Abstract
Our objective was to assess the effects of repeated antenatal corticosteroid treatments on the neonatal auditory brainstem response (ABR), a sensitive measure of neonatal brain maturity and auditory function. To achieve this, we performed and blindly evaluated neonatal ABRs on a subset of infants delivering within a multicenter randomized placebo-controlled clinical trial comparing single versus repeated courses of antenatal corticosteroid treatments for women at 23–31 weeks gestation who remained at increased risk for preterm birth. The women were randomly assigned to either the single or the repeated antenatal corticosteroid treatment group. Women in the repeated antenatal corticosteroid group received weekly antenatal corticosteroid treatments until 34 weeks gestation or until they reached a study-determined limited number of courses, whereas women in the single antenatal corticosteroid group received an initial course of corticosteroid followed by weekly placebo injections. We performed ABR testing on their infants prior to discharge. The latencies of waves I, III and V and the peak-to-trough amplitudes of waves I and V were compared between those in the single (n=27) and repeated antenatal corticosteroid treatment (n=24) groups. The majority of repeated antenatal corticosteroid infants (20 of 24) were exposed to ≥4 antenatal corticosteroid treatments. Even though gestational age was similar between our subset of single and repeated antenatal corticosteroid treatment groups, infant birth weight and length and head circumference were significantly smaller in the repeated antenatal corticosteroid group (p<0.05). Despite these differences in birth sizes, there were no significant group differences in the ABR wave latencies or amplitudes. We concluded that our repeated antenatal corticosteroid treatments, in comparison to a single treatment, did not significantly benefit or harm the neonatal ABR despite significant effects on birth size.
Keywords: Antenatal corticosteroid (AC), Auditory brainstem response (ABR), Betamethasone, Brain development, Hearing, Premature birth, Premature infant, Prenatal glucocorticoid
1. Introduction
Antenatal corticosteroid (AC) therapy reduces the incidence of neonatal mortality, respiratory distress syndrome, intra-ventricular hemorrhage, and necrotizing enterocolitis [10,26]. Consequently, a National Institute of Child Health and Human Development (NICHD) consensus report endorsed the use of AC for fetal lung maturation but emphasized the need to study its effects on other organ systems [1]. Even though a single AC treatment improves neonatal outcome, the benefits of treatment may diminish after one week [1]. Thus, the practice of weekly treatment has emerged.
Repeat dosing for human pregnancies remaining in utero more than 7 days after the initial treatment reduces acute neonatal respiratory morbidities but can reduce fetal growth [12,15,39]. Repeated AC also decreases human placental growth [32], may increase the incidence of cerebral palsy [38] and may cause attention problems [11]; although not all studies have found these effects [14,24,37]. Animal studies of repeated AC treatment have found impaired brain myelination and neurologic development [8,13,29,30], reduced fetal brain [17,20,22] and birth weights [18,19], altered postnatal cortisol and thyroid levels [18] and altered neural growth [4,33].
Thus, there is uncertainty concerning the management for patients who remain pregnant for more than one week after an initial course of corticosteroid therapy. To address this issue, the NICHD Maternal Fetal Medicine Units (MFMU) Network performed a randomized clinical trial in women at increased risk for preterm delivery to test the hypothesis that when compared with a single course, repeated weekly courses of AC would improve neonatal outcome without causing harm. The clinical trial found an improvement in some neonatal pulmonary outcomes, but no difference in overall outcomes and a reduction in birth weight [39].
To further evaluate the impact of repeated corticosteroid exposure to the fetus, we assessed neonatal brain maturation by using the ABR in infants exposed to single versus repeated courses of AC. The ABR is a sensory evoked potential extracted from the electroencephalogram. The ABR provides an objective measure of neural transmission times along the peripheral and brainstem portions of the auditory pathway [27]. As a measure of brain maturation, increasing maturity is associated with decreasing (faster) wave latencies (neural transmission times) and larger wave amplitudes [31]. The ABR is also used to detect peripheral and central hearing disorders [16], to differentially diagnose sensorineural and conductive hearing losses, and to predict neurodevelopmental impairment [7,9,28]. As such, neonatal ABRs can help determine if clinical treatments such as repeated AC treatments have beneficial or harmful effects on infant brain maturation, neural integrity and auditory function. Our study had no specific hypothesis about whether repeated AC treatment would have beneficial or harmful effects on the neonatal ABR.
