Abstract
Neutrophils are the most abundant cell type involved in the innate immune response. They are rapidly recruited to sites of injury or infection where they engulf and kill invading microorganisms. Neutrophil apoptosis, the process of programmed cell death that prevents the release of neutrophil histotoxic contents, is tightly regulated and limits the destructive capacity of neutrophil products to surrounding tissue. The subsequent recognition and phagocytosis of apoptotic cells by phagocytic cells such as macrophages is central to the successful resolution of an inflammatory response and it is increasingly apparent that the dying neutrophil itself exerts an anti-inflammatory effect through modulation of surrounding cell responses, particularly macrophage inflammatory cytokine release. Apoptosis may be delayed, induced or enhanced by micro-organisms dependent on their immune evasion strategies and the health of the host they encounter. There is now an established field of research aimed at understanding the regulation of apoptosis and its potential as a target for therapeutic intervention in inflammatory and infective diseases. This review focuses on the physiological regulation of neutrophil apoptosis with respect to the innate immune system and highlights recent advances in mechanistic understanding of apoptotic pathways and their therapeutic manipulation in appropriate and excessive innate immune responses.
Key Words: Apoptosis, Inflammation, Neutrophils
Introduction
Neutrophils are the most abundant cells of the innate immune system and are major players in the body's fight against infection. Neutrophils release a cytotoxic and proteolytic cocktail that allows effective killing of invading micro-organisms. Neutrophils and their armamentarium contribute significantly to the inflammatory response in normal physiology and there are important regulatory mechanisms that allow limitation and resolution of this response [1,2]. Apoptosis, a programmed cell death that occurs to conserve toxic neutrophil contents, is one such mechanism. The apoptotic cell undergoes several characteristic alterations (fig. 1), including alteration of cell surface markers (e.g. increased expression of phosphatidylserine) that help phagocytes, such as macrophages, to clear them [3]. Apoptosis and the subsequent phagocytosis of apoptotic cells is central to successful resolution of inflammation and there is significant research interest in targeting apoptosis as a means to treat neutrophil-dominant, inflammatory diseases [4]. It is also apparent that in inflammatory disease there are several ways in which this regulatory mechanism can be compromised [1]. There is also increasing evidence that the neutrophil can contribute significantly to the inflammatory process through expression and release of factors that influence the behaviour of other cell types, particularly those involved in promoting resolution of inflammation [5]. The apoptotic neutrophil and the process of cell death exert anti-inflammatory effects that have been shown to be of therapeutic value in inflammatory disease models. In addition to pharmacological manipulation of the apoptotic pathway (e.g. by using cyclin-dependent kinase (CDK) inhibitors [6]), the systemic addition of apoptotic neutrophils (made apoptotic ex vivo) has also enhanced survival in animal models of sepsis [7] as a result of the LPS-binding capacity of apoptotic neutrophils and the subsequent clearance of these cells by macrophages. The benefits of apoptotic neutrophils are twofold as they also stimulate macrophages into a pro-resolution phenotype, reducing the inappropriate inflammatory response further. This is an excellent example of an apoptosis-based strategy proving beneficial in a model of excessive host response to microbial infection. The apoptotic pathway can be targeted by microbes that promote neutrophil death as well as those that extend neutrophil longevity in order to avoid provoking an early immune response [8]. It is increasingly important to consider the mechanistic insights that may be gleaned from an understanding of appropriate innate immune system responses, their subversion and the inappropriate or excessive response which has been described in non-infectious inflammatory diseases. This is especially true in the design of anti-microbial and anti-inflammatory therapeutic agents, and it is clear that there is much to be gained from collaborative work between microbe-centred researchers and those interested in inflammatory cell apoptosis.
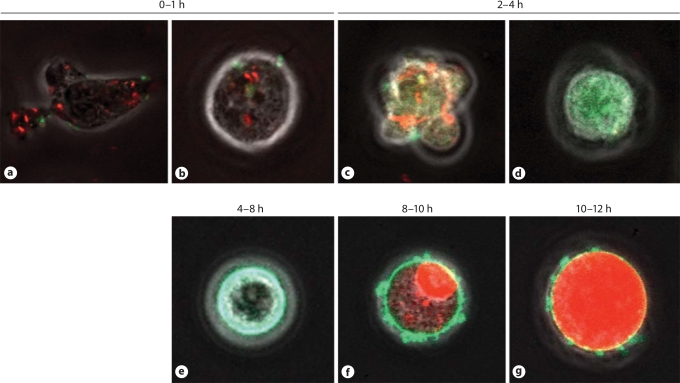
Fig. 1.
Demonstration of neutrophil apoptosis by live-cell imaging using confocal microscopy. Neutrophil apoptosis induced by the cyclin-dependent kinase inhibitor R-roscovitine. Neutrophil with intact mitochondria fluorescing red (a). Neutrophil has rounded up (b) before blebbing occurs (c) and mitochondrial dissipation with green fluorescence throughout the cytoplasm (d). Annexin-V binding to exposed phosphatidylserine residues is shown by green fluorescence at cell periphery (e) followed by secondary necrosis with PI entering cell nucleus (f), and then spreading through the cytoplasm (g), presumably with nuclear membrane disruption.
Role of the Neutrophil in Inflammation
Neutrophils are part of the granulocyte family of leukocytes that also include eosinophils and basophils. They are termed granulocytes because of the small packages (granular in appearance) of preformed proteins that adorn their cytoplasm and help to define their morphology histologically. Several types of granules exist within neutrophils: secretory, primary, secondary, and tertiary granules. Over 300 proteins are contained within the granules that are involved in many neutrophil processes including: adhesion, migration and anti-bacterial activities [9]. The secretory vesicles are of endocytic origin and are the first to be mobilised to facilitate neutrophil recruitment and migration. They contain a reservoir of membrane proteins that are incorporated into the neutrophil outer membrane following vesicle release to promote the inflammatory response. The β-integrins, complement receptor 1 (CR1), CD14, CD16, and formyl peptide receptors are all upregulated on the neutrophil membrane following secretory vesicle activation. Significant concentrations of heparin-binding protein (HBP) can be found following secretory vesicle activation and is thought to act as a monocyte chemoattractant which has important implications for the resolution of the inflammatory response [10].
Tertiary and secondary granules are the next granules to be mobilised, followed finally by the primary granules. This coordinated and timely release of different granule subsets allows for the appropriate response to be mounted at the right time and minimises the destructive potential of the neutrophil. The primary or azurophilic granules are responsible for the majority of anti-microbial effectors, containing alpha-defensins and the serine protease, elastase.
