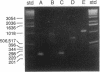
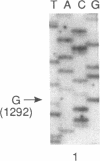
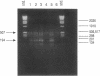
Abstract
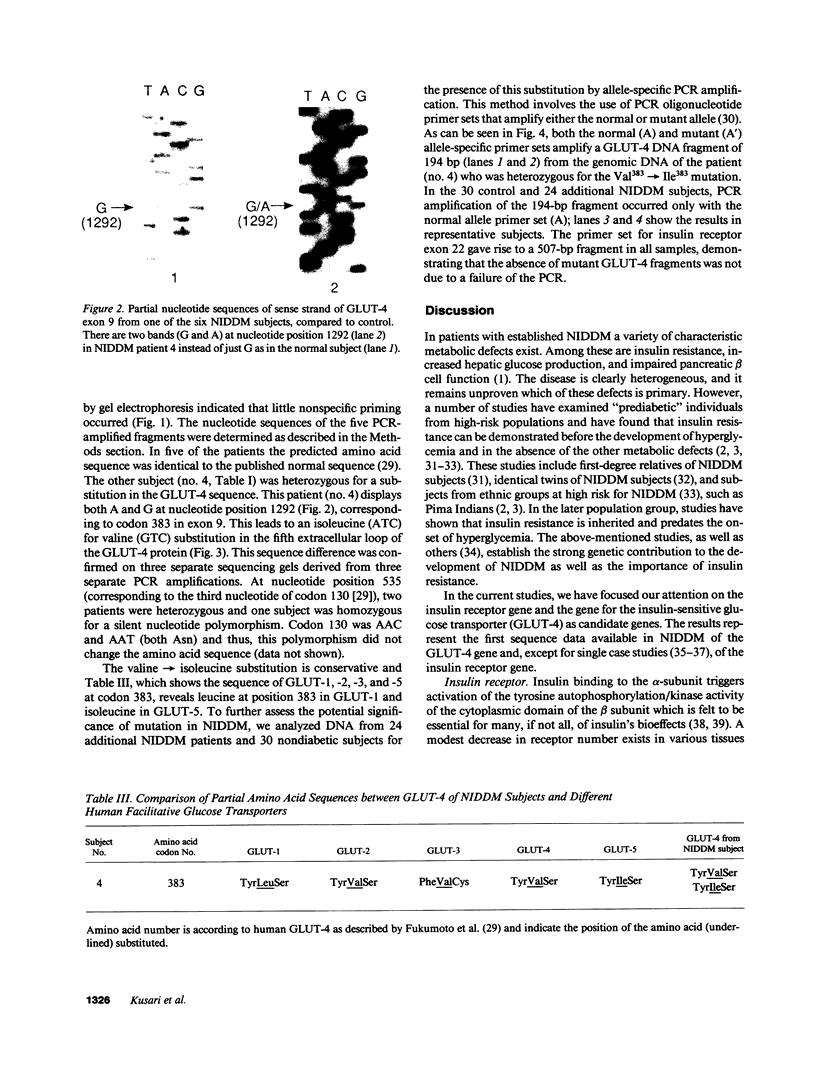
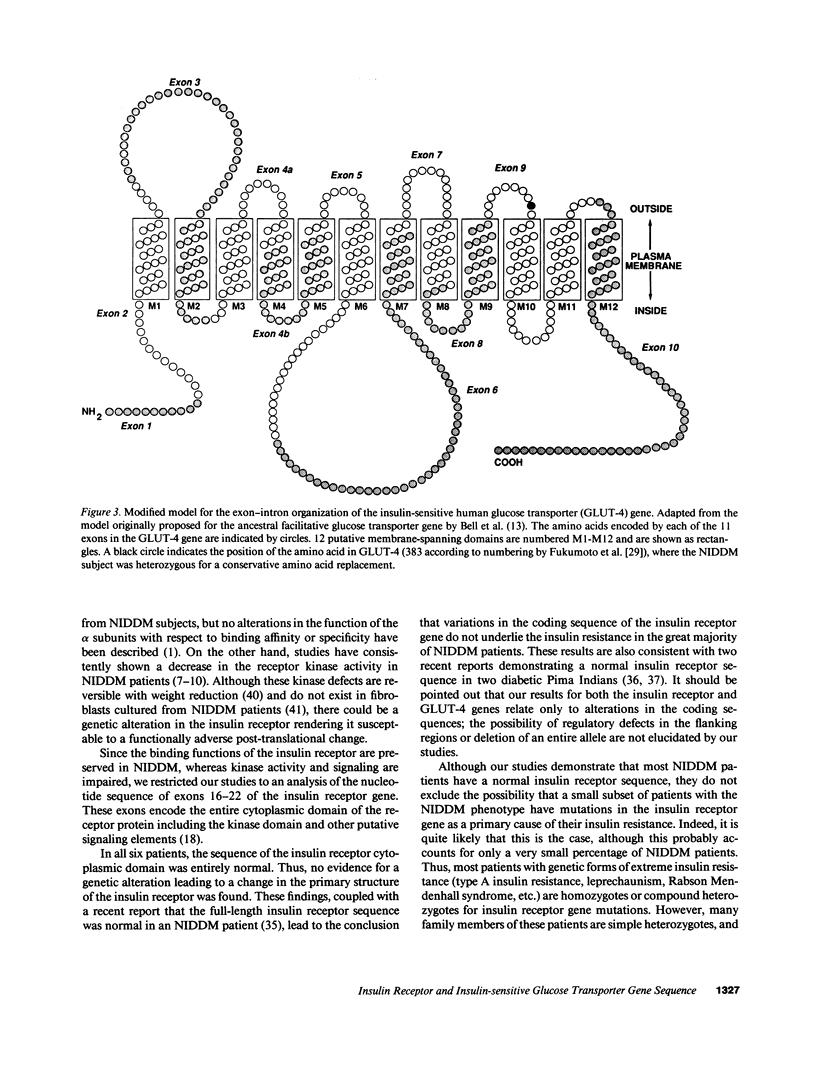
Insulin resistance is a common feature of non-insulin-dependent diabetes mellitus (NIDDM) and "diabetes susceptibility genes" may be involved in this abnormality. Two potential candidate genes are the insulin receptor (IR) and the insulin-sensitive glucose transporter (GLUT-4). To elucidate whether structural defects in the IR and/or GLUT-4 could be a primary cause of insulin resistance in NIDDM, we have sequenced the entire coding region of the GLUT-4 gene from DNA of six NIDDM patients. Since binding properties of the IRs from NIDDM subjects are normal, we also analyzed the sequence of exons 16-22 (encoding the entire cytoplasmic domain of the IR) of the IR gene from the same six patients. When compared with the normal IR sequence, no difference was found in the predicted amino acid sequence of the IR cytoplasmic domain derived from the NIDDM patients. Sequence analysis of the GLUT-4 gene revealed that one patient was heterozygous for a mutation in which isoleucine (ATC) was substituted for valine (GTC) at position 383. Consequently, the GLUT-4 sequence at position 383 was determined in 24 additional NIDDM patients and 30 nondiabetic controls and all showed only the normal sequence. From these studies, we conclude that the insulin resistance seen in the great majority of subjects with the common form of NIDDM is not due to genetic variation in the coding sequence of the IR beta subunit, nor to any single mutation in the GLUT-4 gene. Possibly, a subpopulation of NIDDM patients exists displaying variation in the GLUT-4 gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett A. H., Eff C., Leslie R. D., Pyke D. A. Diabetes in identical twins. A study of 200 pairs. Diabetologia. 1981 Feb;20(2):87–93. doi: 10.1007/BF00262007. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Kayano T., Buse J. B., Burant C. F., Takeda J., Lin D., Fukumoto H., Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990 Mar;13(3):198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- Cama A., Patterson A. P., Kadowaki T., Kadowaki H., Siegel G., D'Ambrosio D., Lillioja S., Roth J., Taylor S. I. The amino acid sequence of the insulin receptor is normal in an insulin-resistant Pima Indian. J Clin Endocrinol Metab. 1990 Apr;70(4):1155–1161. doi: 10.1210/jcem-70-4-1155. [DOI] [PubMed] [Google Scholar]
