Abstract
Dioscorea nipponica and the preparations made from it have been used for long to prevent and treat coronary heart disease in traditional Chinese medicine. A group of steroidal saponins present in the plant are believed to be the active ingredients. It has been a challenge to study the individual saponins separately due to the similarities in their chemical and physical properties. In this work, human serum albumin (HSA) functionalized magnetic nanoparticles (MNPs) were used to isolate and identify saponin ligands that bind to HSA from D. nipponica extract. Electrospray ionization mass spectrometry (ESI-MS) was used for compound identification and semi-quantification. Three saponins, i.e. dioscin, gracillin, and pseudo-protodioscin showed affinity to HSA-MNPs and thus isolated effectively from the extract. The other two saponins detected in the extract (i.e. protodioscin and 26-O-β-D-glucopyranosyl-3β, 20α, 26-triol-25 (R) -Δ5, 22-dienofurostan-3-O-α-L-rhamnopyranosyl (1→2)-[α-L-rhamnopyranosyl (1→4)]-β-D-glucopyranoside) exhibited no affinity at all. Among the three saponins fished out, dioscin bound to HSA much stronger than gracillin and pseudo-protodioscin did. The results indicated that affinity interaction between HSA immobilized on MNPs and small molecule compounds were highly dependent on chemical structures and, potentially, medicinal usefulness. The present work demonstrates a facile and effective way to isolate and identify ligands of receptors from medicinal plants.
Keywords: Ligand fishing, Magnetic nanoparticles, Human serum album, Dioscorea nipponica, Saponins, Dioscin
1. Introduction
Most ligands (e.g. therapeutic agents and neurotransmitters) bind to protein receptors. Receptor theory is one of the basic theories in pharmacodynamics that explains drug action, its mechanism, as well as the relationship between drug action and its molecular structure [1, 2]. Ligand fishing is a method based on the receptor theory to screen biological complex matrices as potential sources for ligands of known or orphan receptors. It has been proven useful for discovering bioactive components from botanical extracts [3 - 6]. Ligand fishing experiments have been carried out by multiple methods including SPR [7, 8], circular dichroism, photochemical fishing [9], and 2D and 3D molecular descriptors [10]. Magnetic nanoparticles (MNPs) are widely used in biological and chemical sciences due to its excellent suspension stability, easy surface modification, and convenient solid-liquid separation. MNP-based ligand fishing has been predominantly applied for protein purification purposes so far. For example, MNPs coated with protein A or protein G were used to isolate proteins expressed in cellular extracts [11 - 13]. Although a few concept-proof studies were carried out on ligand fishing of small molecules [14 - 18], the use of MNP-based ligand fishing on the isolation and identification of bioactive compounds from botanical extracts has not been reported to the best of our knowledge. Drug discovery and drug development are increasingly focused on the identification of unknown interaction partners /complexes from cellular and /or botanical extracts to known targets. High throughput screening (HTS) of drugs based on the use of receptors becomes a conventional method for new drug search, and has been very successful in developing a series of drugs with good efficacies and low side effects. However, HTS is designed to screen pure compounds, which is not suitable for detecting multiple ligands from complex systems.
Albumins are widely used as model proteins in biophysical and physicochemical studies. Human serum albumin (HSA), the most abundant protein in blood plasma, plays a major role in the transport and deposition of endogenous and exogenous ligands [19]. It has typical sites of coordination for different categories of compounds including amino acids, fatty acids, hormones, and drugs [20]. The multiple binding sites warrant HSA's exceptional ability to interact with many substances and make this protein an important regulator of intercellular fluxes and pharmacokinetic behavior of many drugs. The three-dimensional structure of HSA has been determined through X-ray crystallographic measurements [21]. Since the affinity towards HSA influences the overall distribution, metabolism, and efficacy of ligands, the investigation of their binding to HSA is of fundamental significance.
