Abstract
Background
VCL-CB01, a candidate CMV DNA vaccine containing plasmids encoding CMV phosphoprotein 65 (pp65) and glycoprotein B (gB) to induce cellular and humoral immune responses and formulated with poloxamer CRL1005 and benzalkonium chloride to enhance immune responses, was evaluated in a Phase 1 clinical trial.
Methods
VCL-CB01 was evaluated in 44 healthy adult subjects (22 CMV-seronegative, 22 CMV-seropositive) ages 18-43. Thirty-two subjects received 1 mg or 5 mg doses of vaccine on a 0-, 2-, and 8-week schedule, and 12 subjects received 5 mg doses of vaccine on a 0-, 3-, 7-, and 28-day schedule.
Results
Overall, the vaccine was well tolerated with no serious adverse events. Local reactions included mild to moderate injection site pain and tenderness, induration, and erythema. Systemic reactions included mild to moderate malaise and myalgia. All reactions resolved without sequelae. Through Week 16 of the study, immunogenicity, as measured by ELISA and/or ex vivo IFN-γ ELISPOT assay, was documented in 45% of CMV-seronegative subjects and 25% of CMV-seropositive subjects who received the full vaccine series and 68% of CMV-seronegative subjects had memory IFN-γ T-cell responses at Week 32.
Conclusion
The safety and immunogenicity data from this trial support further evaluation of VCL-CB01.
Keywords: DNA vaccine, cytomegalovirus, clinical trial, hematopoietic cell transplant, congenital CMV
Introduction
Cytomegalovirus (CMV), a beta-herpesvirus, infects 50-85% of adults by age 40 [1]. Most healthy individuals who acquire CMV after birth develop few, if any, symptoms; however, CMV disease causes significant morbidity and mortality in immunocompromised individuals, such as recipients of hematopoietic cell transplants (HCT) and solid organ transplants (SOT) [2]. In HIV-infected individuals, CMV infection accelerates progression to AIDS and death, despite antiretroviral therapy [3]. In the U.S., congenital abnormalities due to transplacental infection with CMV lead to death or birth defects, including deafness and mental retardation, in approximately 8000 infants each year [4, 5]. A CMV vaccine is currently not available even though, the Institute of Medicine ranked CMV as the top priority for vaccine development in the U.S. [6].
The incidence of CMV antigenemia in CMV-seropositive HCT recipients who receive no prophylaxis is 50-70% in the first 100 days after transplant [7, 8]. Preemptive antiviral therapy reduces the incidence of CMV-associated disease to approximately 5% [9]; however, drug toxicity, the expense of antiviral treatment, and the possibility of the emergence of drug-resistant viruses are major drawbacks to the use of antivirals for prevention of CMV disease. Even with antiviral therapy, patients may develop viremia, or may develop “late-onset” CMV viremia and disease after therapy is discontinued [10, 11]. A CMV vaccine that enables the patient’s immune system to control CMV infection, resulting in a reduced need for antiviral therapy, would be a valuable therapeutic option for HCT recipients.
Control of CMV in immunocompromised persons is primarily associated with cellular immune responses. Both CD8+ and CD4+ T cells appear to be important for protection against CMV disease [12, 13]. A recent study of CMV specific CD4+ and CD8+ T cells from normal healthy donors used overlapping peptides from 213 CMV open reading frames to identify antigens recognized after CMV infection [14]. The CMV tegument phosphoprotein 65 (pp65) and the major CMV surface glycoprotein B (gB) were the antigens most frequently recognized by CD4+ T cells, and pp65 was also one of the antigens most frequently recognized by CD8+ T cells.
The development of a vaccine for prevention of congenital infection by transplacental transmission of CMV is also a high priority. In contrast to the transplant setting, antibodies to surface glycoproteins, especially gB, appear to be critical for protection against maternal-fetal transfer of CMV [15]. In addition, CMV specific T-cell responses are also likely to play an important role in reducing viral load in the mother and thus exposure of the fetus.
A CMV vaccine that induces protective T-cell and antibody responses has the potential to prevent infection or ameliorate CMV disease due to congenital infection or Totransplantation. To that end, we developed a CMV DNA vaccine, VCL-CB01, composed of two plasmids with human codon-optimized CMV genes, pp65 and gB [16]. CMV pp65 was included to induce T-cell responses; gB was included to induce antibodies and T-cell responses. VCL-CB01 was formulated with poloxamer CRL1005 and benzalkonium chloride (BAK) to increase immunogenicity.
Materials and Methods
VCL-CB01 Vaccine
VCL-CB01, a bivalent CMV DNA vaccine consisting of two plasmids, VCL-6368 and VCL-6365 formulated with poloxamer CRL1005 and BAK in PBS, was previously described [16]. VCL-6368 encodes pp65 from AD169 with the putative protein kinase domain removed by deletion of amino acids 435-438. VCL-6365 encodes the extracellular domain (amino acids 1-713) of CMV gB. Formulation of the two plasmids with CRL1005 and BAK produces a thermodynamically stable, self-assembled particulate system with a defined particle size, surface charge, and stability profile.
Trial design
VCL-CB01 was evaluated for safety and immunogenicity in a Phase 1, multi-center, open-label, dose-escalating trial in healthy CMV-seropositive and CMV-seronegative adults. Subjects received intramuscular (deltoid) injections of 1 mg or 5 mg doses of VCL-CB01 on a 0-, 2-, and 8-week schedule (Groups 1 and 2, respectively) or 5 mg doses of VCL-CB01 on a 0-, 3-, 7-, and 28-day schedule (Group 3). Blood was collected for assessment of immune responses at baseline and Weeks 2, 4, 8, 10, 16, 24 and 32 for Groups 1 and 2 and at baseline and Weeks 1, 2, 4, 6, 12, and 16 for Group 3. The endpoints of the trial were safety and immunogenicity, as defined by gB antibody responses measured by ELISA and pp65 IFN-γ T-cell responses measured by an ex vivo ELISPOT assay. An additional assay, the cultured IFN-γ ELISPOT assay, was used to further evaluate immunogenicity.
