Abstract
IL-10 is an important immunoregulatory factor. However, our understanding of IL-10 gene regulation remains very limited. In this study, following up on our previous novel finding that the protooncogene c-Maf of the basic leucine zipper family of transcription factors is expressed in monocytes and macrophages, we investigate the role of c-Maf in the transcriptional regulation of IL-10 and the under-lying molecular mechanism in macrophages. c-Maf-null macrophages exhibit strongly impaired IL-10 protein production and mRNA expression upon LPS stimulation. Ectopic expression of c-Maf stimulates not only exogenously transfected IL-10 promoter-driven luciferase activity in a dose-dependent manner but also enhances endogenous IL-10 gene expression stimulated by LPS. Both in vitro and in vivo experiments identify a c-Maf response element localized to nucleotides −196/−184 relative to the transcription initiation site in the IL-10 promoter. This site represents an atypical 12-O-tetradecanoate-13-acetate-responsive element for musculoaponeurotic fibrosarcoma recognition and functions as an enhancer element in a heterologous and orientation-independent manner. Furthermore, c-Maf is expressed constitutively in resting monocytes/macrophages. IL-4 can up-regulate c-Maf expression, its binding to IL-10 promoter, and dose dependently enhance IL-10 production induced by LPS; moreover, IL-4 failed to enhance LPS-induced IL-10 production in c-Maf-null macrophages. Taken together, these data demonstrate that c-Maf is an indispensable yet constitutive transcription factor for IL-10 gene expression in LPS-activated macrophages, and IL-4 modulates IL-10 production in inflammatory macrophages likely via its ability to induce c-Maf expression. Thus, this study uncovers a novel and important function of c-Maf in macrophages and elucidates its transcriptional mechanism in the regulation of IL-10 gene expression.
Insult by an invading pathogen induces both innate and adaptive immune responses. The innate immune response pertains to those cells that are preprogrammed to respond to pathogen-associated molecular patterns or danger signals (1), in which the monocyte/macrophage lineage, as both the sentinels and the first line of defense against infection, plays a crucial role. In contrast, the adaptive immune response involves the selection and expansion of immune cells, such as T and B cells, with the development of immunologic memory. Cells of the innate immune response not only serve as the first line of defense but, via providing signal 1 (Ag-specific TCR triggering), signal 2 (costimulation), and signal 3 (polarization, e.g., cytokines), shape the nature of the subsequent adaptive immune response by influencing the T cell cytokine pattern (2).
Although the inflammatory response is critical in controlling the growth of pathogenic microorganisms, excessive cytokine production is harmful to the host and can even be fatal (3). Thus, the challenge faced by the immune system of an infected host is to respond with sufficient intensity and duration to control and eliminate the infection while minimizing nonspecific injury to host tissues. Monocytes are recognized as one of the key promoters of acute and chronic inflammatory responses. One intriguing feature of macrophage biology is the ability of activated macrophage populations to initially produce proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-12 (4), and subsequently, the second phase of the macrophage response involves the production of IL-10, a potent immunosuppressive cytokine, which down-regulates the production of proinflammatory cytokines (5). The equilibrium of pro- and anti-inflammatory cytokine expression is of central importance for understanding how the immune system regulates responses to pathogenic infection (6).
IL-10 is one of the most potent and significant anti-inflammatory cytokines. It mediates the down-regulation of Th1 responses by inhibiting the production of most immune-activating cytokines (7, 8), thereby limiting and ultimately terminating inflammatory responses (9). This function is illustrated readily by the phenotype of IL-10-null mice, which acquires autoimmune manifestations of colitis due to an overabundance of IL-12 and IFN-γ (9, 10) and exhibits increased resistance to microbial infection (11). The beneficial effect of IL-10 treatment for inflammatory conditions in both animal models and clinical diseases (recent review; Ref. 9) suggests that the key functions of IL-10 are to maintain homeostasis of the immune system and to protect the host from excessive inflammation.
IL-10 gene regulation can occur both at the transcriptional and posttranscriptional levels (9, 12). However, current understanding of the mechanisms in IL-10 gene regulation remains rudimentary. A recent study from our laboratory (13) revealed that IL-10 and the protooncogene c-Maf induce their mutual expression in inflammatory macrophages, and c-Maf is a strong suppressor of IL-12 p40 gene transcription. c-Maf is the cellular counterpart of oncogenic v-Maf, which was identified originally in the genome of the acute transforming avian retrovirus AS42 and induces musculoaponeurotic fibrosarcoma (Maf)3 in vivo and transforms primary chicken embryo fibroblast in vitro (14). c-Maf belongs to the family of basic region leucine zipper domain transcription factors, and the cell lineage-specific targets of c-Maf have been identified; some examples include the following: IL-4 in Th2 cells (15), the crystalline genes in lens fiber cells (16, 17), insulin gene in islet β cells (18), p53 (19), and L7 (20). In all these genes, c-Maf exerts its transcriptional role through binding to a Maf recognition element (MARE) (21). There are two forms of human c-Maf mRNA, c-Maf-long and c-Maf-short, as the result of differential splicing (22, 23). The first direct in vivo demonstration of a physiological role of c-Maf was provided by studies in c-Maf-null mice (16, 17, 24). Disruption of the c-Maf gene affected both intrauterine and postnatal survival (17). In addition, it has been shown that c-Maf is one of the master regulators for Th2 differentiation (15, 25), Therefore, c-Maf is both a developmentally and immunologically important gene. In the present study, we further investigated the molecular mechanisms by which c-Maf regulates IL-10 gene transcription in macrophages.
Materials and Methods
Mice
c-Maf −/− mice were kindly provided by Dr. I. Cheng Ho of Harvard School of Public Health (Boston, MA) (17). Fetal liver-derived macrophages were prepared from gestation day 14.5 embryos by intercrossing c-Maf +/− mice as described previously (13).
Cells and reagents
RAW264.7 murine macrophage cell line (ATCC TIB-71), human monocytes isolation, and culturing were conducted as described previously (26). LPS (Escherichia coli serotype 0127:B8) was purchased from Sigma-Al-drich (catalog no. L-3129). The Abs were as follows: c-Maf (catalog no. A300-613A; Bethyl Laboratories), c-Maf (M153, sc-7866x; Santa Cruz Biotechnology), and c-fos (sc-52x; Santa Cruz Biotechnology). Recombinant human IL-4 and IFN-γ were purchased from Genzyme.
Reporter plasmids
The human IL-10 promoter-luciferase construct (pIL-10 (−1044/+30)-luc) was generously provided by Dr. L. Zaiegler-Heitbrock of University of Leicester (Leicester, U.K.) (27). For generation of the 5′ successive deletion series, the upstream and downstream PCR primers were designed to include BamHI and XhoI restriction sites, respectively. The PCR products were ligated directly into pCR2.1 T/A vector and then subcloned into the same backbone of pIL-10 (−1044/+30)-luc plasmid by using BamHI/XhoI digestion. For the substitution mutants, an overlapping PCR procedure was used (28) by using a pair of overlapping internal primers that contain the mutant sequences. All constructs are relative to the transcriptional initiation site and confirmed by sequencing against the human IL-10 promoter (GenBank accession no. Z30175; Ref. 29). The murine IL-4 promoter-driven luciferase construct, pIL-4 (−157/+68)-luc, was kindly provided by Dr. Richard Flavell of Yale University (New Haven, CT) (15). For the chimeric promoter constructs, the consensus MARE, 5′-TGCTGACTCAGCAACATTGTTGCTGACTCAGCAC-3′; −203/−170 wild type, 5′-TCATTTTTGCTTACGATGCAAAAATTGAAAACTA-3′; and −203/−170 mutant (M7), 5′-TCATTTTGTAGGCATCGTACAAAATTGAAAACTA-3′ (core element underlined) were ligated to the upstream of the minimal herpes simplex virus thymidine kinase (TK) promoter (−109/+18)-luciferase vector, pTKluc (30), in both forward and reverse orientations. All plasmids were isolated using the Qiagen EndoFree Maxi kit (Qiagen).