- Caro J. F., Ittoop O., Pories W. J., Meelheim D., Flickinger E. G., Thomas F., Jenquin M., Silverman J. F., Khazanie P. G., Sinha M. K. Studies on the mechanism of insulin resistance in the liver from humans with noninsulin-dependent diabetes. Insulin action and binding in isolated hepatocytes, insulin receptor structure, and kinase activity. J Clin Invest. 1986 Jul;78(1):249–258. doi: 10.1172/JCI112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J. F., Sinha M. K., Raju S. M., Ittoop O., Pories W. J., Flickinger E. G., Meelheim D., Dohm G. L. Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. J Clin Invest. 1987 May;79(5):1330–1337. doi: 10.1172/JCI112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaraldi T. P., Kolterman O. G., Scarlett J. A., Kao M., Olefsky J. M. Role of glucose transport in the postreceptor defect of non-insulin-dependent diabetes mellitus. Diabetes. 1982 Nov;31(11):1016–1022. doi: 10.2337/diacare.31.11.1016. [DOI] [PubMed] [Google Scholar]
- Comi R. J., Grunberger G., Gorden P. Relationship of insulin binding and insulin-stimulated tyrosine kinase activity is altered in type II diabetes. J Clin Invest. 1987 Feb;79(2):453–462. doi: 10.1172/JCI112833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm G. L., Tapscott E. B., Pories W. J., Dabbs D. J., Flickinger E. G., Meelheim D., Fushiki T., Atkinson S. M., Elton C. W., Caro J. F. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest. 1988 Aug;82(2):486–494. doi: 10.1172/JCI113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J., Franssila-Kallunki A., Ekstrand A., Saloranta C., Widén E., Schalin C., Groop L. Early metabolic defects in persons at increased risk for non-insulin-dependent diabetes mellitus. N Engl J Med. 1989 Aug 10;321(6):337–343. doi: 10.1056/NEJM198908103210601. [DOI] [PubMed] [Google Scholar]
- Freidenberg G. R., Henry R. R., Klein H. H., Reichart D. R., Olefsky J. M. Decreased kinase activity of insulin receptors from adipocytes of non-insulin-dependent diabetic subjects. J Clin Invest. 1987 Jan;79(1):240–250. doi: 10.1172/JCI112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Henry R. R., Wallace P., Olefsky J. M. Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes. 1986 Sep;35(9):990–998. doi: 10.2337/diab.35.9.990. [DOI] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Kadowaki H., Taylor S. I. A nonsense mutation causing decreased levels of insulin receptor mRNA: detection by a simplified technique for direct sequencing of genomic DNA amplified by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Jan;87(2):658–662. doi: 10.1073/pnas.87.