An Institutional Review Board approved the clinical protocol and informed consent at each of 4 sites, and written informed consent was obtained at enrollment from each volunteer prior to any procedures.
Safety assessment
Safety was assessed by measurement of vital signs, laboratory tests, review of reactogenicity 30 minutes after each injection, symptom-directed clinical evaluations, post-injection subject diaries, adverse event monitoring, and review of concomitant medication usage. Toxicity tables and grades established by the NIAID were used for evaluating adverse events.
Ex vivo ELISPOT assay
Peripheral blood mononuclear cells (PBMC) isolated from blood shipped overnight from the study sites were cryopreserved after each blood collection. The ex vivo ELISPOT assay for detection of IFN-γ secreting T cells was developed and qualified in the Vical Clinical Immunoassay Laboratory. The assay was performed with thawed PBMC from multiple time points, including baseline, in the same plate. In our experience, cryopreservation of PBMC for less than one year does not reduce responses relative to fresh PBMC in the ex vivo ELISPOT assay (authors’ unpublished data); however, the use of cryopreserved PBMC allows batching of PBMC from various time points in a single assay to minimize the effects of assay variability.
For the ex vivo ELISPOT assay, PBMC at 200,000 cells/well in 96-well plates were stimulated overnight at 37°C in 5% CO2 with pools of overlapping 15-mer peptides (Biosynthesis, Lewisville, TX). CMV pp65 and gB peptides, derived from sequences of antigens encoded in VCL-CB01, were in a single pool of 137 or 176 peptides, respectively, at 7.5 μg/mL for each peptide, or split into 2 pools at 10 μg/mL for each peptide. Wells were coated with anti-human IFN-γ antibody (BD Pharmingen, San Diego, CA) and were developed by sequential addition of biotinylated anti-human IFN-γ antibody (BD Pharmingen), avidin-HRP (Vector Laboratories, Burlingame, CA) and AEC substrate (BD Pharmingen). Spot forming units (SFU) were counted with an ImmunoSpot Analyzer (C.T.L., Cleveland, OH). Results were expressed as SFU/106 PBMC after subtraction of SFU of wells without peptides. Controls included phytohemagglutinin (PHA)-stimulated PBMC, wells without peptide, a pool of 98 overlapping peptides derived from CMV immediate early antigen 1 (IE1, not encoded in VCL-CB01), and control PBMC with established ranges for pp65, IE1 and gB.
Cultured ELISPOT assay
PBMC were seeded at 2 × 106 cells/well in 24-well plates and cultured for 10 days at 37°C in 5% CO2 with overlapping peptides (described above) at 0.2 μg/mL for each peptide. Separate wells were cultured with pp65, gB or IE1 peptides. Recombinant human IL-2 (eBioscience, San Diego, CA) at 900 U/mL was added on Days 3 and 7. After 10 days, cells were washed and rested overnight in media without peptides. The IFN-γ ELISPOT assay was performed as described above except cells were plated at 40,000 cells/well. Data were expressed as SFU/106 cultured cells after subtraction of SFU of wells without peptide. Controls included wells without peptide, wells with IE1 peptides and control PBMC cultured in the same manner as the test samples.
CMV gB antibody ELISA
Serum gB specific IgG antibodies were detected in a gB binding ELISA developed and qualified in the Vical Clinical Immunoassay Laboratory. The assay uses 96-well plates coated with 2 μg/mL recombinant gB purified from stably transfected CHO cells (a generous gift from Sanofi-Aventis, Lyon, France). Sera were screened for gB binding at 1:100 and if positive, serial dilutions of the sera were assayed with a reference serum to determine anti-gB antibody levels in ELISA Units/mL (EU/mL). Antibody binding was detected with an HRP-conjugated goat anti-human IgG Fc antibody (Jackson ImmunoResearch, West Grove, PA), followed by ABTS substrate (KPL, Gaithersburg, MD) and stop solution (KPL). Absorbance was read at 405 nm with a reference at 490 nm and gB antibody levels were interpolated from a standard curve. Controls in the assay included positive and negative sera with established EU/mL ranges and reagent control wells.
Statistical analyses
The primary analysis was the incidence of adverse events. Descriptive summary statistics for continuous variables, including the mean, standard deviation, median, minimum, and maximum, were used to summarize safety data. Summaries for categorical variables were presented as counts and percentages. Fisher’s exact test was used to test for significant group differences.
Results
Trial subjects
A total of 44 subjects (22 CMV-seropositive and 22 CMV-seronegative) were enrolled, including 18 men (40.9%) and 26 women (59.1 %) (Table 1). The race characteristics of the subject population were as follows: 37 White (84.1%); 3 African American (6.8%); 1 Asian (2.3%); 3 other/unknown (6.8%). Seven subjects (15.9%) were Hispanic or Latino and 37 (84.1%) were not. Subjects’ mean age was 28.6 years, and ranged from 18 to 43. Four subjects discontinued the study; 2 discontinued before receiving the third dose of vaccine and 2 discontinued after receiving all of the vaccine doses. No subject discontinued due to vaccine related AEs.
Table 1.