Expression vectors for transcription factors
c-Maf expression vector (pCEFL/c-Maf) and its control vector (pCEFL) have been described before (23). There are two forms of c-Maf proteins as a result of alternative splicing: c-Maf-long and c-Maf-short (22, 23). The v-Maf dominant-negative mutant (DNM), Mxi-v-Maf, was kindly provided by Dr. K. Katoka (Tokyo Institute of Technology, Tokyo, Japan). Mxi-v-Maf is a fusion protein consisting of amino acids 1–70 of human Mxi1 that contains the Sin3 interaction domain fused to amino acids 19 –369 of v-Maf, and this chimeric protein can bind to MARE sequence; however, Mxi1-Sin3 interaction domain masked the function of the Maf transactivation domain, thus acting as a DNM (31, 32). The Mxi-v-Maf is in the pGEM-4 vector (31).
Transient transfection and reporter assays
RAW264.7 cell transfections were performed by electroporation and followed by luciferase assay as described before (13). Results are expressed generally as mean ± SD from two to three independent experiments. All statistical analyses were performed with two-tailed Student’s t tests. Data were considered significant if p was <0.05.
Adenovirus-based expression vectors
Adenoviruses harboring human c-Maf-internal ribosomal entry site-enhanced green fluorescent protein (EGFP) or EGFP alone and their transduction of human monocyte-derived macrophages were as described before (13).
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed by following the protocol in the ChIP Assay kit (Upstate Biotechnology). The presence of a selected DNA sequence of the human IL-10 gene was assessed by PCR. The primers used were sense (−206/−188), 5′-CAATCATTTTTGCTTACGA-3′, and antisense (−147/−132), 5′-CCTTAGAGCTCCTCCT-3′ (65-bp product). As a negative control, a separate region of the human IL-10 promoter was also included in the ChIP experiment. It is located between −3158 and −2947 upstream of the −206/−132 region. The pair of PCR primers used in this control were sense, 5′-AGTGAGAAGGCAGGCACCTA-3′, and antisense, 5′-ATCCCCCACTGGAAAAATTC-3′. The PCR cycles were as follows: 95°C for 3 min, 60°C for 40 s, and 72°C for 30 s, 1 cycle; 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s, 29 cycles; and 72°C for 7 min, 1 cycle.
RNase protection assay (RPA)
Total RNA was purified using Ultraspec RNA isolation system (Biotecx Laboratories). RPA was performed by using the Riboquant kit (BD Pharmingen), according to the manufacturer’s instructions. The mCK-2b and hCK2 template sets were used. The respective cytokine mRNAs were quantified by using the PhosphorImager and ImageQuant (ImageQuant 5.0; Molecular Dynamics) and normalized to the corresponding L32 and GAPDH controls.
Cytokine production by ELISA
BD Pharmingen OptEIA sets for mouse IL10, human IL-10, and human IL-12p40 were used.
Nuclear extract and recombinant human c-Maf
Nuclear extracts were isolated as described previously (33). The recombinant human c-Maf protein was synthesized by using pTNT Quick Coupled transcription/Translation System (Promega). In brief, both human c-Maf-long and -short form cDNA were cloned individually into pTNT vector under the T7 promoter and named pTNT/c-Maf and then proceeded to the pTNT reaction following the instruction.
EMSA
EMSA, competitive EMSA, and supershifts were performed as described previously (34). The following oligonucleotides were used as probes: MARE consensus, 5′-GGAATTGCTGACTCAGCATTACT-3′; −203/−170, 5′-TCATTTTTGCTTACGATGCAAAAATTGAAAACTA-3′ (core element underlined); and NF-κB consensus, 5′-ATGTGAGGGGACTTTCCCAGGC-3′.
Computational analysis of the IL-10 promoter region
Human IL-10 promoter region −1044/+30 was analyzed by the MatIn-spector V2.2 professional program (35) to predict transcription factor binding sites.
RT-PCR analysis of gene expression
Reverse transcription reactions were performed as previously described (13), and the following primers were used for PCR: human IL-10 (GenBank accession no. M57627) forward (position 104 –129), 5′-AGTCTGAGAACAGCTGCACCCACTTC-3′, and reverse (position 297–318), 5′-GGGCATCACCTCCTCCAGGTAA-3′ (215-bp product, annealing temperature 60°C, 30 cycles). Because the cDNA sequences of c-Maf-short and c-Maf-long are identical until the position 1925 (22), the same up-stream PCR primer for the two forms of c-Maf was used: sense (position 1571–1592), 5′-TGCACTTCGACGACCGCTTCTC-3′; antisense-short form (position 1957–1938), 5′-GGTGGCTAGCTGGAATCGCG-3′ (Gen-Bank accession no. AF055376, 386-bp product, annealing temperature 60°C, ~30 cycles); and antisense-long form (position 2040 –2011), 5′- TGTACAGCTCTCACACAAATTTCATTTTGT-3′ (GenBank accession no. AF055377, 469-bp product, annealing temperature 60°C, 30 cycles). Human GAPDH (GenBank accession no. M33197) was used as an internal control: sense (position 80–99), 5′-GAGTCAACGGATTTGGTCGT-3′; and antisense (position 298 –317), 5′-TTGATTTTGGAGGGATCTCG-3′ (238-bp product, annealing temperature 60°C, ~28 cycles).
Results
IL-10 gene expression in c-Maf −/− macrophages is greatly impaired
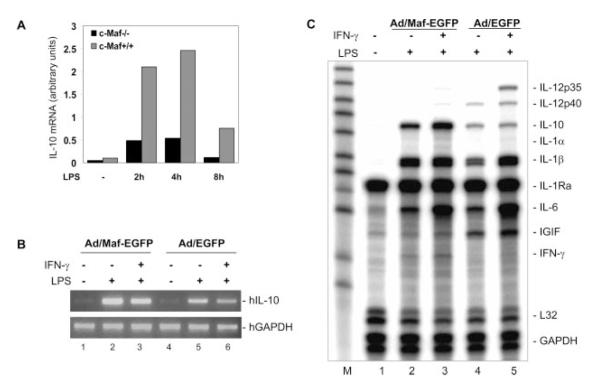
To characterize the role of c-Maf in the IL-10 gene expression in macrophages, we first examined the IL-10 mRNA expression in c-Maf −/− macrophages stimulated with LPS over a time course from 0 to 8 h. c-Maf mutant macrophages showed significantly impaired IL-10 mRNA expression relative to wild-type littermate macrophages after LPS stimulation throughout the time course (Fig. 1A), confirming our previous reporting (13). To address the question of whether c-Maf can activate the transcription of endogenous IL-10 gene in macrophages, a c-Maf overexpression approach was used. Human monocyte-derived macrophages were transduced with adenovirus-expressing human c-Maf or EGFP (as control).
FIGURE 1.
Impaired IL-10 production by c-Maf−/− macrophages upon LPS stimulation. A, Fetal liver-derived macrophages from c-Maf−/− and wild-type littermate embryos of gestation day 14.5 were plated into 24-well plates at a density of 0.5 × 106 cells/ml/well. Cells were stimulated with 1 μg/ml LPS. At different time points as indicated, IL-10 mRNA level was analyzed by RPA. One representative of five embryos for each group (n = 5) in two independent experiments with similar results is shown. B and C, Human monocyte-derived macrophages were transduced with adenovirus-expressing c-Maf or EGFP 24 h after the transduction cells were treated with LPS (1 μg/ml) for4hor primed with rhIFN-γ (10 ng/ml) for 16 h, followed by LPS for 4 h. Total RNA was isolated for analysis by RT-PCR (B) or by RPA (C) to detect IL-10 mRNA expression. hIL, human IL; hGAPDH, human GAPDH.
