Abstract
Although the complement system is centrally involved in host defense, its overactivation or deregulation (e.g., due to inherent host genetic defects or due to pathogen subversion) may excessively amplify inflammation and contribute to immunopathology. Periodontitis is an oral infection-driven chronic inflammatory disease which exerts a systemic impact on health. This paper reviews evidence linking complement to periodontal inflammation and pathogenesis. Clinical and histological observations show a correlation between periodontal inflammatory activity and local complement activation. Certain genetic polymorphisms or deficiencies in specific complement components appear to predispose to increased susceptibility to periodontitis. Animal model studies and in vitro experiments indicate that periodontal bacteria can either inhibit or activate distinct components of the complement cascade. Porphyromonas gingivalis, a keystone species in periodontitis, subverts complement receptor 3 and C5a anaphylatoxin receptor signaling in ways that promote its adaptive fitness in the presence of non-productive inflammation. Overall, available evidence suggests that complement activation or subversion contributes to periodontal pathogenesis, although not all complement pathways or functions are necessarily destructive. Effective complement-targeted therapeutic intervention in periodontitis would require determining the precise roles of the various inductive or effector complement pathways. This information is essential as it may reveal which specific pathways need to be blocked to counteract microbial evasion and inflammatory pathology or, conversely, be enhanced to promote host immunity.
1. Introduction
Host defense and inflammation is fundamentally dependent on the complement system, which orchestrates critical events in this regard. These include recruitment and activation of inflammatory cells, microbial opsonization, phagocytosis, and lysis, as well as crosstalk and regulation of other systems, including Toll-like receptors (TLR) [1, 2]. In the latter context, the TLR4-mediated inflammatory response to in vivo bacterial lipopolysaccharide (LPS) challenge is amplified by complement [3]. In addition, complement inhibition protects against experimental sepsis induced by high doses of LPS or by cecal ligation and puncture (CLP) peritonitis [4]. Moreover, complement bridges innate to adaptive immunity by regulating the activation of both B cells and T cells, either directly or through effects on antigen-presenting cells [5–8]. Its protective role notwithstanding, complement may cause or exacerbate inflammatory tissue damage when overactivated or deregulated, either by pathogens or due to inherent host genetic defects [9–12]. Indeed, complement pathways constitute a major link between infection and various local or systemic inflammatory or autoimmune diseases [1, 9, 13, 14]. This communication reviews evidence implicating complement in periodontal inflammation and pathogenesis. Furthermore, it discusses interventional strategies that could complement current clinical periodontal treatment, which is often not sufficient by itself to reverse destructive inflammation [15, 16].
2. Periodontal inflammation
Periodontitis is a highly prevalent chronic inflammatory disease that causes destruction of the tooth-supporting tissues. In its severe form, periodontitis may eventually lead to tooth loss and/or exert a significant systemic impact on health [17]. The annual cost of periodontal therapy in the U.S. exceeds $14 billion [18] and the suspected association of periodontitis with certain systemic diseases (e.g., atherosclerosis, aspiration pneumonia, diabetes, adverse pregnancy outcomes, and rheumatoid arthritis [17, 19–24]) underscores the importance of implementing new and effective treatments. Moreover, the fact that many treated patients develop recurrent disease for reasons that are not clear necessitates better understanding of the underlying immunopathology [15, 16].
Although bacteria populating the tooth-associated biofilm are essential for the initiation of periodontitis, it is actually the host inflammatory response to this challenge, rather than direct bacterial action, that primarily causes periodontal tissue damage [25, 26]. The role of certain proinflammatory cytokines (e.g., interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), and IL-6) in destructive periodontal inflammation is well established [26, 27]. Moreover, recent clinical observations also implicate IL-17 [25, 28–31]. The periodontitis-associated bacteria comprise a group of gram-negative anaerobic organisms, among which more prominent are the so-called “red complex” pathogens, i.e., Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia [32, 33]. The reason(s) as to why the host response often fails to control periodontal infection and reverse disease progression are not well understood, although disruption of host homeostasis by periodontal pathogens may be a major contributory factor [34]. In this respect, pathogen manipulation of pattern-recognition and response mechanisms may perturb otherwise homeostatic host-bacterial interactions, thereby leading to non-protective and non-resolving chronic inflammation. One such example involves P. gingivalis, a master of immune subversion in the oral cavity and a keystone species in periodontitis, which is thought to promote the survival and virulence of the whole biofilm community [35, 36]. It, therefore, seems reasonable that periodontal disease could be prevented or mitigated by interventions aiming to control inflammation and counteract microbial subversion of the host response.
3. Complement and its role in periodontal pathogenesis
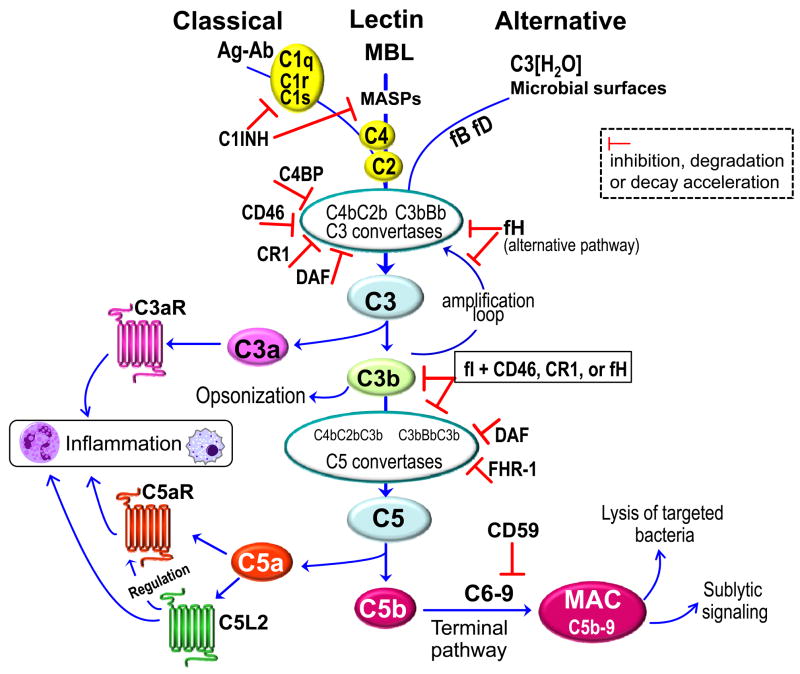
The triggering of the complement cascade involves sequential activation and proteolytic cleavage of a series of serum proteins, via three distinct mechanisms, namely the classical, lectin, and alternative [1] (Fig. 1). The activation of the classical pathway is initiated by antigen-antibody complexes recognized by the C1q subunit of C1, whereas the lectin pathway is triggered through interaction of a secreted pattern-recognition receptor (the mannose-binding lectin; MBL) with specific carbohydrate groups on the surface of a variety of microorganisms. Both the classical and the lectin pathways then proceed through C4 and C2 cleavage for the generation of the classical/lectin C3 convertase (C4bC2b) (Fig. 1). It should be noted that C4bC2b and C4bC2a are interchangeably used in the literature to refer to the classical/lectin pathway C3 convertase. In this review, this convertase is referred to as C4bC2b by designating the small and non-proteolytic C2 fragment that drifts away as C2a (in analogy to C3a), and the protease segment as C2b. The alternative pathway is initiated by low-level, spontaneous hydrolysis of C3 to C3(H2O), which is a C3b analog and forms the initial alternative pathway C3 convertase in the presence of factors B and D (C3[H2O]Bb). As long as there is no sufficient negative regulation (e.g., in the case of non-self surfaces such as bacteria), this initiation is followed by rapid propagation of the alternative pathway involving an amplification loop and the formation of the predominant alternative pathway C3 convertase (C3bBb) [6] (Fig. 1). Moreover, the alternative pathway can be induced by bacterial lipopolysacharide and lipooligosacharide molecules in a way that strictly requires the participation of the plasma protein properdin attached to microbial surfaces [37, 38]. The alternative pathway also serves as a positive amplification loop of complement activation through the classical and lectin pathways, and thereby an initially weak stimulus can get markedly amplified [6]. Despite what might be implied by its name, the “alternative” pathway may potentially contribute to ≥ 80% of total complement activation, even when the initial trigger is provided through the other two pathways [39]. All three pathways converge at the third component of complement (C3) which, upon activation by pathway-specific C3 convertases, leads to the generation of a number of effector molecules. These include the C3a and C5a anaphylatoxins which activate specific G-protein–coupled receptors (C3aR and C5aR, respectively) and mediate mobilization and activation of leukocytes. Also important are the C3b and iC3b opsonins that promote phagocytosis through complement receptors (CR1 and CR3, respectively), and the C5b-9 membrane attack complex (MAC) which can lyse targeted pathogens [1] (Fig. 1). In addition to the classic C5aR (CD88), C5a also interacts with an alternative but quite enigmatic G protein-independent receptor, the C5a-like receptor 2 (C5L2; GPR77), which has been assigned both regulatory and proinflammatory roles [40–44].
Fig. 1. Activation and regulation of the complement system.
