Abstract
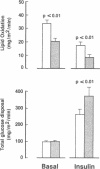
Increased nonesterified fatty acid (NEFA) levels may be important in causing insulin resistance in skeletal muscles in patients with non-insulin-dependent diabetes mellitus (NIDDM). The acute effect of the antilipolytic nicotinic acid analogue Acipimox (2 X 250 mg) on basal and insulin-stimulated (3 h, 40 mU/m2 per min) glucose metabolism was therefore studied in 12 patients with NIDDM. Whole-body glucose metabolism was assessed using [3-3H]glucose and indirect calorimetry. Biopsies were taken from the vastus lateralis muscle during basal and insulin-stimulated steady-state periods. Acipimox reduced NEFA in the basal state and during insulin stimulation. Lipid oxidation was inhibited by Acipimox in all patients in the basal state (20 +/- 2 vs. 33 +/- 3 mg/m2 per min, P less than 0.01) and during insulin infusion (8 +/- 2 vs. 17 +/- 2 mg/m2 per min, P less than 0.01). Acipimox increased the insulin-stimulated glucose disposal rate (369 +/- 49 vs. 262 +/- 31 mg/m2 per min, P less than 0.01), whereas the glucose disposal rate was unaffected by Acipimox in the basal state. Acipimox increased glucose oxidation in the basal state (76 +/- 4 vs. 50 +/- 4 mg/m2 per min, P less than 0.01). During insulin infusion Acipimox increased both glucose oxidation (121 +/- 7 vs. 95 +/- 4 mg/m2 per min, P less than 0.01) and nonoxidative glucose disposal (248 +/- 47 vs. 167 +/- 29 mg/m2 per min, P less than 0.01). Acipimox enhanced basal and insulin-stimulated muscle fractional glycogen synthase activities (32 +/- 2 vs. 25 +/- 3%, P less than 0.05, and 50 +/- 5 vs. 41 +/- 4%, P less than 0.05). Activities of muscle pyruvate dehydrogenase and phosphofructokinase were unaffected by Acipimox. In conclusion, Acipimox acutely improved insulin action in patients with NIDDM by increasing both glucose oxidation and nonoxidative glucose disposal. This supports the hypothesis that elevated NEFA concentrations may be important for the insulin resistance in NIDDM. The mechanism responsible for the increased insulin-stimulated nonoxidative glucose disposal may be a stimulatory effect of Acipimox on glycogen synthase activity in skeletal muscles.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argyraki M., Wright P. D., Venables C. W., Proud G., Taylor R. In vitro study of human skeletal muscle strips: effect of nonesterified fatty acid supply on glucose storage. Metabolism. 1989 Dec;38(12):1183–1187. doi: 10.1016/0026-0495(89)90157-1. [DOI] [PubMed] [Google Scholar]
- Beatty C. H., Bocek R. M. Interrelation of carbohydrate and palmitate metabolism in skeletal muscle. Am J Physiol. 1971 Jun;220(6):1928–1934. doi: 10.1152/ajplegacy.1971.220.6.1928. [DOI] [PubMed] [Google Scholar]
- Bevilacqua S., Bonadonna R., Buzzigoli G., Boni C., Ciociaro D., Maccari F., Giorico M. A., Ferrannini E. Acute elevation of free fatty acid levels leads to hepatic insulin resistance in obese subjects. Metabolism. 1987 May;36(5):502–506. doi: 10.1016/0026-0495(87)90051-5. [DOI] [PubMed] [Google Scholar]
- Bevilacqua S., Buzzigoli G., Bonadonna R., Brandi L. S., Oleggini M., Boni C., Geloni M., Ferrannini E. Operation of Randle's cycle in patients with NIDDM. Diabetes. 1990 Mar;39(3):383–389. doi: 10.2337/diab.39.3.383. [DOI] [PubMed] [Google Scholar]