2. Patients and Methods
2.1. Design
A randomized, double-masked, placebo-controlled, multi-center clinical trial was performed at 18 centers of the NICHD MFMU Network between 2000 and 2003. The clinical trial was approved by the Human Investigation Committees at each center. The materials, methods, patient flowchart and results for the clinical trial’s parent study and chief outcome variables are detailed elsewhere [32,38,39]. The study enrolled pregnant women between 23 weeks 0 days and 31 weeks 6 days were at high risk for spontaneous preterm birth or those diagnosed with placenta previa or chronic abruption who had received a single course of AC therapy. Gestational age was determined either from the last menstrual period or the dating sonogram. The women were randomized to receive additional weekly courses of Betamethasone (the Repeated AC group) or placebo (the Single AC group). Each AC course was comprised of two injections (IM) of 12 mg Betamethasone (as 6 mg Betamethasone phosphate and 6 mg Betamethasone acetate) given 24 hr apart. Each course of placebo was comprised of two isovolumetric injections (IM) of normal saline given 24 hr apart [32,38,39]. Weekly treatments continued until 33 weeks 6 days gestation or until delivery, whichever occurred first. After 67 women had been enrolled in the main trial, the maximum number of additional courses was limited to 4. This change was due to literature suggesting possible harmful effects from multiple courses of antenatal corticosteroids [12,15]. The clinical trial was terminated early by the Data and Safety Monitoring Committee (DSMC) for safety reasons unrelated to the ABR.
Exclusion criteria included preterm premature rupture of the membranes (PROM), chorioamnionitis, major fetal anomaly, non-reassuring fetal status, insulin-dependent diabetes, and systemic corticosteroid use in the current pregnancy. There were no additional inclusion or exclusion criteria for the ABR portion of the clinical trial.
The ABR component of the trial was limited to 6 centers having the necessary equipment and expertise to perform the ABR testing (Wayne State University, Drexel University, University of Miami, University of Utah, University of Cincinnati and University of Chicago). The ABR was a pre-specified secondary outcome of the clinical trial’s initial design. A separate consent form was used for ABR collection in addition to the primary consent form for the main clinical trial. It was explained to each mother that she could elect not to have her infant participate in the ABR study and that this would not influence the infant’s participation in the rest of the clinical trial.
2.2. ABR Procedure
The infants’ ABRs from five participating centers were collected on Bio-logic sensory evoked potential systems (Bio-logic Corp, Mundelein, IL 60060, USA) whereas one center (n=7 infants) used a Nicolet Spirit (Nicolet Corp, 5225 Verona Road, Madison, WI 53711, USA). All centers used standard recording techniques [6,31]. The stimuli were 45 and 70 dB Normal Hearing Level (NHL) clicks, where 0 dB NHL=35 dB Sound Pressure Level on Bio-logic equipment (personal communication with Karen Morris, Bio-logic Corp., 17 December 2002). Other stimulus parameters were: click duration=0.1 milliseconds, polarity=rarefaction, rate=31 clicks/sec. The stimuli were presented to the right and left ears separately through TDH-39 headphones (Telephonics Corp, Farmingdale, NY 11735, USA) or ER-3 insert earphones (Etymotic Research Inc, Elk Grove Village, IL 60007, USA). Fifty-five of the 60 infants had their click stimuli transduced by the TDH-39 headphones and 5 infants by the ER-3 insert earphones. We found no significant difference in published normative ABRs produced by TDH-39 headphones versus those collected in our clinic using ER-3 insert earphones [6]. The surface recording electrodes were placed on the infant’s upper forehead (Fz) and left (A1) and right (A2) lower mastoid regions. The ABRs were recorded using an ipsilateral recording montage of Fz-A1 for the left ear and Fz-A2 for the right ear, where Fz was the active (non-inverting) electrode and the A1 or A2 were the reference (inverting) electrodes. The contralateral electrode served as the common (ground) electrode. Electrode impedances were ≤5 kOhms whenever possible, but never >10 kOhms. At least two ABR traces were collected at both stimulus intensities and from each ear. These replication traces ensured the reproducibility and reliability of the ABRs. The recording parameters were: bandpass filter = 100 to 3000 Hz, 60 Hz notch filter = In, time window = 20 millisecond, number of stimuli = 2048 per trace. The ABRs were collected from sleeping or otherwise quiet infants. Stimulus artifact rejections from the infant’s movement activity were ≤10% of the total stimuli presented.
Infants were tested within 36 hours before discharge and were clinically stable when evaluated. All infants were at least 32 weeks gestational age when tested because the neonatal ABR is not well developed before this age [16,31]. If the initial ABR recordings were not reproducible or questionable for any reason, a repeat session was attempted the same day or the next day. The ABRs were scored for the latencies of waves I, III, and V and for the peak-to-trough amplitudes of waves I and V by qualified audiologists at each participating site who were unaware of each infant’s antenatal steroid status. Of the two replication ABRs collected for each ear at each stimulus intensity, the ABR trace with the shortest latencies were used for statistical analyses.
2.3. Sample Size
The primary ABR parameter that indicates a neurologic disorder or altered brain maturation is an abnormally prolonged wave I–V interpeak latency (IPL). We determined that 65 patients per group would be required to have 80% power to detect a latency difference of 0.5 standard deviations between the Repeated and Single AC treatment groups, with a 2-sided Type 1 error of 5%. Thus, the goal of the study was to test a total of 130 infants with the ABR. The parent study was terminated after the second interim analysis at the recommendation of the external Data and Safety Monitoring Committee (DSMC) because of a tendency towards decreased birth weight in the Repeated AC group without any reduction in the composite primary morbidity outcome, as defined elsewhere [39]. At the time of the study termination, there were 49 mothers consented to the ABR study, 11 of whom carried twins, resulting in 60 infants being ABR tested. Fifty-one infants had complete sets of ABR data in response to the 70 dB clicks (i.e., two ABRs from each ear) with 24 for the Repeated and 27 for the Single AC groups. Failure to get complete sets of ABRs on 9 infants was due to awakening and excess movement activity.