Mature neutrophils are terminally differentiated, non-dividing cells that develop from CD34+ pluripotent stem cells in the bone marrow under the direction of various colony stimulating factors (CSFs) including IL-3, granulocyte-CSF (G-CSF) and granulocyte macrophage-CSF (GM-CSF) [11]. Many of these continue to influence neutrophil longevity outside of the bone marrow and Kuijpers et al. [12] have shown changes at gene level promoted by G-CSF in terminally differentiated neutrophils. Neutrophils are released into the systemic circulation where they form the majority of the circulating leukocyte cell population. There is also a ‘marginated’ population of mature neutrophils found in the microvasculature of many tissues including, for instance, the lung as well as a population resident in the spleen (the ‘splenic pool’). Under normal conditions the number of mature neutrophils remains fairly constant, but during pro-inflammatory episodes (e.g. infection), these can be increased in the circulation by up to 10-fold. Rapid demargination, along with increased bone marrow production, occurs in response to increased levels of soluble inflammatory factors. Upon encountering inflammatory signals, neutrophils change their responsiveness dramatically to allow directed migration and enhancement of microbicidal capacity. Once at the site of infection, neutrophils unleash their arsenal of toxic products to kill and clear invading pathogens by the processes of phagocytosis, degranulation and generation of reactive oxygen metabolites (e.g. superoxide anion). In addition, there is substantial evidence that neutrophils change their phenotype following a successful response from a pro-inflammatory state where they produce and release pro-inflammatory mediators such as LTB4 and PAF to a more anti-inflammatory pro-resolution state whereby they release products (e.g. lipoxins) that can influence the resolution phase of inflammation [13,14]. Indeed, further recent evidence to suggest that the neutrophil is more plastic than once appreciated has been described. For example, tumour-associated neutrophils can polarize into two phenotypes; an anti-tumorigenic (N1) or pro-tumorigenic (N2) phenotype under the regulation of TGF-β [15]. In addition, it has been suggested that mature neutrophils may transdifferentiate into macrophage-like cells [16]. A controversial finding that is nonetheless intriguing despite being reliant on quite specific in vitro conditions.
Under normal conditions, circulating neutrophils have a short half-life (7–12 h in vivo) and are generally functionally quiescent. It is believed that circulating neutrophils either home to the spleen, liver or back to the bone marrow to be destroyed [17] or alternatively leave the circulation to infiltrate tissues. Neutrophil life-span is dynamically influenced during inflammation to enhance the anti-microbial action of the neutrophil. This is typified by the increased half-life of neutrophils stimulated with inflammatory agents such as LPS [18,19]. Once their physiological function has been fulfilled in the tissues (e.g. destruction of invading organisms), they undergo spontaneous apoptosis, a programmed cell death that occurs to preserve neutrophil membrane integrity and prevent the uncontrolled release of toxic cell contents. Apoptosis-specific cell changes promote the recognition and uptake of cells by phagocytes such as macrophages. The resolution of inflammation therefore relies on the effective ‘switching off’ of the neutrophil, the promotion of apoptosis and the successful clearance of these cells.
There are two main mechanisms that account for the powerful microbicidal properties of neutrophils: the production and reaction of free radical species, and the coordinated release of proteolytic and anti-microbial granule contents. Neutrophils and other phagocytes are uniquely equipped with the NADPH oxidase enzyme that when activated, catalyses the transfer of electrons to molecular oxygen to produce the superoxide anion, a highly reactive free radical with powerful antimicrobial properties. This process, referred to as the respiratory burst, occurs in response to soluble inflammatory factors or following phagocytosis. The free radicals can be released outside the cell, or within the cell in the phagosome, the internalised compartment containing ingested material. Other reactive oxygen species are readily formed following superoxide release. In the phagosome, superoxide rapidly reacts to form hydrogen peroxide, another powerful oxidant with antimicrobial effects. Other reactive oxygen metabolites, such as nitric oxide and peroxynitrite, can also form depending on the presence of substrates and/or quenching anti-oxidant load within the inflammatory milieu. The importance of the respiratory burst to the microbicidal effects of neutrophils is underpinned by the clinical features of chronic granulomatous disease (CGD), which is caused by a rare, inherited mutation in one of the subunits of the NADPH oxidase enzyme that renders the cell incapable of producing superoxide. As a result, these patients suffer from recurrent bacterial and fungal infections and develop characteristic granulomas (typical of unresolved antimicrobial response) in their organs.
The release of granule contents, particularly those of the primary and secondary granules have significant antimicrobial actions. The granules contain an abundance of proteins and enzymes including myeloperoxidase (MPO), lysozyme, lactoferrin, hCAP18 and NGAL. The cathelicidins are an evolutionary conserved family of peptides that have well characterised antimicrobial and immunomodulatory actions. The human cathelicidin, LL-37, formed from proteolytic cleavage of hCAP-18 following its release from neutrophil-specific granules, is found in high concentrations during infection. It is readily detectable in saliva and lung secretions reflecting its importance in the first line of defence against pathogens in these ‘high contact’ areas. As well as antimicrobial action against bacteria, fungi and viruses, LL-37 has also been shown to have important immunomodulatory effects including chemotactic and endotoxin-neutralising properties (see below). The most abundant granule enzyme is MPO, which forms cytotoxic hypochlorous acid (HOCl) from the reaction of chloride anion with hydrogen peroxide produced after the respiratory burst. MPO also oxidises tyrosine residue to form another cytotoxic species, the tyrosyl radical. This enzyme has a heme prosthetic group, which gives it a green pigment that can be seen in neutrophil-rich secretions such as pus and mucous. There is now evidence that suggests MPO can act as a paracrine signalling molecule, promoting neutrophil survival through its binding to the β2 integrin and subsequent activation of second messenger pathways [20].