All three pathways converge at the third component of complement (C3) which is activated by pathway-specific C3 convertases [1]. The classical pathway is initiated by antigen-antibody (Ag-Ab) complexes and requires the participation of C1, C2, and C4. The lectin pathway is triggered through interaction of the mannose-binding lectin (MBL) with specific microbial carbohydrate groups, followed by activation of MBL-associated serine proteases (MASPs) and cleavage of C2 and C4. The alternative pathway is initiated by spontaneously hydrolyzed C3 [C3(H2O)] which can thereby form a complex with factor B (fB), followed by fB cleavage by factor D (fD) and formation of the initial alternative pathway C3 convertase [1]. Morerover, the alternative pathway can be induced by bacterial lipopolysacharide and lipooligosacharide in a properdin-dependent way [38]. Proteolytic cleavage of a series of proteins downstream of C3 leads to the generation of potent effector molecules. These include the inflammatory anaphylatoxins C3a and C5a, which activate specific receptors (C3aR and C5aR, respectively), although C5a also interacts with the so-called C5a receptor-like 2 (C5L2), which appears to mediate both regulatory and proinflammatory effects [40–44]. In the terminal pathway, C5b initiates the assembly of the C5b-9 membrane attack complex (MAC), which in turn induces microbial cell lysis [1] or host cell signaling at sublytic concentrations [78, 168]. Complement activation is regulated at multiple steps by various regulatory proteins, as indicated by the characteristic inhibitory symbols. C1NH, C1 inhibitor; C4BP, C4b-binding protein; CR1; complement receptor 1; DAF, decay accelerating factor; fH, factor H; FHR-1, fH-related protein 1.
Due to its potentially destructive nature for host tissues, complement activation is tightly controlled by membrane-bound and soluble regulatory proteins. Membrane bound regulators include the decay accelerating factor (DAF; CD55) which accelerates the decay of the C3 and C5 convertases, as well as the CD46 (membrane cofactor protein; MCP) and the complement receptor 1 (CR1; CD35). In association with the fluid-phase protease factor I (fI), CD46 and CR1 help degrade C3b and C4b, the latter of which is required for the formation of the classical/lectin C3 convertase [11, 45]. Host cells are protected from MAC-mediated lysis through another membrane-bound regulatory protein, the CD59 (also referred to as protectin), which inhibits the terminal step of MAC formation [11, 45] (Fig. 1). An important fluid-phase regulator is factor H (fH), which controls the alternative pathway by inhibiting the formation and accelerating the decay of the alternative pathway C3 convertase. Moreover, fH contributes to cleavage and inactivation of C3b to iC3b, and thus can also inhibit the formation and amplification of the C5 convertase [11]. On the other hand, the newly described fH-related protein 1 (FHR-1) was shown to directly bind C5 and block the activity of the C5 convertase and downstream formation of MAC [46]. The circulating C4b-binding protein (C4BP), which is another cofactor for fI, accelerates decay of classical and lectin pathway convertases, whereas the C1 inhibitor (C1INH) is an important soluble inhibitor of the classical and lectin pathways [11] (Fig. 1).
Complement activities are not restricted to a linear cascade of events, as briefly outlined above, but involve a network of interactions with other systems for better coordination of the host response to infection or injury. These connections allow complement to coordinate innate immunity through crosstalk with TLRs [2], provide a barrier against the spread of invading bacteria by potentiating local clotting [47], and replenish the immune system through mobilization of hematopoietic stem/progenitor cells from the bone marrow [48, 49]. The elaborate system of complement effectors and regulators (Fig. 1) also impacts on the activation and differentiation of T cell subsets [6, 50–52]. However, deregulation of these finely balanced complement activities may not only lead to failure to protect the host against pathogens, but could also initiate or amplify inflammatory tissue damage [9–12, 53]. As outlined below, complement deregulation or subversion may play an important role in periodontal inflammation and pathogenesis.
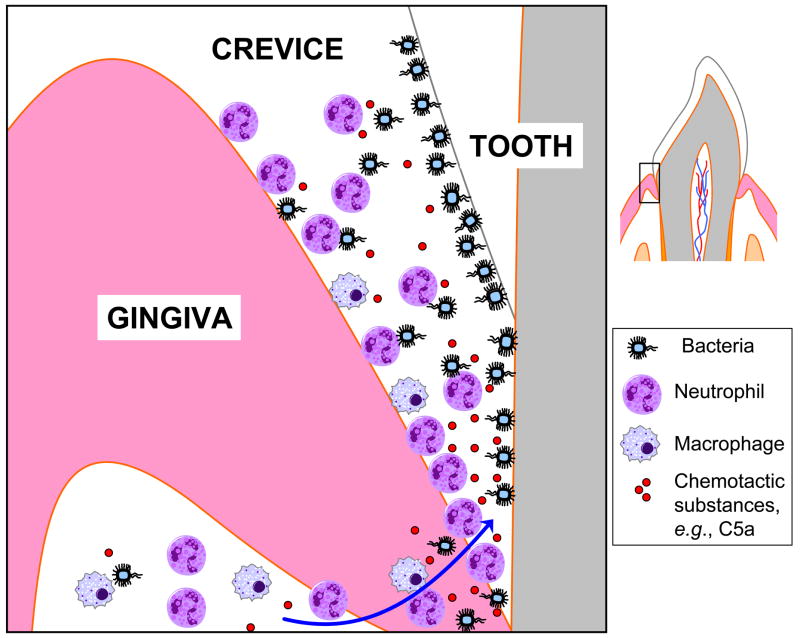
A number of clinical and histological observations suggest complement involvement in periodontitis (Table 1). Activated complement fragments are abundantly found in the gingival crevicular fluid (GCF) of periodontitis patients, whereas they are absent or present in lower concentrations in GCF from healthy individuals [54–58]. GCF represents the inflammatory exudate which bathes the space between the free gingiva and the tooth surfaces, known as gingival crevice [59] (Fig. 2). In general, complement can be found in GCF at up to 70 to 80% of its concentration in serum, but certain activated fragments can be found at much higher levels in GCF reflecting local generation [55, 60–62]. Complement components and cleavage products covering the whole complement cascade (e.g., C1q, factor B, Bb, C3, C3a, C3b, C3c, C3d, C4, C5, C5a, C5b, C9) have been detected in chronically inflamed gingiva or in GCF of patients, although undetected or at lower levels in healthy control samples [54, 55, 61–67]. GCF from periodontitis patients displays complement-dependent hemolytic activity, suggesting the presence of a functional complement system (C1–C9) in gingival inflammatory exudates [63, 67]. Importantly, induction of experimental gingival inflammation in human volunteers causes progressive elevation of complement cleavage products correlating with increased clinical indices of inflammation [58]. Specifically, this study assessed cleavage of factor B, C3, and C4 in GCF samples during the experimental period and detected, respectively, their conversion to Bb and C3c but not to C4c, suggesting preferential activation of the alternative pathway [58]. On the other hand, the central complement component C3 is among the top 5% genes that are most strongly downregulated following periodontal therapy [68]. Moreover, C3 conversion to C3c in GCF decreases dramatically after periodontal therapy [69]. Mechanistically, local complement activation may promote periodontal inflammation predominantly via C5a-induced vasodilation, increased vascular permeability and flow of inflammatory exudate, and chemotactic recruitment of inflammatory cells, especially neutrophils [70, 71] (Fig. 2). Neutrophils are thought to be key players in host-mediated inflammatory tissue injury in periodontitis [72] and can be found in great numbers in the gingival crevice (≥ 95% of total leukocytes) [73]. Extravasating neutrophils enter the gingival crevice through the junctional epithelium which, under inflamed conditions, is largely occupied (by about 60%) by trafficking neutrophils [73, 74].
Table 1.
Complement-related clinical associations in periodontitis
| Observations | Refs. |
|---|---|
| Activated/functional complement components at significantly higher levels in the GCF of patients than in healthy controls (e.g., factor B, Bb, C3, C3b, C3c, C4, C5a, C9) | [54–58, 63, 67, 69, 73, 166] |
| Activated/functional complement components abundantly found in chronically inflamed gingiva (e.g., C1q, factor B, Bb, C3, C3a, C3b, C3c, C3d, C5, C5b, C9); undetected or at lower levels in healthy control samples | [54, 62, 64–67] |
| Induction of experimental human gingivitis causes progressive elevation of complement cleavage fragments (Bb, C3c) correlating with increased clinical indices | [58] |
| C3 among the top 5% genes that are most strongly downregulated after periodontal therapy | [68] |
| C3 conversion to C3c in GCF increases with increasing periodontal pocket depth but decreases dramatically after periodontal therapy | [69, 167] |
| Aggressive periodontitis with severe gingival angioedema linked to C1INH deficiency | [75] |
| Weaker expression of the CD59 regulatory protein in the gingiva of periodontitis patients compared to healthy controls | [66] |
| Partial C4 gene deficiencies significantly more frequent in periodontal patients compared to healthy controls | [82] |
| Single nucleotide polymorphism of C5 (rs17611) significantly more prevalent in periodontitis patients than in healthy controls | [77] |
Fig. 2. Chemotactic recruitment of inflammatory cells in the gingival crevice.
Inflammatory cells, the majority of which are neutrophils, are recruited to the gingival crevice in response to chemotactic signals such as the complement anaphylatoxin C5a [71, 73, 74], which can be generated either immunologically or through microbial action [87, 150]. Although gingival crevicular neutrophils form what looks like a “defense wall” against the tooth-associated bacteria, they largely fail to control the infection and may cause collateral inflammatory tissue damage [71, 73, 142–144]. The cartoon (on the left) represents magnification of the demarcated tooth area on the right.
Interestingly, a case of aggressive periodontitis accompanied by severe gingival angioedema was linked to dysregulated complement function, specifically C1INH deficiency [75]. Moreover, a single nucleotide polymorphism of C5 (rs17611), which is associated with increased serum C5 levels and susceptibility to liver fibrosis (a complement-associated disease) [76], was shown to be more prevalent in periodontitis patients than in healthy controls [77]. An immunohistochemical study showed weaker expression of CD59 in the gingiva of periodontitis patients compared to healthy controls, suggesting reduced protection of diseased tissues against autologous MAC-mediated tissue damage [66]. Intriguingly, even sublytic amounts of the C5b-9 MAC could cause periodontal tissue destruction. In this regard, non-lethal concentrations of C5b-9 (or the intermediate C5b-8 complex) induce activation of phospholipase A2, release of arachidonic acid, and synthesis of prostaglandin E2 [78–80]. This mechanism can potentially cause periodontal bone loss, since complement induces prostaglandin E2-mediated bone resorption in organ culture, in a C6-dependent way [81].