Dioscorea nipponica is a medicinal plant used to treat cardiodynia in traditional Chinese medicine. The extract of D. nipponica is used to prepare “Di-Ao Xin-Xue-Kang” capsule by Chengdu Di'ao Pharmaceutical group [22, 23], which is a popular botanical medicine in China for prevention and treatment of coronary heart disease (CHD). Previous studies showed that the steroid saponins in this plant as a whole had cholesterol-lowering effects and contributed significantly to the bioactivities of “Di-Ao Xin-Xue-Kang” medicine [24, 25]. However, study on the activities of the individual saponins has been inadequate. One of the major difficulties is to obtain large quantities of individual saponins because they are very similar to each other in terms of chemical structure. In this work, we demonstrate for the first time that ligand fishing, based on biological macromolecule functionalized MNPs, offers an effective and convenient way to identify and isolate bioactive small molecules from botanical extracts.
2. Experimental
2.1 Chemicals and reagents
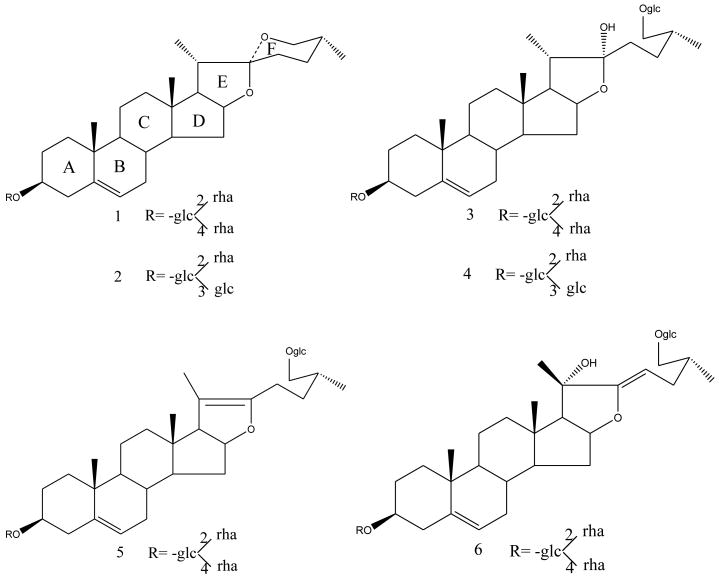
The rhizomes of D. nipponica were obtained from Sichuan province, China. Human serum albumin (HSA) and 25% glutaraldehyde solution (GD) were purchased from Sigma–Aldrich (MO, USA). Ginsenoside Rb1 (internal standard) was from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Tetraethyl orthosilicate (TEOS) and 3-aminopropyltrimethoxysilane (APTMS) were from TCI (Tokyo, Japan). Six authentic saponins: dioscin (compound 1), gracillin (compound 2), protodioscin (compound 3), protogracillin (compound 4), pseudo-protodioscin (compound 5), and 26-O-β-D-glucopyranosyl-3β, 20α, 26-triol-25 (R) - Δ5, 22-dienofurostan-3-O-α-L -rhamnopyranosyl (1→2)- [α-L-rhamnopyranosyl (1→4)]-β-D-glucopyranoside (compound 6) were prepared from D. nipponica in our laboratory and their structures (Fig. 1) were elucidated by MS, 1H NMR and 13C NMR. The purity of each compound was determined to be > 98% by HPLC–ELSD. HPLC grade acetonitrile (Fisher, USA) and purified water from a Milli-Q water system (Millipore Corp., Bedford, MA, USA) were used for sample preparation and analysis. Other chemicals and solvents were of analytical reagent grade.
Fig.1.
Chemical structures of the test compounds: (1) dioscin; (2) gracillin; (3) protodioscin; (4) protogracillin; (5) pseudo-protodioscin; and (6) 26-O-β-D-glucopyranosyl-3β, 20α, 26-triol-25 (R) - Δ5, 22-dienofurostan-3-O-α-L -rhamnopyranosyl (1→2)-[α-L-rhamnopyranosyl (1→4)]-β-D-glucopyranoside.
2.2 Mass spectrometry
ThermoQuest Finnigan LCQDECA system equipped with an electrospray ionization source (ThermoQuest LC/MS Division, San Jose, CA, USA) was used for mass spectrometric analysis. The operating conditions were optimized in negative mode as following: sheath gas flow rate, 35 units; auxiliary gas flow rate, 0 units; capillary temperature, 250 °C; capillary voltage, 4.5 kV. Samples were introduced into the ESI source by continuous infusion at a flow-rate of 5 μL/min using a syringe pump.
