Abstract
Regulatory T cells (Tregs) are committed to suppressive functions. Recently, it was proposed that Tregs could produce IL-17 under proinflammatory, polarizing conditions.We studied the role of Tregs on IL-17 production in the absence of exogenous cytokines and insults. Using in vitro and in vivo approaches, we determined that under neutral conditions, simultaneous activation of Tregs and naive CD4+ conventional T cells in the presence of APCs resulted in conversion of Tregs into IL-17–producing cells, and endogenous IL-1β was mandatory in this process. Mechanistic analysis revealed that the IL-1R1 was highly expressed on Tregs and that IL-1β induced marked activation of p38 and JNK, which were involved in IL-17 production. These observations could have important implications on therapeutic strategies using Tregs.
The differentiation and function of effector CD4+ T cells allows effective immune responses to diverse insults. It has long been appreciated that naive CD4 T cells can differentiate into IFN-γ producing Th1 cells and IL-4 producing Th2 cells (1). Recently, the Th1/Th2 paradigm of Th cell differentiation has been expanded following the discovery of effector Th cells that produce IL-17 (Th17) and exhibit effector functions distinct from Th1 and Th2 (2–4). Several groups have shown that polarizing culture with a combination of TGF-β and the proinflammatory cytokine IL-6 is required to induce the differentiation of naive CD4+ T cells into Th17 cells (2–4). Subsequent studies indicated that IL-1β plays a critical role in early Th17 cell differentiation (5).
Tight regulation of effector T cell responses is required to avoid autoimmunity. Regulatory T cells (Tregs) prevent differentiation of naive T cells into Th1 and Th2 (6) and suppress immune responses driven by these two cell types (7). The role of Tregs in controlling Th17 cells is less clear. Studies have indicated that Th17 cells are not suppressed but rather enhanced by Treg (4, 8). In contrast, other studies support an inhibitory role of Tregs on Th17 differentiation and IL-17 production (9).
These discrepant conclusions could be due to the fact that Treg can be converted into IL-17–producing cells (10–13). Notably, factors involved in differentiation of Th17 cells also affect the function of Tregs. Exposure of naive conventional T cells (Tconvs) to IL-6 along with TGF-β results in RORγt expression and IL-17 production (14). IL-6 may also downregulate Foxp3 and alter the suppressive function of Tregs (15). Furthermore, in the presence of IL-6, Tregs can be converted into IL-17–producing cells (13). Most studies on this subject have used inflammatory settings by modulating levels of polarizing cytokines in vitro or in vivo. However, this approach might not be ideal to evaluate the physiologic role of Tregs on IL-17 production during immune responses.
We studied the role of Tregs on IL-17 production in the absence of exogenous polarizing cytokines or insults. We determined that under neutral conditions, simultaneous activation of naive Tconv and Tregs in the presence of APC induced differentiation of Tregs but not Tconvs into IL-17 producers, and IL-1β was mandatory for this function. These observations could have important implications on therapeutic strategies using Tregs.
Materials and Methods
Mice and reagents
Mouse strains used were: C57BL/6 (Charles River Laboratories, Wilmington, MA); B6.PL, IL-1R1−/−, OT-II TCR-transgenic (H-2b) specific for chicken OVA323–339 (The Jackson Laboratory, Bar Harbor, ME); 2D2 TCR-transgenic (H-2b) specific for myelin oligodendrocyte glycoprotein (MOG)35–55 (The Jackson Laboratory); and Foxp3-GFP knock-in (from Dr. Mohamed Oukka, Brigham and Women’s Hospital, Boston, MA). Abs used were: anti-CD4 (GK1.5), anti-TCRvα2 (B20.1), anti-IL-17A (eBio17B7), anti-RORγt (AFKJS-9), anti-Foxp3 (FJK-16s; eBioscience, San Diego, CA), anti-Thy1.1 (HIS5; Biolegend, San Diego, CA), anti- TCRvβ5 (MR9-4), anti-TCRvα3.2 (KJ23) and anti-TCRvβ11 (RR3–15; BD Biosciences, San Jose, CA); anti–IL-1R1 (JAMA-147; Santa Cruz Biotechnology); neutralizing Abs for TGF-β, IL-6, and IL-1β; mouse IgG1, rat IgG, goat IgG isotype controls, and blocking Ab for IL-6Rα (R&D Systems, Minneapolis, MN); anti–p-IκB, IκB, p-p38, p38, p-JNK (Cell Signaling Technology); and anti–β-actin (Santa Cruz Biotechnology). Recombinant cytokines and pharmacologic compounds used were as follows: TGF-β (R&D Systems), IL-1β and IL-6 (eBioscience); keyhole limpet hemocyanin (KLH), SB203580 and SP600125 (EMD4Biscoences); PS1145 (Sigma, St. Louis, MO).
