In the hemostatic and thrombotic processes, thrombin is generated at the activated platelet surface through the concerted assembly and function of the macromolecular, enzymatic complexes, intrinsic tenase and prothrombinase, to effect the generation of factor Xa and thrombin, respectively [1, 2]. Platelet activation can be accomplished by nanomolar concentrations of thrombin and is central to appropriate coagulation complex assembly and function [3, 4]. Assembly of a functional intrinsic tenase [for review see 5] or prothrombinase [for review see 6] complex is dependent upon several protein/protein and protein/membrane interactions. However, platelet activation and complex formation may not be sufficient. Several laboratories have identified discrete subpopulations of platelets that differentially regulate procoagulant enzyme complex and function [7-11]. When all platelets are thrombin-activated to a level consistent with maximal procoagulant activity, only a subpopulation of the activated platelets are capable of prothrombinase [9] or intrinsic tenase complex assembly [7, 11]. In these studies, factor Va and factor Xa binding colocalize to the same subset of activated platelets [9], as do components of the intrinsic tenase complex (factor VIIIa, factor IXa, and factor X) [11]. Likewise, platelet activation with low levels of thrombin plus collagen, or thrombin plus a glycoprotein VI agonist, convulxin, generates a subpopulation of platelets, referred to as COATED-platelets, that express high levels of membrane bound α-granule proteins, such as factor Va [10], which are thought to be covalently bound to the platelet surface via serotonin [12]. COATED-platelets can also bind high levels of factor Xa [10].
Currently, it is not clear if platelet activation with high concentrations of thrombin alone generates a subpopulation of platelets similar to COATED-platelets. Monroe and colleagues extended these observations in their cell-based model of blood coagulation and demonstrated that following activation with thrombin and convulxin the same population of platelets that binds factors Va and Xa, also binds factors VIIIa and IXa, the components of the intrinsic tenase complex [8]. Thus, it can be hypothesized that the extent of thrombin generated at a site of vascular injury is not only dependent upon coagulation factor levels, or the presence or absence of a specific coagulation factor polymorphism, but is also dependent upon the number of procoagulant platelets recruited to the site of vascular injury. Once formed, thrombin is rapidly inactivated in the vasculature [13]. Thus, an individual's propensity to bleed or clot is directly related to the amount of thrombin produced locally, and the ability of this thrombin to propagate the hemostatic response through its activation of various coagulation cofactors and proteases and to recruit activated platelets into the growing thrombus [14]. Therefore, measures of platelet activation alone, either direct, or by following release of α-granule contents, may not be adequate to predict platelet procoagulant potential.
Previous studies demonstrated that thrombin generation parallels 125I-factor Xa binding to activated platelets [3]. Therefore, an assay quantifying the subpopulation of platelets binding factor Xa may be useful in determining an individual's ability to generate thrombin. Indeed, in a washed platelet system, the percent of activated platelets binding factor Xa could be correlated in concurrent kinetic experiments with thrombin generation (r=0.802, n=18) (P. Tracy, personal communication). However, this assay requires the isolation of platelets from whole blood, which limits its utility in population-based studies. Our goal is to create an easy to use, stable subpopulation platelet assay using minimal blood. Thus, in this communication, we performed a pilot study to see if a flow cytometric assay of prothrombinase complex assembly on platelets in whole blood was feasible, and to determine how well its results correlate with those seen using washed platelets.
