, producing long-lasting alterations in gene expression (i.e., epigenetic effects; Zeisel & Blusztajn, 1994; McGowan et al., 2008; Davison et al., 2009). This type of mechanism has been shown to underlie the long-lasting effect of maternal behavior on stress reactivity and cognitive functioning of the offspring (Szyf et al., 2007, McGowan et al., 2008), as well as the enduring effects of maternal diet on coat color and adiposity of the offspring (Wolff et al., 1998).
Although we hypothesize that these observed beneficial cognitive effects of perinatal choline supplementation in the Ts65Dn mice reflect protection against the neurodegeneration of CBF neurons, it is also possible that this early nutritional supplement normalizes brain development of these mice. In addition to being a dietary source of methyl groups, choline is also a precursor of phospholipid components of membranes, including those of neurons and glia, and as a result, its availability during early development may alter various ontogenetic processes, including cell division and proliferation, myelination, and synaptogenesis (Zeisel and Blusztajn,1994; Albright et al., 1999a,b, 2003).
Conclusions and Implications
Supplementing the maternal diet with excess choline during pregnancy and lactation substantially improved attentional function of the trisomic offspring, and partially normalized the heightened emotional response exhibited by these mice following an error or omission of an expected reward. These findings offer the exciting possibility that increasing the choline intake of pregnant and lactating women might significantly lessen the cognitive and affective dysfunction in offspring who have DS as well as, more generally, reduce the offspring’s risk of aging-related cognitive decline, including that caused by AD. It should be emphasized that this suggestion pertains specifically to the benefits of increased choline intake during early development, not later in life. Choline supplements have not generally been found to improve cognitive functioning when given to young (Mohs & Davis, 1980) or aged (Mohs et al., 1980) humans, and there is no evidence that this therapy would be useful to older children or adults with DS. Rather, the observed lasting cognitive benefit of pre- and/or early postnatal choline supplementation observed in the present study as well as in prior studies involving normal rodents (reviewed above) or FAS (Ryan et al., 2008; Thomas et al., 2000; 2007) are likely to reflect epigenetic effects (described above), initiated during early development. Thus, it is likely that there is a critical period during early development when choline availability can exert this type of lasting effect in DS individuals, as has been demonstrated for the lasting effects of choline availability on memory function in normal rodents (Meck et al. 2007). However, it should be noted that there is one report demonstrating that dietary choline supplementation from PND 35–63, following kainic acid -induced status epilepticus, can protect rats from the memory deficits normally induced by this insult (Holmes et al., 2002), indicating that choline supplementation can exert certain types of neuroprotective effects even when implemented later in development.
Finally, the benefit seen for the 2N mice in the present study, albeit more circumscribed, adds to the already substantial evidence that choline supplementation during pregnancy and lactation in rodents improves cognitive functioning in the adult offspring, an effect that is most pronounced during aging (Meck and Williams, 2003; Meck et al., 2007). Collectively, these findings suggest that the current dietary guidelines for choline, which correspond to the level of intake deemed necessary to prevent maternal liver damage (Inst. Med., Natl. Acad. Sci. USA. 1998), may not be optimal for brain development, and that higher levels of choline intake during pregnancy and lactation might enhance cognitive functioning of the offspring throughout their lives.
