Abstract
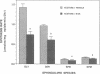
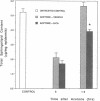
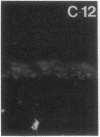
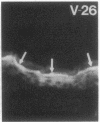
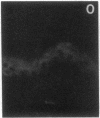
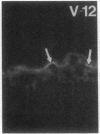
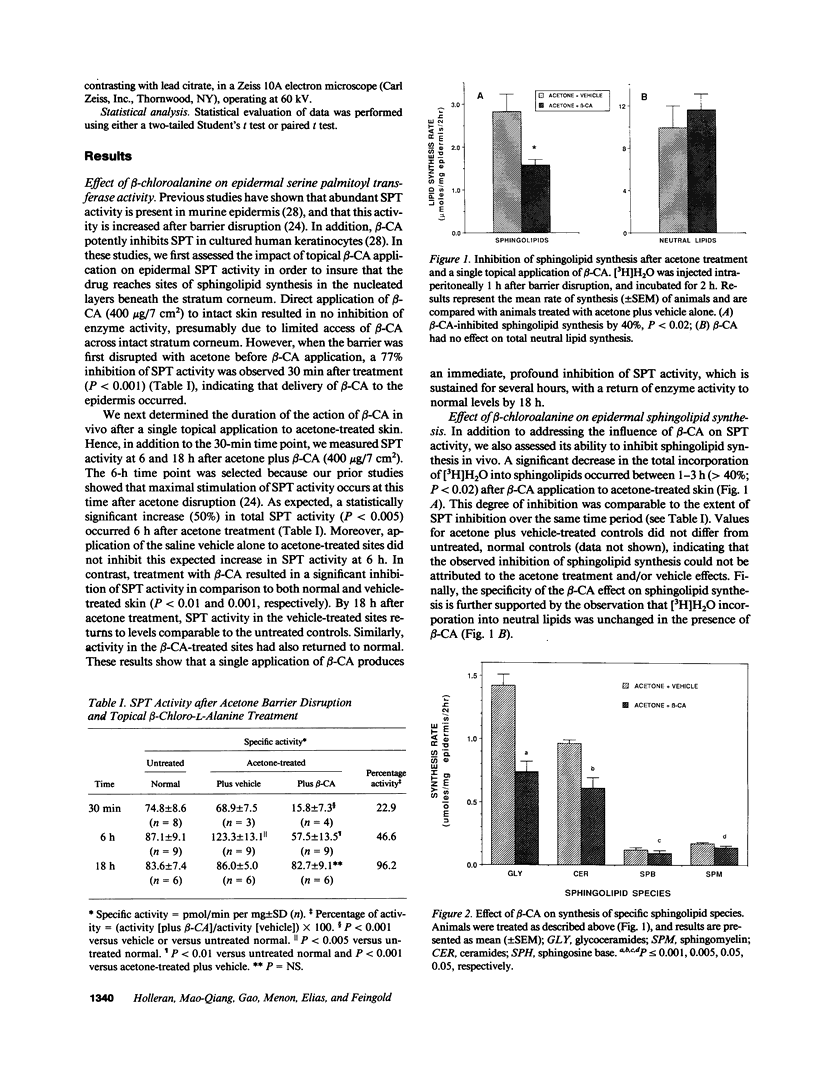
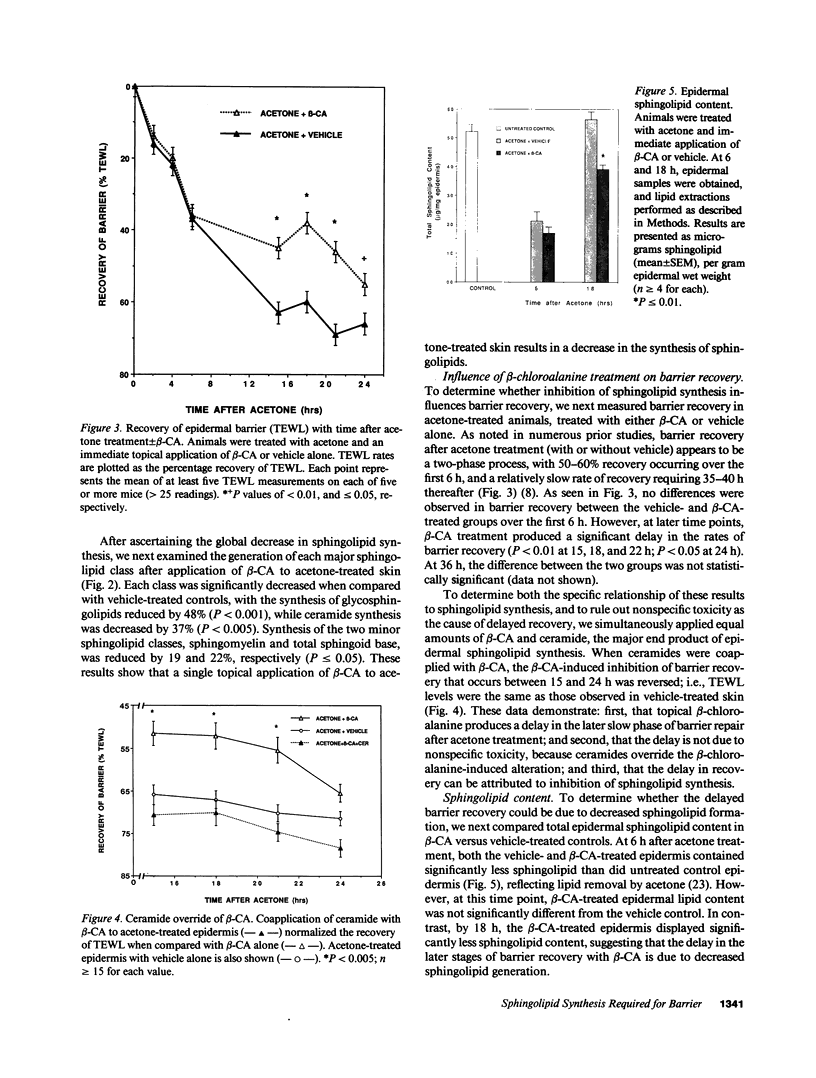
Stratum corneum lipids comprise an approximately equimolar mixture of sphingolipids, cholesterol, and free fatty acids, arranged as intercellular membrane bilayers that are presumed to mediate the epidermal permeability barrier. Prior studies have shown that alterations in epidermal barrier function lead to a rapid increase in cholesterol and fatty acid synthesis which parallels the early stages of the repair process. Despite an abundance of indirect evidence for their role in the barrier, the importance of sphingolipids has yet to be demonstrated directly. Whereas sphingolipid synthesis also increases during barrier repair, this response is delayed in comparison to cholesterol and fatty acid synthesis (Holleran, W.M., et al. 1991. J. Lipid Res. 32:1151-1158). To further delineate the role of sphingolipids in barrier homeostasis, we assessed the impact of inhibition of sphingolipid synthesis on epidermal barrier recovery. A single topical application of beta-chloro-L-alanine (beta-CA), an irreversible inhibitor of serine-palmitoyl transferase (SPT), applied to acetone-treated skin of hairless mice resulted in: (a) greater than 75% inhibition of SPT activity at 30 min (P less than 0.001); (b) a global decrease in sphingolipid synthesis between 1 and 3 h (P less than 0.02); (c) reduction of epidermal sphingolipid content at 18 h (P less than 0.01); (d) delayed reaccumulation of histochemical staining for sphingolipids in the stratum corneum; and (e) reduced numbers and contents of lamellar bodies in the stratum granulosum. Finally, despite its immediate, marked diminution of sphingolipid synthesis, beta-CA slowed barrier recovery only at late time points (greater than 6 h) after acetone treatment. This inhibition was overridden by coapplications of ceramides (the distal SPT product), indicating that the delay in repair was not due to non-specific toxicity. These studies demonstrate a distinctive role for epidermal sphingolipids in permeability barrier homeostasis.