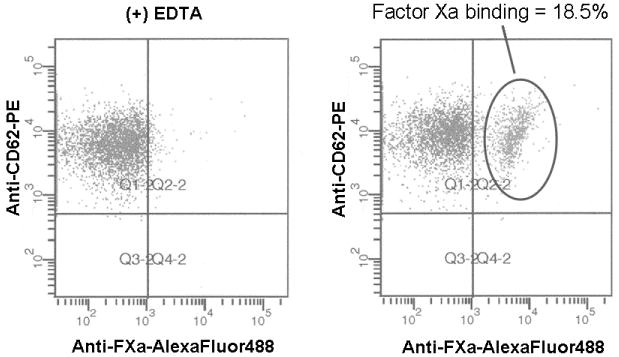
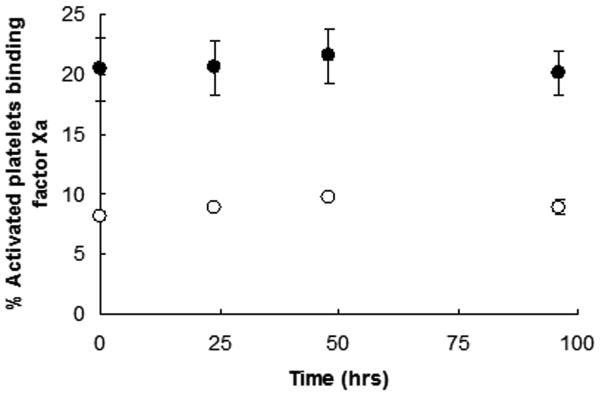
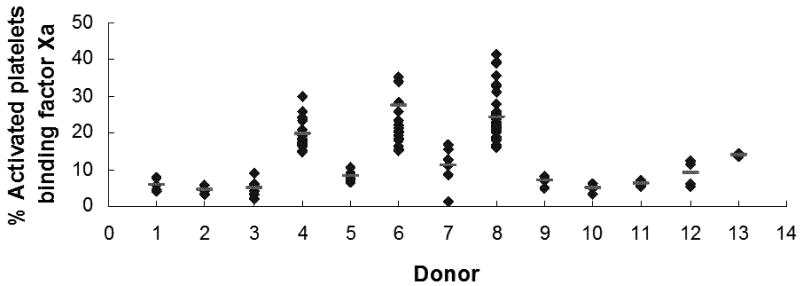
For these studies, all individuals were recruited and advised according to a protocol for obtaining blood samples from normal donors approved by the University of Vermont Human IRB and provided informed written consent. All donors were requested to abstain from use of aspirin and NSAIDs for at least 10 days prior to blood donation. To prevent artificial platelet activation, blood was drawn without venous constriction with a 19-gauge butterfly needle using a two syringe technique. After discard of the first 2-3 mL of drawn blood, 1 mL of blood was drawn into corn trypsin inhibitor (100μg/mL) (Haematologic Technologies Inc., Essex Junction, VT) and heparin (50 U/mL) to suppress the contact pathway of blood coagulation [15]. Aliquots of blood were added to concentrations of protease activated receptor (PAR) 1 and PAR4 agonist peptides (100 and 500μM, respectively) (synthesized by the Protein Core Facility, Department of Biochemistry, University of Vermont), that generate maximal prothrombinase complex assembly and function (P. Tracy, personal communication), as well as plasma factor Va (40nM) (prepared as described previously [3]) and EGRck-factor Xa (40nM) (Haematologic Technologies Inc.) to allow for prothrombinase assembly. Some reactions contained 10 mM EDTA to distinguish between functional and non-functional factor Xa binding. Factor Xa binding was assessed using an AlexaFluor-488-conjugated (Invitrogen, Carlsbad, CA) anti-factor Xa monoclonal antibody (0.5 μM) generously provided by Dr. Kenneth Mann (Department of Biochemistry, University of Vermont), which does not inhibit prothrombinase assembly or function. Following incubation with Optilyse C (1:1 dilution) (Beckman Coulter, Brea, CA) to effect red blood cell lysis and cell fixation, samples were stored at 4°C until flow cytometric analysis. Functional factor Xa binding was defined as positive reactivity with the anti-factor Xa antibody that was greater than that observed with EDTA (Figure 1A). When immunostained and fixed samples were stored at 4°C, the percent platelets binding factor Xa was stable was 96 hr (Figure 1B). Statistical analysis of one individual assayed multiple times indicated that in this assay the percent platelets binding factor Xa (19.7±4.2%, n=16) (see Donor 4, Figure 1C) was not different than the percent thrombin-activated, washed platelets capable of binding factor Xa (20.7±7.0%, n=84) (P=0.56) (P. Tracy, personal communication). These results show that measuring platelet subpopulations in whole blood is feasible and comparable to results obtained using a washed platelet system.
Figure 1. Flow cytomteric assay of factor Xa binding to platelets in whole blood.