Acknowledgments
This work was supported by funding from NIH [R01 HD045224, R01 HD057564, P30 HD04024-36 (University of Colorado IDDRC)], the Anna and John J. Sie Foundation, and KOSEF grant 2009-0069193. We are grateful to Linda Crnic, Ph.D., for initiating our collaborative work in this area, and to Stephen D. Ginsberg, Ph.D. and Elliott J. Mufson, Ph.D., who are collaborating on ongoing studies concerning the underlying mechanism of action of these observed beneficial effects.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res Dev Brain Res. 1999a;115:123–129. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res Dev Brain Res. 1999b;113:13–20. doi: 10.1016/s0165-3806(98)00183-7. [DOI] [PubMed] [Google Scholar]
- Albright CD, Siwek DF, Craciunescu CN, Mar MH, Kowall NW, Williams CL, Zeisel SH. Choline availability during embryonic development alters the localization of calretinin in developing and aging mouse hippocampus. Nutr Neurosci. 2003;6:129–134. doi: 10.1080/1028415031000084418. [DOI] [PubMed] [Google Scholar]
- Antonarakis SE, Lyle R, Chrast R, Scott HS. Differential gene expression studies to explore the molecular pathophysiology of Down syndrome. Brain Res Brain Res Rev. 2001;36:265–274. doi: 10.1016/s0165-0173(01)00103-5. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm AC. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav Brain Res. 2003;139:47–57. doi: 10.1016/s0166-4328(02)00082-7. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK. Choline, a vital amine. Science. 1998;281:794–795. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Cermak JM, Holler T, Jackson DA. Imprinting of hippocampal metabolism of choline by its availability during gestation: implications for cholinergic neurotransmission. J Physiol Paris. 1998;92:199–203. doi: 10.1016/s0928-4257(98)80010-7. [DOI] [PubMed] [Google Scholar]
- Bowes C, Li T, Frankel WN, Danciger M, Coffin JM, Applebury ML, Farber DB. Localization of a retroviral element within the rd gene coding for the beta subunit of cGMP phosphodiesterase. Proc Natl Acad Sci U S A. 1993;90:2955–2959. doi: 10.1073/pnas.90.7.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone GT. Down syndrome: advances in molecular biology and the neurosciences. J Dev Behav Pediatr. 2001;22:40–59. doi: 10.1097/00004703-200102000-00007. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Petanceska S, Peterhoff CM, Terio NB, Epstein CJ, Villar A, Carlson EJ, Staufenbiel M, Nixon RA. App gene dosage modulates endosomal abnormalities of Alzheimer’s disease in a segmental trisomy 16 mouse model of down syndrome. J Neurosci. 2003;23(17):6788–6792. doi: 10.1523/JNEUROSCI.23-17-06788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak JM, Blusztajn JK, Meck WH, Williams CL, Fitzgerald CM, Rosene DL, Loy R. Prenatal availability of choline alters the development of acetylcholinesterase in the rat hippocampus. Dev Neurosci. 1999;21:94–104. doi: 10.1159/000017371. [DOI] [PubMed] [Google Scholar]
- Cheng RK, MacDonald CJ, Williams CL, Meck WH. Prenatal choline supplementation alters the timing, emotion, and memory performance (TEMP) of adult male and female rats as indexed by differential reinforcement of low-rate schedule behavior. Learn Mem. 2008;5;15(3):153–62. doi: 10.1101/lm.729408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RK, Scott AC, Penney TB, Williams CL, Meck WH. Prenatal-choline supplementation differentially modulates timing of auditory and visual stimuli in aged rats. Brain Res. 2008;1237:167–175. doi: 10.1016/j.brainres.2008.08.062. [DOI] [PubMed] [Google Scholar]
- Cooper JD, Salehi A, Delcroix JD, Howe CL, Belichenko PV, Chua-Couzens J, Kilbridge JF, Carlson EJ, Epstein CJ, Mobley WC. Failed retrograde transport of NGF in a mouse model of Down’s syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proc Natl Acad Sci U S A. 2001;98:10439–10444. doi: 10.1073/pnas.181219298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crnic LS, Pennington B. Down Syndrome: neuropsychology and animal models. Mahway, NJ: US: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- Davison JM, Mellott TJ, Kovacheva VP, Blusztajn JK. Gestational choline supply regulates methylation of histone H3, expression of histone methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA methylation of their genes in rat fetal liver and brain. J Biol Chem. 2009;284:1982–1989. doi: 10.1074/jbc.M807651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davisson MT, Schmidt C, Akeson EC. Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog Clin Biol Res. 1990;360:263–280. [PubMed] [Google Scholar]