- Blass J. P., Avigan J., Uhlendorf B. W. A defect in pyruvate decarboxylase in a child with an intermittent movement disorder. J Clin Invest. 1970 Mar;49(3):423–432. doi: 10.1172/JCI106251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Howard B. V., Reaven G., Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984 Oct;74(4):1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Stone K., Mott D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. J Clin Invest. 1984 Apr;73(4):1185–1190. doi: 10.1172/JCI111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterson I. D., Fuller S. J., Randle P. J. Effect of the fatty acid oxidation inhibitor 2-tetradecylglycidic acid on pyruvate dehydrogenase complex activity in starved and alloxan-diabetic rats. Biochem J. 1982 Oct 15;208(1):53–60. doi: 10.1042/bj2080053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobelli C., Mari A., Ferrannini E. Non-steady state: error analysis of Steele's model and developments for glucose kinetics. Am J Physiol. 1987 May;252(5 Pt 1):E679–E689. doi: 10.1152/ajpendo.1987.252.5.E679. [DOI] [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Eriksson J., Franssila-Kallunki A., Ekstrand A., Saloranta C., Widén E., Schalin C., Groop L. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med. 1989 Aug 10;321(6):337–343. doi: 10.1056/NEJM198908103210601. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Barrett E. J., Bevilacqua S., DeFronzo R. A. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983 Nov;72(5):1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988 Mar;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- Frayn K. N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Fuccella L. M., Goldaniga G., Lovisolo P., Maggi E., Musatti L., Mandelli V., Sirtori C. R. Inhibition of lipolysis by nicotinic acid and by acipimox. Clin Pharmacol Ther. 1980 Dec;28(6):790–795. doi: 10.1038/clpt.1980.236. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 9. Effects of fatty acids and ketone bodies, and of alloxan-diabetes and starvation, on pyruvate metabolism and on lactate-pyruvate and L-glycerol 3-phosphate-dihydroxyacetone phosphate concentration ratios in rat heart and rat diaphragm muscles. Biochem J. 1964 Dec;93(3):665–678. doi: 10.1042/bj0930665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golay A., Chen Y. D., Reaven G. M. Effect of differences in glucose tolerance on insulin's ability to regulate carbohydrate and free fatty acid metabolism in obese individuals. J Clin Endocrinol Metab. 1986 Jun;62(6):1081–1088. doi: 10.1210/jcem-62-6-1081. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Berger M., Ruderman N. B. Glucose metabolism in rat skeletal muscle at rest. Effect of starvation, diabetes, ketone bodies and free fatty acids. Diabetes. 1974 Nov;23(11):881–888. doi: 10.2337/diab.23.11.881. [DOI] [PubMed] [Google Scholar]
- Greenfield M., Kolterman O., Olefsky J., Reaven G. M. Mechanism of hypertriglyceridaemia in diabetic patients with fasting hyperglycaemia. Diabetologia. 1980 Jun;18(6):441–446. doi: 10.1007/BF00261698. [DOI] [PubMed] [Google Scholar]
- Gross R. C., Carlson L. A. Metabolic effects of nicotinic acid in acute insulin deficiency in the rat. Diabetes. 1968 Jun;17(6):353–361. doi: 10.2337/diab.17.6.353. [DOI] [PubMed] [Google Scholar]
- Hagg S. A., Taylor S. I., Ruberman N. B. Glucose metabolism in perfused skeletal muscle. Pyruvate dehydrogenase activity in starvation, diabetes and exercise. Biochem J. 1976 Aug 15;158(2):203–210. doi: 10.1042/bj1580203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heding L. G. Determination of total serum insulin (IRI) in insulin-treated diabetic patients. Diabetologia. 1972 Aug;8(4):260–266. doi: 10.1007/BF01225569. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Radioimmunological determination of human C-peptide in serum. Diabetologia. 1975 Dec;11(6):541–548. doi: 10.1007/BF01222104. [DOI] [PubMed] [Google Scholar]