Twenty-four h after the transduction, the macrophages were treated with LPS for4hor primed with human IFN-γ for 16 h, then treated with LPS for 4 h. The endogenous IL-10 mRNA expression was detected by RT-PCR (Fig. 1B) and RPA (Fig. 1C). Compared with the control adenovirus, c-Maf transduction of primary macrophages significantly enhanced LPS- or IFN-γ/LPS-stimulated IL-10 mRNA expression (compare lanes 2 and 3 to 4 and 5, respectively, in Fig. 1, B and C). c-Maf adenovirus transduction alone did not induce the endogenous IL-10 gene transcription (Fig. 1A, lane 1).
Ectopic c-Maf expression can activate IL-10 gene transcription
Additionally, to demonstrate the transcriptional role of c-Maf in the regulation of IL-10 gene expression, the human IL-10 promoter (−1044/+30)-driven luciferase reporter was cotransfected with the human c-Maf expression plasmid into the mouse macrophage cell line RAW264.7, which has been used extensively to study macrophage cytokine gene expression (36, 37). The result shows that ectopically introduced c-Maf dose dependently up-regulated the IL-10 promoter activity (Fig. 2A). The short and long forms of c-Maf worked at similar efficiencies in activating the IL-10 promoter activity (Fig. 2B). Thus, the c-Maf-long form was used for all subsequent experiments. Next, we wanted to define the role of the endogenous c-Maf in IL-10 transcription. To address this issue, we used the well-characterized v-Maf-DNM (38). Protein blast analysis (⟨www.ncbi.nlm.nih.gov/BLAST/⟩) indicated that the bZIP-Maf identity between v-Maf (GenBank accession no. NP062191) and mouse c-Maf (GenBank accession no. I57555) or human c-Maf-short form (GenBank accession no. AAC27037) or human c-Maf-long form (GenBank accession no. AAC27038) is 98, 99, and 98%, respectively. These high degrees of homology suggest that v-Maf-DNM may be able to functionally block mammalian c-Maf. To verify whether the v-Maf-DNM, Mxi-v-Maf, could block the function of mammalian c-Maf, we first tested its activity on a mouse IL-4 promoter-driven luciferase reporter that has been shown to respond to c-Maf (15). The cotransfection of pIL-4 (−157/+68)-luc with c-Maf or c-Maf plus Mxi-v-Maf into RAW264.7 cells showed that Mxi-v-Maf strongly blocked the c-Maf-induced IL-4 transcription (data not shown), confirming its cross-species efficacy. We then cotransfected the pIL-10(−1044/+30)-luc with c-Maf or c-Maf plus Mxi-v-Maf. As shown in Fig. 2C, Mxi-v-Maf completely blocked ectopic c-Maf-induced IL-10 transcription. Moreover, when we cotransfected pIL-10(−1044/+30)-luc with Mxi-v-Maf into RAW264.7 cells, it also completely inhibited the constitutive as well as LPS-induced IL-10 promoter activity (Fig. 2D), suggesting that the endogenous c-Maf present in RAW264.7 cells (13) is not only required for LPS-induced IL-10 gene expression but also contributes to the basal level of IL-10 transcription.
FIGURE 2.
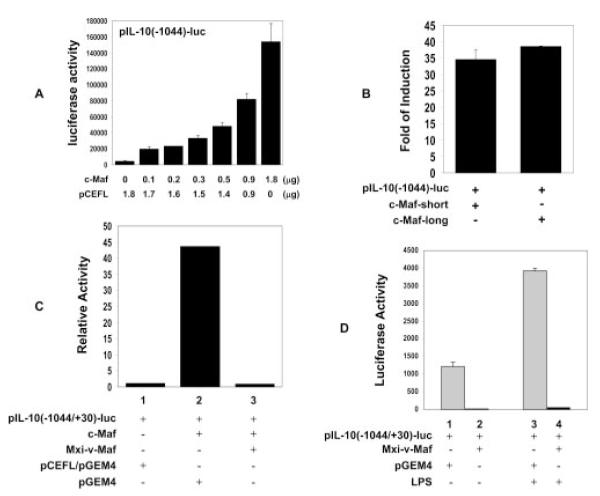
Ectopic c-Maf expression activates IL-10 gene transcription. A, RAW264.7 cells were transfected by electroporation using 6.5 μg of pIL-10 (−1044/+30)-luc reporter construct together with indicated amounts of expression vector c-Maf (long form) or its control vector pCEFL. The total amount of the effector plasmids (pCEFL/c-Maf) was maintained at 1.8 μg with pCEFL. The result shown here is the summary of two independent experiments (mean ± SD). B, The long and short forms of c-Maf were cotransfected with the IL-10 promoter as described in A. Their respective luciferase activities were expressed as fold induction compared with the activity of pCEFL plus IL-10 promoter, which was set as 1. C, RAW264.7 cells were cotransfected with 6.5 μg of pIL-10 (−1044/ +30)-luc with control vectors or pIL-10 (−1044/+30)-luc plus 1.8 μgof c-Maf expression vector (reporter:effector = 1:1/4 in molarity) or pIL-10 (−1044/+30)-luc and c-Maf expression vector plus v-Maf-DNM (reporter:effector:Mxi-v-Maf = 1:1/4:1 in molarity). Luciferase activity was expressed as fold induction (relative luciferase activity) by arbitrarily setting the activity of pIL-10 (−1044/+30)-luc as 1. Statistical analysis indicated that c-Maf significantly up-regulated IL-10 promoter-driven luciferase activity (p < 0.001, lane 1 vs 2), and Mxi-v-Maf completely blocked the exogenously transfected c-Maf on the induction of pIL-10(−1044/+30)-luc (p < 0.001, lane 2 vs 3; p > 0.05, lane 1 vs 3). D, RAW264.7 cells were cotransfected with 6.5 μg of pIL-10 (−1044/+30)-luc with control vector or with Mxi-v-Maf. Transfected cells were either left alone (medium) or treated with 1 μg/ml LPS for 20 h. Statistical analysis indicated that Mxi-v-Maf significantly blocked the endogenous c-Maf-induced basal (p < 0.05, lane 1 vs 2) and the LPS-induced luciferase activity of pIL-10(−1044/+30)-luc (p < 0.05, lane 3 vs 4). One representative of three independent experiments is shown.
Localization of the c-Maf response region in human IL-10 promoter
To identify the c-Maf response element(s) in the IL-10 promoter, a series of the 5′ deletion mutants of IL-10 promoter-driven luciferase constructs were cotransfected with the c-Maf expression plasmid into RAW264.7 cells, and the luciferase activities were plotted relative to the activity of the full-length promoter (−1044/+30), which was set arbitrarily as 100%. Two constructs, −408 (no. 8) and −171 (no. 12) (Fig. 3), showed considerable decrease in their response to c-Maf, suggesting that the regions −511/−408 and −206/−171 may harbor c-Maf-responsive element(s). The complete loss of promoter activity in the −61 construct (no. 16) was likely due to the fact that the truncation was in the proximity of TATA-box, which is located at −59/−54 (see Fig. 5A for details).
FIGURE 3.
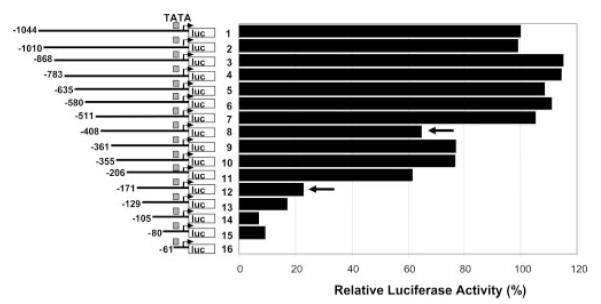
Identification of c-Maf-responsive regions in the IL-10 promoter. The full-length (−1044/+30) and a series of 5′ deletion mutants of the human IL-10 promoter-luciferase constructs were cotransfected with c-Maf expression vector into RAW264.7 cells at a molar ratio of 1:1/4 (reporter:effector). The relative luciferase activities were plotted against that of the −1044/+30 promoter, which is set as 100%. A representative of two experiments with similar results are shown.
FIGURE 5.