Granulocyte Apoptosis
The mechanisms that drive granulocyte apoptosis have been extensively reviewed [1,2,21]. There are a number of key distinguishing features of neutrophil apoptosis. The pre-eminence of the Bcl-2 homologue Mcl-1 [22] as a survival protein preventing intrinsic apoptosis is central to the neutrophil's capacity to undergo rapid apoptosis and is probably responsible for the limitation of neutrophil longevity to 24–36 h. Mcl-1 is an unusual bcl-2 protein in that it has a PEST domain (rich in proline (P), glutamic acid (E), serine (S), and threonine (T)) which means it can be rapidly turned over in the proteasome giving it a very short half-life of approximately 2 h [23,24,25]. This is in contrast to the key pro-apoptotic bcl-2 homologues, thought to be Bax [26,27], Bid [28] and Bim [29,30] which can persist in the cell beyond 12 h. Bim has recently and somewhat counter-intuitively been shown to be upregulated by pro-survival factors such as GM-CSF and IL-3, the presumption being that it acts as a physiological brake on cytokine-enhanced longevity [29]. It is not surprising that a critical excess of pro- as opposed to anti-apoptotic bcl-2 homologues is a perpetual Damocles’ sword hanging over neutrophil fate. This eventuality leads to loss of mitochondrial potential (probably achieved by the formation of pores in the outer mitochondrial membrane by pro-apoptotic bcl-2 homologues) and the progression of apoptosis as shown in figures 1 and 2[31]. Other interesting facets of neutrophil apoptosis include the dual effects of TNF-α on survival and extrinsic apoptosis [32,33]. TNF-α drives early neutrophil apoptosis (as early as 2 h after treatment) by triggering the extrinsic pathway (fig. 2) but promotes late survival (beyond 24 h) by initiating the NF-κB pathway, the sine qua non of neutrophil inflammatory signalling, leading to transcription of survival proteins including XIAP and A1 [34]. The extrinsic and intrinsic apoptotic pathways are linked by caspase-8 cleavage of Bid (fig. 2) in type II apoptosis cells, such as neutrophils, which has important implications for the experimental demonstration of apoptotic mechanism. Traditionally, it has been assumed that this led to activation of procaspase 9 via the apoptosome, though a recent study has suggested that the key feature may be inhibition of XIAP by Smac leading to procaspase-3 activation [35]. It has been suggested that this may be the critical determinant of type II apoptosis as opposed to type I. For a more comprehensive overview of the investigation of apoptosis and appropriate nomenclature, see the following reviews [36,37]. Other points of interest include the role given to reactive oxygen species (ROS) in initiating apoptosis by activation of pre-formed lipid raft clustered apoptotic machinery [38] and the existence of neutrophil deficiency syndromes such as severe congenital neutropenia or disorders of neutrophil impairment where apoptosis is affected by the absence of ROS generation (chronic granulomatous disease), or granule formation (Chediak-Higashi) [39]. Additionally, neutrophils are now noted to undergo a novel and NADPH oxidase-dependent form of cell death ‘NET-osis’ which was thought to be the inextricable sequelae of the production of antimicrobial NETs [40]. An alternative outcome has been suggested by a study showing that neutrophils can form NETs from mitochondrial DNA and subsequently remain viable [41]. Neutrophil death is highly regulated on a constitutive basis and in response to pathogens the complexity increases, so that it is often difficult to decide which death-modality is most favourable from a host perspective.
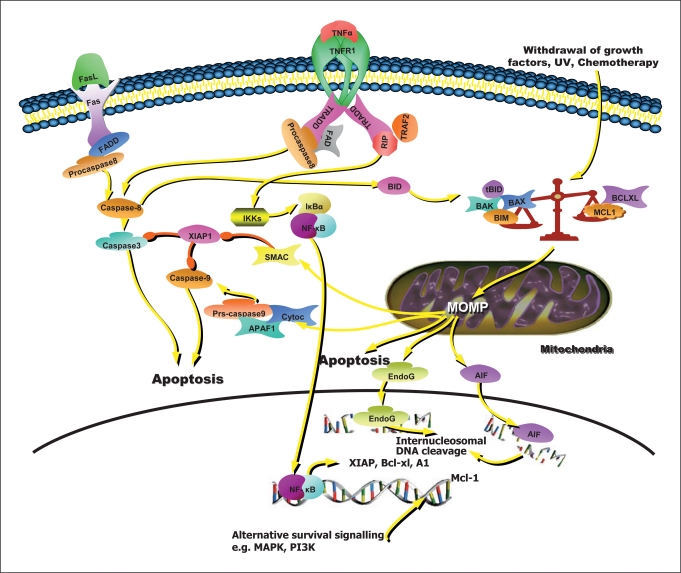
Fig. 2.
Neutrophil apoptosis. Extrinsic apoptosis as typified by apoptotic pathway triggering by TNF-α or Fas ligand (TNF-α depicted). Extrinsic apoptosis proceeds via clustering and cleavage of procaspase-8. In neutrophils (and other type II apoptotic cells) it is linked to the intrinsic apoptotic (or mitochondrial) death pathway by cleavage of the pro-apoptotic bcl-2 homologue Bid. The intrinsic pathway is initiated when the tightly regulated balance of bcl-2 homologue proteins tips in favour of pro-apoptotic proteins such as Bim, Bad, Bax and Bak. Also shown is the antiapoptotic function of TNF-α which mediates production of survival proteins via NF-κB signalling. The key anti-apoptotic Bcl-2 homologue in neutrophils is Mcl-1 which is regulated by a number of signalling pathways (created using SABIOscience presentation tools).
Pathogens and Neutrophil Apoptosis
The physiological role of neutrophils is directed towards the eradication of invading pathogens that have breached front-line structural immune defence [1]. A massive neutrophil influx may occur in response to the detection of pathogen elements or signalling from tissue cells that have encountered pathogens (mainly tissue macrophages). A successful response should result in the eradication of the culprit micro-organism and the restoration of tissue homeostasis. Neutrophil apoptosis can play a decisive role in either a positive or negative outcome as might be expected from the ancient and intricate interplay of host-pathogen response interaction [8,42].
A model in which neutrophil apoptosis can play a strong positive role is that of Streptococcus pneumoniae pneumonia [43]. In this condition, a comprehensive but contained neutrophil influx results in the phagocytosis of the bacteria and destruction within the neutrophil phagosome. Phagocytosis primes the neutrophil to undergo apoptosis; a response that has been termed phagocytosis-induced cell death (PICD) [44]. This is a beneficial occurrence as it effectively prevents further pathogen movement. A dead cell should be more difficult to hijack and use as a Trojan horse by hostile invaders [45]. Apoptotic neutrophils are then cleared by resident and recruited macrophages as well as tissue epithelial cells. This clearance prevents neutrophils from progressing to secondary necrosis and the potential for bystander tissue damage. It also provides a second opportunity for pathogen destruction as apoptotic cells and debris are further digested and disposed of.