- Hennig G., Löffler G., Wieland O. H. Active and inactive forms of pyruvatedehydrogenase in skeletal muscle as related to the metabolic and functional state of the muscle cell. FEBS Lett. 1975 Nov 15;59(2):142–145. doi: 10.1016/0014-5793(75)80361-9. [DOI] [PubMed] [Google Scholar]
- Hother-Nielsen O., Beck-Nielsen H. On the determination of basal glucose production rate in patients with type 2 (non-insulin-dependent) diabetes mellitus using primed-continuous 3-3H-glucose infusion. Diabetologia. 1990 Oct;33(10):603–610. doi: 10.1007/BF00400204. [DOI] [PubMed] [Google Scholar]
- ITAYA K., UI M. COLORIMETRIC DETERMINATION OF FREE FATTY ACIDS IN BIOLOGICAL FLUIDS. J Lipid Res. 1965 Jan;6:16–20. [PubMed] [Google Scholar]
- Jefferson L. S., Koehler J. O., Morgan H. E. Effect of insulin on protein synthesis in skeletal muscle of an isolated perfused preparation of rat hemicorpus. Proc Natl Acad Sci U S A. 1972 Apr;69(4):816–820. doi: 10.1073/pnas.69.4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. E., Mandarino L. J. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. J Clin Invest. 1990 Dec;86(6):1999–2007. doi: 10.1172/JCI114935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey A. L., Radcliffe P. M., Randle P. J., Sugden P. H. Regulation of kinase reactions in pig heart pyruvate dehydrogenase complex. Biochem J. 1979 Aug 1;181(2):427–433. doi: 10.1042/bj1810427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lovisolo P. P., Briatico-Vangosa G., Orsini G., Ronchi R., Angelucci R., Valzelli G. Pharmacological profile of a new anti-lipolytic agent: 5-methyl-pyrazine-2-carboxylic acid 4-oxide (acipimox) (1) I - Mechanism of action. Pharmacol Res Commun. 1981 Feb;13(2):151–161. doi: 10.1016/s0031-6989(81)80016-1. [DOI] [PubMed] [Google Scholar]
- Maizels E. Z., Ruderman N. B., Goodman M. N., Lau D. Effect of acetoacetate on glucose metabolism in the soleus and extensor digitorum longus muscles of the rat. Biochem J. 1977 Mar 15;162(3):557–568. doi: 10.1042/bj1620557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandarino L. J., Wright K. S., Verity L. S., Nichols J., Bell J. M., Kolterman O. G., Beck-Nielsen H. Effects of insulin infusion on human skeletal muscle pyruvate dehydrogenase, phosphofructokinase, and glycogen synthase. Evidence for their role in oxidative and nonoxidative glucose metabolism. J Clin Invest. 1987 Sep;80(3):655–663. doi: 10.1172/JCI113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen H. B. Quantitative determination of hemoglobin A1c by thin-layer isoelectric focusing. J Chromatogr. 1980 Jun 13;182(3-4):325–333. doi: 10.1016/s0378-4347(00)81481-4. [DOI] [PubMed] [Google Scholar]
- Musatti L., Maggi E., Moro E., Valzelli G., Tamassia V. Bioavailability and pharmacokinetics in man of acipimox, a new antilipolytic and hypolipemic agent. J Int Med Res. 1981;9(5):381–386. doi: 10.1177/030006058100900515. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Sugden P. H., Williams T. Effect of citrate on the activities of 6-phosphofructokinase from nervous and muscle tissues from different animals and its relationships to the regulation of glycolysis. Biochem J. 1977 Jul 15;166(1):123–129. doi: 10.1042/bj1660123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- ROOT M. A., ASHMORE J. THE HYPOGLYCEMIC ACTIVITY OF NICOTINIC ACID IN RATS. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964 May 11;248:117–123. doi: 10.1007/BF00246667. [DOI] [PubMed] [Google Scholar]
- Randle P. J., England P. J., Denton R. M. Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem J. 1970 May;117(4):677–695. doi: 10.1042/bj1170677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle P. J., Kerbey A. L., Espinal J. Mechanisms decreasing glucose oxidation in diabetes and starvation: role of lipid fuels and hormones. Diabetes Metab Rev. 1988 Nov;4(7):623–638. doi: 10.1002/dmr.5610040702. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Sugden P. H., Kerbey A. L., Radcliffe P. M., Hutson N. J. Regulation of pyruvate oxidation and the conservation of glucose. Biochem Soc Symp. 1978;(43):47–67. [PubMed] [Google Scholar]