2.4. Statistical analyses
For discrete neonatal outcome variables, a pregnancy was credited with an outcome if either or both twins experienced that outcome and Chi Square tests were based on pregnancies rather than infants. The Wei-Lachin procedure [40], an extension of the Wilcoxon Sum test that can adjust for the correlation between twins, was used to compare the Repeated and Single AC treatment groups for continuous variables.
The ABR variables were analyzed both before and after adjustments for covariates, using Generalized Estimating Equations (GEE) [25], a regression model that can be used to adjust for the association between twins. Covariates were selected for either conceptual reasons or because they were closely correlated with the ABR’s wave I–V interpeak latency. Covariates considered in the adjusted analyses were treatment group, gestational age (GA) at birth, day of life at the ABR test, gestational age (GA) at the ABR test, and gender.
Nominal two-sided p-values are reported. For this analysis, p<0.05 was considered significant.
All statistical analyses were performed by the George Washington University Biostatistics Control Center (BCC), Rockville, Maryland, USA 20852.
3. Results
3.1. Maternal and infant characteristics
Table 1 shows that the maternal characteristics were similar between the Repeated and Single AC treatment groups. The majority of women (19 of 22 = 86.4 %) received 3 or more repeated courses of AC or placebo in addition to the initial course for a total of ≥4 treatment courses.
Table 1.
Maternal characteristics of the repeated antenatal corticosteroid (AC) and single AC groups presented as mean and standard deviation (SD), number (N) and percent (%) or median and 2.5th –97.5th percentile.
| Variable | Repeated (n=22) | Single (n=27) | P value |
|---|---|---|---|
| Maternal age (yrs) | 23.9 (6.2) | 24.8 (6.0) | 0.559 |
| Gestational age at enrollment (wks) | 28.6 (2.0) | 28.7 (2.3) | 0.864 |
| Gestational age at delivery (wks) | 35.5 (3.1) | 35.8 (3.5) | 0.778 |
| Predominant race (%) | 0.664 | ||
| African-American | 19 (86.4) | 20 (74.1) | |
| Caucasian | 2 (9.1) | 4 (14.8) | |
| Other | 1 (4.5) | 3 (11.1) | |
| Marital status (%) | 0.478 | ||
| Married | 6 (27.3) | 12 (44.4) | |
| Divorced | 3 (13.6) | 3 (11.1) | |
| Never married | 13 (59.1) | 12 (44.4) | |
| Years of school | 11.8 (1.9) | 12.4 (1.3) | 0.247 |
| Smoked during pregnancy (%) | 2 (9.1) | 4 (14.8) | 0.678 |
| Alcohol during pregnancy (%) | 2 (9.1) | 1 (3.7) | 0.581 |
| Street drugs during pregnancy (%) | 1 (4.5) | 2 (7.4) | 1.000 |
| Twin pregnancy (%) | 5 (22.7) | 6 (22.2) | 1.000 |
| Nulliparity (%) | 3 (13.6) | 1 (3.7) | 0.314 |
| Previous spontaneous birth (%) | 7 (31.8) | 10 (37.0) | 0.703 |
| Pre-randomization infection (%) | 11 (50.0) | 15 (55.6) | 0.698 |
| Gestational Diabetes (%) | 0 (0.0) | 1 (3.7) | 1.000 |
| Study courses received (%)a | |||
| 1 | 3 (13.6) | 3 (11.1) | |
| 2 | 0 (0.0) | 0 (0.0) | |
| 3 | 1 (4.5) | 3 (11.1) | |
| 4 | 16 (72.7) | 17 (63.0) | |
| 5 | 1 (4.5) | 1 (3.7) | |
| 6 | 0 (0.0) | 1 (3.7) | |
| 7 | 1 (4.5) | 0 (0.0) | |
| 8 | 0 (0.0) | 2 (7.4) | |
| Study courses received (median [2.5th–97.5th percentiles])a | 4 (1–7) | 4 (1–8) | 0.866 |
Number of courses given in addition to the initial antenatal corticosteroid course that was given to women in both groups
The characteristics from all ABR-tested infants (n=60) are shown in Table 2. None of the infants in the ABR study had craniofacial anomalies, chromosomal anomalies, toxoplasmosis, rubella, cytomegalovirus or hyperbilirubinemia. The majority of infants with complete ABR data (20 of 24 = 83.3%) received 3 or more repeated courses of AC or placebo in addition to the initial course for a total of ≥4 treatment courses. The Repeated AC group had significantly smaller birth weights, lengths, and head circumferences than the Single AC group, even though these two groups had the same average gestational age at delivery. There were no group differences in gestational age at the time of ABR testing (i.e., the GA at birth plus the post-partum age), Apgar scores, bilirubin levels or morbidities.