(A) Contact pathway-suppressed whole blood was incubated with PAR1 and PAR4 peptides, and factor Va and EGRck-factor Xa at ambient temperature. Platelets were identified using forward scatter and side scatter measurements, and immunostaining with anti-CD61-PerCP (1 nM) (Becton Dickinson, Franklin Lakes, NJ). Activated platelets were identified by immunostaining with anti-P-selectin-PE (0.5 nM) (Becton Dickinson). Factor Xa binding to platelets was determined by immunostaining with anti-factor Xa (FXa)-AlexaFluor488 in the presence or absence of EDTA. Multi-parameter flow cytometric analysis was performed using a BD LSR II Flow Cytometer (Becton Dickinson). The flow cytometry scattergrams shown are representative of those obtained in the presence (left) or absence of EDTA (right). The positive gate was set such that ≤ 2% of the platelets immunostained with the anti-factor Xa antibody in the presence of EDTA were positive. Functional factor Xa binding is reported as the % activated platelets binding factor Xa. (B) Flow cytometric analyses up to 96 hrs subsequent to immunostaining indicated that the percent platelets binding factor Xa was stable when the samples were stored at 4°C. Shown are the results obtained with two individuals. (C) Factor Xa binding to the platelets of 13 healthy individuals was determined repeatedly over a 6 week period as described above. The black diamonds represent single measurements while the light gray horizontal lines indicate the mean of the measurements for that individual.
To test the stability of the assay, we evaluated whole blood obtained by phlebotomy, in 13 healthy, nonmedicated individuals (8 men and 5 women, 27.2±6.4 years old), with no known bleeding or thrombotic tendencies, over a 6 week period. The percent activated platelets binding factor Xa in this normal population varied from 1.5-41.5% (n=136) (Figure 1B). Analytical and biological variability was assessed using a nested ANOVA as described [16]. Three coefficients of variation (CVs) were determined: analytical (CVa), within-subject (CVi), and between-subject (CVg). The index of individuality (CVi/CVg), a measure of how individuals vary relative to the population distribution, was also calculated. Assuming reasonable analytic precision (e.g. CVa<15%), the smaller the value for this index, the more likely a single measure will accurately represent a person's rank over time. For factor Xa binding, the analytical variability of the assay is low (11.6%), and the ratio of the within-subject (34.0%) to the between subject (49.9%) variability (0.68) indicates that this measurement will prove useful for identifying differences between individuals.
This study is the first to demonstrate that procoagulant platelet subpopulations can be measured reproducibly in whole blood using a rapid, flow cytometry-based assay. Since this assay does not require washed platelets and can be performed using as little as 1mL of whole blood, it is more amenable to onsite collection. Corn trypsin inhibitor was used in this assay as it inhibits the contact pathway of blood coagulation [17]. As blood coagulation in vivo is most likely initiated by tissue factor/factor VIIa, activation of platelets in blood supplemented with CTI in the absence of a vascular injury that exposes blood to tissue factor is more likely to simulate in vivo conditions.
Measuring factor Xa binding to activated platelets in whole blood has the potential to be useful for identifying differences in thrombin generation between individuals in large, population-based studies. Such studies are currently underway to get an insight into how platelets regulate thrombin formation in pathologic states by assessing platelet subpopulation formation in men with varying degrees of factor VIII-deficiency [18, 19], as well as in individuals in a large protein C-deficient kindred (protein C Vermont) [20]. As an individual's ability to generate these subpopulations (i.e. bind factor Xa) is related directly to thrombin formation, and thus, may be predictive of their propensity to bleed or clot, analyses will be performed to determine the relationship between platelet factor Xa binding and hemostatic and/or thrombotic phenotypes.
Acknowledgments
The authors would like to thank Paula B. Tracy, Ph.D., Department of Biochemistry, University of Vermont, for her insightful discussions during the development of this assay. This work was supported by NIH Grant HL46703 (Project 5) (to K.B.Z.).