Subject Distribution
| Group | Injection Schedule |
Dose per Injection (mg) |
CMV Seropositive Subjects |
CMV Seronegative Subjects |
|---|---|---|---|---|
| 1 | Week 0, 2, 8 | 1 | 8a | 8b |
| 2 | Week 0, 2, 8 | 5 | 8c | 8 |
| 3 | Day 0, 3, 7, 28 | 5 | 6 | 6 |
|
|
||||
| Total | 22 | 22 | ||
One subject, who received only a single injection, discontinued the trial after Week 2; a second subject, who received all 3 injections, discontinued the trial after Week 10
One subject, who received all 3 injections, discontinued the trial after Week 24
One subject, who received only 2 injections, discontinued the trial after Week 2
Safety
In general, VCL-CB01 was well-tolerated. No serious adverse events (SAEs) were reported. The most common vaccine-related AEs consisted of injection site pain (82%), myalgia (55%), headache (41%), and malaise (41%). Adverse events were generally of mild to moderate severity. Thirty-six subjects (81.8%) experienced at least 1 related Grade 1 AE. Eighteen subjects (40.9%) developed at least 1 related Grade 2 AE. One related Grade 3 AE, pain at the injection site, was experienced by one subject after the second 1-mg injection (Day 14), which abated to a Grade 2 level within 1 day and resolved within 10 days. There were no Grade 4 AEs. The maximum toxicity grades of related AEs, sorted by treatment group are shown in Table 2. The duration of the most commonly reported related AEs ranged from 1-4 days, with the exception of one grade 2 AE, “feeling hot or cold”, in one subject, with a duration of 7 days.
Table 2.
| Group 1 N=16 |
Group 2 N=16 |
Group 3 N=12 |
Total N=44 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Maximum Toxicity Grade |
Maximum Toxicity Grade |
Maximum Toxicity Grade |
Subjects with Related Adverse Events (%) |
|||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||
| General Disorders and Administration Site Conditions | ||||||||||
| Injection Site Pain | 7 | 2 | 1 | 10 | 4 | 0 | 4 | 8 | 0 | 36 (82) |
| Malaise | 4 | 1 | 0 | 5 | 1 | 0 | 5 | 2 | 0 | 18 (41) |
| Feeling Hot and Cold | 1 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 5 (11) |
| Injection Site Induration | 0 | 0 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 5 (11) |
| Fatigue | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (7) |
| Injection Site Erythema | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 3 (7) |
| Injection Site Pruritis | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 3 (7) |
| Pyrexia | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 (7) |
| Feeling Hot | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (5) |
| Injection Site Swelling | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 (5) |
| Rigors | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 (5) |
| Musculoskeletal and Connective Tissue | ||||||||||
| Myalgia | 3 | 3 | 0 | 8 | 3 | 0 | 6 | 1 | 0 | 24 (55) |
| Nervous system | ||||||||||
| Headache | 5 | 1 | 0 | 7 | 0 | 0 | 5 | 0 | 0 | 18 (41) |
| Gastrointestinal | ||||||||||
| Nausea | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 (7) |
| Skin and Subcutaneous Tissue Disorders | ||||||||||
| Erythema | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 (5) |
| Pruritis | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 (5) |
| Rash | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 (5) |
| Investigations | ||||||||||
| Haemoglobin Decreased | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 (5) |
No Toxicity Grade 4 related adverse events were reported
Subjects may have had more than one occurrence of the same event, however only one occurrence was counted per subject.
Group 2 and 3 subjects generally experienced more local reactogenicity than Group 1 subjects. In particular, there was more injection site pain with the higher dose (Group 2, 87.5%) and accelerated schedule (Group 3, 100%) versus Group 1 (62.5%). Similarly, only subjects in Groups 2 and 3 experienced injection site erythema (6.2% and 16.7%, respectively) and injection site induration (18.8% and 16.7%, respectively). Injection site swelling was reported for subjects in Group 3 only (16.7%). Dose and injection schedule had no consistent relationship to the frequency of systemic symptoms. In addition, the onset of the immunogenic response did not correlate with an increase in the number of local or systemic AEs.
Immunogenicity
The immunogenicity of VCL-CB01 was initially assessed through Week 16 by the pp65 ex vivo ELISPOT assay for T-cell responses and at all time points by gB binding ELISA for antibody responses. In addition, gB T-cell responses were assessed for CMV-seronegative subjects in Group 2 at Week 10 and in Group 3 at Week 12 by IFN-γ ELISPOT assay. As indicated in Table 3, CMV-seronegative subjects in all groups had vaccine-induced pp65 and/or gB T-cell responses (25-50%) and gB antibody responses (16.7-25%). The gB antibody responses detected with the gB ELISA were not likely to be induced as a result of CMV infection during the course of the trial as all CMV-seronegative subjects were negative by CMV ELISA (Diamedix CMV IgG and IgM ELISA), at the last time point in the trial (authors’ unpublished data). CMV-seropositive subjects in all groups had vaccine-induced increases in pp65-specific T cells (12.5-37.5%), but for CMV-seropositive subjects in any group, gB antibody levels did not increase more than 2-fold over baseline after vaccination.
Table 3.