Table 2.
Infant characteristics of the repeated antenatal corticosteroid (AC) and single AC groups presented as mean and standard deviation (SD), number (N) and percent (%) or median and 2.5th –97.5th percentile.
| Variable | Repeated (n=27) | Single (n=33) | P value |
|---|---|---|---|
| Gender | 0.991 | ||
| Males (%) | 14 (51.9) | 17 (51.5) | |
| Females (%) | 13 (48.1) | 16 (48.5) | |
| Singletons (%) | 17 (77.3) | 21 (77.8) | |
| Pairs of Twins (%) | 5 (22.7) | 6 (22.2) | |
| Birth weight (grams) | 2121 (586) | 2539 (727) | 0.027 |
| Birth length (cm) | 43.9 (4.3) | 46.6 (4.3) | 0.035 |
| Birth head circumference (cm) | 31.0 (2.4) | 32.1 (2.6) | 0.036 |
| 1-minute Apgar < 5 (%) | 0 (0) | 3 (9.1) | 0.242 |
| 5-minute Apgar < 7 (%) | 0 (0) | 1 (3.0) | 1.000 |
| Morbidities: | |||
| Days in NICU (median [2.5th–97.5th percentiles]) | 6 (0–117) | 0 (0–78) | 0.063 |
| Supplemental oxygen 36 wks corrected age (%) | 2 (7.4) | 1 (3.0) | 0.581 |
| Highest total bilirubin level (mg/dL) | 10.1 (3.3) | 11.3 (3.3) | 0.670 |
| Respiratory distress syndrome | 3 (11.1) | 3 (9.1) | 1.000 |
| Necrotizing enterocolitis (%) | 0 (0) | 1 (3.0) | 1.000 |
| Intraventricular hemorrhage (% grades 3–4) | 0 (0) | 0 (0) | |
| Patent ductus arteriosus (%) | 2 (7.4) | 0 (0) | 0.449 |
| Retinopathy of prematurity (%) | 1 (3.7) | 0 (0) | 0.449 |
| Seizures (%) | 0 (0) | 0 (0) | |
| Age at discharge (days) (median [2.5th–97.5th percentiles]) | 6 (3–117) | 4 (2–78) | 0.055 |
| Age at ABR (days) (median [2.5th–97.5th percentiles]) | 5 (2–62) | 3 (1–66) | 0.051 |
| Age at ABR (days) (%) | |||
| 1–2 days (%) | 2 (7.4) | 9 (27.3) | |
| 3–7 days (%) | 15 (55.6) | 15 (45.5) | |
| 8–14 days (%) | 5 (18.5) | 7 (21.2) | |
| 15–22 days (%) | 5 (18.5) | 2 (6.1) | |
| Gestational age at ABR (weeks) | 36.7 (2.2) | 36.9 (2.5) | 0.900 |
ABR, auditory brainstem response; NICU, Neonatal Intensive Care Unit
3.2. Auditory brainstem responses
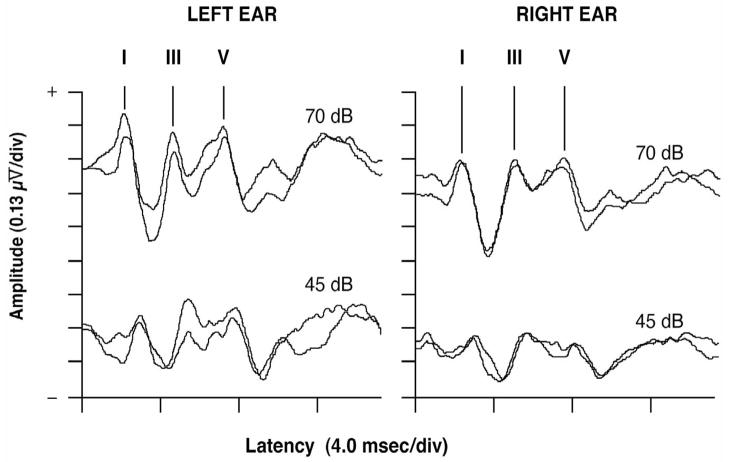
Figure 1 shows typical neonatal ABRs for a single infant. The ABR results from infants with complete data from right and left ear stimulation at 70 dB are in Table 3. Although the mean latencies of all waves were longer (prolonged) in the Repeated AC group as compared to the Single AC group (suggesting slower neural transmission times), none of these findings reached statistical significance. Similarly, there were no group differences in the ABRs elicited by the 45 dB click stimuli. The right ear wave I–V IPLs were 5.29±0.44 and 5.31±0.52 msec for the Repeated (n=20) and Single AC (n=26) groups, respectively (p=0.89). The left ear wave I–V IPLs were 5.43±0.50 and 5.42±0.62 msec for the Repeated (n=25) and Single AC (n=26) groups respectively (p=0.96).