Abbreviations
- PAR
protease activated receptor
- CV
coefficient of variation
Footnotes
Presented at the XXIInd Congress of the International Society of Thrombosis & Haemostasis, Boston MA, July 2009.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Hoffman M, Monroe DM, Oliver JA, Roberts HR. Factors IXa and Xa play distinct roles in tissue factor-dependent initiation of coagulation. Blood. 1995;86:1794–801. [PubMed] [Google Scholar]
- 2.Tracy PB, Nesheim ME, Mann KG. Platelet factor Xa receptor. Methods Enzymol. 1992;215:329–60. doi: 10.1016/0076-6879(92)15075-n. [DOI] [PubMed] [Google Scholar]
- 3.Bouchard BA, Catcher CS, Thrash BR, Adida C, Tracy PB. Effector cell protease receptor-1, a platelet activation-dependent membrane protein, regulates prothrombinase-catalyzed thrombin generation. J Biol Chem. 1997;272:9244–51. doi: 10.1074/jbc.272.14.9244. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad SS, Rawala-Sheikh R, Walsh PN. Platelet receptor occupancy with factor IXa promotes factor X activation. J Biol Chem. 1989;264:20012–6. [PubMed] [Google Scholar]
- 5.Ahmad SS, London FS, Walsh PN. The assembly of the factor X-activating complex on activated human platelets. J Thromb Haemost. 2003;1:48–59. doi: 10.1046/j.1538-7836.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 6.Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol. 2002;22:1381–9. doi: 10.1161/01.atv.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]
- 7.London FS, Marcinkiewicz M, Walsh PN. A subpopulation of platelets responds to thrombin- or SFLLRN-stimulation with binding sites for factor IXa. J Biol Chem. 2004;279:19854–9. doi: 10.1074/jbc.M310624200. [DOI] [PubMed] [Google Scholar]
- 8.Kempton CL, Hoffman M, Roberts HR, Monroe DM. Platelet heterogeneity: variation in coagulation complexes on platelet subpopulations. Arterioscler Thromb Vasc Biol. 2005;25:861–6. doi: 10.1161/01.ATV.0000155987.26583.9b. [DOI] [PubMed] [Google Scholar]
- 9.Feng P, Tracy PB. Not all platelets are equal procoagulants? Blood. 1998;92:1441a. [Google Scholar]
- 10.Alberio L, Safa O, Clemetson KJ, Esmon CT, Dale GL. Surface expression and functional characterization of alpha-granule factor V in human platelets: effects of ionophore A23187, thrombin, collagen, and convulxin. Blood. 2000;95:1694–702. [PubMed] [Google Scholar]
- 11.Panteleev MA, Ananyeva NM, Greco NJ, Ataullakhanov FI, Saenko EL. Two subpopulations of thrombin-activated platelets differ in their binding of the components of the intrinsic factor X-activating complex. J Thromb Haemost. 2005;3:2545–53. doi: 10.1111/j.1538-7836.2005.01616.x. [DOI] [PubMed] [Google Scholar]
- 12.Dale GL, Friese P, Batar P, Hamilton SF, Reed GL, Jackson KW, Clemetson KJ, Alberio L. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature. 2002;415:175–9. doi: 10.1038/415175a. [DOI] [PubMed] [Google Scholar]
- 13.Goldsack NR, Chambers RC, Dabbagh K, Laurent GJ. Thrombin. Int J Biochem Cell Biol. 1998;30:641–6. doi: 10.1016/s1357-2725(98)00011-9. [DOI] [PubMed] [Google Scholar]
- 14.Shah R. Protease-activated receptors in cardiovascular health and diseases. American heart journal. 2009;157:253–62. doi: 10.1016/j.ahj.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Cawthern KM, van't Veer C, Lock JB, DiLorenzo ME, Branda RF, Mann KG. Blood coagulation in hemophilia A and hemophilia C. Blood. 1998;91:4581–92. [PubMed] [Google Scholar]
- 16.Sakkinen PA, Macy EM, Callas PW, Cornell ES, Hayes TE, Kuller LH, Tracy RP. Analytical and biologic variability in measures of hemostasis, fibrinolysis, and inflammation: assessment and implications for epidemiology. American journal of epidemiology. 1999;149:261–7. doi: 10.1093/oxfordjournals.aje.a009801. [DOI] [PubMed] [Google Scholar]
- 17.Rand MD, Lock JB, van't Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88:3432–45. [PubMed] [Google Scholar]
- 18.Bouchard BA, Paradis AK, Rivard GE, Brummel-Ziedins KE. Procoagulant platelet subpopulation formation in whole blood: Correlation with whole blood clotting time and 5-year mean bleeding score in individuals with factor VIII deficiency. Blood. 2009;114(Suppl. 1):4022. [Google Scholar]
- 19.Brummel-Ziedins KE, Whelihan MF, Gissel M, Mann KG, Rivard GE. Thrombin generation and bleeding in hemophilia A. Haemophilia. 2009;15:1118–25. doi: 10.1111/j.1365-2516.2009.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bovill EG, Tomczak JA, Grant B, Bhushan F, Pillemer E, Rainville IR, Long GL. Protein CVermont: symptomatic type II protein C deficiency associated with two GLA domain mutations. Blood. 1992;79:1456–65. [PubMed] [Google Scholar]