However, there are striking examples of subversion of this process by many pathogens including: Chlamydia pneumoniae [46,47], Pseudomonas aeruginosa[48] and Staphylococcus aureus[49]. It is important to note that the effects of pathogens on neutrophils in experimental models are dependent on a number of variables which can mean that directly opposing (and potentially equally valid) results may be obtained. Key variables like bacterial multiplicity of infection (MOI), bacterial strain, duration of exposure, cell preparation and purity may influence neutrophil longevity. It has previously been shown that high MOIs lead to neutrophil apoptosis whilst low MOIs stimulate longevity [50].
C. pneumoniae, an obligate intracellular pathogen and cause of community-acquired pneumonia can survive and even multiply inside human neutrophil granulocytes. Once inside the neutrophil, C. pneumoniae is unwilling to relinquish its obliging host and compels the neutrophil to an extended longevity of up to 90 h [46]. This effect appears to be mediated both by bacterial LPS and enhanced neutrophil production of IL-8 acting in both an autocrine and paracrine manner. These effects are not permanent and the neutrophil does eventually undergo apoptosis and phagocytosis by a macrophage. Unfortunately, the macrophage becomes the unwitting host of the C. pneumoniae organism, and because it has been internalised without exposure to surface receptors, an inappropriate response is generated [47]. Instead of recognising the micro-organism as non-self and responding phlogistically, the macrophage merely registers uptake of an apoptotic cell, an input that generates an anti-inflammatory phenotype and prevents macrophage activation [51]. It has now been shown that this process probably facilitates infection of epithelial cells by C. pneumoniae as bugs that were directly ingested by macrophages, as opposed to consumed within an apoptotic cell, were at least contained, even if not killed [47].
Another organism that is increasingly recognised to modulate neutrophil apoptosis is S. aureus. Despite clinical evidence, arising from the increased susceptibility of patients with the immune-deficiency syndromes discussed above, suggesting that the main effector arm of the immune response to S. aureus is the neutrophil, this organism can induce survival, apoptosis or necrosis in different contexts [49]. Survival appears to be conferred by enhanced production of the cytokines IL-6 and IL-1β which suggests that there may be a paracrine effect on surrounding tissue cells with feedback of survival signals [50,52,53].
While the neutrophil proves relatively resistant to staphylococcal haemolysins, delta haemolysin has been shown to prime and even directly activate (in the presence of LPS/TNF) neutrophils which (depending on the extent of activation) may prolong or curtail neutrophil life-span. Currently, the most infamous staphylococcal toxin is Panton-Valentine leucocidin toxin (PVL) because of MRSA strains bearing this toxin that can cause severe necrotising pneumonia in previously healthy individuals. PVL at low concentrations promotes the formation of pores in neutrophil mitochondrial membranes promoting classical mitochondrial apoptosis, whilst at high concentrations cell membranes are rendered porous promoting necrosis [55]. It has also been suggested that PVL might promote neutrophil activation. This combination of excessive, ineffectual activation and decreased longevity with a further suggestion that the bactericidal activity of neutrophils is simultaneously disarmed by downregulation of oxidative burst capacity makes for a ruinous mix of persistent infection and inflammation-driven tissue necrosis [49].
Pseudomonas aeruginosa, a pathogen associated with cystic fibrosis and ventilator-associated pneumonia, almost completely dismantles the innate immune defence paradigm discussed earlier. In addition to evading killing by promoting neutrophil apoptosis through production of the toxin pyocyanin, it also inhibits macrophage clearance of apoptotic cells leading to secondary necrosis and tissue destruction [48,54]. The immune system is then forced to defend the indefensible as the tissue environment is subverted to the ends of the bacterium. P. aeruginosa can effectively hide away from subsequent immune sallies through the synthesis of a protective biofilm. It is clear that specific micro-organisms utilise a variety of immune evasion/immune thwarting strategies and it will always be important to consider this when designing novel anti-microbial and anti-inflammatory strategies.
Many different organisms may be implicated in the development of sepsis (pathogens present in peripheral circulation with characteristic physiological changes) and the potential sequelae of septic shock and adult respiratory distress syndrome (ARDS) [1]. Neutrophils have been shown to be functionally downregulated in a recent study of septic ITU patients where various causative pathogens were isolated [56]. In this study, C5a was implicated mechanistically in the inhibition of wide-ranging neutrophil functions including: phagocytosis, chemotaxis and superoxide anion production. This is in almost complete contrast to previous work on the role of this component of the complement cascade which is usually thought of as a chemoattractant and neutrophil activator. The ARDS is arguably an example of an excessive, even inappropriate activation of the innate immune response with potentially fatal consequences. Inflammation is a necessary immune defence mechanism; however, the inflammatory response can become sustained or self-perpetuating if it fails to remove the provoking stimulus or if the mechanisms of resolution become dysfunctional. Increased production of cytokines and continued release of proteolytic and cytotoxic contents by abundant cell infiltrates increase tissue damage associated with acute and chronic inflammatory disorders including: ARDS, ILD, cystic fibrosis, COPD, and arthritis. It is clear that inducing the resolving phase of inflammation through cytokine-neutralising therapies (e.g. anti-TNF-α, anti-CD20 in inflammatory skin disease and arthritis) can be of therapeutic benefit [57,58]. There is now encouraging evidence from animal models that the promotion of neutrophil apoptosis may drive resolution of inflammation both by promoting a favourable pro-resolution cytokine mix and removing the responsible inflammatory effector cell [6,7]. In addition, there is increasing understanding of the mechanisms used by pathogens to subvert apoptotic machinery in neutrophils and evidence that a combination of anti-bacterial therapy and an apoptosis-inducing drug can prevent serious pathogen-driven disease.