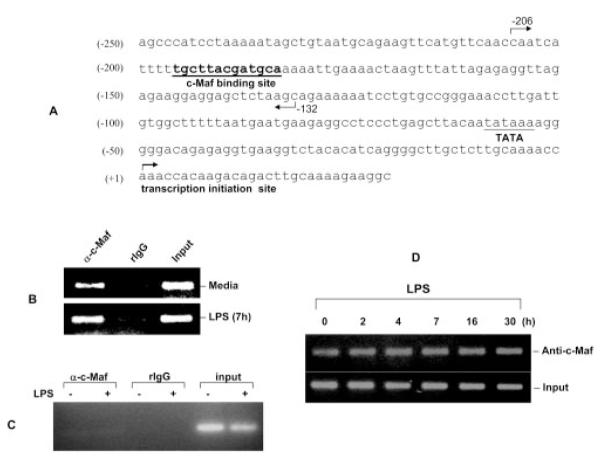
Binding of c-Maf to IL-10 promoter in vivo. A, Partial sequence of the human IL-10 promoter that covers the c-Maf binding site (−196/−184, boldfaced and underlined). The region from −206 to −132 amplified by PCR in the ChIP assay is marked by the two directional arrows. B and C, Human monocyte-derived macrophages, either unstimulated or treated, with LPS (1 μg/ml) for different times as indicated and then subjected to the ChIP procedure with anti-c-Maf Ab (Santa Cruz Biotechnology) or its isotype control (normal rabbit IgG). Two pairs of primers were used to amplify the −203/−132 region (B) and a separate region 3 kb upstream (C). The PCR amplified a 75-bp product in B and a 211-bp product in C. D, Kinetics of c-Maf binding to the IL-10 promoter in LPS-activated human macrophages.
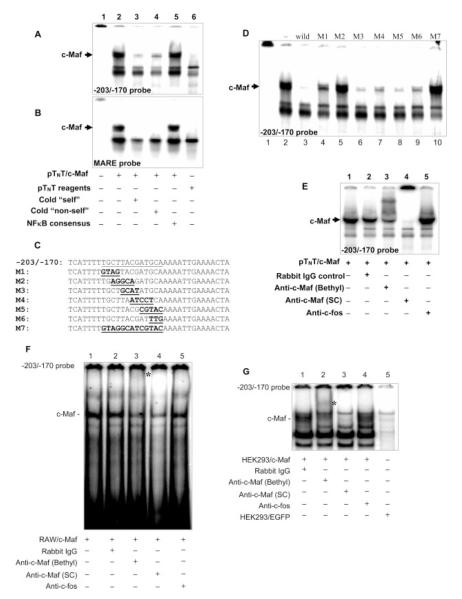
c-Maf binds to the −206/−171 region in the IL-10 promoter in vitro
The above 5′ deletion mutants have narrowed down the c-Maf-responsive elements to two possible regions on the IL-10 promoter. We then localized the exact c-Maf binding site on the IL-10 promoter by EMSA. Kataoka et al. have identified two types of DNA consensus sequences that are bound by v/c-Maf homodimers, a 13-bp TRE-type MARE and a 14-bp cyclic AMP-responsive element-type MARE. The former MARE contains an AP-1 (TGCTGACTCAGCA) binding site, whereas the latter harbors a cyclic AMP-responsive element (TGCTGACGTCAGCA) site (core element underlined). Computational analysis with the MatInspector V2.2 program (35) indicated that there is a putative Maf binding site located at −196/−184. To determine whether c-Maf could bind to the −206/−171 region, recombinant c-Maf protein (long form) was synthesized using the pTNT system, and it has been reported that the synthesized c-Maf protein from homodimers (15). A reciprocal competitive EMSA was used, i.e., by using 32P-labeled double-strand oligos in IL-10 promoter as probes and competed by the MARE consensus sequence, or the other way around, using the 32P-labeled MARE consensus sequence as the probe, then competed with the double-strand oligos derived from the IL-10 promoter. Three sets of double-strand oligos (−490/−470, −467/−435, and −430/−402) were used to cover the first c-Maf-responsive region (−511/−408) and one set of oligos to cover the second responsive region (−203/−170) based on the computational analysis. The three pairs of oligos (−490/−470, −467/−435, and −430/−402) that cover the first potential region (−511/−408) didn’t show any specific binding that could be competed off by cold MARE competitor, nor could they compete for binding to the MARE sequence (data not shown). However, for the second region, the −203/−170 probe formed a specific complex with recombinant c-Maf (Fig. 4A, lane 2, indicated by a black arrow), which could be efficiently competed off by an excess of cold −203/−170 sequence (Fig. 4A, lane 3, “self”) or the consensus MARE sequence (Fig. 4A, lane 4, “non-self”) but not by an irrelevant sequence (NF-κB consensus; Fig. 4A, lane 5). Conversely, the MARE consensus sequence probe displayed very similar binding properties (Fig. 4B), indicating that the binding activities to the −203/−170 in human IL-10 promoter were comparable to the MARE consensus sequences. To further corroborate that this 13-bp (−196/−184) was a c-Maf-specific binding element, a series of 4- or 5-bp substitution mutants (M1–M6) or the entire 13-bp (−196/−184) substitution mutant (M7) were used as competitors against the wild-type −203/−170 probe for EMSA (Fig. 4C). As shown in Fig. 4D, M1 and M3–M6 oligos were able to compete off the majority of the c-Maf-binding activity against the wild-type probe, whereas M2 and the more extensive M7 oligos completely lost their ability to bind c-Maf. To verify the identity of the binding activity to the −203/−170 probe, a supershift assay was conducted using two sources of anti-c-Maf Abs. A c-Maf Ab (Bethyl Laboratories) strongly supershifted the complex (Fig. 4E, lane3), whereas the second c-Maf Ab (Santa Cruz Biotechnology) completely ablated this binding (Fig. 4E, lane 4), consistent with the reported behavior of these Abs (22). However, an anti-c-fos Ab did not have any effect on this complex (Fig. 4E, lane 5). Thus, these results confirm that c-Maf binds to the 13-bp MARE-like sequence located at −196/−184 of the IL-10 promoter with the most critical sequence motif of CTTAC. Similar results were obtained in the reciprocal competitive EMSA using RAW264.7 or human monocyte nuclear exact (data not shown). However, supershift with the nuclear extracts did not yield a clear confirmation (data not shown). We reasoned that this failure might be due to the low levels of endogenous c-Maf. Indeed, when we used nuclear extracts from RAW264.7 cells transiently transfected with the c-Maf expression vector to overexpress c-Maf, we did see a clear supershift (Fig. 4F, lane 3, indicated by the asterisk) or diminished the binding (Fig. 4F, lane 4). When we used nuclear extracts from HEK293 cells transduced with c-Maf-expressing adenovirus, which could render much higher gene expression in target cells, a very clear supershift or abrogation was observed by two anti-c-Maf Abs (Bethyl Laboratories and Santa Cruz Biotechnology; Fig. 4G, lanes 2 and 3, respectively) but not of anti-c-fos Ab (Fig. 4G, lane 4).
FIGURE 4.
Binding of c-Maf to IL-10 promoter in vitro. A and B, Recombinant human c-Maf-long protein, generated by the PTNT system, was used in EMSA with 32P-labeled −203/−170 sequence from the IL-10 promoter (A) or the MARE consensus (B). Competition for c-Maf binding was done by adding cold −203/−170 or MARE or the NF-κB consensus sequences in 50× molar excess. The terms “self” and “non-self” refer reciprocally to −203/−170 and MARE, respectively. Lane 6 in A and B is mock transcription-translation controls. C, A series of consecutive mutant probes of every 4- to 5-bp mutations (M1–M6) or a 13-bp mutation (M7) of the predicted c-MARE were generated in the context of the −203/−170 sequence (mutated sequences are in boldface and underlined). D, The mutant oligos described in C were used as cold competitors (50× molar excess) in competitive EMSA with the wild-type −203/−170 probe and recombinant human c-Maf protein. E, Supershift EMSA was conducted with the −203/−170 probe and recombinant human c-Maf protein. Anti-c-Maf Abs (Bethyl Laboratories or Santa Cruz Biotechnology (SC)), control rabbit IgG, and anti-c-fos Ab were used. F and G, Supershift EMSA was conducted using the −203/−170 probe and the nuclear extract isolated from RAW264.7 cells, which had been transfected with the c-Maf expression vector for 48 h, followed by4hof LPS stimulation (1 μg/ml; F) or nuclear extract isolated from HEK 293 cells transduced with c-Maf-expressing adenovirus (G). The same Abs were used as in E. The supershifted bands are indicated by an asterisk (lane 3 in E; lane 2 in F).