Fig. 1.
Neonatal auditory brainstem responses (ABR) from the left and right ears in response to stimulus click intensities of 70 and 45 dB NHL with replication traces taken at each stimulus intensity to confirm reproducibility. The neonatal ABR is comprised primarily of waves I, III, and V. A wave’s latency is the time from stimulus onset (time = 0 msec) to a wave’s positive peak.
Table 3.
Auditory brainstem responses (ABR) at 70 dB NHL for the repeated antenatal corticosteroid (AC) and single AC groups presented as mean (standard deviation) latency and amplitude of each wave.
| Variable | Repeated (n=24) | Single (n=27) | P value | Cohen’s D |
|---|---|---|---|---|
| Right ear ABR latency (msec) | ||||
| Wave I | 2.31 (0.54) | 2.21 (0.39) | 0.272 | 0.22 |
| Wave III | 5.34 (0.48) | 5.23 (0.52) | 0.209 | 0.23 |
| Wave V | 7.93 (0.60) | 7.63 (0.65) | 0.079 | 0.48 |
| Wave I–V | 5.62 (0.55) | 5.42 (0.54) | 0.305 | 0.37 |
| Right ear ABR amplitude (μV) | ||||
| Wave I | 0.25 (0.08) | 0.21 (0.08) | 0.076 | 0.48 |
| Wave V | 0.18 (0.06) | 0.19 (0.07) | 0.599 | 0.08 |
| Left ear ABR latency (msec) | ||||
| Wave I | 2.13 (0.36) | 2.08 (0.36) | 0.334 | 0.16 |
| Wave III | 5.18 (0.37) | 5.13 (0.38) | 0.779 | 0.13 |
| Wave V | 7.74 (0.60) | 7.62 (0.52) | 0.406 | 0.22 |
| Wave I–V | 5.60 (0.50) | 5.54 (0.60) | 0.953 | 0.11 |
| Left ear ABR amplitude (μV) | ||||
| Wave I | 0.26 (0.11) | 0.23 (0.08) | 0.169 | 0.41 |
| Wave V | 0.21 (0.08) | 0.20 (0.08) | 0.760 | 0.12 |
ABR, auditory brainstem response; dB NHL, decibels normal hearing level
Using the infant’s longest (worst) wave I–V IPL from either ear, a regression analysis examined the association between the I–V IPL and treatment group while adjusting for the possible confounding effects of gestational age at birth and gender. Gestational age at birth was highly associated with the I–V IPL (p=0.004). Treatment group was not significantly associated with the I–V IPL (p=0.372). Addition of age (day of life) at the ABR test and birth weight to the regression model gave similar results.
Whereas brain size can influence ABR latencies (i.e., smaller brain = shorter neural transmission times), a regression model using the ratio of Wave I–V IPL to head circumference was used to help control for such an influence. Head circumference was measured within the first 3 days after birth and the ABR was typically taken within the first 7 days after birth (Table 2). This analysis indicated that gestational age at birth was highly associated with the ratio of the I–V IPL to head circumference (p<0.0001) but that treatment group was not (p=0.254).
4. Discussion
We found no significant differences in the ABR latencies or amplitudes of infants prenatally exposed to repeated courses of AC in comparison with infants who only received a single course. This finding occurred despite the repeated betamethasone group being born with significantly smaller birth weights, lengths and head circumferences that were equivalent to a two week maturational delay; results similar to those of the parent study [39].
Our ABR results are additive to those from a recently published study that found no significant effects of antenatal Betamethasone when infants predominantly exposed to a single course were compared to those who were unexposed [3]. In contrast, our study was specifically designed to address the issue of single versus multiple AC treatments and still demonstrated no significant ABR changes.
A more recent study found no benefit or harm from multiple AC treatments [2]. However, there are noteworthy differences between our respective studies: (A) Our multiple AC treatment group had a median of 5 AC courses, resulting in significant reductions in birth weight, head circumference and body length; whereas the other study had a median of 3 AC courses with no reductions in birth weight, head circumference or body length [5]. This observation is consistent with both animal [18,19] and human studies [38,39] suggesting a threshold for untoward effects. In our primary, study significant alterations of fetal growth were only seen in pregnancies exposed to 4 or more AC courses [38,39]. (B) Our study was a multi-center double-blind randomized placebo-controlled clinical trial overcoming the potential confounders of a single center retrospective cohort study. (C) We specifically assessed the possible confounding effects of birth weight, head circumference, gender, gestational age at birth, and day of life at testing. Although these variables correlated with the ABR, statistical control for their possible confounding influence still showed no treatment group effect. Our study also reported on other maternal and neonatal confounders including maternal alcohol and cigarette consumption, NEC, IVH, PDA, ROP and bilirubin levels. (D) Our study also benefitted from its prospective acquisition of patients being studied specifically to evaluate the impact of steroids on fetal development. The two previous studies by Amin and colleagues involved only retrospective data gathered from infants enrolled in a bilirubin study [2,3,5].