Anti-Inflammatory Potential of Apoptotic Neutrophils
The apoptotic neutrophil is generally functionally quiescent. The major secretory and transcriptional processes are shut down during apoptosis, which reflects the anti-inflammatory nature of the apoptotic process [59]. However, the apoptotic neutrophil itself represents an important anti-inflammatory stimulus to other cells involved in resolution of inflammation. The phenotype of the apoptotic neutrophil changes to produce ‘eat me’ signals that are recognised by surrounding phagocytes. Godson and collaborators have identified the release of annexin 1 by apoptotic cells as a soluble signal to promote phagocytosis of these cells by macrophages through a lipoxin receptor (lipoxin A4 receptors)-mediated pathway [61]. The exposure of phosphatidylserine (PS) residues on the apoptotic neutrophil membrane allows for greater recognition by macrophages, thereby enhancing the clearance of the cells. Interaction of PS with its corresponding, newly identified receptors on macrophages not only initiates phagocytosis but also modifies the transcriptional profile of the macrophage, increasing production of IL-10 and TGF-β, cytokines associated with resolving the inflammatory response and promoting tissue repair [61,52]. These processes partly help explain the benefits associated with extracorporeal photophoresis, where a patient's own leukocytes are isolated, induced to undergo apoptosis with the use of photoactivated drugs and re-administered via the circulation. This therapy is used to enhance the cytotoxic T cell response in the treatment of T cell lymphoma, but clinical benefits have been observed in a wide variety of diseases including rheumatoid arthritis, inflammatory bowel disease and graft-versus-host disease, presumably due to the multifaceted role of apoptotic cells in the modulation of cytokine response and promotion of immune tolerance [63,64].
It is also apparent that the apoptotic neutrophil has excellent LPS-binding capacity thereby reducing the LPS stimulation of viable, responding cells. This function of apoptotic neutrophils has been exploited in an interesting study that elegantly demonstrated the beneficial effect of systemic administration of apoptotic human neutrophils in murine models of septic shock [7]. These mice were protected from endotoxin lethality in concordance with decreased pro-inflammatory cytokine production, reduced PMN infiltration to the organs and a reduction in serum LPS concentrations. Apoptotic cells bound LPS and were removed by macrophages promoting an IL-10-driven pro-resolution of inflammation response from macrophages and other cells influenced by the altered cytokine milieu. This is an excellent example of an apoptosis-based strategy proving beneficial in a model of excessive host response to microbial infection.
The apoptotic death process, as opposed to the necrotic death process, maintains membrane integrity to limit release of harmful neutrophil contents. If clearance does not follow apoptosis, secondary necrosis can occur, leading to exacerbated inflammation. Indeed, dysfunctioning clearance mechanisms are linked with the pathogenesis of many inflammatory and autoimmune diseases such as systemic lupus erythematosus. Recently, it has been proposed that the dying and necrotic neutrophil has anti-inflammatory potential linked to the liberation of alpha defensins [65]. These molecules have complex immunomodulatory and anti-microbial effects and are actively released by activated neutrophils during degranulation at inflammatory sites. Neutrophil necrosis occurs by direct cytotoxicity induced by noxious stimuli, by apoptotic neutrophils undergoing secondary necrosis resulting from failed clearance or by pore-forming toxins such as PVL. The cathelicidin LL-37 has also been shown to induce rapid secondary necrosis of neutrophils that can potentiate the anti-inflammatory effect of macrophages without compromising clearance mechanisms [66]. Although these studies did not show any adverse effects on macrophage function with suggestion of promotion of a less inflammatory macrophage response, this must be weighed against the presence of toxic granule contents in the inflammatory milieu that previously had always been thought to contribute to host tissue damage. These studies clearly demonstrate the balance between the pro- and anti-inflammatory effects of neutrophils is a precarious one.
Not only do Gram-negative and Gram-positive bacteria and their products have an ability to influence apoptosis and functional longevity of neutrophils, there is abundant evidence indicating that other organisms such as viruses and fungi have similar capabilities. For example, gliotoxin, an abundant product liberated from fungi such as Aspergillus fumigatus, can induce apoptosis of neutrophils by mechanisms including inhibition of NF-κB [67]. Neutrophil apoptosis or survival during viral infections may have potentially important consequences in either promoting or impairing the ability of the host to clear infection but also contribute or reduce infection-associated pathology. Such examples include the ability of respiratory syncytial virus to inhibit neutrophil apoptosis, whilst on the other hand viral infections such as HIV and influenza A can promote neutrophil apoptosis [68].
Therapeutic Interventions Targeting Neutrophil Apoptosis
Advances in knowledge of neutrophil biology over the past few decades have identified the neutrophil as a key immune defence cell, but it is also becoming clear that even in death neutrophils have important immunomodulatory functions. There is much left to discover about the mechanisms regulating neutrophil life and death. Work performed in our laboratory has focussed on the use of pharmacological inhibitors of cyclin-dependent kinases (CDKi) to drive neutrophil apoptosis in an attempt to resolve inflammatory conditions [6]. The cyclin-dependent kinases are enzymes associated with the progression and regulation of the cell cycle and the CDKi drugs have been in development as treatments for cancer, where the cell cycle is clearly an important target [69]. Neutrophils are terminally differentiated cells that one would normally consider not requiring of CDKs; however, measurable levels of various CDKs have been reported. Furthermore, treatment of neutrophils with CDKi drugs accelerates the rate of apoptosis through a caspase-dependent mechanism [6]. This has led to some exciting research in inflammatory pharmacology, focussing on novel therapeutics to resolve chronic inflammatory conditions by promoting cell-specific apoptotic death. In murine models of inflammation, including passively induced arthritis, bleomycin-induced lung injury and carrageenan-elicited acute pleurisy, the CDKi R-roscovitine significantly improved resolution of inflammation that was associated with an increase in neutrophil apoptosis. The benefit of inducing neutrophil apoptosis during pneumococcal meningitis was comprehensively shown by Koedel et al. [70]. Neurological deficits caused by brain tissue damage are a common finding in survivors of severe meningitis and are linked to increased longevity of the neutrophil population recruited to the brain. The induction of neutrophil apoptosis with R-roscovitine in conjunction with antibiotic therapy (ceftriaxone) reduced markers of neuronal damage in murine models of pneumococcal meningitis. This suggests a possible new strategy for the limitation of detrimental neurological side effects of meningeal infection as well as opening up a more general approach to the management of severe infective disease where a causative role for neutrophils in disease pathology can be demonstrated. A dual strategy directed against both the causative organism and the inflammatory response generated toward that organism may be more efficacious than traditional microbe-directed treatment.