Specific in vivo c-Maf binding to the IL-10 promoter in human macrophages
To determine whether c-Maf could bind to the MARE-like sequence in the IL-10 promoter in vivo, ChIP assay was performed. This technique can establish whether a known transcription factor binds in the vicinity of a known regulatory element in living cells (39). As Fig. 5 demonstrates, in primary human monocyte-derived macrophages, c-Maf specifically bound to the IL-10 promoter in this region in vivo between −206 and −132 (Fig. 5B) but not to a separate region 3 kb upstream (Fig. 5C). Surprisingly, the binding was not inducible by LPS stimulation over a period of 30 h (Fig. 5D). The above data collectively suggest that c-Maf can bind to the −203/−170 sequence both in vitro and in vivo, and the core element for this binding resides in the 13-bp region at −196 to −184 in human IL-10 promoter.
The −196/−184 sequence acts as an enhancer
The above results provided evidence that −196/−184 in human IL-10 promoter is a MARE-like sequence. To test the function of this element in the induction of IL-10 promoter activity by c-Maf, a reporter construct with a 13-bp substitution (M7) was constructed on the backbone of −206/+30 of the IL-10 promoter. Compared with pIL-10 (−206)-luc, the response of pIL-10(−206)M7-luc to c-Maf was decreased by >50% and down to the level of the −171/+30 construct (Fig. 6A). Additionally, to characterize the function of this c-Maf response element, chimeric promoters were generated (Fig. 6B). Transient transfection of these constructs with c-Maf expression plasmid was performed in RAW264.7 cells. As shown in Fig. 6B, in the forward orientation, although the wild-type −206/−170 sequence, which contains the MARE-like element, was less potent in the response to c-Maf than that of the consensus MARE-containing TK promoter, the 13-bp M7 mutation resulted in a complete loss of its c-Maf response, decreasing to the basal level (Fig. 6B, upper panel). In the reverse orientation, although wild-type −206/−170 TK chimeric promoter showed an even greater response to c-Maf, the M7 mutant was still nonresponsive (Fig. 6B, lower panel). Taken together, these data additionally demonstrate that the 13-bp MARE-like sequence is a functional c-Maf response element; moreover, because it works in an orientation-independent manner, it may act as an enhancer element for IL-10 transcription.
FIGURE 6.
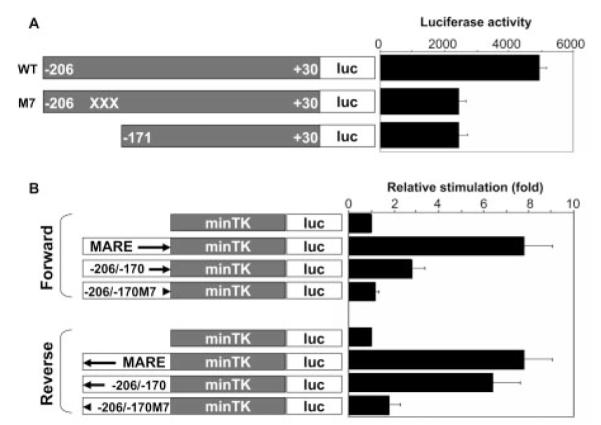
Enhancer activity of the MARE-like sequence. A, RAW264.7 cells were cotransfected with pIL-10(−206/+30)-luc, pIL-10(−206/+30)M4-luc, or pIL-10(−171/+30)-luc with the c-Maf expression vector (reporter:effector is 1:1/4 in molarity). The crosses in the M4 construct denote the 13-bp mutations. Statistic analysis indicates that, compared with pIL-10(−206/+30)-luc, pIL-10 (−206/+30)M4-luc significantly lost its response to c-Maf induction (p < 0.001) and decreased to the level comparable with the downstream deletion mutant reporter pIL-10(−171/+30)-luc (p > 0.05). Mean values ± SD of three experiments are shown. B, The sequences of −206/−170, −206/−171M4 (mutant), and MARE consensus were cloned into a minimal TK promoter in both orientations (forward and reverse indicated by directional arrows). RAW264.7 cells were cotransfected with these chimeric reporter constructs or the parental pTK-luc, with c-Maf expression vector (reporter:effector = 1:1/4 in molarity). Luciferase activity was expressed as relative stimulation to that of the minimal pTK-luc reporter, which is arbitrarily set as 1. Compared with the minimal pTK-luc reporter, c-Maf significantly induced the response of p(MARE)-TK-luc and p(−206/−170)-TK-luc (p < 0.01 and p < 0.05, respectively) in both forward and reverse orientations; however, p(−206/−170)M4-TK-luc lost its response to c-Maf induction in both orientations (p > 0.05 and p > 0.05, respectively). Mean values ± SD of three experiments are shown.
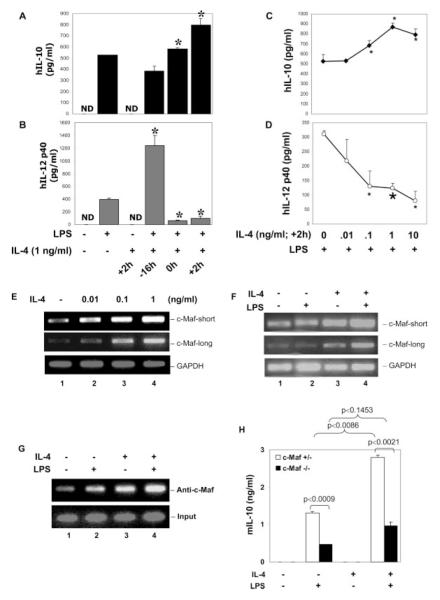
IL-4 enhances c-Maf expression and IL-10 production
Additionally, we explored the physiological relevance of this transcription regulation, i.e., under what circumstance(s) is this c-Maf-mediated transcriptional regulation invoked? It is well known that inflammatory macrophages can be deactivated by IL-4 or IL-13 and play important roles in humoral immunity, homeostasis, and tissue injury repair (40, 41). On the basis of the established role of IL-4 in transcriptional regulation of c-Maf in Th cells (42, 43), we asked the two following questions: 1) Does IL-4 influence IL-10 production in human monocytes/macrophages? 2) Does IL-4 modulate c-Maf expression in human monocytes/macrophages?