Vaccine Respondersa by Group and CMV Serostatus
| Response | Group 1 1 mg at 0, 2, and 8 weeks |
Group 2 5 mg at 0, 2, and 8 weeks |
Group 3 5 mg at 0, 3, 7, 28 days |
|||
|---|---|---|---|---|---|---|
| Seronegative (N= 8) N (%) |
Seropositive (N= 8) N (%) |
Seronegative (N= 8) N (%) |
Seropositive (N= 8) N (%) |
Seronegative (N= 6) N (%) |
Seropositive (N= 6) N (%) |
|
| gB antibody | 2 (25.0) |
0 (0.0) |
2 (25.0) |
0 (0.0) |
1 (16.7) |
0 (0.0) |
| pp65 ex vivo IFN-γ ELISPOT |
2 (25.0) |
3 ( 37.5) |
3 (37.5) |
1 (12.5) |
3 (50.0) |
1 (16.7) |
| gB ex vivo IFN-γ ELISPOT |
n.d.b | n.d. | 2 (25) |
n.d. | 2 (33) |
n.d. |
| Total | 3 (37.5) |
3 (37.5) |
4 (50.0) |
1 (12.5) |
3 (50.0) |
1 (16.7) |
Subjects with a vaccine induced antibody or T-cell response prior to or at Week 16 (Day 112) are reported Antibody responders to the cytomegalovirus (CMV) glycoprotein B (gB) are those with sera that were negative in the gB ELISA prior to vaccination and positive in the assay after vaccination or, those with sera that were positive in the assay at baseline, and had a postvaccine increase in gB antibody levels of >4-fold relative to baseline.
Ex vivo gamma interferon (IFN-γ) ELISPOT assay responders to the CMV phosphoprotein 65 (pp65) or gB are those with negative results in the ex vivo IFN-γ ELISPOT assay prior to vaccination and positive results in the assay after vaccination or those with positive results in the ex vivo IFN-γ ELISPOT assay prior to vaccination and a postvaccine increase in pp65 specific IFN-γ secreting T cells of >2.4-fold.
not done
Immune response kinetics
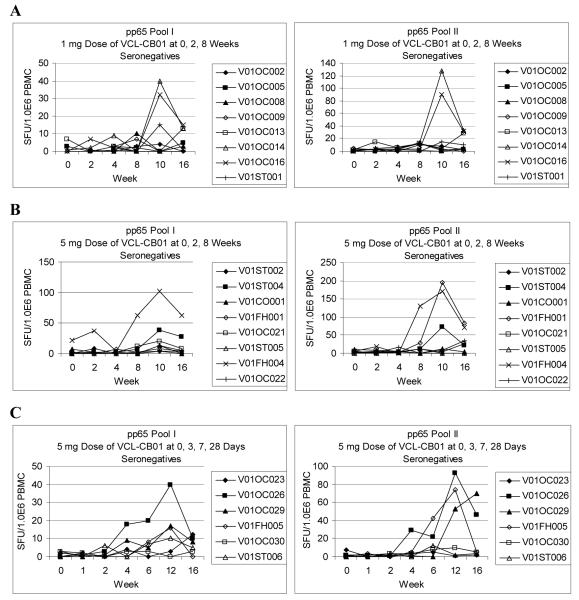
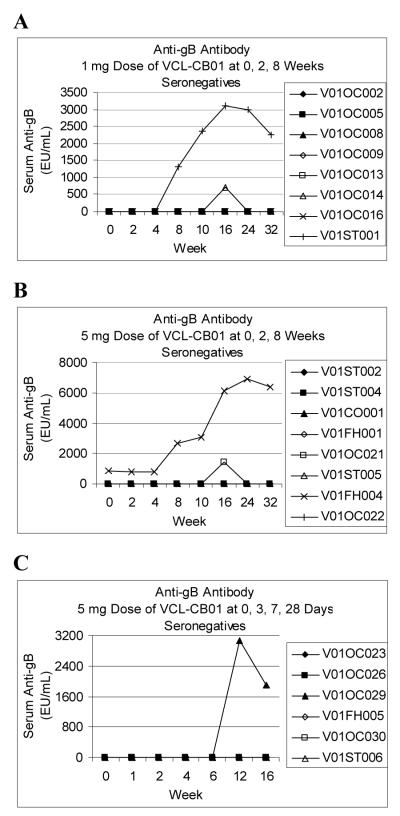
Individual results and time courses for T-cell and antibody responses through Week 16 for CMV-seronegative subjects are shown in Figures 1 and 2. In general, relative to the initial injection, T-cell responses peaked by Weeks 10-12 and antibody responses peaked by Weeks 12-16. The kinetics of the responses relative to the first injection appear to be similar for both injection schedules.
Figure 1. T-cell Responses for CMV-Seronegative Subjects.
Ex vivo gamma interferon (IFN-γ) ELISPOT assays were performed as described in Materials and Methods. Individual cytomegalovirus (CMV) phosphoprotein 65 (pp65) T-cell responses are shown for CMV-seronegative subjects vaccinated with 1 mg (A), or 5 mg (B) doses of VCL-CB01 at 0, 2, and 8 weeks and CMV-seronegative subjects vaccinated with 5 mg doses of VCL-CB01 at 0, 3, 7, and 28 days (C).
Figure 2. Antibody Responses for CMV-Seronegative Subjects.
Cytomegalovirus (CMV) glycoprotein B (gB) ELISAs were performed as described in Materials and Methods. A serum specimen was positive in the assay if the absorbance for serum at a 1:100 dilution was greater than a reactivity threshold calculated as 2.5 times the absorbance of pooled CMV-seronegative sera at a 1:100 dilution. Anti-gB levels in EU/mL were interpolated from a standard curve using serum with well defined reactivity to CMV gB. In a survey of 76 CMV-seronegative sera and 55 CMV-seropositive sera the assay had a sensitivity of 100% and specificity of 95%. The results for the CMV-seropositive sera ranged from 16,350 EU/mL to 413,440 EU/mL with a median value of 154,560 EU/mL (authors’ unpublished data).
Individual gB antibody responses are shown for CMV-seronegative subjects vaccinated with 1 mg (A), or 5 mg (B) doses of VCL-CB01 at 0, 2, and 8 weeks and CMV-seronegative subjects vaccinated with 5 mg doses of VCL-CB01 at 0, 3, 7, and 28 days (C).