- Davisson MT, Schmidt C, Reeves RH, Irving NG, Akeson EC, Harris BS, Bronson RT. Segmental trisomy as a mouse model for Down syndrome. Prog Clin Biol Res. 1993;384:117–133. [PubMed] [Google Scholar]
- Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr. 2008;87:424–430. doi: 10.1093/ajcn/87.2.424. [DOI] [PubMed] [Google Scholar]
- Driscoll LL, Carroll JC, Moon J, Crnic LS, Levitsky DA, Strupp BJ. Impaired sustained attention and error-induced stereotypy in the aged Ts65Dn mouse: a mouse model of Down syndrome and Alzheimer’s disease. Behav Neurosci. 2004;118:1196–1205. doi: 10.1037/0735-7044.118.6.1196. [DOI] [PubMed] [Google Scholar]
- Escorihuela RM, Fernandez-Teruel A, Vallina IF, Baamonde C, Lumbreras MA, Dierssen M, Tobena A, Florez J. A behavioral assessment of Ts65Dn mice: a putative Down syndrome model. Neurosci Lett. 1995;199:143–146. doi: 10.1016/0304-3940(95)12052-6. [DOI] [PubMed] [Google Scholar]
- Fischer W, Bjorklund A, Chen K, Gage FH. NGF improves spatial memory in aged rodents as a function of age. J Neurosci. 1991;11:1889–1906. doi: 10.1523/JNEUROSCI.11-07-01889.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner K, Fortna A, Bechtel L, Davisson MT. Mouse models of Down syndrome: how useful can they be? Comparison of the gene content of human chromosome 21 with orthologous mouse genomic regions. Gene. 2003;318:137–147. doi: 10.1016/s0378-1119(03)00769-8. [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Erickson JD, Varoqui H, Hersh LB, Bennett DA, Cochran EJ, Mufson EJ, Levey AI. Preservation of nucleus basalis neurons containing choline acetyltransferase and the vesicular acetylcholine transporter in the elderly with mild cognitive impairment and early Alzheimer’s disease. J Comp Neurol. 1999;411:693–704. [PubMed] [Google Scholar]
- Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur J Neurosci. 2007;25:2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn MJ, Kirby ED, Gibson EM, Wong-Goodrich SJ, Mellott TJ, Blusztajn JK, Williams CL. Age-related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats. Brain Res. 2008;1237:110–123. doi: 10.1016/j.brainres.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm AC, Ford KA, Hyde LA, Bimonte HA, Hunter CL, Nelson M, Albeck D, Sanders LA, Mufson EJ, Crnic LS. Estrogen restores cognition and cholinergic phenotype in an animal model of Down syndrome. Physiol Behav. 2002;77:371–385. doi: 10.1016/s0031-9384(02)00884-3. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Sanders LA, Crnic LS. Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down’s syndrome. Exp Neurol. 2000;161:647–663. doi: 10.1006/exnr.1999.7289. [DOI] [PubMed] [Google Scholar]
- Guo-Ross SX, Clark S, Montoya DA, Jones KH, Obernier J, Shetty AK, White AM, Blusztajn JK, Wilson WA, Swartzwelder HS. Prenatal choline supplementation protects against postnatal neurotoxicity. J Neurosci. 2002;22:RC195. doi: 10.1523/JNEUROSCI.22-01-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986;6:2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL, Yang Y, Liu Z, Cermak JM, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK. Seizure-induced memory impairment is reduced by choline supplementation before or after status epilepticus. Epilepsy Res. 2002;48(1–2):3–13. doi: 10.1016/s0920-1211(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Li YW, DeArmond SJ, McKinley MP, Gage FH, Epstein CJ, Mobley WC. Mouse model of neurodegeneration: atrophy of basal forebrain cholinergic neurons in trisomy 16 transplants. Proc Natl Acad Sci U S A. 1992;89:1383–1387. doi: 10.1073/pnas.89.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Santucci D, Kilbridge J, Chua-Couzens J, Fontana DJ, Daniels SE, Johnson RM, Chen K, Sun Y, Carlson E, Alleva E, Epstein CJ, Mobley WC. Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome. Proc Natl Acad Sci U S A. 1996;93:13333–13338. doi: 10.1073/pnas.93.23.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humby T, Laird FM, Davies W, Wilkinson LS. Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur J Neurosci. 1999;11:2813–2823. doi: 10.1046/j.1460-9568.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- Hunter CL, Bimonte HA, Granholm AC. Behavioral comparison of 4 and 6 month-old Ts65Dn mice: age-related impairments in working and reference memory. Behav Brain Res. 2003;138:121–131. doi: 10.1016/s0166-4328(02)00275-9. [DOI] [PubMed] [Google Scholar]