To address the first question, human monocytes were treated with LPS or LPS + IL-4 and added at different time points relative to LPS. There was a differential impact of the IL-4 exposure on LPS-induced IL-10 and IL-12 p40 production. Addition of IL-4 together or 2 h after LPS stimulation enhanced IL-10 production (Fig. 7A) while strongly inhibiting IL-12 p40 production (Fig. 7B). IL-4 added at 16 h before LPS strongly enhanced IL-12 p40 and p70 (data not shown) synthesis, which is consistent with our previous report of the priming effect of IL-4 on IL-12 production (44). Furthermore, IL-4 added at +2 h dose dependently enhanced LPS-induced IL-10 production (Fig. 7C) while strongly suppressing IL-12 p40 production (Fig. 7D). These results are consistent with the reported effects of IL-4 on endotoxin-induced IL-10 production in murine macrophages (45). To address the second question, we examined the effect of IL-4 on the regulation of c-Maf mRNA expression. Human monocytes were treated with IL-4 in different doses for 2 h, and the total RNA was isolated for RT-PCR. Fig. 7E shows that IL-4 dose dependently stimulated c-Maf expression (both short and long forms) in resting monocytes, indicating that, as with Th cells (42, 43), IL-4 regulates c-Maf transcription also in macrophages. Control experiments in the nonadherent portion of the human PBMC with the same treatment indicated that the c-Maf mRNA expression observed was derived predominantly from monocytes and not from lymphocytes (data not shown). Additionally, we characterized the effect of LPS, IL-4, and LPS + IL-4 on the c-Maf mRNA expression. Human monocytes were treated with LPS, IL-4, or LPS + IL-4 for 8 h (at this time point, IL-10 mRNA reaches its peak (data not shown)). The total RNA was isolated for RT-PCR. Fig. 7F shows the following: 1) c-Maf was expressed constitutively in resting monocytes; 2) at 8 h, LPS did not stimulate an enhancement in c-Maf mRNA expression; and 3) IL-4 treatment enhanced and IL-4 + LPS further augmented c-Maf expression. ChIP assay in human monocytes also showed that IL-4 could by itself induce endogenous c-Maf binding to the IL-10 promoter at the MARE, even to a greater extent than LPS (compare lane 3 to lane 2 of Fig. 7G), and the LPS + IL-4 combination had an additive effect (Fig. 7G, lane 4). An important point to note is that although IL-4 alone stimulates high c-Maf expression, it is insufficient to induce IL-10 production by itself, and LPS treatment is required (Fig. 7A). This observation is consistent with the data shown in Fig. 1B, where we found that c-Maf adenovirus-mediated c-Maf overexpression alone did not induce IL-10 gene expression. Only by working together with LPS can the endogenous IL-10 gene be turned on. Furthermore, to investigate the role of c-Maf in IL-4-enhanced IL-10 expression, c-Maf-null macrophages were treated with LPS, IL-4, or LPS + IL-4. As shown in Fig. 7H, LPS-induced IL-10 production was enhanced strongly by IL-4 in cells heterozygous for c-Maf ( p = 0.0086) but not in c-Maf−/− littermate-derived cells ( p = 0.1453), demonstrating that c-Maf is at least partially required for IL-4-enhancement of LPS-induced IL-10 production.
FIGURE 7.
Modulation of LPS-induced IL-10, IL-12 p40, and c-Maf expression by IL-4. A and B, Primary human monocytes were treated with LPS (1 μg/ ml) alone or recombinant human IL (hIL)-4 (1 ng/ml at +2 h) alone or LPS plus IL-4 added at different times relative to LPS. Twelve hours later, cell culture supernatants were collected for IL-10 (A) or IL-12 p40 (B) production by ELISA. *, A value of p < 0.05 compared with LPS treatment alone. C and D, Human monocytes were treated with different doses of rhIL-4 and added 2 h after LPS stimulation. Cell culture supernatants were collected 12 h after LPS stimulation for IL-10 and IL-12 p40 secretion by ELISA. *, A value of p < 0.05 and  , p < 0.01, compared with LPS treatment alone. E, Human monocytes were treated with different concentrations of IL-4 as indicated for 2 h, and total RNA was isolated for RT-PCR analysis of c-Maf expression (both short and long forms). GAPDH was analyzed as the internal control. F, Human monocytes were treated with IL-4 (1 ng/ml) or LPS (1 μg/ml) or both (IL-4 was added together with LPS) for 8 h, and total RNA was isolated for RT-PCR to examine the long- and short-form c-Maf mRNA expression. GAPDH was analyzed as the internal control. G, Human monocytes were treated with recombinant murine IL-4 (1 ng/ml) or LPS (1 μg/ml) or both (IL-4 was added at +2h) for 8 h, and ChIP assay was conducted as described in Fig. 5B. H, Fetal liver-derived macrophages from c-Maf−/− and c-Maf+/− littermate embryos of gestation day 14.5 were plated into 48-well plates at a density of 0.2 × 106 cells/ml/well. Cells were stimulated with 1 μg/ml LPS, murine IL-4 (1 ng/ml), or LPS + IL-4 (added at +2 h). Supernatants were collected 24 h after LPS treatment for ELISA. Results shown are the summary of four heterozygous and three homozygous knockout embryos. ND, nondetectable.
, p < 0.01, compared with LPS treatment alone. E, Human monocytes were treated with different concentrations of IL-4 as indicated for 2 h, and total RNA was isolated for RT-PCR analysis of c-Maf expression (both short and long forms). GAPDH was analyzed as the internal control. F, Human monocytes were treated with IL-4 (1 ng/ml) or LPS (1 μg/ml) or both (IL-4 was added together with LPS) for 8 h, and total RNA was isolated for RT-PCR to examine the long- and short-form c-Maf mRNA expression. GAPDH was analyzed as the internal control. G, Human monocytes were treated with recombinant murine IL-4 (1 ng/ml) or LPS (1 μg/ml) or both (IL-4 was added at +2h) for 8 h, and ChIP assay was conducted as described in Fig. 5B. H, Fetal liver-derived macrophages from c-Maf−/− and c-Maf+/− littermate embryos of gestation day 14.5 were plated into 48-well plates at a density of 0.2 × 106 cells/ml/well. Cells were stimulated with 1 μg/ml LPS, murine IL-4 (1 ng/ml), or LPS + IL-4 (added at +2 h). Supernatants were collected 24 h after LPS treatment for ELISA. Results shown are the summary of four heterozygous and three homozygous knockout embryos. ND, nondetectable.
Discussion
Upon LPS stimulation, the kinetics of cytokine production in macrophages is characterized by rapid induction of proinflammatory cytokines and followed by the production of anti-inflammatory cytokines, e.g., IL-10, hence, dampening the exaggeration of proinflammatory cytokine production and associated pathology. This kinetic cytokine production pattern is illustrated in the sepsis syndrome in vivo (46). IL-10, as a potent anti-inflammatory cytokine, inhibits the expression of a number of inducible genes in macrophages, including several proinflammatory cytokines (47). Despite its biological importance, relatively little is known about the IL-10 gene regulation. In the present study, we report that protooncogene c-Maf is an essential transcription factor for IL-10 gene expression in macrophages. There are four sets of experiments that point to this conclusion. First, c-Maf-null macrophages exhibit impaired IL-10 production upon LPS stimulation. Second, c-Maf up-regulates both the exogenous IL-10 promoter activity, as well as the endogenous IL-10 gene transcription stimulated by LPS. Third, a c-Maf binding site is localized in the IL-10 promoter to −196/−184, which resembles a TRE-type MARE, demonstrated by both in vitro (EMSA) and in vivo (ChIP assay) experiments. Fourth, functional studies reveal that this site is operational in the response to c-Maf induction by mutagenesis and chimeric promoter approaches. All of these results indicate that c-Maf is a nonredundant transcription factor and regulates IL-10 gene expression through physically binding to the IL-10 promoter. Transcriptional regulation is dependent on the interaction among transcription factors and cis-elements. In addition, the cis-elements are wrapped by histones and not always accessible by the transcription factors or transcription factor complexes. Therefore, modification of histones determines their state—loose or condense—and then determines the accessibility of these cis-elements and then determines the gene transcription state (histone codon theory). The observation that c-Maf is expressed constitutively in resting macrophages initially prompted us to postulate that c-Maf may not bind the MARE-like site in IL-10 promoter until later time points after LPS stimulation, which is known to induce chromatin changes (48). The ChIP analyses surprisingly revealed constitutive c-Maf binding to the MARE sequence in both the resting state and LPS-activated human macrophages (Fig. 5, B and C). There are a few possible explanations: firstly, c-Maf is constantly present and active, and LPS stimulation induces additional factors that, together with c-Maf, contribute to transcriptional activation of IL-10 in macrophages. Secondly, c-Maf may undergo posttranslational modifications. It is reported that c-Maf activity is regulated negatively by the protein kinase A-, protein kinase C-, and MAPK/ERK-signaling cascades; however, mutation of the two most likely phosphorylation sites didn’t change its transactivation ability (49). Thirdly, c-Maf can interact with transcriptional coactivators CREB-binding protein/p300, as shown in the case of αA-crystallin promoter. The coactivation of c-Maf by CREB-binding protein/p300 requires histone acetyltransferase activity for crystalline gene expression (50), and their recruitment could modulate the chromatin structure, exposing the cis-elements for access by LPS-induced transcription factors and then turn on the IL-10 gene.