Despite such differences, these two previous studies and our current study concur that there is no significant benefit or harm to the infant ABR from various AC treatments. Since none of these studies found group differences in the neonatal ABRs, it is unlikely that potentially confounding factors such as acidosis, oxidative stress, middle ear effusion or exposure to ototoxic medication influenced the ABR outcomes. These studies were limited however in that they used the ABR only as a neurological assessment tool; none of them fully used the ABR to assess possible hearing loss.
One concern of the present study is the failure to recruit to the projected sample size (65/group) because the main trial was prematurely terminated. This raises the possibility that our lack of effect on the ABR was secondary to a type II error. Our initial estimate of sample size was rather conservative, being based on an ability to detect a 0.5 standard deviation difference in the I–V IPL. Given the sample tested, we had only 40% power to detect a 0.5 standard deviation group difference and >90% power to detect one standard deviation. The differences we observed were considerably less than originally hypothesized. The observed group differences were 0.20 and 0.06 msec for the right and left ear I–V IPLs and 0.30 and 0.12 msec for the right and left wave V latencies, respectively (Cohen’s d=0.11 to 0.48). These differences are comparable to about a one-week maturational delay for the Repeated AC group [23]. This contrasts with the significant group differences in birth weights and head circumferences. We conclude therefore that the slightly longer I–V IPLs and wave V latencies in the Repeated AC treatment group were clinically mild effects at best, that there was some brain sparing and that low statistical power was probably not a major factor in our failure to find group differences.
Our study had a high proportion of twins (22–23%) in the two treatment groups. This is consistent with other studies which found that twins account for 12–28% of preterm births [35,36]. We also used the Wei-Lachin procedure to adjust for the correlation between twins [40]. Thus, the proportion of twins in our study was neither unusual nor a biasing factor.
In conclusion, the relatively small effect on the ABR from repeated AC treatments suggests that despite significant alterations of fetal growth, there was relative sparing of the neural mechanisms that generate the ABR. There are some caveats to this conclusion. For example, there is insufficient information to project the impact of our neonatal findings on later life. Follow-up studies from the primary network trial have demonstrated that despite weight and size differences at birth, catch-up growth occurs in the multiple steroid exposed infants by 2 to 3 years of age. While no neurocognitive differences between the Single and Repeated AC cohorts were identifiable at age 3 years, there was a suggestion of an increased risk of cerebral palsy in infants exposed to 4 or more AC courses [38]. As pertains to the ABR, a recent animal study found that elevated maternal-fetal corticosteroid levels induced by handling stress resulted in ABR abnormalities indicative of a hearing loss in the adult offspring [21]. Elevated corticosteroid levels can cause the “fetal programming” of adult-onset diseases [34] and adverse neurodevelopmental effects can occur as a consequence of repeated neonatal corticosteroid treatments [41]. These findings raise concerns about adverse effects occurring in humans from too much AC treatment and raise the question about how much AC treatment is too much.
Acknowledgments
This study was funded by grants HD27917, HD27905, HD27861, HD27915, HD34122, HD34208, HD34136, HD40512, HD36801 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. There were no conflicts of interest to report. Other participating members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
Drexel University; M. DiVito, A. Sciscione, V. Berghella, P. Trauffer, M. Pollock, M. Talucci, M. Goodman
Wayne State University; Y. Sorokin, M. Dombrowski, G. Norman, A. Millinder, C. Sudz, D. Driscoll
George Washington University, Biostatistics Center; E. Thom, F. Galbis-Reig, A. Das, L. Leuchtenburg, D. Johnson
Ohio State University; J. Iams, M. Landon, S. Meadows, P. Shubert
University of Utah; M. Varner, K. Anderson, A. Guzman, A. Crowley, M. Fuller, S. Bohning
The National Institute of Child Health and Human Development; D. McNellis, K. Howell, S. Pagliaro
Northwestern University; G. Mallett
University of Chicago; P. Jones, G. Mallett, J. Perkins
University of Miami; D. Martin, F. Doyle, R. Fifer
University of Cincinnati; H. How, N. Elder, B. Alexander, W. Girdler, E. Johnson, R. Keith
Vanderbilt University, S. Gabbe
In addition to the authors, the following subcommittee members participated in protocol development and coordination between clinical research centers (Michelle DiVito, MSN), protocol/data management and statistical analysis (Elizabeth A. Thom, PhD), and protocol development and oversight (Yoram Sorokin, MD).