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., White M. F. The insulin receptor and the molecular mechanism of insulin action. J Clin Invest. 1988 Oct;82(4):1151–1156. doi: 10.1172/JCI113711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanicki M. A., Pilch P. F. Regulation of glucose-transporter function. Diabetes Care. 1990 Mar;13(3):219–227. doi: 10.2337/diacare.13.3.219. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusari J., Olefsky J. M., Strahl C., McClain D. A. Insulin-receptor cDNA sequence in NIDDM patient homozygous for insulin-receptor gene RFLP. Diabetes. 1991 Feb;40(2):249–254. doi: 10.2337/diab.40.2.249. [DOI] [PubMed] [Google Scholar]
- Moller D. E., Yokota A., Flier J. S. Normal insulin-receptor cDNA sequence in Pima Indians with NIDDM. Diabetes. 1989 Nov;38(11):1496–1500. doi: 10.2337/diab.38.11.1496. [DOI] [PubMed] [Google Scholar]
- Newman B., Selby J. V., King M. C., Slemenda C., Fabsitz R., Friedman G. D. Concordance for type 2 (non-insulin-dependent) diabetes mellitus in male twins. Diabetologia. 1987 Oct;30(10):763–768. doi: 10.1007/BF00275741. [DOI] [PubMed] [Google Scholar]
- O'Rahilly S., Spivey R. S., Holman R. R., Nugent Z., Clark A., Turner R. C. Type II diabetes of early onset: a distinct clinical and genetic syndrome? Br Med J (Clin Res Ed) 1987 Apr 11;294(6577):923–928. doi: 10.1136/bmj.294.6577.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Garvey W. T., Henry R. R., Brillon D., Matthaei S., Freidenberg G. R. Cellular mechanisms of insulin resistance in non-insulin-dependent (type II) diabetes. Am J Med. 1988 Nov 28;85(5A):86–105. doi: 10.1016/0002-9343(88)90401-9. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Kolterman O. G., Scarlett J. A. Insulin action and resistance in obesity and noninsulin-dependent type II diabetes mellitus. Am J Physiol. 1982 Jul;243(1):E15–E30. doi: 10.1152/ajpendo.1982.243.1.E15. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. The insulin receptor. A multifunctional protein. Diabetes. 1990 Sep;39(9):1009–1016. doi: 10.2337/diab.39.9.1009. [DOI] [PubMed] [Google Scholar]
- Pedersen O., Bak J. F., Andersen P. H., Lund S., Moller D. E., Flier J. S., Kahn B. B. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM. Diabetes. 1990 Jul;39(7):865–870. doi: 10.2337/diab.39.7.865. [DOI] [PubMed] [Google Scholar]