Cell purification and culture
CD4+CD25+ Tregs were prepared using a specific isolation kit (Miltenyi Biotec, Auburn, CA). Tregs from Foxp3-GFP knock-in mice were isolated by sorting GFP+ cells. CD4+CD25−CD62Lhi naive Tconvs were prepared using CD4+ isolation kit and CD62L-magnetic beads (Miltenyi Biotech). Naive Tconvs (5 × 104 cells/well), Tregs (5 × 104 cells/well), and effector Tconvs (5 × 104cells/well) were cultured in 96-well round-bottom plates with anti-CD3 (1 µg/ml) and mitomycin C-treated APCs (2.5 × 105 cells/well) or with anti-CD3 (1 µg/ml) and anti-CD28 (1 µg/ml) mAbs. When indicated, TGF-β (2 ng/ml), IL-6 (20 ng/ml), neutralizing Abs for TGF-β (0.2–10 µg/ml), IL-6 (4 µg/ml), IL-1β (5 µg/ml), or blocking Abs for IL-6Rα (4 µg/ml) were added.
Cell surface and intracellular staining
For IL-17A detection, cells were incubated with PMA (50 ng/ml), ionomycin (1 µM) and Golgiplug (BD Pharmingen, San Diego, CA) for 5 h, stained with anti–CD4-Cy7 and anti–Thy1.1-perCP, fixed, permeabilized in cytofix-permeab (eBioscience) followed by labeling of IL-17-PE. For Foxp3 and RORγt detection cells were stained with CD4-Cy7, fixed, permeabilized, and labeled with Foxp3-APC and RORγt-PE and analyzed by flow cytometry.
Cytokine ELISA
Cytokine levels were measured with ELISA kits (eBioscience).
Adoptive transfer and immunization
B6.PL or IL-1R1−/− Tregs were transferred i.v. (8 × 106 cells per mouse) into nonirradiated syngeneic IL-1R1−/− mice, which 24 h later were immunized s.c. with 100 µg KLH in IFA as described (5).
Results
Simultaneous activation of naive Tconvs and Tregs in the presence of APCs promotes IL-17 production
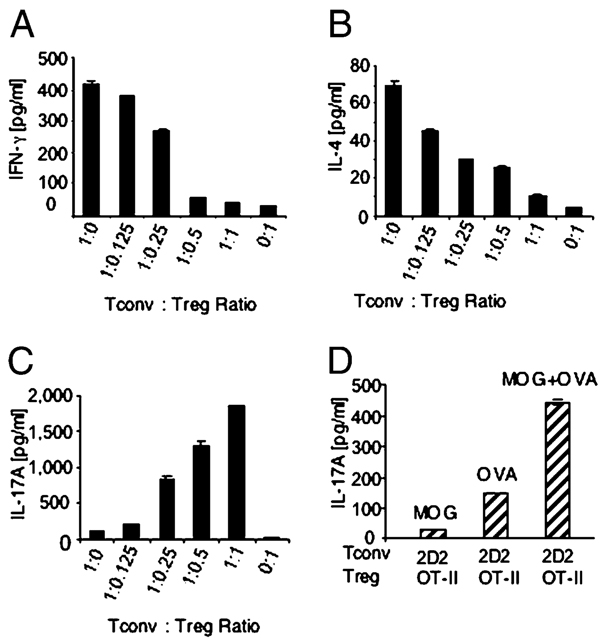
It is well established that supplementing T cell cultures with polarizing cytokines, such as TGF-β and IL-6, leads to the differentiation of Th17 cells (2–4, 14). Moreover, in the presence of exogenous IL-6, Tregs can be converted into IL-17–producing cells (13). To evaluate the physiologic role of Tregs on IL-17 production, we used neutral culture conditions in which no exogenous polarizing cytokines were supplied. Graded numbers of Tregs were cocultured with naive Tconvs during T cell activation, and production of cytokines was analyzed. The addition of Tregs during culture of naive Tconvs with anti-CD3 mAb and APCs suppressed production of IFN-γ and IL-4 (Fig. 1A–B) in a dose-dependent manner consistent with previous reports (6). In sharp contrast, considerable amounts of IL-17 that displayed a positive correlation with the number of Tregs were detected in the same cultures (Fig. 1C). Naive Tconvs and Tregs cultured alone with APCs were incapable of producing IL-17.