Footnotes
Clinical trial registry name: A Randomized Placebo-Controlled Trial of Antenatal Corticosteroid Regimens (NCT00015002)
Clinical trial registered at website URL: http://clinicaltrials.gov/ct/show/NCT00015002?order=2
Conflicts of Interest
There were no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NIH Consensus Develpoment Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. Effect of corticosteroids for fetal maturation on perinatal outcomes. J Am Med Assoc. 1995;273:413–8. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 2.Amin SB, Guillet R. Auditory neural maturation after exposure to multiple courses of antenatal betamethasone in premature infants as evaluated by auditory brainstem response. Pediatrics. 2007;119:502–8. doi: 10.1542/peds.2006-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin SB, Orlando MS, Dalzell LE, Merle KS, Guillet R. Brainstem maturation after antenatal steroids exposure in premature infants as evaluated by auditory brainstem-evoked response. J Perinatol. 2003;23:307–11. doi: 10.1038/sj.jp.7210898. [DOI] [PubMed] [Google Scholar]
- 4.Antonow-Schlorke I, Muller T, Brodhun M, Wicher C, Schubert H, Nathanielsz PW, Witte OW, Schwab M. Betamethasone-related acute alterations of microtubule-associated proteins in the fetal sheep brain are reversible and independent of age during the last one-third of gestation. Am J Obstet Gynecol. 2007;196:553 e1–6. doi: 10.1016/j.ajog.2006.10.898. [DOI] [PubMed] [Google Scholar]
- 5.Church MW. Effects of multiple courses of antenatal betamethasone on the auditory brainstem responses of premature infants. Pediatrics. 2007;120:450. doi: 10.1542/peds.2007-1125. author reply 450–1. [DOI] [PubMed] [Google Scholar]
- 6.Church MW, Parent-Jenkins L, Rozzelle AA, Eldis FE, Kazzi SN. Auditory brainstem response abnormalities and hearing loss in children with craniosynostosis. Pediatrics. 2007;119:e1351–60. doi: 10.1542/peds.2006-3009. [DOI] [PubMed] [Google Scholar]
- 7.Cone-Wesson B, Vohr BR, Sininger YS, Widen JE, Folsom RC, Gorga MP, Norton SJ. Identification of neonatal hearing impairment: infants with hearing loss. Ear Hear. 2000;21:488–507. doi: 10.1097/00003446-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Cotterrell M, Balazs R, Johnson AL. Effects of corticosteroids on the biochemical maturation of rat brain: postnatal cell formation. J Neurochem. 1972;19:2151–67. doi: 10.1111/j.1471-4159.1972.tb05124.x. [DOI] [PubMed] [Google Scholar]
- 9.Cox C, Hack M, Aram D, Borawski E. Neonatal auditory brainstem response failure of very low birth weight infants: 8-year outcome. Pediatr Res. 1992;31:68–72. doi: 10.1203/00006450-199201000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173:322–35. doi: 10.1016/0002-9378(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 11.Crowther CA, Doyle LW, Haslam RR, Hiller JE, Harding JE, Robinson JS. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179–89. doi: 10.1056/NEJMoa071152. [DOI] [PubMed] [Google Scholar]
- 12.Dirnberger DR, Yoder BA, Gordon MC. Single versus repeated-course antenatal corticosteroids: outcomes in singleton and multiple-gestation pregnancies. Am J Perinatol. 2001;18:267–7. doi: 10.1055/s-2001-16989. [DOI] [PubMed] [Google Scholar]
- 13.Dunlop SA, Archer MA, Quinlivan JA, Beazley LD, Newnham JP. Repeated prenatal corticosteroids delay myelination in the ovine central nervous system. J Matern Fetal Med. 1997;6:309–13. doi: 10.1002/(SICI)1520-6661(199711/12)6:6<309::AID-MFM1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Elimian A, Verma U, Visintainer P, Tejani N. Effectiveness of multidose antenatal steroids. Obstet Gynecol. 2000;95:34–6. doi: 10.1016/s0029-7844(99)00471-8. [DOI] [PubMed] [Google Scholar]
- 15.French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180:114–21. doi: 10.1016/s0002-9378(99)70160-2. [DOI] [PubMed] [Google Scholar]
- 16.Galambos R, Wilson MJ, Silva PD. Identifying hearing loss in the intensive care nursery: a 20-year summary. J Am Acad Audiol. 1994;5:151–62. [PubMed] [Google Scholar]
- 17.Huang WL, Beazley LD, Quinlivan JA, Evans SF, Newnham JP, Dunlop SA. Effect of corticosteroids on brain growth in fetal sheep. Obstet Gynecol. 1999;94:213–8. doi: 10.1016/s0029-7844(99)00265-3. [DOI] [PubMed] [Google Scholar]
- 18.Ikegami M, Jobe AH, Newnham J, Polk DH, Willet KE, Sly P. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. Am J Respir Crit Care Med. 1997;156:178–84. doi: 10.1164/ajrccm.156.1.9612036. [DOI] [PubMed] [Google Scholar]
- 19.Jobe AH, Wada N, Berry LM, Ikegami M, Ervin MG. Single and repetitive maternal glucocorticoid exposures reduce fetal growth in sheep. Am J Obstet Gynecol. 1998;178:880–5. doi: 10.1016/s0002-9378(98)70518-6. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JW, Mitzner W, Beck JC, London WT, Sly DL, Lee PA, Khouzami VA, Cavalieri RL. Long-term effects of betamethasone on fetal development. Am J Obstet Gynecol. 1981;141:1053–64. doi: 10.1016/s0002-9378(16)32697-7. [DOI] [PubMed] [Google Scholar]