- Hunter CL, Bimonte-Nelson HA, Nelson M, Eckman CB, Granholm AC. Behavioral and neurobiological markers of Alzheimer’s disease in Ts65Dn mice: effects of estrogen. Neurobiol Aging. 2004;25:873–884. doi: 10.1016/j.neurobiolaging.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Crnic LS. Age-related deficits in context discrimination learning in Ts65Dn mice that model Down syndrome and Alzheimer’s disease. Behav Neurosci. 2001;115:1239–1246. [PubMed] [Google Scholar]
- Hyde LA, Frisone DF, Crnic LS. Ts65Dn mice, a model for Down syndrome, have deficits in context discrimination learning suggesting impaired hippocampal function. Behav Brain Res. 2001;118:53–60. doi: 10.1016/s0166-4328(00)00313-2. [DOI] [PubMed] [Google Scholar]
- Isacson O, Seo H, Lin L, Albeck D, Granholm AC. Alzheimer’s disease and Down’s syndrome: roles of APP, trophic factors and ACh. Trends Neurosci. 2002;25:79–84. doi: 10.1016/s0166-2236(02)02037-4. [DOI] [PubMed] [Google Scholar]
- Kaisho Y, Ohta H, Miyamoto M, Igarashi K. Nerve growth factor promoter driven neurotrophin-3 overexpression in the mouse and the protective effect of transgene on age-related behavioral deficits. Neurosci Lett. 1999;277(3):181–4. doi: 10.1016/s0304-3940(99)00874-5. [DOI] [PubMed] [Google Scholar]
- Korenberg JR, Chen XN, Devon KL, Noya D, Oster-Granite ML, Birren BW. Mouse molecular cytogenetic resource: 157 BACs link the chromosomal and genetic maps. Genome Res. 1999;9:514–523. [PMC free article] [PubMed] [Google Scholar]
- Kromer LF. Nerve growth factor treatment after brain injury prevents neuronal death. Science. 1987;235:214–216. doi: 10.1126/science.3798108. [DOI] [PubMed] [Google Scholar]
- Lai F, Williams RS. A prospective study of Alzheimer disease in Down syndrome. Arch Neurol. 1989;46:849–853. doi: 10.1001/archneur.1989.00520440031017. [DOI] [PubMed] [Google Scholar]
- Li Q, Guo-Ross S, Lewis DV, Turner D, White AM, Wilson WA, Swartzwelder HS. Dietary prenatal choline supplementation alters postnatal hippocampal structure and function. J Neurophysiol. 2004;91:1545–1555. doi: 10.1152/jn.00785.2003. [DOI] [PubMed] [Google Scholar]
- Loy R, Heyer D, Williams CL, Meck WH. Choline-induced spatial memory facilitation correlates with altered distribution and morphology of septal neurons. Adv Exp Med Biol. 1991;295:373–382. doi: 10.1007/978-1-4757-0145-6_21. [DOI] [PubMed] [Google Scholar]
- Mann DM. Alzheimer’s disease and Down’s syndrome. Histopathology. 1988;13:125–137. doi: 10.1111/j.1365-2559.1988.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Marston HM, Spratt C, Kelly JS. Phenotyping complex behaviours: assessment of circadian control and 5-choice serial reaction learning in the mouse. Behav Brain Res. 2001;125:189–193. doi: 10.1016/s0166-4328(01)00300-x. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Meaney MJ, Szyf M. Diet and the epigenetic (re)programming of phenotypic differences in behavior. Brain Res. 2008;1237:12–24. doi: 10.1016/j.brainres.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. Neuroreport. 1997;8:3045–3051. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol. 1988;21:339–353. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav Neurosci. 1989;103:1234–1241. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front Integr Neurosci. 2007;1:7. doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Brain Res Cogn Brain Res. 1998;6(3):233. doi: 10.1016/s0926-6410(97)00031-1. [DOI] [PubMed] [Google Scholar]
- Mellott TJ, Follettie MT, Diesl V, Hill AA, Lopez-Coviella I, Blusztajn JK. Prenatal choline availability modulates hippocampal and cerebral cortical gene expression. Faseb J. 2007;21:1311–1323. doi: 10.1096/fj.06-6597com. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mohs RC, Davis KL. Choline chloride effects on memory: correlation with the effects ofphysostigmine. Psychiatry Res. 1980;2(2):149–56. doi: 10.1016/0165-1781(80)90071-2. [DOI] [PubMed] [Google Scholar]
- Mohs RC, Davis KL, Tinklenberg JR, Hollister LE. Choline chloride effects on memory in the elderly. Neurobiol Aging. 1980;1(1):21–5. doi: 10.1016/0197-4580(80)90020-2. [DOI] [PubMed] [Google Scholar]