Targeting the apoptotic pathway to promote resolution has also been investigated at the level of MAPK signalling and by targeting proteins of the Bcl-2 family. Specific pharmacological inhibition of ERK1/2 involved in MAPK signalling augmented resolution of inflammation in a pleurisy model that was linked indirectly (by inhibition of the production of neutrophil survival factors at the site of inflammation) to an increase in neutrophil apoptosis [71]. Conversely, inhibition of the pro-apoptotic protein Bax and non-specific inhibition of ERK (and COX-2) augmented inflammation in the same model by increasing neutrophil longevity. PI3 kinase signalling has been implicated in granulocyte recruitment but also has a role in the persistence of neutrophil and eosinophil-driven inflammation and is thus recognised as a target for resolution therapies [72]. The relatively new characterisation of endogenously found lipid-derived resolvin molecules (aspirin-triggered 15-epi-lipoxin A4) have also recently been found to influence neutrophil apoptosis by suppressing MPO-induced survival mechanisms with improved resolution of inflammation in lung-injury models [12,13]. Macrophage clearance of apoptotic neutrophils is another therapeutic target for resolvin compounds that have shown anti-inflammatory and pro-resolution effects linked to enhancement of phagocytosis of apoptotic cells and attenuation of macrophage pro-inflammatory cytokine and chemokine release [73]. This demonstrates the multi-factorial role these compounds can play in promoting resolution. Our knowledge of apoptotic regulation in neutrophils is increasing and continues to underline the importance of the balance of pro- and anti-apoptotic Bcl-2 protein family members in the kinetics of apoptosis and ultimately the persistence or resolution of inflammation. The importance of the key survival protein, Mcl-1, is demonstrated by the effect of CDKi compounds, as it is down-regulated both in inflammatory and cancer models [6,74]. Modulation of the pathways leading to NF-κB activation has been implicated in the resulting destabilisation of levels of Mcl-1 in cancer cells [75,76]. However, this is not mirrored in inflammatory neutrophils where it has been shown that direct activation of NF-κB is unaffected by CDKi treatment [77]. Inflammatory mediators that prolong neutrophil survival often work by stabilising Mcl-1 protein levels; therefore, it is pertinent that the promotion of apoptosis can overcome these survival signals that would be in abundance during chronic inflammation. Indeed, accelerated neutrophil apoptosis was still observed in the presence of survival factors LPS, GM-CSF, TNF-α and dcAMP [6]. The effect of CDKis on other cell types involved in inflammation must also be considered, in particular with regard to the clearance of apoptotic cells by macrophages. Uncompromised clearance mechanisms are essential to the success of the paradigm of resolution especially where the proposed strategy involves increasing the apoptotic cell burden. So far, we have shown that macrophage recruitment is not adversely affected by CDKi treatment [unpubl. data]. Furthermore, the effect of CDK inhibition may prove to have beneficial anti-inflammatory actions as shown by various studies that demonstrate a decrease in LPS-stimulated pro-inflammatory cytokine production by macrophages over-expressing the endogenous CDK inhibitor, p21 [78,79]. These findings complement current work in our laboratory showing a similar reduction in cytokine production in human macrophages following pharmacological CDK inhibition [unpubl. data]. Eosinophils, like neutrophils, are of the granulocyte lineage and contain many of the same regulatory proteins involved in apoptotic responses. Mcl-1 has been demonstrated to be an important survival protein in these cells, and recent work by our group has shown that eosinophils are also susceptible to accelerated apoptosis following CDKi treatment through a similar, mitochondrial (intrinsic) death pathway requiring caspase activation [80]. Taken together, small-molecular inhibitors of CDKs offer a promising target for anti-inflammatory, anti-allergic and anti-infective therapies.
Conclusion
We have attempted to consider the importance of neutrophil apoptosis within the contexts of the appropriate immune response to invading pathogens and the inappropriate or excessive inflammation in inflammatory disease. We have highlighted that the intricate interplay between pathogens and the innate immune response mean that it is possible to see therapeutic value in the promotion of neutrophil apoptosis even in situations where intuitively one would deem a neutrophil response appropriate. Additionally, we have mentioned the potential of combining an anti-bacterial strategy with a pro-apoptotic approach in order to achieve bacterial killing and dampen potentially harmful excesses of inflammation. Our message is that there is much to learn and much to be gained from collaboration between microbe-oriented researchers and inflammatory cell biologists.
Acknowledgements
This work was funded by grants from the MRC (G0601481) and Wellcome Trust (WT082181).
Footnotes
S. Fox and A.E. Leitch contributed equally.
References
- 1.Leitch AE, Duffin R, Haslett C, Rossi AG. Relevance of granulocyte apoptosis to resolution of inflammation at the respiratory mucosa. Mucosal Immunol. 2008;5:350–363. doi: 10.1038/mi.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallett JM, Leitch AE, Riley NA, Duffin R, Haslett C, Rossi AG. Novel pharmacological strategies for driving inflammatory cell apoptosis and enhancing the resolution of inflammation. Trends Pharmacol Sci. 2008;29:250–257. doi: 10.1016/j.tips.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation: programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan CN, Brain SD, Buckley CD, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 6.Rossi AG, Sawatzky DA, Walker A, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 7.Ren Y, Xie Y, Jiang G, et al. Apoptotic cells protect mice against lipopolysaccharide-induced shock. J Immunol. 2008;180:4978–4985. doi: 10.4049/jimmunol.180.7.4978. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy AD, DeLeo FR. Neutrophil apoptosis and the resolution of infection. Immunol Res. 2009;43:25–61. doi: 10.1007/s12026-008-8049-6. [DOI] [PubMed] [Google Scholar]
- 9.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 10.Soehnlein O, Xie X, Ulbrich H, et al. Neutrophil-derived heparin-binding protein (HBP/CAP37) deposited on endothelium enhances monocyte arrest under flow conditions. J Immunol. 2005;174:6399–6405. doi: 10.4049/jimmunol.174.10.6399. [DOI] [PubMed] [Google Scholar]