These observations collectively suggest a role for complement activation in periodontal inflammation and pathogenesis. However, they do not necessarily rule out possible protective functions by at least some complement pathways. In this regard, partial C4 gene deficiencies are significantly more frequent in periodontal patients relative to healthy controls [82], thus suggesting that not all complement pathways or functions necessarily mediate destructive effects. For instance, C3b generated via the classical and/or the lectin pathway could promote opsonization and phagocytosis of periodontal bacteria, thereby contributing to control of infection and of bacterial-induced inflammation.
In vitro studies have shown that periodontal bacteria, such as P. gingivalis, T. denticola, and Prevotella intermedia, interact with the complement system in complex ways that either inhibit or activate specific complement components [60, 71, 83–87] (Table 2). P. gingivalis attenuates the activation of the complement cascade, regardless of the initiation pathway involved (classical, lectin, or alternative), via its ability to degrade and inactivate the central complement component C3 [88, 89]. This proteolytic activity is mediated by its cysteine proteases, known as gingipains. All three gingipain enzymes participate in complement inactivation, although the Arg-specific enzymes (HRgpA and RgpB) are more potent than the Lys-specific gingipain (Kgp) [60]. A similar mechanism is shared by P. intermedia which by means of a cysteine protease, termed interpain A (InpA), can degrade C3 and thereby acquire resistance against the antibacterial activity of complement [84]. Interestingly, P. intermedia not only co-aggregates with P. gingivalis [90] but its interpain synergizes with P. gingivalis gingipains in complement attenuation [84]. This P. gingivalis-P. intermedia synergism may also protect otherwise complement-susceptible bystander bacterial species in the dental plaque biofilm. As a further safety precaution against complement, P. gingivalis employs its HRgpA to capture fluid-phase C4BP on the bacterial cell surface, thereby acquiring the ability to negatively regulate the classical/lectin pathway C3 convertase [91]. In a related context, T. denticola expresses a 11.4-kDa cell surface lipoprotein which can bind fH, and could thus protect the organism against the alternative pathway [86].
Table 2.
Interactions of periodontal pathogens with complement
| Interaction | Pathogens (effector molecules) | Refs. |
|---|---|---|
| Inhibition of complement activation through digestion of the central C3 component |
P. gingivalis (HRgpA, RgpB) termedia (InpA) |
[60, 84] |
| Hijacking complement regulatory proteins (C4BP, Factor H) |
P. gingivalis (HRgpA) T. denticola (11.4-kDa lipoprotein) |
[86, 91] |
| Proteolytic shedding of complement regulatory proteins (CD46) from host cell surface | P. gingivalis (Kgp) | [93] |
| Microbial enzyme-dependent generation of specific complement fragments (anaphylatoxins, iC3b) |
P. gingivalis (HRgpA, RgpB) P. intermedia (InpA) T. denticola (dentilisin) |
[84, 87, 92, 150] |
| Direct binding of complement receptors (CR3) | P. gingivalis (fimbriae) | [119, 120] |
These anti-complement mechanisms notwithstanding, P. gingivalis, P. intermedia, and T. denticola appear to generate specific complement activation fragments through direct enzymatic action on complement proteins [60, 84, 87, 92]. Superficially, these activities seem counterproductive for the adaptive fitness of the bacteria. Moreover, despite their demonstrated ability to inhibit complement at relatively high concentrations, both P. intermedia interpain and P. gingivalis gingipains are able to activate the C1 complex (and thus the classical pathway) at low enzyme concentrations. A possible interpretation of these puzzling findings is that pathogens may better promote their survival by sophisticated manipulation of the complement system rather than by its wholesale inhibition. In this context, P. gingivalis and P. intermedia appear to inhibit critical antimicrobial responses that could eliminate them, whereas they stimulate local inflammatory responses that result in nutrient acquisition (e.g., GCF-derived peptides and hemin, a source of essential iron) and, furthermore, cause collateral tissue damage [71]. Thus, the induced inflammation is non-productive from the host point of view and may consequently become non-resolving and chronic. P. gingivalis may additionally contribute to host tissue damage by causing proteolytic shedding of CD46 from the surface of oral epithelial cells, thus rendering them potentially susceptible to unintended complement attack [93]. Therefore, periodontal pathogens appear to have evolved in ways that allow them to not only endure inflammation but also exploit it for promoting their survival and, collaterally, causing tissue injury.
From the above discussion, it becomes necessary to identify the precise roles, protective or destructive, of the various complement pathways and components before rational therapeutic intervention is applied for the treatment of periodontal disease. It is also important to identify which pathways/components are subverted by bacteria in ways that deregulate the host response. These objectives would necessitate a systematic approach in preclinical models of this disease, employing mechanistic and interventional studies, before confirmation can be pursued in human studies. Indeed, causal mechanistic relationships cannot normally be addressed in human studies due to important ethical considerations [94]. However, once a safe and effective therapeutic compound has been identified in preclinical models, it could justifiably move into human clinical trials.
4. Inflammatory diseases and potential for complementary therapy
In addition to periodontitis, complement is activated in a variety of systemic or local autoimmune or inflammatory conditions, including systemic lupus erythematosus, rheumatoid arthritis, sepsis, ischemia/reperfusion injury, myocardial infarction and atherosclerosis, allergy and asthma, inflammatory bowel disease, Alzheimer s disease, multiple sclerosis, organ graft rejection, and age-related macular degeneration [9, 10, 13]. Complement is often activated locally at sites of tissue destruction but it can also cause disease through systemic activation as in sepsis [10]. Although complement may be overactivated in a subset of patients as a result of inadequate complement regulation (polymorphisms or abnormalities of complement control proteins), the precise role of complement in immune pathology is largely unknown; therefore, animal models are often employed to offer useful mechanistic insights [1, 2, 12, 13, 42, 95, 96].
Since C3 is the central complement component in which all three activation pathways converge, therapeutic inhibition of C3 could be a reasonably effective approach to treat complement-related diseases. However, it cannot be assumed that all three activation mechanisms are harmful in a given disease. It is possible that a certain pathway is overactivated and contributes to unwarranted inflammation, while another pathway is activated in a controlled manner and contributes to host defense. For example, the classical pathway appears to be specifically implicated in certain inflammatory conditions (e.g., hyperacute xenograft rejection or acute myocardial infarction), whereas the lectin pathway has been associated with protection against upper respiratory infections and community-acquired pneumonia [97–100]. On the other hand, the classical pathway, but not the lectin pathway, is important for innate immunity to group B Streptococcus [101]. The alternative pathway may represent up to 80–90% of complement activation [1, 39] and is important for protective immunity against certain pathogens (e.g., Streptococcus pyogenes [102]). However, the alternative pathway is thought to be heavily involved in several complement-dependent pathologic conditions [14]. Thus, there is strong rationale for selective inhibition of specific complement pathways (or defined components) implicated in pathology, in order to keep intact those pathways or components that may mediate protective functions [99, 103]. In fact, complete complement inhibition at the C3 level to prevent inflammatory tissue damage may compromise host defense and thus increase the risk of infections. However, in case of topical interventions to treat local inflammatory diseases, such risks should be relatively minimal. Risks should also be reduced in diseases requiring short-term treatment, such as myocardial infarction or stroke. Several complement-specific drugs are currently under clinical development or have received approval, although this topic is outside the purview of this paper and the reader is referred to specialized reviews [9, 13]. Briefly, the first complement-targeted drugs, including recombinant C1-INH (for the treatment of hereditary angioedema) and a blocking anti-C5 antibody (for paroxysmal nocturnal hemoglobulinuria) have already been approved and reached the market. Moreover, other drugs are in clinical trials for various diseases, including the C3 inhibitor compstatin (POT-4) which completed phase I trials (for age-related macular degeneration) and the C5aR antagonist PMX-53, which completed phase II trials (for rheumatoid arthritis and psoriasis) [9, 13]. Following below is discussion on potential complement-targeted therapeutic interventions in periodontitis on the basis of data from preclinical models.
5. CR3 in periodontal pathogenesis and implications for therapeutic intervention
CR3 is a β2 integrin (CD11b/CD18) that plays diverse roles in immunity and inflammation, including iC3b-mediated phagocytosis, promotion of leukocyte migration to sites of extravascular inflammation, and induction of cytokine responses [104]. In this latter context, CR3 was shown to co-associate with pattern-recognition receptors, such as CD14 and TLRS (TLR2 and TLR4), in membrane lipid rafts of activated phagocytes [105, 106]. In addition to its binding affinity for host molecules (iC3b, fibrinogen, and intercellular adhesion molecule-1 [ICAM-1]), CR3 can also interact with diverse microbial molecules. These include enterobacterial LPS, Bordetella pertussis filamentous hemagglutinin, Leishmania gp63, and P. gingivalis fimbriae [107–111]. The adhesive interactions of CR3 are tightly regulated through inside-out signaling. Indeed, whereas the default conformation of CR3 in resting cells is of low affinity, a rapid and transient shift to a high-affinity binding state can be triggered by inside-out signals generated by other receptors, such as chemokine receptors or TLRs [112, 113].