Full text
PDF

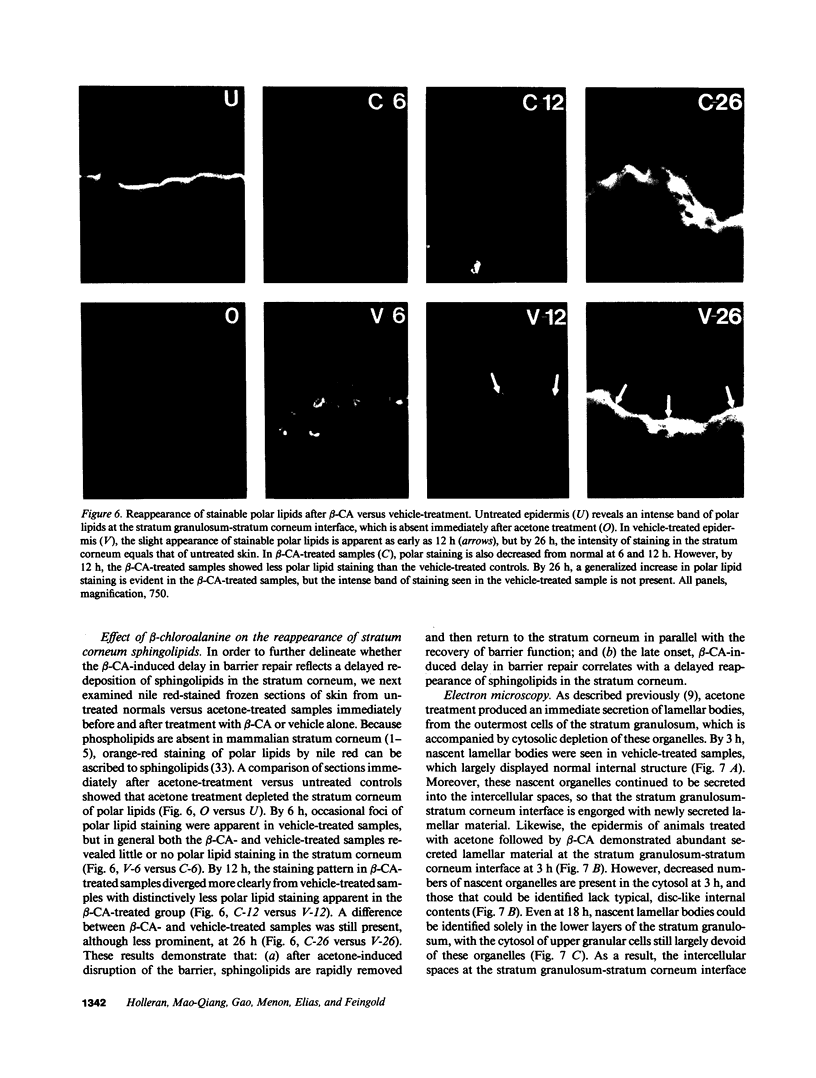



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham W., Wertz P. W., Downing D. T. Linoleate-rich acylglucosylceramides of pig epidermis: structure determination by proton magnetic resonance. J Lipid Res. 1985 Jun;26(6):761–766. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bowser P. A., Nugteren D. H., White R. J., Houtsmuller U. M., Prottey C. Identification, isolation and characterization of epidermal lipids containing linoleic acid. Biochim Biophys Acta. 1985 May 17;834(3):419–428. doi: 10.1016/0005-2760(85)90016-5. [DOI] [PubMed] [Google Scholar]
- Bowser P. A., White R. J. Isolation, barrier properties and lipid analysis of stratum compactum, a discrete region of the stratum corneum. Br J Dermatol. 1985 Jan;112(1):1–14. doi: 10.1111/j.1365-2133.1985.tb02285.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braun P. E., Morell P., Radin N. S. Synthesis of C18- and C20-dihydrosphingosines, ketodihydrosphingosines, and ceramides by microsomal preparations from mouse brain. J Biol Chem. 1970 Jan 25;245(2):335–341. [PubMed] [Google Scholar]
- Cox P., Squier C. A. Variations in lipids in different layers of porcine epidermis. J Invest Dermatol. 1986 Dec;87(6):741–744. doi: 10.1111/1523-1747.ep12456872. [DOI] [PubMed] [Google Scholar]
- Dykes P. J., Marks R., Davies M. G., Reynolds D. J. Epidermal metabolism in heredopathia atactica polyneuritiformis (Refsum's disease). J Invest Dermatol. 1978 Mar;70(3):126–129. doi: 10.1111/1523-1747.ep12258532. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Brown B. E., Fritsch P., Goerke J., Gray G. M., White R. J. Localization and composition of lipids in neonatal mouse stratum granulosum and stratum corneum. J Invest Dermatol. 1979 Nov;73(5):339–348. doi: 10.1111/1523-1747.ep12550377. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Brown B. E. The mammalian cutaneous permeability barrier: defective barrier function is essential fatty acid deficiency correlates with abnormal intercellular lipid deposition. Lab Invest. 1978 Dec;39(6):574–583. [PubMed] [Google Scholar]
- Elias P. M., Brown B. E., Ziboh V. A. The permeability barrier in essential fatty acid deficiency: evidence for a direct role for linoleic acid in barrier function. J Invest Dermatol. 1980 Apr;74(4):230–233. doi: 10.1111/1523-1747.ep12541775. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Menon G. K., Grayson S., Brown B. E., Rehfeld S. J. Avian sebokeratocytes and marine mammal lipokeratinocytes: structural, lipid biochemical, and functional considerations. Am J Anat. 1987 Oct;180(2):161–177. doi: 10.1002/aja.1001800206. [DOI] [PubMed] [Google Scholar]
- Elias P. M., Williams M. L. Neutral lipid storage disease with ichthyosis. Defective lamellar body contents and intracellular dispersion. Arch Dermatol. 1985 Aug;121(8):1000–1008. [PubMed] [Google Scholar]