- 11.Opferman JT. Life and death during hematopoietic differentiation. Curr Opin Immunol. 2007;19:497–502. doi: 10.1016/j.coi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Drewniak A, van Raam BJ, Geissler J, Tool AT, Mook OR, van den Berg TK, Baas F, Kuijpers TW. Changes in gene expression of granulocytes during in vivo granulocyte colony-stimulating factor/dexamethasone mobilization for transfusion purposes. Blood. 2009;113:5979–5998. doi: 10.1182/blood-2008-10-182147. [DOI] [PubMed] [Google Scholar]
- 13.El Kebir, Jozsef L, Pan W, et al. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med. 2009;180:311–319. doi: 10.1164/rccm.200810-1601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 15.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: ‘N1' versus ‘N2’ TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasmono RT, Ehrnsperger A, Cronau SL, et al. Mouse neutrophilic granulocytes express mRNA encoding the macrophage colony-stimulating factor receptor (CSF-1R) as well as many other macrophage-specific transcripts and can transdifferentiate into macrophages in vitro in response to CSF-1. J Leukoc Biol. 2007;82:111–123. doi: 10.1189/jlb.1206713. [DOI] [PubMed] [Google Scholar]
- 17.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 18.Haslett C, Guthrie LA, Kopaniak MM, Johnston RB, Jr, Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985;119:101–110. [PMC free article] [PubMed] [Google Scholar]
- 19.Haslett C, Lee A, Savill JS, Meagher L, Whyte MK. Apoptosis (programmed cell death) and functional changes in aging neutrophils: modulation by inflammatory mediators. Chest. 1991;99:6S. [PubMed] [Google Scholar]
- 20.El Kebir D, József L, Pan W, Filep JG. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ Res. 2008;103:352–359. doi: 10.1161/01.RES.0000326772.76822.7a. [DOI] [PubMed] [Google Scholar]
- 21.Simon HU. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. 2003;193:101–110. doi: 10.1034/j.1600-065x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 22.Edwards SW, Derouet M, Howse M, Moots RJ. Regulation of neutrophil apoptosis by Mcl-1. Biochem Soc Trans. 2004;32:489–492. doi: 10.1042/BST0320489. [DOI] [PubMed] [Google Scholar]
- 23.Akgul C, Moulding DA, White MR, Edwards SW. In vivo localisation and stability of human Mcl-1 using green fluorescent protein (GFP) fusion proteins. FEBS Lett. 2000;478:72–76. doi: 10.1016/s0014-5793(00)01809-3. [DOI] [PubMed] [Google Scholar]
- 24.Dzhagalov I, St JA, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–1626. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leuenroth SJ, Grutkoski PS, Ayala A, Simms HH. The loss of Mcl-1 expression in human polymorphonuclear leukocytes promotes apoptosis. J Leukoc Biol. 2000;68:158–166. [PubMed] [Google Scholar]
- 26.Lovell JF, Billen LP, Bindner S, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Weinmann P, Gaehtgens P, Walzog B. Bcl-Xl- and Bax-alpha-mediated regulation of apoptosis of human neutrophils via caspase-3. Blood. 1999;93:3106–3115. [PubMed] [Google Scholar]
- 28.Scatizzi JC, Hutcheson J, Bickel E, Haines GK, III, Perlman H. Pro-apoptotic Bid is required for the resolution of the effector phase of inflammatory arthritis. Arthritis Res Ther. 2007;9:R49. doi: 10.1186/ar2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andina N, Conus S, Schneider EM, Fey MF, Simon HU. Induction of Bim limits cytokine-mediated prolonged survival of neutrophils. Cell Death Differ. 2009;16:1248–1255. doi: 10.1038/cdd.2009.50. [DOI] [PubMed] [Google Scholar]
- 30.Villunger A, Scott C, Bouillet P, Strasser A. Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival. Blood. 2003;101:2393–2400. doi: 10.1182/blood-2002-07-2132. [DOI] [PubMed] [Google Scholar]
- 31.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 32.Cross A, Moots RJ, Edwards SW. The dual effects of TNFalpha on neutrophil apoptosis are mediated via differential effects on expression of Mcl-1 and Bfl-1. Blood. 2008;111:878–884. doi: 10.1182/blood-2007-05-087833. [DOI] [PubMed] [Google Scholar]
- 33.Murray J, Barbara JA, Dunkley SA, et al. Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood. 1997;90:2772–2783. [PubMed] [Google Scholar]
- 34.Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13:738–747. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- 35.Jost PJ, Grabow S, Gray D, et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460:1035–1039. doi: 10.1038/nature08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galluzzi L, Aaronson SA, Abrams J, et al. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 2009;16:1093–1107. doi: 10.1038/cdd.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheel-Toellner D, Wang K, Assi LK, et al. Clustering of death receptors in lipid rafts initiates neutrophil spontaneous apoptosis. Biochem Soc Trans. 2004;32:679–681. doi: 10.1042/BST0320679. [DOI] [PubMed] [Google Scholar]
- 39.Fischer A. Human primary immunodeficiency diseases. Immunity. 2007;27:835–845. doi: 10.1016/j.immuni.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 41.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 42.Anwar S, Whyte MK. Neutrophil apoptosis in infectious disease. Exp Lung Res. 2007;33:519–528. doi: 10.1080/01902140701756620. [DOI] [PubMed] [Google Scholar]
- 43.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med. 1999;160:S5–S11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi SD, Braughton KR, Whitney AR, et al. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc Natl Acad Sci USA. 2003;100:10948–10953. doi: 10.1073/pnas.1833375100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laskay T, van ZG, Solbach W. Neutrophil granulocytes as host cells and transport vehicles for intracellular pathogens: apoptosis as infection-promoting factor. Immunobiology. 2008;213:183–191. doi: 10.1016/j.imbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 46.van ZG, Gieffers J, Kothe H, et al. Chlamydia pneumoniae multiply in neutrophil granulocytes and delay their spontaneous apoptosis. J Immunol. 2004;172:1768–1776. doi: 10.4049/jimmunol.172.3.1768. [DOI] [PubMed] [Google Scholar]
- 47.Rupp J, Pfleiderer L, Jugert C, et al. Chlamydia pneumoniae hides inside apoptotic neutrophils to silently infect and propagate in macrophages. PLoS One. 2009;4:e6020. doi: 10.1371/journal.pone.0006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabroe I, Whyte MK. Incapacitating the immune system in cystic fibrosis. Nat Med. 2007;13:1417–1418. doi: 10.1038/nm1207-1417. [DOI] [PubMed] [Google Scholar]