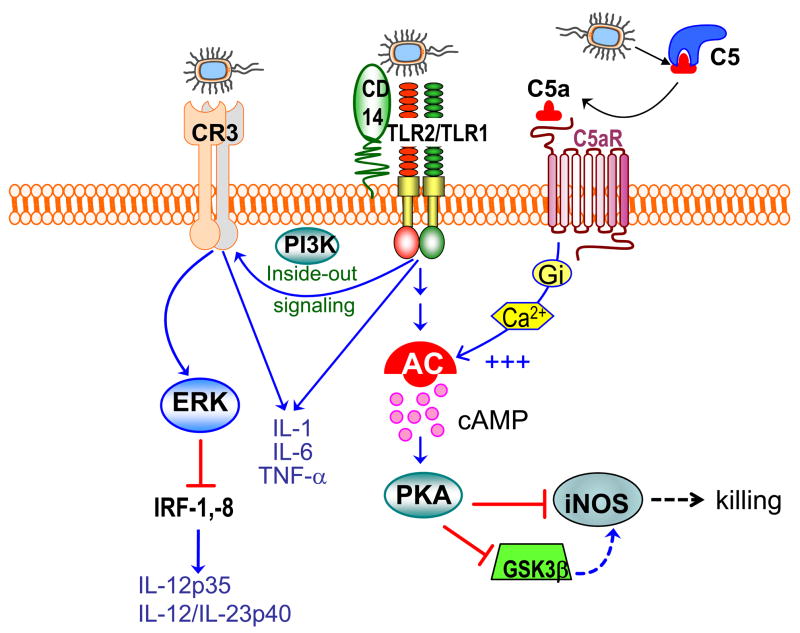
P. gingivalis is particularly strong in activating CR3. Specifically, the fimbriae of P. gingivalis bind CD14 and activate TLR2- and phosphatidylinositol 3-kinase (PI3K)-mediated inside-out signaling leading to activation of the ligand-binding capacity of CR3 [113]. Activated CR3 can in turn interact directly with this bacterium [114] (Fig. 3). The interactions of CR3 on monocytes or macrophages with P. gingivalis lead to inflammatory responses, such as induction of certain cytokines (TNF-α, IL-1β, and IL-6) [106, 110, 115] and stimulation of monocyte adhesion to endothelial ICAM-1, leading to transmigration across endothelial cell monolayers [116].
Fig. 3. Complement cross-talk pathways and their exploitation by P. gingivalis.
P. gingivalis is recognized by the CD14/TLR2/TLR1 receptor complex [106]. This interaction induces PI3K-dependent inside-out signaling, which induces the high-affinity conformation of CR3 [113, 169]. Once in the high-affinity state, CR3 binds P. gingivalis leading to induction of ERK1/2 signaling. This in turn downregulates IL-12 p35 and p40 mRNA expression [119], possibly through suppression of critical transcription factors (the interferon regulatory factors 1 and 8; IRF-1, −8), required for the expression of IL-12 family cytokines [129]. This suppressive effect is specific for IL-12 since induction of proinflammatory cytokines (e.g., IL-1β, IL-6, and TNF-α) is upregulated. Inhibition of bioactive IL-12 through this mechanism in vivo results in impaired immune clearance of P. gingivalis [119]. Moreover, P. gingivalis uses its gingipains to attack C5 and release biologically C5a [87, 150]. Upon C5aR binding, C5a stimulates Gαi-dependent intracellular Ca2+ signaling which synergistically enhances the otherwise weak cAMP responses induced by TLR2/TLR1 activation alone. The ensuing activation of the cAMP-dependent protein kinase A (PKA) pathway inactivates glycogen synthase kinase-3β (GSK3β) and impairs the inducible nitrogen synthase (iNOS)-dependent killing of the pathogen in macrophages in vitro and in vivo [87] .
These P. gingivalis-induced CR3-dependent responses could potentially contribute to innate host defense. However, it is also possible that they may have pathophysiological consequences in periodontal disease. In this regard, CR3-induced cytokines such as TNF-α, IL-1β, and IL-6 can cause periodontal bone resorption [26], whereas CR3-dependent migration of inflammatory cells could amplify periodontal inflammation. Neutrophils, which are implicated as major effectors of inflammation-induced tissue damage in periodontitis [72], express high levels of CR3 which facilitates their trafficking to sites of extravascular inflammation [117, 118]. It is thus plausible that P. gingivalis may promote periodontal tissue recruitment of neutrophils or other inflammatory phagocytes through activation of CR3, thereby exacerbating periodontal inflammation.
Although the above mechanistic scenario for CR3-mediated inflammation and tissue destruction in periodontitis is hypothetical, it was nonetheless shown that CR3 indeed participates in periodontal pathogenesis. Specifically, in a mouse model of P. gingivalis-induced periodontitis, CR3 blockade through local application of a small-molecule antagonist (XVA143) inhibits induction of periodontal bone loss [119]. However, it is not known whether the beneficial effects of CR3 inhibition are mediated via direct control of periodontal inflammation. An alternative (or additional) possibility involves a mechanism that may enhance P. gingivalis adaptive fitness.
The alternative possibility is related to the capacity of P. gingivalis to exploit CR3 for entering macrophages in a way that promotes its persistence. Indeed, the intracellular survival of P. gingivalis is significantly reduced in CR3-deficient (CD11b−/−) mouse macrophages, suggesting that CR3-dependent phagocytosis of P. gingivalis does not promote its killing [120]. This could be attributed to the fact that CR3 is not linked to vigorous microbicidal mechanisms, in contrast to certain other phagocytic receptors such as the Fcγ receptor III (CD16) [121–125]. In macrophages, for instance, CD16-derived phagosomes fuse more readily with lysosomes than CR3-derived phagosomes [126]. A plausible interpretation for the relatively mild CR3 post-phagocytic events is that CR3 is heavily committed with phagocytosis of iC3b-opsonized apoptotic cells, which normally pose minimal danger and thus do not warrant strong inflammatory responses [127, 128]. Thus, P. gingivalis appears to co-opt this relatively non-inflammatory phagocytic mechanism. Intriguingly, although T. denticola is not known to directly bind CR3, it expresses a serine protease (dentilisin) which generates iC3b upon hydrolysis of the α chain of C3 [92]. This is likely to promote iC3b-mediated CR3 uptake of T. denticola, which might thereby promote its survival, like P. gingivalis does; however, direct experimental evidence is currently lacking.
There are additional CR3-associated benefits for P. gingivalis. Although P. gingivalis ligation of monocyte/macrophage CR3 promotes the induction of several proinflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8), the induction of IL-12 in those same cells is downregulated [110, 119]. Specifically, CR3 ligation by P. gingivalis activates outside in signaling and extracellular signal-related kinase 1/2 (ERK1/2) activation, which in turn selectively inhibits mRNA expression of the IL-12 p35 and p40 subunits and production of IL-12 protein [119]; this mechanism possibly involves suppression of the interferon regulatory factors 1 and 8, which are critical for the transcription of IL-12 family cytokine gens [129] (Fig. 3). Additionally, P. gingivalis blocks IL-12 induction by other bacterial stimuli (e.g., LPS from Aggregatibacter actinomycetemcomitans, another important periodontal pathogen), suggesting that this immune subversion strategy may also benefit co-habiting bacteria occupying the same niche [119]. The selective CR3-dependent inhibition of IL-12 is not specific to P. gingivalis activation, but is a general feature of CR3 outside-in signaling [130], also occurring during phagocytosis of apoptotic cells [127]. As a result of this mechanism (Fig. 3), CR3-deficient mice elicit higher levels of IL-12 (and, secondarily, IFN-γ) leading to enhanced clearance of P. gingivalis infection compared to wild-type mice [119]. Importantly, similar host-defense promoting effects are seen in wild-type mice in which CR3 is pharmacologically blocked, suggesting the utility of CR3 inhibitors for therapeutic control of P. gingivalis infections [119].
Like many other chronic inflammatory diseases, the prevalence and severity of periodontitis increases with aging, although it is not clear whether, or what kind of, age-related changes in innate immunity are responsible [131]. Interestingly, although phagocytosis generally declines with aging [132, 133], CR3-dependent phagocytosis remains intact [134]. Specifically, unlike CD16-mediated phagocytosis which declines in elderly individuals owing to age-associated downregulation of CD16 expression, CD11b expression is preserved in old age [134]. It could thus be expected that CR3-mediated phagocytosis of P. gingivalis may be preserved with aging, whereas alternative uptake of the pathogen by strongly microbicidal pathways may decline. In relative terms, therefore, CR3-mediated internalization of P. gingivalis may increase with aging. Interestingly, not only CR3 but also other receptors involved in the inside-out pathway for CR3 activation (CD14 and TLR2) display increased expression in the gingiva of old mice relative to young controls [135]. This suggests that the microbial exploitability of CR3 and its impact on periodontitis could increase with aging. In the same study, only a subset of investigated innate immune receptors exhibited age-associated differential expression. Among them is C5aR [135], which could contribute to heightened periodontal inflammation, owing to its involvement in the amplification of the host inflammatory response [10].
6. Rationale for targeting the C5a-C5aR axis in periodontitis
The complement-activated fragment C5a is perhaps the most powerful effector of the complement cascade, mediating, among other functions, chemotactic recruitment and activation of neutrophils and other inflammatory cells [136]. Although the immunostimulatory and inflammatory properties of C5a can potentially protect the host against pathogens, they can also contribute to the pathogenesis of a number of acute or chronic inflammatory diseases, such as sepsis, acute lung injury, ischemia-reperfusion injury, and rheumatoid arthritis [136, 137]. C5a-induced inflammation is predominantly mediated via C5aR (CD88), although recent evidence suggests that C5L2 (GPR77) may synergize with C5aR at least in septic inflammation [42].
In addition to their role in acute inflammation, neutrophils have been implicated in chronic inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel disease, chronic obstructive pulmonary disease, and periodontitis [72, 138–141]. Intriguingly, although gingival crevicular neutrophils form what looks like a “defense wall” against the advancing microbial hordes (Fig. 2), they largely fail to control the bacteria despite being viable and capable of eliciting responses, including release of reactive oxygen species [73, 142–145]. Since reactive oxygen species do not discriminate between microbial and host cells, they are more likely to cause collateral damage to periodontal tissues [146–148] than to control the infection. In this regard, many oral bacteria, including P. gingivalis, are resistant to oxidative burst killing [71, 149]. The reasons for the relative impotence of neutrophils to control periodontal infection (and in failing to do so to promote non-resolving inflammation) are largely unexplored. However, P. gingivalis may contribute to subversion of leukocyte function by employing specific C5 convertase-like enzymes to generate high levels of C5a, which the bacterium exploits to promote its survival and non-productive inflammation that is destructive for the host [71, 87, 150].