2.3 Preparation of HSA-MNPs
HSA functionalized MNPs were prepared following the procedures reported previously [14, 26, 27]. Briefly, Fe3O4 nanoparticles (MNPs) were prepared by co-precipitation of Fe2+ and Fe3+ with ammonia water under vigorous stirring. The MNPs were then coated using TEOS. SiO2 shelled MNPs were treated with APTMS to obtain -NH2 group terminated MNPs. HSA -MNP coupling was achieved by means of glutaraldehyde reaction. The HSA-MNPs prepared (∼20 nm in diameter) were characterized by transmission electron microscopy (TEM). HSA-MNPs were suspended in NH4Ac solution and kept at 4 °C before use.
2.4 Preparation of D. nipponica Extract [28]
Dried rhizomes of D. nipponica (100 g) were sliced and then boiled in 1.2 L water for 2 hours. The extraction was repeated for three times. The liquid phases were combined and passed through a macroporous resin column. The 75% ethanol fraction was collected and concentrated. The residue was re-dissolved in 80% ethanol and the solution was filtrated. The filtrate was concentrated and subjected to spray drying to obtain the extract that contains total steroidal saponins of D. nipponica.
2.5 Ligand Fishing
2.5.1 Ligand fishing of D. nipponica extract
A 1.3 mg/mL solution of the extract (S0) was prepared in ammonium acetate buffer solution (10 mM/L, pH 7.4). Portions of S0 (1 mL) and HSA-MNPs suspension (100 μL) were transferred to a 4-mL eppendorf tube. The tube was vigorously shaken for 30 min on a vortex oscillator, and then put on a magnet for 5 min to achieve a liquid-solid separation. The supernatant (S1) was carefully transferred to a test tube and saved. The HSA-MNPs was washed three times (using 1 mL buffer each time) with vigorously shaking for 2 min. After liquid-solid separation, the supernatants were carefully collected and saved as solutions S2, S3 and S4, respectively. The fourth wash of the HSA-MNPs was carried out with 1 mL buffer containing 50% ACN for 2 min. The supernatant was collected and saved as solution S5. In order to monitor the change of the components binding to HSA- MNPs, all the solutions were analyzed by mass spectrometry.
2.5.2 Ligand fishing of the known compounds isolated from D. nipponica
A standard mixture solution (M0) was prepared to contain dioscin (1) at 4.6 μmol/L, gracillin (2) at 5.4 μmol/L, protodioscin (3) at 5.3 μmol/L, and protogracillin (4) 4.9 μmol/L. Portions of 1 mL M0 and 100 μL HSA-MNPs suspension were transferred to a 4-mL eppendorf tube. Similarly, M1, M2, M3, M4, and M5 solutions were obtained as described above.
2.6 Preparation of standard mixture solutions for semi-quantitative analysis by ESI-MS
Ginsenoside Rb1 was added as the internal standard into eleven standard solutions to investigate the change in quantities of dioscin (1) and pseudo-protodioscin (5). Three authentic compounds (i.e. dioscin (1) 100 μg/mL, pseudo- protodioscin (5) 100 μg/mL, and ginsenoside Rb1 350 μg/mL) were mixed to obtain eleven solutions with the concentration ratio of dioscin to pseudo- protodioscin ranging from 8:1 to 1:128 while keeping ginsenoside Rb1 concentration constant at 116.7 μg/mL as shown in table 1.
Table.1.