- Montoya DA, White AM, Williams CL, Blusztajn JK, Meck WH, Swartzwelder HS. Prenatal choline exposure alters hippocampal responsiveness to cholinergic stimulation in adulthood. Brain Res Dev Brain Res. 2000;123:25–32. doi: 10.1016/s0165-3806(00)00075-4. [DOI] [PubMed] [Google Scholar]
- Mohler E, Wong R, Meck WH, Williams CL. Prenatal choline supplementation improves radial-arm maze performance of C56BL6/J mice. Society for Neuroscience Abstracts. 1998;24:184. [Google Scholar]
- Mohler EG, Meck WH, Williams CL. Sustained attention in adult mice is modulated by prenatal choline availability. Int J Comp Psychol. 2001;14:136–50. [Google Scholar]
- Mufson EJ, Lavine N, Jaffar S, Kordower JH, Quirion R, Saragovi HU. Reduction in p140-TrkA receptor protein within the nucleus basalis and cortex in Alzheimer’s disease. Exp Neurol. 1997;146:91–103. doi: 10.1006/exnr.1997.6504. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Ginsberg SD, Ikonomovic MD, DeKosky ST. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J Chem Neuroanat. 2003;26:233–242. doi: 10.1016/s0891-0618(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Muir JL, Robbins TW, Everitt BJ. Disruptive effects of muscimol infused into the basal forebrain on conditional discrimination and visual attention: differential interactions with cholinergic mechanisms. Psychopharmacology (Berl) 1992;107:541–550. doi: 10.1007/BF02245269. [DOI] [PubMed] [Google Scholar]
- Nag N, Mellott TJ, Berger-Sweeney JE. Effects of postnatal dietary choline supplementation on motor regional brain volume and growth factor expression in a mouse model of Rett syndrome. Brain Res. 2008;27(1237):101–9. doi: 10.1016/j.brainres.2008.08.042. [DOI] [PubMed] [Google Scholar]
- Napoli I, Blusztajn JK, Mellott TJ. Prenatal choline supplementation in rats increases the expression of IGF2 and its receptor IGF2R and enhances IGF2-induced acetylcholine release in hippocampus and frontal cortex. Brain Res. 2008;1237:124–135. doi: 10.1016/j.brainres.2008.08.046. [DOI] [PubMed] [Google Scholar]
- Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, Schmidt C, Bronson RT, Davisson MT. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Berger-Sweeney J. Postnatal choline supplementation in preweanling mice: sexually dimorphic behavioral and neurochemical effects. Behav Neurosci. 1998;112:1387–1392. [PubMed] [Google Scholar]
- Risbrough V, Bontempi B, Menzaghi F. Selective immunolesioning of the basal forebrain cholinergic neurons in rats: effect on attention using the 5-choice serial reaction time task. Psychopharmacology (Berl) 2002;164:71–81. doi: 10.1007/s00213-002-1170-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ, Marston HM, Wilkinson J, Jones GH, Page KJ. Comparative effects of ibotenic acid- and quisqualic acid-induced lesions of the substantia innominata on attentional function in the rat: further implications for the role of the cholinergic neurons of the nucleus basalis in cognitive processes. Behav Brain Res. 1989;35:221–240. doi: 10.1016/s0166-4328(89)80143-3. [DOI] [PubMed] [Google Scholar]
- Roizen NJ, Patterson D. Down’s syndrome. Lancet. 2003;361:1281–1289. doi: 10.1016/S0140-6736(03)12987-X. [DOI] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res. 2008;27(1237):91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto-Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, Xia W, Villar A, Campbell WA, Kulnane LS, Nixon RA, Lamb BT, Epstein CJ, Stokin GB, Goldstein LS, Mobley WC. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Loy R, Williams CL. Prenatal choline supplementation increases NGF levels in the hippocampus and frontal cortex of young and adult rats. Brain Res. 2002;947:9–16. doi: 10.1016/s0006-8993(02)02900-1. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–60. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Scarpa S, Cavallaro RA, D’Anselmi F, Fuso A. Gene silencing through methylation: an epigenetic intervention on Alzheimer disease. J Alzheimers Dis. 2006;9:407–414. doi: 10.3233/jad-2006-9406. [DOI] [PubMed] [Google Scholar]
- Schenk FBC. Indirect effects of peri- and postnatal choline treatment on place-learning abilities in rat. Psychobiology. 1995;23:302–313. [Google Scholar]