- Reaven G. M., Chang H., Ho H., Jeng C. Y., Hoffman B. B. Lowering of plasma glucose in diabetic rats by antilipolytic agents. Am J Physiol. 1988 Jan;254(1 Pt 1):E23–E30. doi: 10.1152/ajpendo.1988.254.1.E23. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Greenfield M. S. Diabetic hypertriglyceridemia: evidence for three clinical syndromes. Diabetes. 1981;30(Suppl 2):66–75. doi: 10.2337/diab.30.2.s66. [DOI] [PubMed] [Google Scholar]
- Reimer F., Löffler G., Hennig G., Wieland O. H. The influence of insulin on glucose and fatty acid metabolism in the isolated perfused rat hind quarter. Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):1055–1066. doi: 10.1515/bchm2.1975.356.s1.1055. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Ruderman N. B., Gavras H., Belur E. R., Galbo H. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. Am J Physiol. 1982 Jan;242(1):E25–E32. doi: 10.1152/ajpendo.1982.242.1.E25. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G., Adams R. P., Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol. 1985 Feb;248(2 Pt 2):R190–R196. doi: 10.1152/ajpregu.1985.248.2.R190. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G., Saltin B. Extra- and intracellular water spaces in muscles of man at rest and with dynamic exercise. Am J Physiol. 1982 Sep;243(3):R271–R280. doi: 10.1152/ajpregu.1982.243.3.R271. [DOI] [PubMed] [Google Scholar]
- Stansbie D., Denton R. M., Bridges B. J., Pask H. T., Randle P. J. Regulation of pyruvate dehydrogenase and pyruvate dehydrogenase phosphate phosphatase activity in rat epididymal fat-pads. Effects of starvation, alloxan-diabetes and high-fat diet. Biochem J. 1976 Jan 15;154(1):225–236. doi: 10.1042/bj1540225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappy L., Owen O. E., Boden G. Effect of hyperinsulinemia on urea pool size and substrate oxidation rates. Diabetes. 1988 Sep;37(9):1212–1216. doi: 10.2337/diab.37.9.1212. [DOI] [PubMed] [Google Scholar]
- Taylor W. M., Halperin M. L. Stimulation of glucose transport in rat adipocytes by insulin, adenosine, nicotinic acid and hydrogen peroxide. Role of adenosine 3':5'-cyclic monophosphate. Biochem J. 1979 Feb 15;178(2):381–389. doi: 10.1042/bj1780381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiébaud D., DeFronzo R. A., Jacot E., Golay A., Acheson K., Maeder E., Jéquier E., Felber J. P. Effect of long chain triglyceride infusion on glucose metabolism in man. Metabolism. 1982 Nov;31(11):1128–1136. doi: 10.1016/0026-0495(82)90163-9. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Wieland O. H., Patzelt C., Löffler G. Active and inactive forms of pyruvate dehydrogenase in rat liver. Effect of starvation and refeeding and of insulin treatment on pyruvate-dehydrogenase interconversion. Eur J Biochem. 1972 Apr 11;26(3):426–433. doi: 10.1111/j.1432-1033.1972.tb01783.x. [DOI] [PubMed] [Google Scholar]
- Wieland O. H. The mammalian pyruvate dehydrogenase complex: structure and regulation. Rev Physiol Biochem Pharmacol. 1983;96:123–170. doi: 10.1007/BFb0031008. [DOI] [PubMed] [Google Scholar]
- Wieland O., Siess E., Schulze-Wethmar F. H., von Funcke H. G., Winton B. Active and inactive forms of pyruvate dehydrogenase in rat heart and kidney: effect of diabetes, fasting, and refeeding on pyruvate dehydrogenase interconversion. Arch Biochem Biophys. 1971 Apr;143(2):593–601. doi: 10.1016/0003-9861(71)90244-x. [DOI] [PubMed] [Google Scholar]