Priming of memory T Cells
To more fully evaluate the ability of VCL-CB01 to prime for memory T-cell responses, we assayed PBMC from Week 32 for CMV-seronegative subjects in Groups 1 and 2 by the ex vivo ELISPOT assay. In addition, PBMC from Week 32 for CMV-seronegative subjects in Groups 1 and 2 and Week 16 for Group 3 were evaluated in a cultured ELISPOT assay, which may be more sensitive for assessing DNA vaccine priming of memory responses. Distinct from the ex-vivo ELISPOT assay, in which the PBMC are cultured overnight with peptides, the cultured ELISPOT assay involves culturing PBMC with peptides and recombinant human IL-2 for 10 days prior to use in the IFN-γ ELISPOT assay. Thus, the cultured ELISPOT assay detects antigen specific T cells with the capacity to proliferate and secrete IFN-γ upon restimulation with antigen [17].
As indicated in Table 4, antigen-specific pp65 or gB IFN-γ T-cell responses were detected by ex vivo and/or cultured ELISPOT assay in 15 of 22 CMV-seronegative subjects (68.1%) up to 24 weeks after the last injection. No memory T-cell responses were detected after culture with the IE1 specificity control peptides and IL-2. Group 1 responders that were initially identified by ex vivo ELISPOT assay at earlier time points failed to demonstrate responses in the same ex vivo assay by Week 32. All subjects in Group 2 who had T-cell responses by Week 16 in the ex vivo ELISPOT assay also had detectable responses in the same assay at Week 32. Interestingly, 2 additional subjects in Group 2 (V01OC021 and V01OC022) had detectable pp65 and/or gB responses by ex vivo ELISPOT assay at Week 32 even though T-cell responses were not previously detected in PBMC from those subjects through Week 16. Two of 3 responders in Group 3 had detectable pp65 T-cell responses by ex vivo ELISPOT assay at Week 16.
Table 4.
Ex Vivo and Cultured IFN-γ ELISPOT Assay Responses for CMV-Seronegative Subjects
| Ex vivo ELISPOT Assay Results (SFU/106 PBMC)a |
Cultured IFN-γ ELISPOT Assay Results (SFU/106 cultured cells)b |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| pp65 | gB | IE1 | pp65 | gB | IE1 | ||||
| Group 1 | Wk 32 | Wk 0 | Wk 32 | Wk 0 | Wk 32 | Wk 0 | Wk 32 | ||
|
| |||||||||
| V01OC002 | 0 | 0 | 0 | 100 | 242 | 6 | 1475 | 75 | 14 |
| V01OC005 | 8 | 10 | 12 | 0 | 42 | 50 | 83 | 42 | 25 |
| V01OC008c | 3 | 3 | 8 | 36 | 792 | 0 | 92 | 17 | 183 |
| V01OC009 | 3 | 1 | 8 | 0 | 6 | 50 | 6 | 250 | 192 |
| V01OC013 | 18 | 29 | 1 | 125 | 2258 | 92 | 125 | 0 | 42 |
| V01OC014 | 29 | 31 | 4 | 11 | 1967 | 50 | 4467 | 0 | 0 |
| V01OC016 | 32 | 22 | 8 | 11 | 2417 | 47 | 783 | 14 | 100 |
| V01ST001 | 12 | 23 | 17 | 6 | 358 | 217 | 608 | 75 | 89 |
|
| |||||||||
| Group 2 | Wk 32 | Wk 0 | Wk 32 | Wk 0 | Wk 32 | Wk 0 | Wk 32 | ||
|
| |||||||||
| V01ST002 | 0 | 3 | 0 | 11 | 0 | 8 | 33 | 0 | 0 |
| V01ST004 | 52 | 25 | 15 | 75 | 5017 | 56 | 775 | 14 | 58 |
| V01CO001 | 3 | 9 | 7 | 17 | 39 | 53 | 117 | 36 | 0 |
| V01FH001 | 70 | 40 | 2 | 33 | 2433 | 6 | 542 | 50 | 17 |
| V01OC021d | 90 | 92 | 25 | 100 | 6067 | 217 | 5575 | 111 | 150 |
| V01ST005 | 0 | 3 | 6 | 0 | 0 | 8 | 14 | 17 | 17 |
| V01FH004 | 96 | 33 | 27 | 61 | 417 | 8 | 6375 | 19 | 33 |
| V01OC022 | 27 | 53 | 0 | 3 | 4517 | 383 | 1600 | 42 | 50 |
|
| |||||||||
| Group 3 | Wk 16e | Wk 0 | Wk 16 | Wk 0 | Wk 16 | Wk 0 | Wk 16 | ||
|
| |||||||||
| V01OC023 | 14 | n.d.f | 0 | 33 | 58 | 333 | 538 | 8 | 11 |
| V01OC026 | 56 | n.d. | 0 | 28 | 1900 | 542 | 1658 | 19 | 17 |
| V01OC029 | 78 | n.d. | 0 | 53 | 1267 | 0 | 792 | 67 | 50 |
| V01FH005 | 5 | n.d. | 5 | 17 | 1542 | 58 | 2100 | 25 | 108 |
| V01OC030 | 8 | n.d. | 5 | 8 | 0 | 0 | 3 | 0 | 6 |
| V01ST006 | 8 | n.d. | 0 | 11 | 583 | 17 | 950 | 33 | 92 |
A subject was considered to be a responder to the cytomegalovirus (CMV) proteins phosphoprotein 65 (pp65), glycoprotein B (gB) or immediate early antigen 1 (IE1) by ex vivo gamma interferon (IFN-γ) ELISPOT assay if the mean spot forming units per 106 peripheral blood mononuclear cells (SFU/106 PBMC) for replicate wells was greater than or equal to 50 and the mean SFU/well for replicate wells was greater than twice the mean background (without peptide) SFU/well. Positive responses are in bold type. Using the established cut-off, 63 CMV-seronegative subjects were all negative in the assay (100% specificity) and 50 CMV-seropositive subjects were all positive for at least one of the three antigens in the assay (100% sensitivity); positive responses for the CMV-seropositive subjects ranged from 50 SFU/106 PBMC to the upper limit of the assay, 6000 SFU/106 PBMC (authors’ unpublished data).