FIGURE 1.
Simultaneous activation of Tconvs and Tregs in the presence of APCs promotes IL-17 production. A–C, Tconvs from C57BL/6 mice were cultured with or without Tregs from Foxp3-GFP knock-in mice with anti-CD3 mAb and APC, supernatants were harvested on day 3, and cytokines were measured by ELISA. Representative results of five experiments are shown. D, 2D2 Tconvs were cultured with OT-II Tregs and APCs in the presence of MOG35–55, OVA323–339, or both peptides. Representative results of two experiments are shown.
These results suggest that coexistence of both Tregs and naive Tconvs in the culture during T cell activation was required for IL-17 production. To investigate whether TCR stimulation of Tconvs and Tregs was mandatory for IL-17 production, we used naive Tconvs from 2D2 (MOG35–55-specific) mice and Tregs from OT-II (OVA323–339-specific) mice. Very low levels of IL-17 were produced when either MOG or OVA peptide was present (Fig. 1D). In contrast, significant amounts of IL-17 were produced by addition of both peptides (Fig. 1D) indicating that simultaneous activation of Tconv and Treg was required.
APCs integrate signals required for production of IL-17 (16). To investigate whether APCs were indispensable for IL-17 production in our system, we stimulated Tconvs and Tregs with anti-CD3 and anti-CD28 mAbs without APCs. Under these conditions, IL-17 was not detected (Supplemental Fig. 1A–C), indicating that APCs were mandatory for IL-17 production under nonpolarizing conditions.
Tregs acquire the ability to produce IL-17 during coculture with Tconvs and APCs
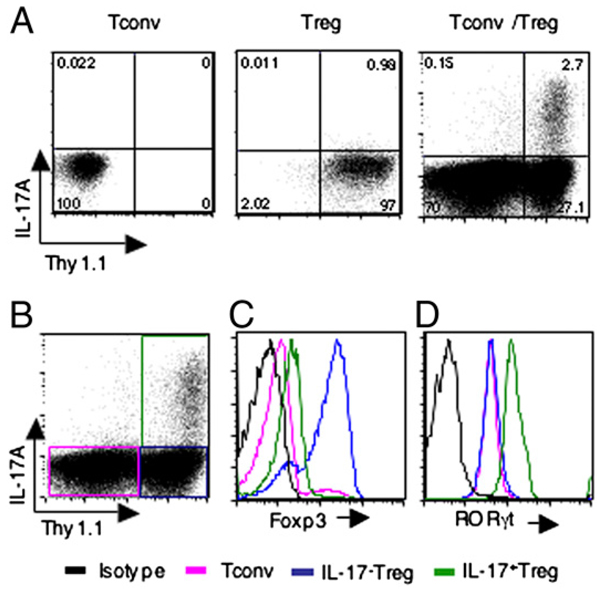
To identify the source of IL-17, we used naive Tconvs from C57BL/6 mice and Tregs from B6.PL C57BL/6 congenic mice that carry the Thy1a (Thy1.1) allele and can be recognized easily by flow cytometric analysis using a Thy1.1-specific Ab. Naive Tconv and Treg populations used in this study were confirmed by assessing Foxp3 expression (data not shown). Culture of Tconvs with anti-CD3 mAb and APCs did not induce IL-17–producing cells (Fig. 2A, left panel). Few IL-17–producing cells were observed during stimulation of Tregs (Fig. 2A, middle panel). Coculture of Tregs and Tconvs with anti-CD3 mAb and APCs did not result in robust differentiation of Tconvs (Thy1.1−) into Th17 cells, in contrast to what was observed in the inflammatory milieu (4, 13). Instead, IL-17–producing cells developed predominantly from Tregs (Thy1.1+; Fig. 2A, right panel). The predominant IL-17 production from Tregs was sustained for at least 5 d of culture (Supplemental Fig. 2A). Thus, without exogenous polarizing cytokines, Tregs are more efficient than Tconvs in producing IL-17.