Compositions of the standard mixtures of dioscin and pseudo-protodioscin for semi-quantitative ESI-MS analysis
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dioscin (mL) |
400 | 400 | 400 | 400 | 200 | 100 | 50 | 25 | 12.5 | 6.25 | 3.125 |
|
pseudo-Protodioscin (mL) |
50 | 100 | 200 | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 400 |
| Ginsenoside Rb1 (mL) |
450 | 450 | 450 | 450 | 450 | 450 | 450 | 450 | 450 | 450 | 450 |
| Methanol (mL) |
450 | 400 | 300 | 100 | 300 | 400 | 450 | 475 | 487.5 | 493.75 | 496.875 |
| Total (mL) |
1350 | ||||||||||
3. Results and discussions
3.1. Detection of saponins in D. nipponica extract by ESI-MS
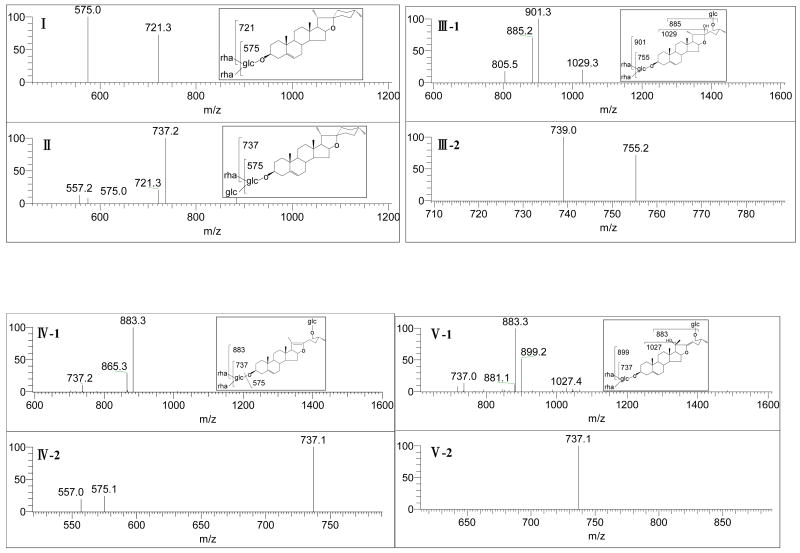
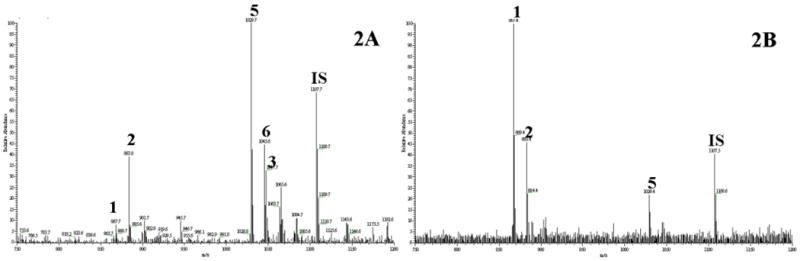
Steroidal saponins in D. nipponica contribute to its bioactivity for the prevention and treatment of CHD [29]. In our previous phytochemical study on its chemical constituents, thirteen steroidal saponins belonging to two categories, namely isospirostanols type and furostanols type, were isolated by using a lengthy chromatographic procedure [30]. In this work, the extract was analyzed by using ESI-MS. Five saponins were detected. As shown in Fig. 2A, five peaks in the TIC spectrum could be assigned as following: m/z 867 [M-H]- to dioscin (1), m/z 883 [M-H]- to gracillin (2), m/z 1047 [M-H]- protodioscin (3), m/z 1029 [M-H]- to pseudo-protodioscin (5), and m/z 1045 [M-H]- to compound 6. The peak identifications were confirmed by MSn as shown in Fig. 3.
Fig. 2.
ESI-MS analysis of D. nipponica extract (A) and the 50% ACN eluent from HAS-MNPs (B). Peak identifications: 1, dioscin; 2, gracillin; 3, protodioscin; 5, pseudo-protodioscin; and 6, 26-O-β-D-glucopyranosyl-3β, 20α, 26-triol-25 (R) - Δ5, 22-dienofurostan-3-O-α-L -rhamnopyranosyl (1→2)-[α-L-rhamnopyranosyl (1→4)]- β-D-glucopyranoside).
Fig. 3.
Structure identification by MSn for compounds 1, 2, 3, 5 and 6: (I) MS/MS spectrum of m/z 867 of compound 1; (II) MS/MS spectrum of m/z 883 of compound 2; (III-1): MS/MS spectrum of m/z 1047 of compound 3; (III-2): MS/MS/MS spectrum of m/z 901 for compound 3; (IV-1): MS/MS spectrum of m/z 1029 of compound 5; (IV-2): MS/MS/MS spectrum of m/z 883 for compound 5; (V-1): MS/MS spectrum of m/z 1045 of compound 6; and (V-2): MS/MS/MS spectrum of m/z 883 for compound 6.