A subject was considered to be a responder to pp65, gB or IE1 by the cultured ELISPOT assay if the SFU/106 cultured cells were >250 and the SFU/well was >2X the SFU of wells without peptide. Data from the analysis of PBMC from CMV-seronegative subjects prior to administration of the CMV DNA vaccine suggested that some subjects may have low levels of crossreactive T-cell responses to peptides in the pools [data not shown]; therefore, the criteria for a positive response to the vaccine included the requirement that the SFU/106 cultured cells was >2X SFU/106 cultured cells at baseline. Positive responses are in bold type. Using these criteria, no positive responses were detected for PBMC stimulated with the IE1 specificity control peptides (IE1 is not encoded in the vaccine, n=22 CMV-seronegative subjects vaccinated with VCL-CB01, 100% specificity for vaccine response). The lack of IE1 responses in PBMC from CMV-seronegative subjects up to Week 32 of the study suggests that none of those subjects acquired CMV during the course of the trial.
Wk 0 PBMC were not available, Wk 2 PBMC were used in the assay
Wk 32 PBMC were not available, Wk 24 PBMC were used in the assay
Results from initial evaluation in the ex vivo ELISPOT assay in which pp65 peptides are split into two pools each are reported. The data reported are the sums of SFU/106 PBMC for pool I and pool II
n.d.,Not done
DNA vaccine induced memory T-cell responses were detected by cultured ELISPOT assay in PBMC from 6 subjects in Group 1 (75.0%), 5 subjects in Group 2 (62.5%) and 4 subjects in Group 3 (66.7%). Furthermore, memory T-cell responses were detected in 5 subjects (4 in Group 1 and 1 in Group 3) who failed to demonstrate T-cell responses by ex vivo ELISPOT assay at any time point. Thus, the cultured ELISPOT assay appears to be more sensitive than the ex-vivo ELISPOT assay for detection of vaccine-induced, antigen-specific T-cell responses.
Discussion
The results from this trial show that the bivalent CMV DNA vaccine, VCL-CB01, was generally well-tolerated and the severity of AEs did not increase significantly with increasing dose or an accelerated vaccination schedule. Through Week 16 of the study, immunogenicity, as measured by ELISA and/or ex vivo ELISPOT assay, was documented in 45% of CMV-seronegative subjects and 25% of CMV-seropositive subjects who received the full vaccine series. Two additional subjects had detectable IFN-γ T-cell responses only at Week 32 and 68% of subjects had memory IFN-γ T-cell responses. In CMV-seropositive subjects, VCL-CB01 boosted existing pp65 T-cell responses, but not gB antibody responses, possibly due to the high baseline levels of gB antibody in subjects with chronic CMV infection.
The protracted time to peak responses was unexpected and may be related to the mechanism of action for DNA vaccines, which is likely to differ from that of conventional vaccines. In theory, in humans, DNA vaccines produce low levels of immunogen for extended periods of time after vaccination [18]. In the absence of a large bolus of antigen, the immune response may not produce large effector responses but could prime antigen specific memory cells. Indeed, DNA vaccines are used for the priming doses of several heterologous prime-boost vaccine regimens currently being evaluated in humans for the prevention of HIV and malaria [19, 20, 21]. In those vaccines, immune responses primed with a DNA vaccine encoding pathogen derived proteins are boosted to higher levels with a viral vectored vaccine encoding the same proteins. In a similar way, a CMV DNA vaccine could prime for a memory response that is boosted upon exposure to CMV during a primary infection or upon reactivation, resulting in rapid control of the virus and protection against CMV associated disease.
Assays that measure only effector cell function directly ex vivo may not be adequate for detecting the priming of immune responses by DNA vaccines or for assessing the potential for mounting a memory response upon exposure to the pathogen targeted by the vaccine. The results from our cultured ELISPOT assay suggest that induction of a directly measurable effector response is not required for priming of antigen-specific memory T-cells. In fact, memory T-cell responses were detected in 5 subjects who failed to demonstrate responses in the ex vivo ELISPOT assay at any time point. Establishment of the cultured ELISPOT assay as a correlate of DNA vaccine priming of immune memory will require evaluation of the assay in the context of a trial in which a memory response could be measured. For example, administration of the live-attenuated CMV Towne strain vaccine (Towne strain) to CMV DNA vaccinated subjects, while not strictly a pathogenic challenge, could be used to demonstrate CMV antigen specific T-cell and B-cell memory responses to CMV infection. The administration of Towne strain was previously used to demonstrate antibody priming by a canarypox virus vaccine encoding CMV gB; antibody responses after administration of Towne strain occurred earlier and were of higher magnitude than those induced in subjects who were not primed with the CMV gB canarypox virus vaccine [22]. A correlation between cultured ELISPOT responses prior to administration of Towne strain to subjects vaccinated with a CMV DNA vaccine and a memory T-cell response to the same antigens after administration of Towne strain would support the use of the cultured ELISPOT assay for evaluation of priming of memory immune responses by DNA vaccines.