FIGURE 2.
Generation of RORgt+IL-17+ Tregs in the presence of Tconvs and APCs. A, Thy1.1− Tconvs and Thy1.1+ Tregs were cultured with APCs and anti-CD3 mAb either independently (left and middle panels) or mixed at a 1:1 ratio (right panel), and production of IL-17A was assessed by intracellular staining on day 3. B, Thy1.1− Tconvs and Thy1.1+ Tregs were cultured at a 1:1 ratio as in A (right panel), and production of IL-17A was assessed by intracellular staining on day 3. Representative results of three experiments are shown. B–D, After gating on Thy1.1−IL-17− Tconvs, Thy1.1+IL-17− Tregs and Thy1.1+IL-17+ Tregs, as indicated in the colored boxes in panel B, expression levels of Foxp3 (C) and RORγt (D) were analyzed on day 3 of culture.
It has been reported that human peripheral and lymphoid tissues contain a significant number of CD4+Foxp3+ T cells that have the capacity to produce IL-17. These cells coexpress Foxp3 and RORγt transcription factors (17). We examined whether the converted IL-17+ Tregs express RORγt and Foxp3 in our culture system. Expression of Foxp3 levels in IL-17+Thy1.1+ cells (the source of Tregs) were higher than in Thy1.1− cells (the source of Tconvs), but was decreased compared with IL-17−Thy1.1+ Tregs (Fig. 2C). In contrast, IL-17+ Tregs expressed higher levels of RORγt compared with both IL-17− Tregs and Tconvs (Fig. 2D). This expression pattern of Foxp3 and RORγt in IL-17+ Tregs, IL-17− Tregs, and Tconvs sustained for at least 5 d of culture (Supplemental Fig. 2B, 2C). These results indicate that converted IL-17–producing Tregs under our in vitro culture conditions co-expressed RORγt and Foxp3. However, Foxp3 was expressed in lower levels in IL-17–producing Tregs compared with Tregs not producing IL-17.
Conversion of Tregs into IL-17–producing cells depends IL-1β
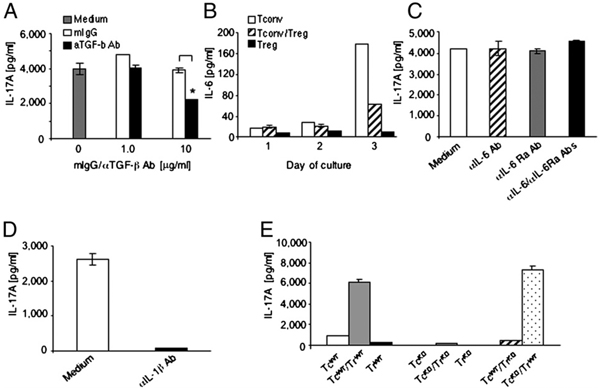
Because our experiments were performed under neutral conditions, we reasoned that endogenous cytokines might be involved in reprogramming Tregs into IL-17–producing cells. The first candidate was TGF-β, which is essential for maintenance of the Treg phenotype (18) and is also required for development of Th17 cells (3). In fact, we determined that substantial amounts of TGF-β (300–400 pg/ml) were produced in the supernatant obtained from coculture of Tconv and Treg (data not shown). We further confirmed that TGF-β was originated from cell populations present in the culture and not from the serum present in the culture media, because IL-17 production was detected in cultures performed in either complete media or serum free media (Supplemental Fig. 3). However, neutralizing TGF-β Ab suppressed but did not eliminate IL-17 production (Fig. 3A), suggesting that TGF-β–independent mechanisms were also involved in this process.