3.2. Ligand fishing from D. nipponica extract with HSA functionalized MNPs
Because the chemical structures of saponins in D. nipponica are very similar as shown in Fig.1, separating them from each other has been a challenge that limits the study on the medicinal benefits of individual saponins. MNP-based ligand fishing may serve as an effective and convenient procedure for the separation. Its high selectivity is based on the receptor theory, and the liquid-solid phase separation involved can be carried out easily. In this work, HSA functionalized MNPs were used to identify ligands from D. nipponica extract. As can be seen in Fig. 2B, three saponins, i.e. dioscin, gracillin, and pseudo-protogracillin, were fished out. That is, they bound to HSA immobilized on MNPs. Interestingly, the other two saponins detected in the extract, i.e. protodioscin and compound 6 showed no affinity to HSA at all. It can be seen by comparing Fig. 2A with Fig. 2B that dioscin (1) was the predominant ligand fished out. It's worth noting that pseudo-protodioscin was the major saponin detected in the extract before ligand fishing. These results indicated that dioscin bound to HSA much stronger than pseudo-protodioscin did.
3.3. Semi-quantitative studies on the binding of saponins to HSA-MNPs
To further investigate the binding of saponins to HSA immobilized on MNPs, a semi-quantitative study was conducted. Ginsenoside Rb1 was used as the internal standard. The semi-quantification was carried out by means of signal ratio of analyte to internal standard. Although no chromatographic separation was performed here, under the selected MS/MS quantification conditions no interference from the coexisting components in D. nipponica extract was observed. Our previous systematic phytochemical [30-31] and LC/MS/MS [32] studies on the components of D. nipponica showed that no isomers of the saponins fished out in this work existed in the extract. Thus, an eleven-point calibration curve was prepared with authentic standards at concentration ratios ranging from 8 : 1 to 1:128 while keeping ginsenoside Rb1 concentration constant at 116.7 μg/mL as shown in table 1. Peak heights were used for the quantification. Linear regression analysis of the results yielded the following equations for the ratio of dioscin (1) to pseudo-protodioscin (5): Y = 8.4247 X + 0.0157, r2 = 0.998, where Y was the peak height ratio of dioscin / ginsenoside Rb1 to pseudo-protodioscin /ginsenoside Rb1, X was the concentration ratio of dioscin to pseudo-protodioscin, and r2 was the correlation coefficient.
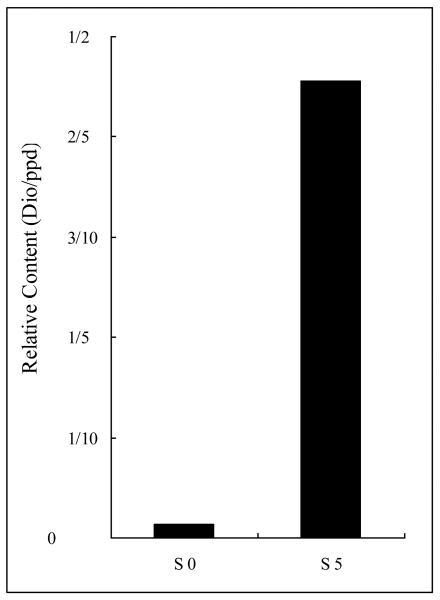
Concentration ratio of dioscin (1) to pseudo-protodioscin (5) was determined semi-quantitatively for solutions S0 and S5. The ratio of dioscin (1) to pseudo-protodioscin (5) was found to be 1:75 in S0 that changed to 5:11 in S5 (shown in Fig. 4), increasing 34 times. The result suggests that both dioscin (1) and pseudo-protodioscin (5) bind to HSA. However, the binding constants differ by 34 times. Dioscin (1) binds much stronger than pseudo-protodioscin (5). Considering the differences in the chemical structures of these two compounds (Fig. 1), we see that dioscin is a saponin with F ring closed while pseudo-protodioscin is a saponin with F ring opened.
Fig.4.
Concentration ratios of dioscin (1) to pseudo-protodioscin (5) in solutions S0 and S5. The results indicated that dioscin bound to HSA about 34 times stronger than pseudo-protodioscin did.