Specifically, P. gingivalis employs its Arg-specific gingpains to generate functional C5a through limited degradation of C5, whereas the C5b remnant is proteolytically destroyed [60, 150], to apparently prevent activation of the terminal complement pathway. The bacterium can actually generate high concentrations of C5a (> 30 nM) after a 30-min incubation in heat-inactivated human serum [87]. This activity is curious and seemingly counterproductive (if not suicidal) for P. gingivalis, especially since this organism goes at great lengths to suppress all three mechanisms of complement activation [60]. It is also antithetical to the strategy of Staphylococcus aureus which actually blocks C5a binding and C5aR activation, via a secreted chemotaxis inhibitory protein [151]. A possible interpretation of this unusual behavior is that proactive release of C5a by P. gingivalis can contribute to stimulation of inflammatory exudate for acquisition of essential nutrients like hemin [71].
Another possible scenario is that local generation of excessive levels of C5a (through gingipain activity and/or immunological means) could paralyze the antimicrobial function of crevicular neutrophils rendering them less effective against P. gingivalis and bystander bacteria. Indeed, neutrophils become immunologically paralyzed, both in vitro and in vivo, when in the presence of high concentrations (10–100 nM) of C5a [10, 152, 153]. Consistent with this, the ability of neutrophils to kill P. gingivalis is inhibited by C5a; however, this occurs even at low C5a concentrations that could not cause paralysis but, on the contrary, enhance the oxidative burst (J.L. Krauss and G. Hajishengallis, unpublished data). Whether the underlying mechanism involves alteration of specific signal transduction pathways is currently under investigation. On the other hand, it has been firmly established that P. gingivalis exploits C5a to impair the killing function of macrophages via manipulation of specific signaling events in the absence of generalized immune suppression [87]. In fact, macrophages express modest levels of C5aR relative to the neutrophils and are, therefore, quite resistant to the deleterious effects of high C5a concentrations [10].
The above alluded C5a-dependent evasive mechanism of P. gingivalis in macrophages involves synergistic production of high and sustained cAMP levels, which inhibit nitric oxide-dependent killing of P. gingivalis [87]. This synergism requires a crosstalk between C5a-activated C5aR and P. gingivalis-activated TLR2, whereas downstream players include cAMP-dependent protein kinase A and glycogen synthase kinase-3β, the interplay of which inhibits the inducible nitric oxide synthase [87] (Fig. 3). Importantly, specific blockade of C5aR with the PMX-53 antagonist abrogates this evasive strategy and facilitates the immune clearance of P. gingivalis in vivo [87]. In the periodontal environment, macrophages can interact with P. gingivalis in the gingival crevice, where they can be chemoattracted (though at lower numbers than neutrophils), and in the underlying connective tissue where P. gingivalis may invade (Fig. 2). Additionally, P. gingivalis-macrophage interactions can also occur in the setting of systemic inflammatory diseases such as atherosclerosis [71, 73, 154].
The above discussed findings suggest that C5aR inhibitors may have important therapeutic implications in periodontitis, and perhaps other infections or inflammatory diseases where P. gingivalis is thought to be implicated (e.g., oral aspiration pneumonia and atherosclerosis [155, 156]). Interestingly, at least in the mouse model, C5aR is expressed at higher levels in aged macrophages or in the periodontal tissues of aged mice, compared to their young counterparts [135, 157]. In addition to its potential exploitation by P. gingivalis for immune evasion, C5a may amplify periodontal tissue damage through its ability to recruit and activate inflammatory cells, and stimulate the induction of reactive oxygen species [136, 148]. On the other hand, P. gingivalis and many other oral bacteria are resistant to oxidative killing [149, 158]. Moreover, C5aR signaling has been implicated in bone immunopathology, at least in rheumatoid arthritis [137, 159]. Therefore, there is sufficient rationale for testing C5aR antagonists in the treatment of periodontitis, although direct implication of C5aR signaling in periodontal tissue destruction remains to be established.
7. Conclusions and future directions
Clinical, animal model-based, and in vitro mechanistic studies strongly suggest an important role for complement in periodontal inflammation and pathogenesis. However, there have not been as yet any systematic approaches to comprehensively identify the precise roles of the various complement pathways in the context of periodontal pathogenesis. Given that complement plays an orchestrating role in host immunity and inflammation [1, 2, 6, 95, 160], its functional mapping in periodontitis will greatly facilitate complement-targeted therapeutic intervention. Indeed, such information will indicate which specific pathways need to be blocked to reverse inflammatory pathology or, conversely, be enhanced to promote host defense. Moreover, neutralization of identified microbial tactics for complement subversion could help restore normal regulation of complement activation. In this translational context, a number of complement-specific drugs are already in clinical trials for other inflammatory diseases [9, 13]. Information on the safety and efficacy of these drugs will also be of relevance in human periodontitis. The current challenge, however, is to use appropriate preclinical models to elucidate the precise roles, protective or destructive, of the various pathways or components of the complement system, in order to rationally apply suitable therapeutic intervention. At a first stage, these studies may take advantage of the availability of a panel of transgenic mice deficient in key complement components [3, 42, 161–163]. Complement knockout mice could be tested against wild-type controls in periodontitis models. These include human pathogen-induced periodontitis in young mice (e.g., inflammatory periodontal bone loss induced by oral infection with P. gingivalis) [94, 119, 164] and naturally occurring periodontitis developed by aged mice, in a way analogous to elderly humans [131, 135]. In the latter model, specific complement-deficient mice could be raised in parallel with normal controls and monitored over time for possible differential susceptibility to periodontitis. The same models could subsequently be used for translational studies, followed by validation of possible safety and efficacy of therapeutic interventions in primate models of periodontitis [27, 165], prior to initiating human clinical trials. If proven effective, complementary therapeutic intervention in parallel with established clinical periodontal treatment could revolutionize the way periodontal patients are managed.
Acknowledgments
Studies performed in the author s laboratory and cited in this paper were supported by U.S. Public Health Service Grants DE015254 and DE018292.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–27. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–63. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, et al. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–36. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo RF, Riedemann NC, Ward PA. Role of C5a-C5aR interaction in sepsis. Shock. 2004;21:1–7. doi: 10.1097/01.shk.0000105502.75189.5e. [DOI] [PubMed] [Google Scholar]
- 5.Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu Rev Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- 6.Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 7.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–35. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver DJ, Jr, Reis ES, Pandey MK, Kohl G, Harris N, Gerard C, et al. C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur J Immunol. 2010;40:710–21. doi: 10.1002/eji.200939333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25:1265–75. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4:133–42. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 11.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–40. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 12.Pettigrew HD, Teuber SS, Gershwin ME. Clinical significance of complement deficiencies. Ann N Y Acad Sci. 2009;1173:108–23. doi: 10.1111/j.1749-6632.2009.04633.x. [DOI] [PubMed] [Google Scholar]
- 13.Wagner E, Frank MM. Therapeutic potential of complement modulation. Nat Rev Drug Discov. 2010;9:43–56. doi: 10.1038/nrd3011. [DOI] [PubMed] [Google Scholar]
- 14.Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol Rev. 2008;223:300–16. doi: 10.1111/j.1600-065X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 15.Armitage GC. Classifying periodontal diseases--a long-standing dilemma. Periodontol 2000. 2002;30:9–23. doi: 10.1034/j.1600-0757.2002.03002.x. [DOI] [PubMed] [Google Scholar]
- 16.Hajishengallis G. Toll gates to periodontal host modulation and vaccine therapy. Periodontol 2000. 2009;51:181–207. doi: 10.1111/j.1600-0757.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 18.Brown LJ, Johns BA, Wall TP. The economics of periodontal diseases. Periodontol 2000. 2002;29:223–34. doi: 10.1034/j.1600-0757.2002.290111.x. [DOI] [PubMed] [Google Scholar]
- 19.Xiong X, Buekens P, Fraser WD, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: a systematic review. Bjog. 2006;113:135–43. doi: 10.1111/j.1471-0528.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- 20.Awano S, Ansai T, Takata Y, Soh I, Akifusa S, Hamasaki T, et al. Oral health and mortality risk from pneumonia in the elderly. J Dent Res. 2008;87:334–9. doi: 10.1177/154405910808700418. [DOI] [PubMed] [Google Scholar]