- Saad M. F., Knowler W. C., Pettitt D. J., Nelson R. G., Mott D. M., Bennett P. H. The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med. 1988 Dec 8;319(23):1500–1506. doi: 10.1056/NEJM198812083192302. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Cassady J., Bottema C. D., Sommer S. S. Characterization of polymerase chain reaction amplification of specific alleles. Anal Biochem. 1990 Apr;186(1):64–68. doi: 10.1016/0003-2697(90)90573-r. [DOI] [PubMed] [Google Scholar]
- Seino S., Seino M., Bell G. I. Human insulin-receptor gene. Partial sequence and amplification of exons by polymerase chain reaction. Diabetes. 1990 Jan;39(1):123–128. doi: 10.2337/diacare.39.1.123. [DOI] [PubMed] [Google Scholar]
- Seino S., Seino M., Nishi S., Bell G. I. Structure of the human insulin receptor gene and characterization of its promoter. Proc Natl Acad Sci U S A. 1989 Jan;86(1):114–118. doi: 10.1073/pnas.86.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M. K., Pories W. J., Flickinger E. G., Meelheim D., Caro J. F. Insulin-receptor kinase activity of adipose tissue from morbidly obese humans with and without NIDDM. Diabetes. 1987 May;36(5):620–625. doi: 10.2337/diab.36.5.620. [DOI] [PubMed] [Google Scholar]
- Sinha M. K., Taylor L. G., Pories W. J., Flickinger E. G., Meelheim D., Atkinson S., Sehgal N. S., Caro J. F. Long-term effect of insulin on glucose transport and insulin binding in cultured adipocytes from normal and obese humans with and without non-insulin-dependent diabetes. J Clin Invest. 1987 Oct;80(4):1073–1081. doi: 10.1172/JCI113163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoflet E. S., Koeberl D. D., Sarkar G., Sommer S. S. Genomic amplification with transcript sequencing. Science. 1988 Jan 29;239(4839):491–494. doi: 10.1126/science.3340835. [DOI] [PubMed] [Google Scholar]
- Truglia J. A., Livingston J. N., Lockwood D. H. Insulin resistance: receptor and post-binding defects in human obesity and non-insulin-dependent diabetes mellitus. Am J Med. 1985 Aug 23;79(2B):13–22. doi: 10.1016/0002-9343(85)90580-7. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Warram J. H., Martin B. C., Krolewski A. S., Soeldner J. S., Kahn C. R. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990 Dec 15;113(12):909–915. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- Wu D. Y., Ugozzoli L., Pal B. K., Wallace R. B. Allele-specific enzymatic amplification of beta-globin genomic DNA for diagnosis of sickle cell anemia. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2757–2760. doi: 10.1073/pnas.86.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]