- 21.Kadner A, Pressimone VJ, Lally BE, Salm AK, Berrebi AS. Low-frequency hearing loss in prenatally stressed rats. Neuroreport. 2006;17:635–8. doi: 10.1097/00001756-200604240-00015. [DOI] [PubMed] [Google Scholar]
- 22.Kutzler MA, Ruane EK, Coksaygan T, Vincent SE, Nathanielsz PW. Effects of three courses of maternally administered dexamethasone at 0.7, 0.75, and 0.8 of gestation on prenatal and postnatal growth in sheep. Pediatrics. 2004;113:313–9. doi: 10.1542/peds.113.2.313. [DOI] [PubMed] [Google Scholar]
- 23.Lasky RE. A developmental study on the effect of stimulus rate on the auditory evoked brain-stem response. Electroencephalogr Clin Neurophysiol. 1984;59:411–9. doi: 10.1016/0168-5597(84)90042-x. [DOI] [PubMed] [Google Scholar]
- 24.LeFlore JL, Salhab WA, Broyles RS, Engle WD. Association of antenatal and postnatal dexamethasone exposure with outcomes in extremely low birth weight neonates. Pediatrics. 2002;110:275–9. doi: 10.1542/peds.110.2.275. [DOI] [PubMed] [Google Scholar]
- 25.Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- 26.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–25. [PubMed] [Google Scholar]
- 27.Moller AR, Jannetta PJ, Moller MB. Neural generators of brainstem evoked potentials. Results from human intracranial recordings. Ann Otol Rhinol Laryngol. 1981;90:591–6. doi: 10.1177/000348948109000616. [DOI] [PubMed] [Google Scholar]
- 28.Murray AD. Newborn auditory brainstem evoked responses (ABRs): longitudinal correlates in the first year. Child Dev. 1988;59:1542–54. [PubMed] [Google Scholar]
- 29.Quinlivan JA, Archer MA, Evans SF, Newnham JP, Dunlop SA. Fetal sciatic nerve growth is delayed following repeated maternal injections of corticosteroid in sheep. J Perinat Med. 2000;28:26–33. doi: 10.1515/JPM.2000.004. [DOI] [PubMed] [Google Scholar]
- 30.Quinlivan JA, Beazley LD, Evans SF, Newnham JP, Dunlop SA. Retinal maturation is delayed by repeated, but not single, maternal injections of betamethasone in sheep. Eye. 2000;14( Pt 1):93–8. doi: 10.1038/eye.2000.20. [DOI] [PubMed] [Google Scholar]
- 31.Salamy A. Neurodevelopment and auditory function in preterm infants. In: JTJ, editor. Principles & Applications In Auditory Evoked Potentials. Allyn and Bacon; Boston, USA: 1994. pp. 287–312. [Google Scholar]
- 32.Sawady J, Mercer BM, Wapner RJ, Zhao Y, Sorokin Y, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, et al. The National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network Beneficial Effects of Antenatal Repeated Steroids study: impact of repeated doses of antenatal corticosteroids on placental growth and histologic findings. Am J Obstet Gynecol. 2007;197:281 e1–8. doi: 10.1016/j.ajog.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 33.Schwab M, Antonow-Schlorke I, Kuhn B, Muller T, Schubert H, Walter B, Sliwka U, Nathanielsz PW. Effect of antenatal betamethasone treatment on microtubule-associated proteins MAP1B and MAP2 in fetal sheep. J Physiol. 2001;530:497–506. doi: 10.1111/j.1469-7793.2001.0497k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seckl JR. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151(Suppl 3):U49–62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- 35.Slattery MM, Geary M, Morrison JJ. Obstetric antecedents for preterm delivery. J Perinat Med. 2008;36:306–9. doi: 10.1515/JPM.2008.045. [DOI] [PubMed] [Google Scholar]
- 36.Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002;360:1489–97. doi: 10.1016/S0140-6736(02)11476-0. [DOI] [PubMed] [Google Scholar]
- 37.Smith LM, Qureshi N, Chao CR. Effects of single and multiple courses of antenatal glucocorticoids in preterm newborns less than 30 weeks’ gestation. J Matern Fetal Med. 2000;9:131–5. doi: 10.1002/(SICI)1520-6661(200003/04)9:2<131::AID-MFM9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 38.Wapner RJ, Sorokin Y, Mele L, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Malone F, Caritis SN, et al. Long-term outcomes after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1190–8. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- 39.Wapner RJ, Sorokin Y, Thom EA, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Harper M, Caritis SN, et al. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol. 2006;195:633–42. doi: 10.1016/j.ajog.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 40.Wei I, Lachin J. Two sample asymptomatically distribution-free tests for incomplete multivariate observations. J Am Stat Assoc. 1984;79:653–61. [Google Scholar]
- 41.Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, Tsai CH. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350:1304–13. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]