- 21.Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR, Jr, Sacco RL, et al. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Circulation. 2005;111:576–82. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–20. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 23.Paju S, Scannapieco FA. Oral biofilms, periodontitis, and pulmonary infections. Oral Dis. 2007;13:508–12. doi: 10.1111/j.1601-0825.2007.1410a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Pablo P, Chapple IL, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5:218–24. doi: 10.1038/nrrheum.2009.28. [DOI] [PubMed] [Google Scholar]
- 25.Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res. 2008;87:817–28. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79:1585–91. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 27.Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–9. [PubMed] [Google Scholar]
- 28.Ohyama H, Kato-Kogoe N, Kuhara A, Nishimura F, Nakasho K, Yamanegi K, et al. The involvement of IL-23 and the Th17 pathway in periodontitis. J Dent Res. 2009;88:633–8. doi: 10.1177/0022034509339889. [DOI] [PubMed] [Google Scholar]
- 29.Dutzan N, Gamonal J, Silva A, Sanz M, Vernal R. Over-expression of forkhead box P3 and its association with receptor activator of nuclear factor- B ligand, interleukin (IL)-17, IL-10 and transforming growth factor- during the progression of chronic periodontitis. J Clin Periodontol. 2009;36:396–403. doi: 10.1111/j.1600-051X.2009.01390.x. [DOI] [PubMed] [Google Scholar]
- 30.Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Junior WM, Rossi MA, et al. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol. 2009;24:1–6. doi: 10.1111/j.1399-302X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 31.Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol. 2005;32:383–9. doi: 10.1111/j.1600-051X.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- 32.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 33.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the "red complex", a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 34.Darveau RP. Periodontitis: a polymicrobial bacterial-induced disruption of host homeostasis. Nat Rev Microbiol. 2010 doi: 10.1038/nrmicro2337. in press. [DOI] [PubMed] [Google Scholar]
- 35.Hajishengallis G. Porphyromonas gingivalis-host interactions: open war or intelligent guerilla tactics? Microbes Infect. 2009;11:637–45. doi: 10.1016/j.micinf.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darveau RP. The oral microbial consortium's interaction with the periodontal innate defense system. DNA Cell Biol. 2009;28:389–95. doi: 10.1089/dna.2009.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol. 2007;179:2600–8. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- 38.Kimura Y, Miwa T, Zhou L, Song W-C. Activator-specific requirement of properdin in the initiation and amplification of the alternative pathway complement. Blood. 2008;111:732–40. doi: 10.1182/blood-2007-05-089821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harboe M, Mollnes TE. The alternative complement pathway revisited. J Cell Mol Med. 2008;12:1074–84. doi: 10.1111/j.1582-4934.2008.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, et al. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem. 2010;285:7633–44. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen NJ, Mirtsos C, Suh D, Lu YC, Lin WJ, McKerlie C, et al. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature. 2007;446:203–7. doi: 10.1038/nature05559. [DOI] [PubMed] [Google Scholar]
- 42.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, et al. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14:551–7. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scola AM, Johswich KO, Morgan BP, Klos A, Monk PN. The human complement fragment receptor, C5L2, is a recycling decoy receptor. Mol Immunol. 2009;46:1149–62. doi: 10.1016/j.molimm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monk PN, Scola AM, Madala P, Fairlie DP. Function, structure and therapeutic potential of complement C5a receptors. Br J Pharmacol. 2007;152:429–48. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim DD, Song WC. Membrane complement regulatory proteins. Clin Immunol. 2006;118:127–36. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Heinen S, Hartmann A, Lauer N, Wiehl U, Dahse H-M, Schirmer S, et al. Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood. 2009;114:2439–47. doi: 10.1182/blood-2009-02-205641. [DOI] [PubMed] [Google Scholar]
- 47.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–92. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Jalili A, Shirvaikar N, Marquez-Curtis L, Qiu Y, Korol C, Lee H, et al. Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: Further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells. Exp Hematol. 2010;38:321–32. doi: 10.1016/j.exphem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee HM, Wysoczynski M, Liu R, Shin DM, Kucia M, Botto M, et al. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia. 2009;24:573–82. doi: 10.1038/leu.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Longhi MP, Harris CL, Morgan BP, Gallimore A. Holding T cells in check--a new role for complement regulators? Trends Immunol. 2006;27:102–8. doi: 10.1016/j.it.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Wagner C, Ochmann C, Schoels M, Giese T, Stegmaier S, Richter R, et al. The complement receptor 1, CR1 (CD35), mediates inhibitory signals in human T-lymphocytes. Mol Immunol. 2006;43:643–51. doi: 10.1016/j.molimm.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–66. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6:132–42. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Attstrom R, Laurel AB, Lahsson U, Sjoholm A. Complement factors in gingival crevice material from healthy and inflamed gingiva in humans. J Periodont Res. 1975;10:19–27. doi: 10.1111/j.1600-0765.1975.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 55.Schenkein HA, Genco RJ. Gingival fluid and serum in periodontal diseases. II. Evidence for cleavage of complement components C3, C3 proactivator (factor B) and C4 in gingival fluid. J Periodontol. 1977;48:778–84. doi: 10.1902/jop.1977.48.12.778. [DOI] [PubMed] [Google Scholar]
- 56.Schenkein HA, Genco RJ. Gingival fluid and serum in periodontal diseases. I. Quantitative study of immunoglobulins, complement components, and other plasma proteins. J Periodontol. 1977;48:772–7. doi: 10.1902/jop.1977.48.12.772. [DOI] [PubMed] [Google Scholar]
- 57.Challacombe SJ, Shirlaw PJ. Immunology of diseases of the oral cavity. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal Immunology. Elsevier Academic Press; 2005. pp. 1517–46. [Google Scholar]
- 58.Patters MR, Niekrash CE, Lang NP. Assessment of complement cleavage in gingival fluid during experimental gingivitis in man. J Clin Periodontol. 1989;16:33–7. doi: 10.1111/j.1600-051x.1989.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 59.Lamster IB, Ahlo JK. Analysis of gingival crevicular fluid as applied to the diagnosis of oral and systemic diseases. Ann N Y Acad Sci. 2007;1098:216–29. doi: 10.1196/annals.1384.027. [DOI] [PubMed] [Google Scholar]
- 60.Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J Immunol. 2007;178:7242–50. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- 61.Schenkein HA, Genco RJ. Complement cleavage products in inflammatory exudates from patients with periodontal diseases. J Immunol. 1978;120:1796. [Google Scholar]
- 62.Lally ET, McArthur WP, Baehni PC. Biosynthesis of complement components in chronically inflamed gingiva. J Periodontal Res. 1982;17:257–62. doi: 10.1111/j.1600-0765.1982.tb01152.x. [DOI] [PubMed] [Google Scholar]
- 63.Courts FJ, Boackle RJ, Fudenberg HH, Silverman MS. Detection of functional complement components in gingival crevicular fluid from humans with periodontal diseases. J Dent Res. 1977;56:327–31. doi: 10.1177/00220345770560032001. [DOI] [PubMed] [Google Scholar]
- 64.Toto PD, Lin L, Gargiulo A. Identification of C3a, IgG, IgM in inflamed human gingiva. J Dent Res. 1978;57:696. doi: 10.1177/00220345780570050501. [DOI] [PubMed] [Google Scholar]
- 65.Nikolopoulou-Papaconstantinou AA, Johannessen AC, Kristoffersen T. Deposits of immunoglobulins, complement, and immune complexes in inflamed human gingiva. Acta Odontol Scand. 1987;45:187–93. doi: 10.3109/00016358709098858. [DOI] [PubMed] [Google Scholar]
- 66.Rautemaa R, Meri S. Protection of gingival epithelium against complement-mediated damage by strong expression of the membrane attack complex inhibitor protectin (CD59) J Dent Res. 1996;75:568–74. doi: 10.1177/00220345960750010901. [DOI] [PubMed] [Google Scholar]
- 67.Boackle RJ. The interaction of salivary secretions with the human complement system--a model for the study of host defense systems on inflamed mucosal surfaces. Crit Rev Oral Biol Med. 1991;2:355–67. doi: 10.1177/10454411910020030401. [DOI] [PubMed] [Google Scholar]
- 68.Beikler T, Peters U, Prior K, Eisenacher M, Flemmig TF. Gene expression in periodontal tissues following treatment. BMC Med Genomics. 2008;1:30. doi: 10.1186/1755-8794-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niekrash CE, Patters MR. Simultaneous assessment of complement components C3, C4, and B and their cleavage products in human gingival fluid. II. Longitudinal changes during periodontal therapy. J Periodontal Res. 1985;20:268–75. doi: 10.1111/j.1600-0765.1985.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 70.Snyderman R. Role for endotoxin and complement in periodontal tissue destruction. J Dent Res. 1972;51:356–61. doi: 10.1177/00220345720510022201. [DOI] [PubMed] [Google Scholar]
- 71.Krauss JL, Potempa J, Lambris JD, Hajishengallis G. Complementary Tolls in the periodontium: how periodontal bacteria modify complement and Toll-like receptor responses to prevail in the host. Periodontol 2000. 2010;52:141–62. doi: 10.1111/j.1600-0757.2009.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- 73.Delima AJ, Van Dyke TE. Origin and function of the cellular components in gingival crevice fluid. Periodontol 2000. 2003;31:55–76. doi: 10.1034/j.1600-0757.2003.03105.x. [DOI] [PubMed] [Google Scholar]
- 74.Schroeder HE. Transmigration and infiltration of leucocytes in human junctional epithelium. Helv Odontol Acta. 1973;17:6–18. [PubMed] [Google Scholar]