The above results led us to hypothesize that isospirostanol type steroidal saponins with F ring closed have a greater affinity to HSA than furostanol type steroidal saponins with F ring opened. To test this hypothesis, another set of ligand fishing experiments were carried out. A standard mixture solution (M0) containing two isospirostanol type steroidal saponins (i.e. dioscin (1) at 4.6 μmol/L and gracillin (2) at 5.4 μmol/L) and two furostanol typesteroidal saponins (i.e. protodioscin (3) at 5.3 μmol/L and progracillin (4) at 4.9 μmol/L) was prepared. The solution was extracted with HSA-MNPs as described above, obtaining solutions M1 ∼ M5. These solutions were analyzed by the ESI-MS semi-quantitative method. It was found that dioscin (1) and gracillin (2) bound to HSA-MNPs and thus were fished out, but protodioscin (3) and progracillin (4) didn't (shown in Fig.5). These results indicate that the affinity interaction between HSA-MNPs and different compounds of similar structure vary significantly and the ligand fishing method has specific structural characteristics. From the pharmacological point of view, whether F ring is closed or not may determine the biological activities of these compounds. Further study in this direction is under way.
Fig. 5.
ESI-MS analysis of the standard mixture of saponins (M0) and the resultant ligand fishing solution (M5). Peak identifications: 1, dioscin; 2, gracillin; 3, protodioscin; and 4, protogracillin. All of the four saponins in the mixture were detected before ligand fishing (M0), but only the two saponins with F ring closed (dioscin and gracillin) bound to HSA-MNPs and were fished out (M5).
4. Conclusions
Saponin ligands that bind to HSA were successfully isolated and identified from D. nipponica extract using HSA functionalized MNPs. Five saponins were detected in D. nipponica extract by using ESI-MS. Three of them, i.e. dioscin, gracillin, and pseudo-protogracillin were identified as saponin ligands of HSA. The other two saponins present in the extract (i.e. protodioscin and compound 6) showed no affinity at all. Further study confirmed that saponins with F ring closed bound to HSA much stronger than those with F ring opened. Dioscin was the saponin in D. nipponica extract that bound to HSA most strongly. Previous studies showed that dioscin had protective effects against hypoxia injury in cardiomyocytes [33] and gracillin had blocking effects on calcium channel of cardiomyocytes [34]. The present study demonstrates for the first time that ligand fishing based on biological macromolecule functionalized MNPs is an effective and convenient way to identify and isolate bioactive small molecules from botanical extracts, and the process has significant structure-specificity.
Acknowledgments
Financial support from National Natural Science Foundation of China (20872137/B020402 to XL) and US National Institutes of Health (SC1GM089557 to YML) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brenner GM, Stevens CW. Pharmacology. 2nd. Elsevier; Philadelphia: 2006. [Google Scholar]
- 2.Hardman JG, Limbird LE. Alfred Goodman Gilman, Goodman & Gilman's the pharmacological Basis Therapeutics. 11st. McGraw-Hil; New York: 2001. [Google Scholar]
- 3.Sugo T, Tachimoto H, Chikatsu T, Murakami Y, Kikukawa Y, Sato S, et al. Biochem Biophys Res Commun. 2006;341:1078. doi: 10.1016/j.bbrc.2006.01.069. [DOI] [PubMed] [Google Scholar]
- 4.Fermas S, Gonnet F, Varenne A, Gareil P, Daniel R. Anal Chem. 2007;79:4987. doi: 10.1021/ac070146h. [DOI] [PubMed] [Google Scholar]
- 5.Borch J, Roepstorff P. Anal Chem. 2004;76:5243. doi: 10.1021/ac049335f. [DOI] [PubMed] [Google Scholar]