- Feingold K. R., Brown B. E., Lear S. R., Moser A. H., Elias P. M. Effect of essential fatty acid deficiency on cutaneous sterol synthesis. J Invest Dermatol. 1986 Nov;87(5):588–591. doi: 10.1111/1523-1747.ep12455835. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Brown B. E., Lear S. R., Moser A. H., Elias P. M. Localization of de novo sterologenesis in mammalian skin. J Invest Dermatol. 1983 Oct;81(4):365–369. doi: 10.1111/1523-1747.ep12519974. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Man M. Q., Menon G. K., Cho S. S., Brown B. E., Elias P. M. Cholesterol synthesis is required for cutaneous barrier function in mice. J Clin Invest. 1990 Nov;86(5):1738–1745. doi: 10.1172/JCI114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold K. R., Man M. Q., Proksch E., Menon G. K., Brown B. E., Elias P. M. The lovastatin-treated rodent: a new model of barrier disruption and epidermal hyperplasia. J Invest Dermatol. 1991 Feb;96(2):201–209. doi: 10.1111/1523-1747.ep12461153. [DOI] [PubMed] [Google Scholar]
- Gray G. M., Yardley H. J. Different populations of pig epidermal cells: isolation and lipid composition. J Lipid Res. 1975 Nov;16(6):441–447. [PubMed] [Google Scholar]
- Grayson S., Johnson-Winegar A. G., Wintroub B. U., Isseroff R. R., Epstein E. H., Jr, Elias P. M. Lamellar body-enriched fractions from neonatal mice: preparative techniques and partial characterization. J Invest Dermatol. 1985 Oct;85(4):289–294. doi: 10.1111/1523-1747.ep12276826. [DOI] [PubMed] [Google Scholar]
- Grubauer G., Elias P. M., Feingold K. R. Transepidermal water loss: the signal for recovery of barrier structure and function. J Lipid Res. 1989 Mar;30(3):323–333. [PubMed] [Google Scholar]
- Grubauer G., Feingold K. R., Elias P. M. Relationship of epidermal lipogenesis to cutaneous barrier function. J Lipid Res. 1987 Jun;28(6):746–752. [PubMed] [Google Scholar]
- Grubauer G., Feingold K. R., Harris R. M., Elias P. M. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989 Jan;30(1):89–96. [PubMed] [Google Scholar]
- Hamanaka S., Asagami C., Suzuki M., Inagaki F., Suzuki A. Structure determination of glucosyl beta 1-N-(omega-O-linoleoyl)-acylsphingosines of human epidermis. J Biochem. 1989 May;105(5):684–690. doi: 10.1093/oxfordjournals.jbchem.a122727. [DOI] [PubMed] [Google Scholar]
- Holleran W. M., Feingold K. R., Man M. Q., Gao W. N., Lee J. M., Elias P. M. Regulation of epidermal sphingolipid synthesis by permeability barrier function. J Lipid Res. 1991 Jul;32(7):1151–1158. [PubMed] [Google Scholar]
- Holleran W. M., Williams M. L., Gao W. N., Elias P. M. Serine-palmitoyl transferase activity in cultured human keratinocytes. J Lipid Res. 1990 Sep;31(9):1655–1661. [PubMed] [Google Scholar]