- 75.Roberts A, Shah M, Chapple IL. C-1 esterase inhibitor dysfunction localised to the periodontal tissues: clues to the role of stress in the pathogenesis of chronic periodontitis? J Clin Periodontol. 2003;30:271–7. doi: 10.1034/j.1600-051x.2003.01266.x. [DOI] [PubMed] [Google Scholar]
- 76.Hillebrandt S, Wasmuth HE, Weiskirchen R, Hellerbrand C, Keppeler H, Werth A, et al. Complement factor 5 is a quantitative trait gene that modifies liver fibrogenesis in mice and humans. Nat Genet. 2005;37:835–43. doi: 10.1038/ng1599. [DOI] [PubMed] [Google Scholar]
- 77.Chai L, Song Y-Q, Zee K-Y, Leung WK. Single nucleotide polymorphisms of complement component 5 and periodontitis. J Periodont Res. 2010;45:301–8. doi: 10.1111/j.1600-0765.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- 78.Niculescu F, Rus H. Mechanisms of signal transduction activated by sublytic assembly of terminal complement complexes on nucleated cells. Immunol Res. 2001;24:191–9. doi: 10.1385/ir:24:2:191. [DOI] [PubMed] [Google Scholar]
- 79.Cybulsky AV, Takano T, Papillon J, McTavish AJ. Complement-induced phospholipase A2 activation in experimental membranous nephropathy. Kidney Int. 2000;57:1052–62. doi: 10.1046/j.1523-1755.2000.00932.x. [DOI] [PubMed] [Google Scholar]
- 80.Daniels RH, Houston WA, Petersen MM, Williams JD, Williams BD, Morgan BP. Stimulation of human rheumatoid synovial cells by non-lethal complement membrane attack. Immunology. 1990;69:237–42. [PMC free article] [PubMed] [Google Scholar]
- 81.Raisz LG, Sandberg AL, Goodson JM, Simmons HA, Mergenhagen SE. Complement-dependent stimulation of prostaglandin synthesis and bone resorption. Science. 1974;185:789–91. doi: 10.1126/science.185.4153.789. [DOI] [PubMed] [Google Scholar]
- 82.Seppanen M, Lokki ML, Notkola IL, Mattila K, Valtonen V, Nieminen A, et al. Complement and c4 null alleles in severe chronic adult periodontitis. Scand J Immunol. 2007;65:176–81. doi: 10.1111/j.1365-3083.2006.01886.x. [DOI] [PubMed] [Google Scholar]
- 83.Slaney JM, Gallagher A, Aduse-Opoku J, Pell K, Curtis MA. Mechanisms of resistance of Porphyromonas gingivalis to killing by serum complement. Infect Immun. 2006;74:5352–61. doi: 10.1128/IAI.00304-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Potempa M, Potempa J, Kantyka T, Nguyen KA, Wawrzonek K, Manandhar SP, et al. Interpain A, a cysteine proteinase from Prevotella intermedia, inhibits complement by degrading complement factor C3. PLoS Pathog. 2009;5:e1000316. doi: 10.1371/journal.ppat.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hajishengallis G, Wang M, Liang S, Shakhatreh MA, James D, Nishiyama S, et al. Subversion of innate immunity by periodontopathic bacteria via exploitation of complement receptor-3. Adv Exp Med Biol. 2008;632:203–19. doi: 10.1007/978-0-387-78952-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McDowell JV, Huang B, Fenno JC, Marconi RT. Analysis of a unique interaction between the complement regulatory protein factor H and the periodontal pathogen Treponema denticola. Infect Immun. 2009;77:1417–25. doi: 10.1128/IAI.01544-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang M, Krauss JL, Domon H, Hosur KB, Liang S, Magotti P, et al. Microbial hijacking of complement-Toll-like receptor crosstalk. Sci Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Potempa J, Pike RN. Corruption of innate immunity by bacterial proteases. J Innate Immun. 2009;1:70–87. doi: 10.1159/000181144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Slaney JM, Curtis MA. Mechanisms of evasion of complement by Porphyromonas gingivalis. Front Biosci. 2008;13:188–96. doi: 10.2741/2669. [DOI] [PubMed] [Google Scholar]
- 90.Kamaguchi A, Ohyama T, Sakai E, Nakamura R, Watanabe T, Baba H, et al. Adhesins encoded by the gingipain genes of Porphyromonas gingivalis are responsible for co-aggregation with Prevotella intermedia. Microbiology. 2003;149:1257–64. doi: 10.1099/mic.0.25997-0. [DOI] [PubMed] [Google Scholar]
- 91.Potempa M, Potempa J, Okroj M, Popadiak K, Eick S, Nguyen KA, et al. Binding of complement inhibitor C4b-binding protein contributes to serum resistance of Porphyromonas gingivalis. J Immunol. 2008;181:5537–44. doi: 10.4049/jimmunol.181.8.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamazaki T, Miyamoto M, Yamada S, Okuda K, Ishihara K. Surface protease of Treponema denticola hydrolyzes C3 and influences function of polymorphonuclear leukocytes. Microbes Infect. 2006;8:1758–63. doi: 10.1016/j.micinf.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 93.Mahtout H, Chandad F, Rojo JM, Grenier D. Porphyromonas gingivalis mediates the shedding and proteolysis of complement regulatory protein CD46 expressed by oral epithelial cells. Oral Microbiol Immunol. 2009;24:396–400. doi: 10.1111/j.1399-302X.2009.00532.x. [DOI] [PubMed] [Google Scholar]
- 94.Graves DT, Fine D, Teng Y-TA, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Friec GL, Kemper C. Complement: coming full circle. Arch Immunol Ther Exp (Warsz) 2009;57:393–407. doi: 10.1007/s00005-009-0047-4. [DOI] [PubMed] [Google Scholar]
- 96.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, et al. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–35. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Griselli M, Herbert J, Hutchinson WL, Taylor KM, Sohail M, Krausz T, et al. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med. 1999;190:1733–40. doi: 10.1084/jem.190.12.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garcia-Laorden MI, Sole-Violan J, Rodriguez de Castro F, Aspa J, Briones ML, Garcia-Saavedra A, et al. Mannose-binding lectin and mannose-binding lectin-associated serine protease 2 in susceptibility, severity, and outcome of pneumonia in adults. J Allergy Clin Immunol. 2008;122:368–74. e1–2. doi: 10.1016/j.jaci.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 99.Roos A, Nauta AJ, Broers D, Faber-Krol MC, Trouw LA, Drijfhout JW, et al. Specific inhibition of the classical complement pathway by C1q-binding peptides. J Immunol. 2001;167:7052–9. doi: 10.4049/jimmunol.167.12.7052. [DOI] [PubMed] [Google Scholar]
- 100.Frakking FN, Brouwer N, van Eijkelenburg NK, Merkus MP, Kuijpers TW, Offringa M, et al. Low mannose-binding lectin (MBL) levels in neonates with pneumonia and sepsis. Clin Exp Immunol. 2007;150:255–62. doi: 10.1111/j.1365-2249.2007.03479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Butko P, Nicholson-Weller A, Wessels MR. Role of complement component C1q in the IgG-Independent opsonophagocytosis of Group B Streptococcus. J Immunol. 1999;163:2761–8. [PubMed] [Google Scholar]
- 102.Yuste J, Ali S, Sriskandan S, Hyams C, Botto M, Brown JS. Roles of the alternative complement pathway and C1q during innate immunity to Streptococcus pyogenes. J Immunol. 2006;176:6112–20. doi: 10.4049/jimmunol.176.10.6112. [DOI] [PubMed] [Google Scholar]
- 103.Roos A, Ramwadhdoebe TH, Nauta AJ, Hack CE, Daha MR. Therapeutic inhibition of the early phase of complement activation. Immunobiology. 2002;205:595–609. doi: 10.1078/0171-2985-00157. [DOI] [PubMed] [Google Scholar]
- 104.Ehlers MRW. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2000;2:289–94. doi: 10.1016/s1286-4579(00)00299-9. [DOI] [PubMed] [Google Scholar]
- 105.Triantafilou M, Brandenburg K, Kusumoto S, Fukase K, Mackie A, Seydel U, et al. Combinational clustering of receptors following stimulation by bacterial products determines lipopolysaccharide responses. Biochem J. 2004;381:527–36. doi: 10.1042/BJ20040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hajishengallis G, Tapping RI, Harokopakis E, Nishiyama S-I, Ratti P, Schifferle RE, et al. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cell Microbiol. 2006;8:1557–70. doi: 10.1111/j.1462-5822.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 107.Diamond MS, Garcia-Aguilar J, Bickford JK, Corbi AL, Springer TA. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–43. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McGuirk P, Mills KH. Direct anti-inflammatory effect of a bacterial virulence factor: IL-10-dependent suppression of IL-12 production by filamentous hemagglutinin from Bordetella pertussis. Eur J Immunol. 2000;30:415–22. doi: 10.1002/1521-4141(200002)30:2<415::AID-IMMU415>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 109.Russell DG, Wright SD. Complement receptor type 3 (CR3) binds to an Arg-Gly-Asp-containing region of the major surface glycoprotein, gp63, of Leishmania promastigotes. J Exp Med. 1988;168:279–92. doi: 10.1084/jem.168.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hajishengallis G, Ratti P, Harokopakis E. Peptide mapping of bacterial fimbrial epitopes interacting with pattern recognition receptors. J Biol Chem. 2005;280:38902–13. doi: 10.1074/jbc.M507326200. [DOI] [PubMed] [Google Scholar]
- 111.Ingalls RR, Arnaout MA, Golenbock DT. Outside-in signaling by lipopolysaccharide through a tailless integrin. J Immunol. 1997;159:433–8. [PubMed] [Google Scholar]
- 112.Laudanna C, Kim JY, Constantin G, Butcher EC. Rapid leukocyte integrin activation by chemokines. Immunol Rev. 2002;186:37–46. doi: 10.1034/j.1600-065x.2002.18604.x. [DOI] [PubMed] [Google Scholar]
- 113.Harokopakis E, Hajishengallis G. Integrin activation by bacterial fimbriae through a pathway involving CD14, Toll-like receptor 2, and phosphatidylinositol-3-kinase. Eur J Immunol. 2005;35:1201–10. doi: 10.1002/eji.200425883. [DOI] [PubMed] [Google Scholar]
- 114.Hajishengallis G, Wang M, Harokopakis E, Triantafilou M, Triantafilou K. Porphyromonas gingivalis fimbriae proactively modulate β2 integrin adhesive activity and promote binding to and internalization by macrophages. Infect Immun. 2006;74:5658–66. doi: 10.1128/IAI.00784-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hajishengallis G, Sojar H, Genco RJ, DeNardin E. Intracellular signaling and cytokine induction upon interactions of Porphyromonas gingivalis fimbriae with pattern-recognition receptors. Immunol Invest. 2004;33:157–72. doi: 10.1081/imm-120030917. [DOI] [PubMed] [Google Scholar]