- Sendera TJ, Ma SY, Jaffar S, Kozlowski PB, Kordower JH, Mawal Y, Saragovi HU, Mufson EJ. Reduction in TrkA-immunoreactive neurons is not associated with an overexpression of galaninergic fibers within the nucleus basalis in Down’s syndrome. J Neurochem. 2000;74:1185–1196. doi: 10.1046/j.1471-4159.2000.741185.x. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Galletly NP, Isacson O, Svendsen CN. Survival of adult basal forebrain cholinergic neurons after loss of target neurons. Science. 1990;247(4940):338–342. doi: 10.1126/science.1688664. [DOI] [PubMed] [Google Scholar]
- Szyf M, Weaver I, Meaney M. Maternal care, the epigenome and phenotypic differences in behavior. Reprod Toxicol. 2007;24(1):9–19. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Tees RC. The influences of sex, rearing environment, and neonatal choline dietary supplementation on spatial and nonspatial learning and memory in adult rats. Dev Psychobiol. 1999a;35:328–342. doi: 10.1002/(sici)1098-2302(199912)35:4<328::aid-dev7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Tees RC. The influences of rearing environment and neonatal choline dietary supplementation on spatial learning and memory in adult rats. Behav Brain Res. 1999b;105:173–188. doi: 10.1016/s0166-4328(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Tees RC, Mohammadi E. The effects of neonatal choline dietary supplementation on adult spatial and configural learning and memory in rats. Dev Psychobiol. 1999;35:226–240. [PubMed] [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol. 2000;22:703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121:120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Thal L, Pay M, Salmon DPUHS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11(5):551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- Vicari S. Motor development and neuropsychological patterns in persons with Down syndrome. Behav Genet. 2006;36:355–364. doi: 10.1007/s10519-006-9057-8. [DOI] [PubMed] [Google Scholar]
- Villar AJ, Belichenko PV, Gillespie AM, Kozy HM, Mobley WC, Epstein CJ. Identification and characterization of a new Down syndrome model, Ts[Rb(12.1716)]2Cje, resulting from a spontaneous Robertsonian fusion between T(171)65Dn and mouse chromosome 12. Mamm Genome. 2005;16:79–90. doi: 10.1007/s00335-004-2428-7. [DOI] [PubMed] [Google Scholar]
- Visser FE, Aldenkamp AP, van Huffelen AC, Kuilman M, Overweg J, van Wijk J. Prospective study of the prevalence of Alzheimer-type dementia in institutionalized individuals with Down syndrome. Am J Ment Retard. 1997;101:400–412. [PubMed] [Google Scholar]
- Walsh TJ, Gandhi C, Stackman RW. Reversible inactivation of the medial septum or nucleus basalis impairs working memory in rats: a dissociation of memory and performance. Behav Neurosci. 1998;112:1114–1124. doi: 10.1037//0735-7044.112.5.1114. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH, Heyer DD, Loy R. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res. 1998;794:225–238. doi: 10.1016/s0006-8993(98)00229-7. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985a;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Dalton AJ, McLachlan C, Wen GY, Wisniewski HM. Alzheimer’s disease in Down’s syndrome: clinicopathologic studies. Neurology. 1985b;35:957–961. doi: 10.1212/wnl.35.7.957. [DOI] [PubMed] [Google Scholar]
- Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. Faseb J. 1998;12(11):949–957. [PubMed] [Google Scholar]
- Wolfinger R, O’Connell M. Generalized linear mixed models: a pseudo-likelihood approach. Journal of Statistical Computing Simulations. 1993;48:233–243. [Google Scholar]
- Wong-Goodrich SJ, Glenn MJ, Mellott TJ, Blusztajn JK, Meck WH, Williams CL. Spatial memory and hippocampal plasticity are differentially sensitive to the availability of choline in adulthood as a function of choline supply in utero. Brain Res. 2008a;1237:153–166. doi: 10.1016/j.brainres.2008.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Goodrich SJ, Mellott TJ, Glenn MJ, Blusztajn JK, Williams CL. Prenatal choline supplementation attenuates neuropathological response to status epilepticus in the adult rat hippocampus. Neurobiol Dis. 2008b;30:255–269. doi: 10.1016/j.nbd.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu Z, Cermak JM, Tandon P, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK, Holmes GL. Protective effects of prenatal choline supplementation on seizure-induced memory impairment. J Neurosci. 2000;20:RC109. doi: 10.1523/JNEUROSCI.20-22-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14:269–96. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]