Gene regulation is a complex process that involves the coordinated integration of distinct signal transduction pathways. Although c-Maf is essential for IL-10 transcription, c-Maf itself is not enough, and its ability to do so still requires LPS stimulation. This situation is analogous to many other factors that have been shown to be involved in the transcription of IL-10, e.g., CREB-1, activating transcription factor-1 (51, 52), C/EBP (53), SV40 promoter 1 (6, 54), SV40 promoter (55), and STAT3 (27). All these factors require an accompanying LPS stimulation, suggesting that multiple signal transduction pathways are involved in the activation of IL-10 expression (56). Furthermore, LPS-induced IL-10 gene transcription requires de novo protein synthesis because cycloheximide treatment before LPS stimulation can completely block IL-10 gene induction (6). Thus, we think that c-Maf works in concert with LPS-induced, yet-to-be-identified factors to activate the IL-10 gene. In support of this hypothesis, blocking AP-1 or CREB by AP-1-specific DNM (A-Fos) or CREB-DNM (57) strongly interfered with the capacity of c-Maf to activate IL-10 transcription (data not shown).
There is increasing evidence indicating that macrophages are a diverse and dynamic population of cells that can be activated through a number of distinct pathways and participate in a wide range of critical functions (41). In this study, we demonstrated that the IL-10-enhancing effect of IL-4 in LPS-activated human monocytes (Fig. 7, A and C) is similar to that shown in mouse macrophages (45). Then we investigated the modulation of c-Maf by IL-4 in human monocytes because it was reported that IL-4 could up-regulate c-Maf through Stat6 in Th cells (42). Our data demonstrates that IL-4 can regulate c-Maf expression and its binding to IL-10 promoter in human macrophages. Moreover, the characteristics of IL-4-enhanced IL-10 production (Fig. 7C) not only correlate well with its ability to stimulate c-Maf expression (Fig. 7E) in human monocytes but also show direct dependency on c-Maf (Fig. 7H). Considering that c-Maf is an essential transcription activator for IL-10 gene transcription, our data suggests that IL-4, which is derived mainly from activated Th2 cells, may exert its macrophage-regulating activity through stimulation of c-Maf. This idea is supported by the c-Maf overexpression experiment in human monocyte-derived macrophages, in which c-Maf overexpression enhanced LPS-induced IL-10 expression (Fig. 1, B and C), and by the study in c-Maf-null macrophages where rIL-4 failed to enhance LPS-induced IL-10 production (Fig. 7H).
Inflammation is a complex response, and it is an attempt to restore homeostasis (58). Macrophages are one of the main players in inflammatory immune responses. In this study, we demonstrated that c-Maf is an essential transcription factor for IL-10 production in inflammatory macrophages. This observation, together with our previous report that c-Maf is an inhibitor of IL-12 production, suggests that it may be an important factor in the homeostatic regulation. Our findings are one step further to understanding the intriguing and complex mechanisms of macrophage-gene regulation.
Acknowledgments
We thank Dr. I. Cheng Ho for providing the c-Maf−/− mice.
Footnotes
This work was supported by National Institutes of Health Grant CA100223 (to X.M.). S.C. was supported in part by a Susan G. Komen Breast Cancer Foundation Postdoctoral Fellowship.
- Maf
- musculoaponeurotic fibrosarcoma
- MARE
- Maf recognition element
- TK
- thymidine kinase
- DNM
- dominant-negative mutant
- EGFP
- enhanced green fluorescent protein
- ChIP
- chromatin immunoprecipitation
- EMSA
- electrophoretic mobility shift assay
- RPA
- RNase protection assay
- TRE
- 12-O-tetradecanoate-13-acetate-responsive element
Disclosures The authors have no financial conflict of interest.
References
- 1.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 1999;5:1249. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 2.Bialynicki-Birula II, Kalinski M, Eberly JH. Bialynicki-Birula, Kalinski, and Eberly reply. Phys. Rev. Lett. 1995;75:973. doi: 10.1103/PhysRevLett.75.973. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr., Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 4.Glauser MP, Zanetti G, Baumgartner JD, Cohen J. Septic shock: pathogenesis. Lancet. 1991;338:732. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- 5.Brandtzaeg P, Osnes L, Ovstebo R, Joo GB, Westvik AB, Kierulf P. Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of interleukin-10 as a major functional deactivator of human monocytes. J. Exp. Med. 1996;184:51. doi: 10.1084/jem.184.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brightbill HD, Plevy SE, Modlin RL, Smale ST. A prominent role for Sp1 during lipopolysaccharide-mediated induction of the IL-10 promoter in macrophages. J. Immunol. 2000;164:1940. doi: 10.4049/jimmunol.164.4.1940. [DOI] [PubMed] [Google Scholar]
- 7.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991;174:1209. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, Mantovani A. Autocrine production of IL-10 mediates defective IL-12 production and NF-κ B activation in tumor-associated macrophages. J. Immunol. 2000;164:762. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 9.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 11.Dai WJ, Kohler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J. Immunol. 1997;158:2259. [PubMed] [Google Scholar]
- 12.Powell MJ, Thompson SA, Tone Y, Waldmann H, Tone M. Posttranscriptional regulation of IL-10 gene expression through sequences in the 3′-untranslated region. J. Immunol. 2000;165:292. doi: 10.4049/jimmunol.165.1.292. [DOI] [PubMed] [Google Scholar]
- 13.Cao S, Liu J, Chesi M, Bergsagel PL, Ho IC, Donnelly RP, Ma X. Differential regulation of IL-12 and IL-10 gene expression in macrophages by the basic leucine zipper transcription factor c-Maf fibrosarcoma. J. Immunol. 2002;169:5715. doi: 10.4049/jimmunol.169.10.5715. [DOI] [PubMed] [Google Scholar]
- 14.Nishizawa M, Kataoka K, Goto N, Fujiwara KT, Kawai S. v-Maf, a viral oncogene that encodes a “leucine zipper” motif. Proc. Natl. Acad. Sci. USA. 1989;86:7711. doi: 10.1073/pnas.86.20.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho IC, Hodge MR, Rooney JW, Glimcher LH. The protooncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- 16.Kawauchi S, Takahashi S, Nakajima O, Ogino H, Morita M, Nishizawa M, Yasuda K, Yamamoto M. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J. Biol. Chem. 1999;274:19254. doi: 10.1074/jbc.274.27.19254. [DOI] [PubMed] [Google Scholar]
- 17.Kim JI, Li T, Ho IC, Grusby MJ, Glimcher LH. Requirement for the c-Maf transcription factor in crystallin gene regulation and lens development. Proc. Natl. Acad. Sci. USA. 1999;96:3781. doi: 10.1073/pnas.96.7.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuoka TA, Zhao L, Artner I, Jarrett HW, Friedman D, Means A, Stein R. Members of the large Maf transcription family regulate insulin gene transcription in islet β cells. Mol. Cell. Biol. 2003;23:6049. doi: 10.1128/MCB.23.17.6049-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hale TK, Myers C, Maitra R, Kolzau T, Nishizawa M, Braithwaite AW. Maf transcriptionally activates the mouse p53 promoter and causes a p53-dependent cell death. J. Biol. Chem. 2000;275:17991. doi: 10.1074/jbc.M000921200. [DOI] [PubMed] [Google Scholar]
- 20.Kurschner C, Morgan JI. The maf proto-oncogene stimulates transcription from multiple sites in a promoter that directs Purkinje neuron-specific gene expression. Mol. Cell. Biol. 1995;15:246. doi: 10.1128/mcb.15.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blank V, Andrews NC. The Maf transcription factors: regulators of differentiation. Trends Biochem. Sci. 1997;22:437. doi: 10.1016/s0968-0004(97)01105-5. [DOI] [PubMed] [Google Scholar]