- 116.Harokopakis E, Albzreh MH, Martin MH, Hajishengallis G. TLR2 transmodulates monocyte adhesion and transmigration via Rac1- and PI3K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J Immunol. 2006;176:7645–56. doi: 10.4049/jimmunol.176.12.7645. [DOI] [PubMed] [Google Scholar]
- 117.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–90. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 118.Issekutz AC, Rowter D, Springer TA. Role of ICAM-1 and ICAM-2 and alternate CD11/CD18 ligands in neutrophil transendothelial migration. J Leukoc Biol. 1999;65:117–26. doi: 10.1002/jlb.65.1.117. [DOI] [PubMed] [Google Scholar]
- 119.Hajishengallis G, Shakhatreh M-AK, Wang M, Liang S. Complement receptor 3 blockade promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo. J Immunol. 2007;179:2359–67. doi: 10.4049/jimmunol.179.4.2359. [DOI] [PubMed] [Google Scholar]
- 120.Wang M, Shakhatreh M-AK, James D, Liang S, Nishiyama S-i, Yoshimura F, et al. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J Immunol. 2007;179:2349–58. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- 121.Lowell CA. Rewiring phagocytic signal transduction. Immunity. 2006;24:243–5. doi: 10.1016/j.immuni.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 122.Wright SD, Silverstein SC. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983;158:2016–23. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–21. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 124.Rosenberger CM, Finlay BB. Phagocyte sabotage: disruption of macrophage signalling by bacterial pathogens. Nat Rev Mol Cell Biol. 2003;4:385–96. doi: 10.1038/nrm1104. [DOI] [PubMed] [Google Scholar]
- 125.Hellwig SM, van Oirschot HF, Hazenbos WL, van Spriel AB, Mooi FR, van De Winkel JG. Targeting to Fcγ receptors, but not CR3 (CD11b/CD18), increases clearance of Bordetella pertussis. J Infect Dis. 2001;183:871–9. doi: 10.1086/319266. [DOI] [PubMed] [Google Scholar]
- 126.Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: aging gracefully. Biochem J. 2002;366:689–704. doi: 10.1042/BJ20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21:643–53. doi: 10.1016/j.immuni.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 128.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–20. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Kohl J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–26. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 130.Marth T, Kelsall BL. Regulation of interleukin-12 by complement receptor 3 signaling. J Exp Med. 1997;185:1987–95. doi: 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hajishengallis G. Too old to fight? Aging and its toll on innate immunity. Mol Oral Microbiol. 2010;25:25–37. doi: 10.1111/j.2041-1014.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sebastian C, Espia M, Serra M, Celada A, Lloberas J. MacrophAging: a cellular and molecular review. Immunobiology. 2005;210:121–6. doi: 10.1016/j.imbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 133.Butcher S, Chahel H, Lord JM. Ageing and the neutrophil: no appetite for killing? Immunology. 2000;100:411–6. doi: 10.1046/j.1365-2567.2000.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Butcher SK, Chahal H, Nayak L, Sinclair A, Henriquez NV, Sapey E, et al. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol. 2001;70:881–6. [PubMed] [Google Scholar]
- 135.Liang S, Hosur KB, Domon H, Hajishengallis G. Periodontal inflammation and bone loss in aged mice. J Periodont Res. 2010 doi: 10.1111/j.1600-0765.2009.01245.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–52. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 137.Okroj M, Heinegard D, Holmdahl R, Blom AM. Rheumatoid arthritis and the complement system. Ann Med. 2007;39:517–30. doi: 10.1080/07853890701477546. [DOI] [PubMed] [Google Scholar]
- 138.Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:273–9. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- 139.Kanazawa N, Furukawa F. Autoinflammatory syndromes with a dermatological perspective. J Dermatol. 2007;34:601–18. doi: 10.1111/j.1346-8138.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- 140.Simpson JL, Phipps S, Gibson PG. Inflammatory mechanisms and treatment of obstructive airway diseases with neutrophilic bronchitis. Pharmacol Ther. 2009;124:86–95. doi: 10.1016/j.pharmthera.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 141.Kitsis E, Weissmann G. The role of the neutrophil in rheumatoid arthritis. Clin Orthop Relat Res. 1991:63–72. [PubMed] [Google Scholar]
- 142.Lange D, Schroeder HE. Cytochemistry and ultrastructure of gingival sulcus cells. Helv Odontol Acta. 1971;15:65–86. [PubMed] [Google Scholar]
- 143.Newman HN. Neutrophils and IgG at the host-plaque interface on children's teeth. J Periodontol. 1980;51:642–51. doi: 10.1902/jop.1980.51.11.642. [DOI] [PubMed] [Google Scholar]
- 144.Schroeder HE, Listgarten MA. The gingival tissues: the architecture of periodontal protection. Periodontology 2000. 1997;13:91–120. doi: 10.1111/j.1600-0757.1997.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 145.Vitkov L, Klappacher M, Hannig M, Krautgartner WD. Neutrophil fate in gingival crevicular fluid. Ultrastruct Pathol. 2010;34:25–30. doi: 10.3109/01913120903419989. [DOI] [PubMed] [Google Scholar]
- 146.Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 147.Van Dyke TE. The management of inflammation in periodontal disease. J Periodontol. 2008;79:1601–8. doi: 10.1902/jop.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 149.Mydel P, Takahashi Y, Yumoto H, Sztukowska M, Kubica M, Gibson FC, 3rd, et al. Roles of the host oxidative immune response and bacterial antioxidant rubrerythrin during Porphyromonas gingivalis infection. PLoS Pathog. 2006;2:e76, 712–25. doi: 10.1371/journal.ppat.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wingrove JA, DiScipio RG, Chen Z, Potempa J, Travis J, Hugli TE. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J Biol Chem. 1992;267:18902–7. [PubMed] [Google Scholar]
- 151.de Haas CJC, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJB, Heezius ECJM, et al. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med. 2004;199:687–95. doi: 10.1084/jem.20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huber-Lang MS, Younkin EM, Sarma JV, McGuire SR, Lu KT, Guo RF, et al. Complement-induced impairment of innate immunity during sepsis. J Immunol. 2002;169:3223–31. doi: 10.4049/jimmunol.169.6.3223. [DOI] [PubMed] [Google Scholar]
- 153.Riedemann NC, Guo RF, Bernacki KD, Reuben JS, Laudes IJ, Neff TA, et al. Regulation by C5a of neutrophil activation during sepsis. Immunity. 2003;19:193–202. doi: 10.1016/s1074-7613(03)00206-1. [DOI] [PubMed] [Google Scholar]
- 154.Gibson FC, 3rd, Hong C, Chou HH, Yumoto H, Chen J, Lien E, et al. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:2801–6. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]
- 155.Gibson FC, 3rd, Yumoto H, Takahashi Y, Chou HH, Genco CA. Innate immune signaling and Porphyromonas gingivalis-accelerated atherosclerosis. J Dent Res. 2006;85:106–21. doi: 10.1177/154405910608500202. [DOI] [PubMed] [Google Scholar]
- 156.Okuda K, Kimizuka R, Abe S, Kato T, Ishihara K. Involvement of periodontopathic anaerobes in aspiration pneumonia. J Periodontol. 2005;76:2154–60. doi: 10.1902/jop.2005.76.11-S.2154. [DOI] [PubMed] [Google Scholar]
- 157.Liang S, Domon H, Hosur KB, Wang M, Hajishengallis G. Age-related alterations in innate immune receptor expression and ability of macrophages to respond to pathogen challenge in vitro. Mech Ageing Dev. 2009;130:538–46. doi: 10.1016/j.mad.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hajishengallis G, Wang M, Bagby GJ, Nelson S. Importance of TLR2 in early innate immune response to acute pulmonary infection with Porphyromonas gingivalis in mice. J Immunol. 2008;181:4141–9. doi: 10.4049/jimmunol.181.6.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Grant EP, Picarella D, Burwell T, Delaney T, Croci A, Avitahl N, et al. Essential role for the C5a receptor in regulating the effector phase of synovial infiltration and joint destruction in experimental arthritis. J Exp Med. 2002;196:1461–71. doi: 10.1084/jem.20020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ricklin D, Hajishengallis G, Yang K, Lambris JD. A new perspective on complement: a keystone biological system for immune surveillance and homeostasis. Nat Immunol. 2010:11. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hopken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383:86–9. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 162.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 163.Drouin SM, Sinha M, Sfyroera G, Lambris JD, Wetsel RA. A protective role for the fifth complement component (c5) in allergic airway disease. Am J Respir Crit Care Med. 2006;173:852–7. doi: 10.1164/rccm.200503-334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Baker PJ, DuFour L, Dixon M, Roopenian DC. Adhesion molecule deficiencies increase Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect Immun. 2000;68:3103–7. doi: 10.1128/iai.68.6.3103-3107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Genco CA, Van Dyke T, Amar S. Animal models for Porphyromonas gingivalis-mediated periodontal disease. Trends Microbiol. 1998;6:444–9. doi: 10.1016/s0966-842x(98)01363-8. [DOI] [PubMed] [Google Scholar]
- 166.Ebersole JL. Humoral immune responses in gingival crevice fluid: local and systemic implications. Periodontol 2000. 2003;31:135–66. doi: 10.1034/j.1600-0757.2003.03109.x. [DOI] [PubMed] [Google Scholar]
- 167.Niekrash CE, Patters MR. Assessment of complement cleavage in gingival fluid in humans with and without periodontal disease. J Periodontal Res. 1986;21:233–42. doi: 10.1111/j.1600-0765.1986.tb01455.x. [DOI] [PubMed] [Google Scholar]
- 168.Bohana-Kashtan O, Ziporen L, Donin N, Kraus S, Fishelson Z. Cell signals transduced by complement. Mol Immunol. 2004;41:583–97. doi: 10.1016/j.molimm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 169.Hajishengallis G, Harokopakis E. Porphyromonas gingivalis interactions with complement receptor 3 (CR3): innate immunity or immune evasion? Front Biosci. 2007;12:4547–57. doi: 10.2741/2409. [DOI] [PubMed] [Google Scholar]