- 6.Zou HF, Zhang QC, Guo Z, Guo BC, Zhang Q, Chen XM. Angew Chem Int Ed. 2002;41:646. [Google Scholar]
- 7.Zhukov A, Schürenberg M, Jansson Ö, Areskoug D, Buijs J. J Biomol Tech. 2004;15:112. [PMC free article] [PubMed] [Google Scholar]
- 8.Catimel B, Weinstock J, Nerrie M, Domagala T, Nice EC. J Chromatogr A. 2000;869:261. doi: 10.1016/s0021-9673(99)01098-5. [DOI] [PubMed] [Google Scholar]
- 9.Sadakane Y, Hatanaka Y. Anal Sci. 2006;22:209. doi: 10.2116/analsci.22.209. [DOI] [PubMed] [Google Scholar]
- 10.Nettles JH, Jenkins JL, Bender A, Deng Z, Davies JW, Glick MJ. Med Chem. 2006;49:6802. doi: 10.1021/jm060902w. [DOI] [PubMed] [Google Scholar]
- 11.Heddini A, Treutiger CJ, Wahlgren M. Am J Trop Med Hyg. 1998;59:663. doi: 10.4269/ajtmh.1998.59.663. [DOI] [PubMed] [Google Scholar]
- 12.Widjojoatmodjo MN, Fluit AC, Torensma R, Verhoef J Immunol Methods. 1993;165:11. doi: 10.1016/0022-1759(93)90101-c. [DOI] [PubMed] [Google Scholar]
- 13.Ljungquist C, Lunderberg J, Rasmussen AM, Hornes E, Uhlen M. DNA Cell Biol. 1993;12:191. doi: 10.1089/dna.1993.12.191. [DOI] [PubMed] [Google Scholar]
- 14.Moaddel R, Marsza MP, Bighi F, Yang Q, Duan X, Wainer IW. Anal Chem. 2007;79:5414. doi: 10.1021/ac070268+. [DOI] [PubMed] [Google Scholar]
- 15.Lin PC, Tseng MC, Su AK, Chen YJ, Lin CC. Anal Chem. 2007;79:3401. doi: 10.1021/ac070195u. [DOI] [PubMed] [Google Scholar]
- 16.Moaddel R, Kole S, Gandhari M, Bernier M, Wainer IW. Anal Chem. 2008;80:7571. doi: 10.1021/ac801153h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonker N, Kretschmer A, Kool J, Fernandez A, Kloos D, Krabbe JG, Lingeman H, Irth H. Anal Chem. 2009;81:4263. doi: 10.1021/ac9000755. [DOI] [PubMed] [Google Scholar]
- 18.Kelly MA, McLellan TJ, Rosner PJ. Anal Chem. 2002;74:1. doi: 10.1021/ac010569y. [DOI] [PubMed] [Google Scholar]
- 19.Yamasaki K, Maruyama T, Kragh-Hansen U, Otagiri M. Biochim Biophys Acta. 1996;1295:147. doi: 10.1016/0167-4838(96)00013-1. [DOI] [PubMed] [Google Scholar]
- 20.Carter D, Ho JX. Adv Protein Chem. 1994;45:153. doi: 10.1016/s0065-3233(08)60640-3. [DOI] [PubMed] [Google Scholar]
- 21.He XM, Carter DC. Nature. 1992;358:209. doi: 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
- 22.Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China (2005 Ed) People's Medical Publishing House; Beijing: 2005. p. 262. [Google Scholar]
- 23.The People's Republic of China Ministry of Health. Web site. http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohywzc/s3580/200908/42506.htm.
- 24.Sokolova LN, Turova AD, Shreter AI. Rast Resus. 1968;4:43. [Google Scholar]
- 25.Zhang KY, Chang TH, Li BJ. J Chi Medi Unive. 1982;11:10. [Google Scholar]
- 26.Liu B, Wang DP, Huang WH, Yu MJ, Yao AH. Mater Res Bull. 2008;43:2904. [Google Scholar]
- 27.Miriam C, Silvia R, Diego M, Fabio C, Emilio T, Davide P. Anal Biochem. 2009;392:96. [Google Scholar]
- 28.Pharmaceutical composition containing steroidal saponins, the preparation method and use thereof. US patent: US20070254847 A1. Liu Z, Qi W, Fu T, Zou W, Ji Y, Li B, Huang Y.
- 29.Li BG, Zhou Z. New Drugs Clin Remed. 1994;13:75. [Google Scholar]
- 30.Chen HC. Master's thesis. Chengdu Institute of Biology, Chinese Academy of sciences; 2003. Studies on Chemical Constituents of Three Medical Plants. [Google Scholar]
- 31.Chen HC, Fu TJ, Liu ZR, Liao X, Ding LS. Acta Chim Sin. 2005;63:869. [Google Scholar]
- 32.Li R, Zhou Y, Wu ZJ, Ding LS. J Mass Spectrom. 2006;41:1. doi: 10.1002/jms.988. [DOI] [PubMed] [Google Scholar]
- 33.Ni L, X P, Wu XS, Chen F. Shanghai J Trad Chin Med. 2007;41:76. [Google Scholar]
- 34.Wang HY, Yu BY, Yu B, Hui YZ. Chin J Nat Med. 2003;1:41. [Google Scholar]