- 22.Valanciute A, le Gouvello S, Solhonne B, Pawlak A, Grimbert P, Lyonnet L, Hue S, Lang P, Remy P, Salomon R, et al. NF-κ B p65 antagonizes IL-4 induction by c-maf in minimal change nephrotic syndrome. J. Immunol. 2004;172:688. doi: 10.4049/jimmunol.172.1.688. [DOI] [PubMed] [Google Scholar]
- 23.Chesi M, Bergsagel PL, Shonukan OO, Martelli ML, Brents LA, Chen T, Schrock E, Ried T, Kuehl WM. Frequent dysregulation of the c-maf protooncogene at 16q23 by translocation to an Ig locus in multiple myeloma. Blood. 1998;91:4457. [PubMed] [Google Scholar]
- 24.Ring BZ, Cordes SP, Overbeek PA, Barsh GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development. 2000;127:307. doi: 10.1242/dev.127.2.307. [DOI] [PubMed] [Google Scholar]
- 25.Kim JI, Ho IC, Grusby MJ, Glimcher LH. The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity. 1999;10:745. doi: 10.1016/s1074-7613(00)80073-4. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Cao S, Herman LM, Ma X. Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-γ-primed IL-12 production by IFN regulatory factor 1. J. Exp. Med. 2003;198:1265. doi: 10.1084/jem.20030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benkhart EM, Siedlar M, Wedel A, Werner T, Ziegler-Heitbrock HW. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J. Immunol. 2000;165:1612. doi: 10.4049/jimmunol.165.3.1612. [DOI] [PubMed] [Google Scholar]
- 28.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 29.Platzer C, Meisel C, Vogt K, Platzer M, Volk HD. Up-regulation of monocytic IL-10 by tumor necrosis factor-α and cAMP elevating drugs. Int. Immunol. 1995;7:517. doi: 10.1093/intimm/7.4.517. [DOI] [PubMed] [Google Scholar]
- 30.McKnight SL, Gavis ER, Kingsbury R, Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981;25:385. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- 31.Kataoka K, Shioda S, Yoshitomo-Nakagawa K, Handa H, Nishizawa M. Maf and Jun nuclear oncoproteins share downstream target genes for inducing cell transformation. J. Biol. Chem. 2001;276:36849. doi: 10.1074/jbc.M102234200. [DOI] [PubMed] [Google Scholar]
- 32.Kataoka K, Nishizawa M, Kawai S. Structure-function analysis of the maf oncogene product, a member of the b-Zip protein family. J. Virol. 1993;67:2133. doi: 10.1128/jvi.67.4.2133-2141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ’mini-extracts,’ prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma X, Neurath M, Gri G, Trinchieri G. Identification and characterization of a novel Ets-2-related nuclear complex implicated in the activation of the human interleukin-12 p40 gene promoter. J. Biol. Chem. 1997;272:10389. doi: 10.1074/jbc.272.16.10389. [DOI] [PubMed] [Google Scholar]
- 35.Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23:4878. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J. Immunol. 1998;160:5936. [PubMed] [Google Scholar]
- 37.Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF, Dzialo R, Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon γ in monocytic cells. J. Exp. Med. 1996;183:147. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kataoka K, Noda M, Nishizawa M. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol. Cell. Biol. 1994;14:700. doi: 10.1128/mcb.14.1.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parekh BS, Maniatis T. Virus infection leads to localized hyperacetylation of histones H3 and H4 at the IFN-β promoter. Mol. Cell. 1999;3:125. doi: 10.1016/s1097-2765(00)80181-1. [DOI] [PubMed] [Google Scholar]
- 40.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin. Sci. 2003;104:27. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 41.Mosser DM. The many faces of macrophage activation. J. Leukocyte Biol. 2003;73:209. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 42.Kurata H, Lee HJ, O’Garra A, Arai N. Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity. 1999;11:677. doi: 10.1016/s1074-7613(00)80142-9. [DOI] [PubMed] [Google Scholar]
- 43.Nurieva RI, Duong J, Kishikawa H, Dianzani U, Rojo JM, Ho I, Flavell RA, Dong C. Transcriptional regulation of th2 differentiation by inducible costimulator. Immunity. 2003;18:801. doi: 10.1016/s1074-7613(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 44.D’Andrea A, Ma X, Aste-Amezaga M, Paganin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor α production. J. Exp. Med. 1995;181:537. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kambayashi T, Jacob CO, Strassmann G. IL-4 and IL-13 modulate IL-10 release in endotoxin-stimulated murine peritoneal mononuclear phagocytes. Cell. Immunol. 1996;171:153. doi: 10.1006/cimm.1996.0186. [DOI] [PubMed] [Google Scholar]
- 46.Oberholzer A, Oberholzer C, Moldawer LL. Interleukin-10: a complex role in the pathogenesis of sepsis syndromes and its potential as an antiinflammatory drug. Crit. Care Med. 2002;30:S58. [PubMed] [Google Scholar]
- 47.Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J. Interferon Cytokine Res. 1999;19:563. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- 48.Saccani S, Pantano S, Natoli G. p38-dependent marking of inflammatory genes for increased NF-κ B recruitment. Nat. Immunol. 2002;3:69. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 49.Civil A, van Genesen ST, Lubsen NH. c-Maf, the γD-crystallin Maf-responsive element and growth factor regulation. Nucleic Acids Res. 2002;30:975. doi: 10.1093/nar/30.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Q, Dowhan DH, Liang D, Moore DD, Overbeek PA. CREB-binding protein/p300 co-activation of crystallin gene expression. J. Biol. Chem. 2002;277:24081. doi: 10.1074/jbc.M201821200. [DOI] [PubMed] [Google Scholar]
- 51.Platzer C, Fritsch E, Elsner T, Lehmann MH, Volk HD, Prosch S. Cyclic adenosine monophosphate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur. J. Immunol. 1999;29:3098. doi: 10.1002/(SICI)1521-4141(199910)29:10<3098::AID-IMMU3098>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 52.Platzer C, Docke W, Volk H, Prosch S. Catecholamines trigger IL-10 release in acute systemic stress reaction by direct stimulation of its promoter/enhancer activity in monocytic cells. J. Neuroimmunol. 2000;105:31. doi: 10.1016/s0165-5728(00)00205-8. [DOI] [PubMed] [Google Scholar]
- 53.Brenner S, Prosch S, Schenke-Layland K, Riese U, Gausmann U, Platzer C. cAMP-induced interleukin-10 promoter activation depends on CCAAT/enhancer-binding protein expression and monocytic differentiation. J. Biol. Chem. 2003;278:5597. doi: 10.1074/jbc.M207448200. [DOI] [PubMed] [Google Scholar]
- 54.Ma W, Lim W, Gee K, Aucoin S, Nandan D, Kozlowski M, Diaz-Mitoma F, Kumar A. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J. Biol. Chem. 2001;276:13664. doi: 10.1074/jbc.M011157200. [DOI] [PubMed] [Google Scholar]
- 55.Tone M, Powell MJ, Tone Y, Thompson SA, Waldmann H. IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. J. Immunol. 2000;165:286. doi: 10.4049/jimmunol.165.1.286. [DOI] [PubMed] [Google Scholar]
- 56.Meisel C, Vogt K, Platzer C, Randow F, Liebenthal C, Volk HD. Differential regulation of monocytic tumor necrosis factor-α and interleukin-10 expression. Eur. J. Immunol. 1996;26:1580. doi: 10.1002/eji.1830260726. [DOI] [PubMed] [Google Scholar]
- 57.Olive M, Krylov D, Echlin DR, Gardner K, Taparowsky E, Vinson C. A dominant negative to activation protein-1 (AP1) that abolishes DNA binding and inhibits oncogenesis. J. Biol. Chem. 1997;272:18586. doi: 10.1074/jbc.272.30.18586. [DOI] [PubMed] [Google Scholar]
- 58.Muller WA. Leukocyte-endothelial cell interactions in the inflammatory response. Lab. Invest. 2002;82:521. doi: 10.1038/labinvest.3780446. [DOI] [PubMed] [Google Scholar]