- 49.Anwar S, Prince LR, Foster SJ, Whyte MK, Sabroe I. The rise and rise of Staphylococcus aureus: laughing in the face of granulocytes. Clin Exp Immunol. 2009;157:216–224. doi: 10.1111/j.1365-2249.2009.03950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ocana MG, Asensi V, Montes AH, Meana A, Celada A, Valle-Garay E. Autoregulation mechanism of human neutrophil apoptosis during bacterial infection. Mol Immunol. 2008;45:2087–2096. doi: 10.1016/j.molimm.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 51.van ZG, Klinger M, Mueller A, et al. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol. 2004;173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 52.Miller LS, O'Connell RM, Gutierrez MA, et al. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 53.Miller LS, Pietras EM, Uricchio LH, et al. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol. 2007;179:6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- 54.Prince LR, Bianchi SM, Vaughan KM, et al. Subversion of a lysosomal pathway regulating neutrophil apoptosis by a major bacterial toxin, pyocyanin. J Immunol. 2008;180:3502–3511. doi: 10.4049/jimmunol.180.5.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Genestier AL, Michallet MC, Prévost G, et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J Clin Invest. 2005;115:3117–3127. doi: 10.1172/JCI22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conway MA, Kefala K, Wilkinson TS, et al. C5a mediates peripheral blood neutrophil dysfunction in critically ill patients. Am J Respir Crit Care Med. 2009;180:19–28. doi: 10.1164/rccm.200812-1928OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feldmann M, Maini RN. Discovery of TNF-alpha as a therapeutic target in rheumatoid arthritis: preclinical and clinical studies. Joint Bone Spine. 2002;69:12–18. doi: 10.1016/s1297-319x(01)00335-9. [DOI] [PubMed] [Google Scholar]
- 58.Williams RO, Paleolog E, Feldmann M. Cytokine inhibitors in rheumatoid arthritis and other autoimmune diseases. Curr Opin Pharmacol. 2007;7:412–417. doi: 10.1016/j.coph.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Dransfield I, Rossi AG, Brown SB, Hart SP. Neutrophils: dead or effete? Cell surface phenotype and implications for phagocytic clearance. Cell Death Differ. 2005;12:1363–1367. doi: 10.1038/sj.cdd.4401695. [DOI] [PubMed] [Google Scholar]
- 60.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scannell M, Flanagan MB, deStefani A, Wynne KJ, Cagney G, Godson C, Maderna P. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J Immunol. 2007;178:4595–4605. doi: 10.4049/jimmunol.178.7.4595. [DOI] [PubMed] [Google Scholar]
- 62.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 63.Bladon J, Taylor PC. The down-regulation of IL1alpha and IL6, in monocytes exposed to extracorporeal photopheresis (ECP)-treated lymphocytes, is not dependent on lymphocyte phosphatidylserine externalization. Transpl Int. 2006;19:319–324. doi: 10.1111/j.1432-2277.2006.00278.x. [DOI] [PubMed] [Google Scholar]
- 64.Bladon J, Taylor PC. Extracorporeal photopheresis: a focus on apoptosis and cytokines. J Dermatol Sci. 2006;43:85–94. doi: 10.1016/j.jdermsci.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Miles K, Clarke DJ, Lu W, et al. Dying and necrotic neutrophils are anti-inflammatory secondary to the release of alpha-defensins. J Immunol. 2009;183:2122–2132. doi: 10.4049/jimmunol.0804187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li HN, Barlow PG, Bylund J, et al. Secondary necrosis of apoptotic neutrophils induced by the human cathelicidin LL-37 is not proinflammatory to phagocytosing macrophages. J Leukoc Biol. 2009;86:891–902. doi: 10.1189/jlb.0209050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ward C, Chilvers ER, Lawson MF, et al. NF-κB activation is a critical regulator of human granulocyte apoptosis in vitro. J Biol Chem. 1999;274:4309–4318. doi: 10.1074/jbc.274.7.4309. [DOI] [PubMed] [Google Scholar]
- 68.Elbim C, Katsikis PD, Estaquier J. Neutrophil apoptosis during viral infections. Open Virol J. 2009;3:52–59. doi: 10.2174/1874357900903010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knockaert M, Greengard P, Meijer L. Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol Sci. 2002;23:417–425. doi: 10.1016/s0165-6147(02)02071-0. [DOI] [PubMed] [Google Scholar]
- 70.Koedel U, Frankenberg T, Kirschnek S, et al. Apoptosis is essential for neutrophil functional shutdown and determines tissue damage in experimental pneumococcal meningitis. PLoS Pathog. 2009;5:e1000461. doi: 10.1371/journal.ppat.1000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sawatzky DA, Willoughby DA, Colville-Nash PR, Rossi AG. The involvement of the apoptosis-modulating proteins ERK 1/2, Bcl-xL and Bax in the resolution of acute inflammation in vivo. Am J Pathol. 2006;168:33–41. doi: 10.2353/ajpath.2006.050058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pinho V, de Castro RR, Amaral FA, et al. Tissue- and stimulus-dependent role of phosphatidylinositol 3-kinase isoforms for neutrophil recruitment induced by chemoattractants in vivo. J Immunol. 2007;179:7891–7898. doi: 10.4049/jimmunol.179.11.7891. [DOI] [PubMed] [Google Scholar]
- 73.MacCallum DE, Melville J, Frame S, et al. Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase II-dependent transcription and down-regulation of Mcl-1. Cancer Res. 2005;65:5399–5407. doi: 10.1158/0008-5472.CAN-05-0233. [DOI] [PubMed] [Google Scholar]
- 74.Dey A, Wong ET, Cheok CF, Tergaonkar V, Lane DP. R-roscovitine simultaneously targets both the p53 and NF-κB pathways and causes potentiation of apoptosis: implications in cancer therapy. Cell Death Differ. 2008;15:263–273. doi: 10.1038/sj.cdd.4402257. [DOI] [PubMed] [Google Scholar]
- 75.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;64:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 76.Mitchell S, Thomas G, Harvey K, et al. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- 77.Leitch AE, Riley NA: The cyclin-dependent kinase inhibitor R-roscovitine down-regulates Mcl-1 to override pro-inflammatory signalling and drive neutrophil apoptosis. Eur J Immunol DOI: 10.1002/eji.200939664. [DOI] [PubMed]
- 78.Lloberas J, Celada A. p21(waf1/CIP1), a CDK inhibitor and a negative feedback system that controls macrophage activation. Eur J Immunol. 2009;39:691–694. doi: 10.1002/eji.200939262. [DOI] [PubMed] [Google Scholar]
- 79.Scatizzi JC, Mavers M, Hutcheson J, et al. The CDK domain of p21 is a suppressor of IL-1beta-mediated inflammation in activated macrophages. Eur J Immunol. 2009;39:820–825. doi: 10.1002/eji.200838683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duffin R, Leitch AE, Sheldrake TA, et al. The CDK inhibitor, R-roscovitine, promotes eosinophil apoptosis by down-regulation of Mcl-1. FEBS Lett. 2009;583:2540–2546. doi: 10.1016/j.febslet.2009.07.017. [DOI] [PubMed] [Google Scholar]