- Hou S. Y., Mitra A. K., White S. H., Menon G. K., Ghadially R., Elias P. M. Membrane structures in normal and essential fatty acid-deficient stratum corneum: characterization by ruthenium tetroxide staining and x-ray diffraction. J Invest Dermatol. 1991 Feb;96(2):215–223. doi: 10.1111/1523-1747.ep12461361. [DOI] [PubMed] [Google Scholar]
- Houtsmuller U. M., van der Beek A. Effects of topical application of fatty acids. Prog Lipid Res. 1981;20:219–224. doi: 10.1016/0163-7827(81)90041-2. [DOI] [PubMed] [Google Scholar]
- Lampe M. A., Burlingame A. L., Whitney J., Williams M. L., Brown B. E., Roitman E., Elias P. M. Human stratum corneum lipids: characterization and regional variations. J Lipid Res. 1983 Feb;24(2):120–130. [PubMed] [Google Scholar]
- Medlock K. A., Merrill A. H., Jr Inhibition of serine palmitoyltransferase in vitro and long-chain base biosynthesis in intact Chinese hamster ovary cells by beta-chloroalanine. Biochemistry. 1988 Sep 6;27(18):7079–7084. doi: 10.1021/bi00418a061. [DOI] [PubMed] [Google Scholar]
- Menon G. K., Feingold K. R., Moser A. H., Brown B. E., Elias P. M. De novo sterologenesis in the skin. II. Regulation by cutaneous barrier requirements. J Lipid Res. 1985 Apr;26(4):418–427. [PubMed] [Google Scholar]
- Ponec M., Weerheim A., Kempenaar J., Mommaas A. M., Nugteren D. H. Lipid composition of cultured human keratinocytes in relation to their differentiation. J Lipid Res. 1988 Jul;29(7):949–961. [PubMed] [Google Scholar]
- Proksch E., Elias P. M., Feingold K. R. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in murine epidermis. Modulation of enzyme content and activation state by barrier requirements. J Clin Invest. 1990 Mar;85(3):874–882. doi: 10.1172/JCI114514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prottey C. Essential fatty acids and the skin. Br J Dermatol. 1976 May;94(5):579–585. doi: 10.1111/j.1365-2133.1976.tb05151.x. [DOI] [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- Wertz P. W., Cho E. S., Downing D. T. Effect of essential fatty acid deficiency on the epidermal sphingolipids of the rat. Biochim Biophys Acta. 1983 Oct 11;753(3):350–355. doi: 10.1016/0005-2760(83)90058-9. [DOI] [PubMed] [Google Scholar]
- Wertz P. W., Downing D. T. Acylglucosylceramides of pig epidermis: structure determination. J Lipid Res. 1983 Jun;24(6):753–758. [PubMed] [Google Scholar]
- Wertz P. W., Downing D. T., Freinkel R. K., Traczyk T. N. Sphingolipids of the stratum corneum and lamellar granules of fetal rat epidermis. J Invest Dermatol. 1984 Sep;83(3):193–195. doi: 10.1111/1523-1747.ep12263553. [DOI] [PubMed] [Google Scholar]
- Williams R. D., Wang E., Merrill A. H., Jr Enzymology of long-chain base synthesis by liver: characterization of serine palmitoyltransferase in rat liver microsomes. Arch Biochem Biophys. 1984 Jan;228(1):282–291. doi: 10.1016/0003-9861(84)90069-9. [DOI] [PubMed] [Google Scholar]