The CMV DNA vaccine evaluated in these studies was more effective for inducing CMV antigen specific T-cells than gB-specific antibody. The likelihood of success for a CMV DNA vaccine focused primarily on the induction of cellular immune responses in the transplant population is supported by several investigations. First, disease severity following allogeneic transplants was decreased by infusing transplant recipients with CMV-specific T cells expanded ex vivo from the donors [13, 23, 24]. Second, a live-attenuated vaccine (Towne) was used to ameliorate disease severity in CMV-seronegative renal transplant recipients who received an organ from a CMV-seropositive donor [25]. In these studies, the emphasis was on the prevention of disease rather than infection. Lastly, strong inverse correlations between the magnitude of both CD4+ and CD8+ T-cell responses and the development of disease in transplant patients have been reported [12, 26].
Unlike the transplant setting, antibody responses directed to the major surface glycoproteins, especially gB, appear critical in the prevention of maternal-fetal transmission, but antibody responses do not provide complete protection against maternal-fetal transmission of CMV [15]. Infection with other CMV genotypes or reactivation of latent CMV can occur with subsequent transplacental transmission to the fetus. Cell-mediated immune responses to CMV immunogens such as pp65 are also likely to play an important role in reducing maternal viral load, which could reduce maternal-fetal transmission or disease occurrence.
The potential efficacy of a plasmid-based vaccine approach to prevention of maternal-fetal transmission was recently demonstrated in a guinea pig model of transplacental transmission of guinea pig CMV (GPCMV). Administration of a DNA vaccine encoding the GPCMV gB prior to conception induced gB antibody responses and decreased viral loads in live-born guinea pig pups of vaccinated dams challenged with GPCMV during pregnancy [27]. A more recent study using an alphavirus replicon vaccine encoding the GPCMV homolog of pp65 in the guinea pig model indicated that T-cell responses to pp65 correlated with reduction of viral load in the peripheral blood of the dams and reduction of pup mortality [28]. Thus, the results in the guinea pig model suggest that DNA vaccine-induced antibody and cell-mediated immune responses could reduce CMV infection and disease.
A vaccine against CMV is currently not available; however, live-attenuated CMV vaccines, canarypox-vectored vaccines, and recombinant gB protein vaccines have been or are currently in clinical trials [29]. The use of a CMV DNA vaccine in immunocompromised subjects would eliminate the safety concerns of live-attenuated CMV or live recombinant viral-vectored vaccines. In addition, a CMV DNA vaccine has the advantage of delivering CMV antigens, while avoiding the many CMV-encoded products involved in immune evasion [30].
In summary, the results of this Phase 1 clinical trial suggest that VCL-CB01, a bivalent CMV DNA vaccine was well tolerated and immunogenic. The vaccine induced both gB antibodies and T-cell responses to both pp65 and gB at 1 mg or 5 mg doses on either injection schedule and priming of memory T cells in a majority of CMV-seronegative subjects. The safety and immunogenicity data from this trial support further clinical investigation of VCL-CB01.
Acknowledgements
The authors thank the clinical trial study coordinators at the Orlando Clinical Research Center, Stanford University, the University of Washington Virology Research Clinic and the City of Hope National Medical Center. The authors also thank Ron Moss and Gary Hermanson for critical reading of the manuscript and Alice Chu for statistical analysis of the safety and immunogenicity data.
Funding: This work was supported by National Institutes of Health Grants R01 AI 060159 and R44 AI058386.
Footnotes
Conflict of Interest: Mary K. Wloch; Vical shareholder
Larry R. Smith; Vical shareholder
Souphaphone Boutsaboualoy; no conflict
Luane Reyes; no conflict
Christina Han; no conflict
Jackie Kehler; no conflict
Heather D. Smith; no conflict
Linda Selk; Vical shareholder
Ryotaro Nakamura; no conflict
Janice M. Brown; no conflict
Thomas Marbury; no conflict
Anna Wald; received research support from Vical
Alain Rolland; Vical shareholder
David Kaslow; Vical shareholder
Thomas Evans; no conflict
Michael Boeckh; received research support and consulting fees from Vical
Meetings where the information has previously been presented: Partial, preliminary safety and immunogenicity results were presented by JB (poster #10.23) and DK (oral presentation #10.08) at the 10th International CMV/Betaherpes Workshop in Williamsburg VA, April 27, 2005 and by MW (poster #1039) at the IDSA 43rd Annual Meeting in San Francisco, CA, October 8, 2005. Partial, preliminary results of the cultured ELISPOT assay were presented by MW (poster #355) at the Keystone Symposium on Immunologic Memory, Santa Fe, NM, March 6, 2007 and the 11th International CMV & Betaherpes Workshop in Toulouse, France, May 17, 2007 (session #S7, poster #9). Development and partial, preliminary results of the cultured ELISPOT assay were presented by SB at the 32nd Annual International Herpesvirus Workshop in Asheville, NC, July 2007.
References
- 1.Gershon AA, Gold E, Nankervis GA. Cytomegalovirus. In: Evans AS, Kaslow RA, editors. Viral infections of humans. 4th ed Plenum Press; New York: 1997. pp. 229–51. [Google Scholar]
- 2.Pass RF. Cytomegalovirus. In: Knipe DM, Howley PM, editors. Fields virology. 4th ed Lippincott Williams & Wilkens; Philadelphia: 2001. pp. 2675–705. [Google Scholar]
- 3.Deayton JR, Sabin CA, Johnson MA, Emery VC, Wilson P, Griffiths PD. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet. 2004;363:2116–21. doi: 10.1016/S0140-6736(04)16500-8. [DOI] [PubMed] [Google Scholar]
- 4.Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904–8. [PubMed] [Google Scholar]
- 5.Stagno S. Cytomegalovirus. In: Remington JS, Klein JO, editors. Infectious diseases of the fetus and newborn infant. W.B. Saunders Company; Philadelphia: 2001. pp. 389–424. [Google Scholar]
- 6.Stratton KR, Durch JS, Lawrence RS. Vaccines for the 21st century: a tool for decision making. National Academy Press; Washington: 2000. pp. 1–10.pp. 165–171. [PubMed] [Google Scholar]
- 7.Junghanss C, Boeckh M, Carter RA, et al. Incidence and outcome of cytomegalovirus infections following nonmyeloblative compared with myeloblative allogeneic stem cell transplantation, a matched control study. Blood. 2002;99:1978–85. doi: 10.1182/blood.v99.6.1978. [DOI] [PubMed] [Google Scholar]
- 8.Junghanss C, Storb R, Maris MB, et al. Impact of unrelated donor status on the incidence and outcome of cytomegalovirus infections after non-myeloblative allogeneic stem cell transplantation. Br J Haematol. 2003;123:662–70. doi: 10.1046/j.1365-2141.2003.04671.x. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch MS. Cytomegalovirus and human herpesvirus types 6, 7, and 8. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s principles of internal medicine. 16th eds McGraw-Hill; New York: 2005. pp. 1049–53. [Google Scholar]
- 10.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis. 2002;185:273–82. doi: 10.1086/338624. [DOI] [PubMed] [Google Scholar]
- 11.Boeckh M, Fries B, Nichols WG. Recent advances in the prevention of CMV infection and disease after hematopoietic stem cell transplantation. Pediatr Transplant. 2004;8(Suppl 5):19–27. doi: 10.1111/j.1398-2265.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 12.Gamadia LE, Remmerswaal EBM, Weel JF, Bemelman F, van Lier RAW, Ten Berge IJM. Primary immune responses to human CMV: a critical role for IFN-γ-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101:2686–92. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 13.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202:379–86. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–85. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler KB, Stagno S, Pass RF. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA. 2003;289:1008–11. doi: 10.1001/jama.289.8.1008. [DOI] [PubMed] [Google Scholar]
- 16.Selinsky C, Luke C, Wloch M, et al. A DNA-based vaccine for the prevention of human cytomegalovirus-associated diseases. Hum Vaccin. 2005;1:16–23. doi: 10.4161/hv.1.1.1335. [DOI] [PubMed] [Google Scholar]
- 17.Goonetilleke N, Moore S, Dally L, et al. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J Virol. 2006;80:4717–28. doi: 10.1128/JVI.80.10.4717-4728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doh SG, Vahlsing HL, Hartikka J, Liang X, Manthorpe M. Spatial-temporal patterns of gene expression in mouse skeletal muscle after injection of lacZ plasmid DNA. Gene Ther. 1997;4:648–63. doi: 10.1038/sj.gt.3300460. [DOI] [PubMed] [Google Scholar]
- 19.Douek DC, Kwong PD, Nabel GJ. The rational design of an AIDS vaccine. Cell. 2006;124:677–81. doi: 10.1016/j.cell.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Hanke T, McMichael AJ, Dorrell L. Clinical experience with plasmid DNA- and modified vaccinia virus Ankara-vectored human immunodeficiency virus type 1 clade A vaccine focusing on T-cell induction. J Gen Virol. 2007;88:1–12. doi: 10.1099/vir.0.82493-0. [DOI] [PubMed] [Google Scholar]
- 21.Keating SM, Bejon P, Berthoud T, et al. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J Immunol. 2005;175:5675–80. doi: 10.4049/jimmunol.175.9.5675. [DOI] [PubMed] [Google Scholar]
- 22.Adler SP, Plotkin SA, Gonczol E, et al. A canarypox vector expressing cytomegalovirus (CMV) glycoprotein B primes for antibody responses to a live-attenuated CMV vaccine (Towne) J Infect Dis. 1999;180:843–6. doi: 10.1086/314951. [DOI] [PubMed] [Google Scholar]
- 23.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–7. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 24.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–44. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 25.Plotkin SA, Higgins R, Kurtz JB, et al. Multicenter trial of Towne strain attenuated virus vaccine in seronegative renal transplant recipients. Transplantation. 1994;58:1176–8. [PubMed] [Google Scholar]
- 26.Reusser P, Cathomas G, Attenhofer R, Tamm M, Thiel G. Cytomegalovirus (CMV)-specific T-cell immunity after renal transplantation mediates protection from CMV disease by limiting the systemic virus load. J Infect Dis. 1999;180:247–53. doi: 10.1086/314879. [DOI] [PubMed] [Google Scholar]
- 27.Schleiss MR, Bourne N, Bernstein DI. Preconception vaccination with a glycoprotein B (gB) DNA vaccine protects against cytomegalovirus (CMV) transmission in the guinea pig model of congenital CMV infection. J Infect Dis. 2003;188:1868–74. doi: 10.1086/379839. [DOI] [PubMed] [Google Scholar]
- 28.Schleiss MR, Lacayo JC, Belkaid Y, et al. Preconceptual administration of an alphavirus replicon UL83 (pp65 homolog) vaccine induces humoral and cellular immunity and improves pregnancy outcome in the guinea pig model of congenital cytomegalovirus infection. J Infect Dis. 2007;195:789–98. doi: 10.1086/511982. [DOI] [PubMed] [Google Scholar]
- 29.Schleiss M. Progress in cytomegalovirus vaccine development. Herpes. 2005;12:66–75. [PubMed] [Google Scholar]
- 30.Mocarski ES., Jr Immumodulation by cytomegalovirus: manipulative strategies beyond evasion. Trends Microbiol. 2002;10:332–9. doi: 10.1016/s0966-842x(02)02393-4. [DOI] [PubMed] [Google Scholar]