FIGURE 3.
Conversion of Tregs into IL-17–producing cells requires TGF-β and IL-1β. A, Tconvs and Tregs were cultured with anti-CD3 mAb and APCs. TGF-β neutralizing Ab or isotype control was added, and production of IL-17A was measured on day 3. *p = 0.003. Representative results of four experiments are shown. B, Tconvs and Tregs were cultured with anti-CD3 mAb and APCs, either individually or mixed at a 1:1 ratio, and IL-6 was measured. C, Tconvs and Tregs were mixed at a 1:1 ratio, cultured with anti-CD3 mAb and APCs in the presence of media, anti-IL-6 neutralizing Ab, anti-IL-6 receptor blocking Ab, or their combination, and IL-17A was assessed. Representative results of three experiments are shown. D, Tconvs and Tregs were cultured with anti-CD3 mAb and APCs in the absence or presence of IL-1β neutralizing Ab. Results are representative of four experiments. E, TcWT or TcKO were cultured with Tregs from TrWT or from TrKO with anti-CD3 mAb and APCs, and production of IL-17A was assessed on day 3. Representative results of two experiments are shown. TcKO, Tconvs from IL-1R1−/− mice; TcWT, Tconvs from wild type mice; TrWT, Tregs from wild type mice; TrKO, Tregs from IL-1R1−/− mice.
Our data revealed that APCs were required for induction of IL-17 production by Tregs. IL-6, which is mainly produced by APCs, can induce production of IL-17 by both Tregs and Tconvs when exogenously added in polarizing cultures (13, 19). For this reason, we evaluated the potential role of endogenous IL-6 in the conversion process. In our experimental setting, IL-6 was detected readily in culture supernatants (Fig. 3B). The highest amount of IL-6 was observed when APCs were cultured with Tconvs and reduced by the addition of Tregs (Fig. 3B). However, a combination of anti–IL-6 neutralizing and IL-6 receptor blocking Abs did not affect IL-17 levels (Fig. 3C), although this Ab combination blocked IL-17 production in polarizing cultures (Supplemental Fig. 4).
IL-1–mediated signals are required for early differentiation of Th17 cells and can mediate production of IL-17 by Foxp3+ Tregs (5, 10, 11). To determine whether IL-β was involved in the conversion process observed in our system, we used IL-1β neutralizing Ab. As shown in Fig. 3D, addition of IL-1β neutralizing Ab to the coculture led to the reduction of IL-17 to almost undetectable levels (Fig. 3D), whereas goat IgG isotype control Ab had no effect (Supplemental Fig. 5). Thus, IL-1β but not IL-6 was indispensable for production of IL-17 by Tregs during stimulation with Tconvs and APCs in nonpolarizing conditions.
Because both Tconvs and Tregs might respond to IL-1β (5), we examined whether IL-1β selectively mediated differentiation of Tregs into IL-17–producing cells in our system using T cells from IL-1R–deficient mice. Individual culture of IL-1R−/− Tconvs or IL-1R−/− Tregs with APCs, and coculture of IL-1R−/− Tconvs and IL-1R−/− Tregs with APCs did not result in detectable IL-17. Similarly, no IL-17 production was observed when wild type Tconvs, instead of IL-1R−/− Tconvs, were used in the coculture system. In contrast, replacement of IL-1R−/− Tregs with wild type Tregs resulted in abundant IL-17 production (Fig. 3E). These results provide compelling evidence that under nonpolarizing conditions, Tregs are the main source of IL-17, and IL-1R–mediated signals are indispensable for this function.
Tregs produce IL-17 in vivo after immunization
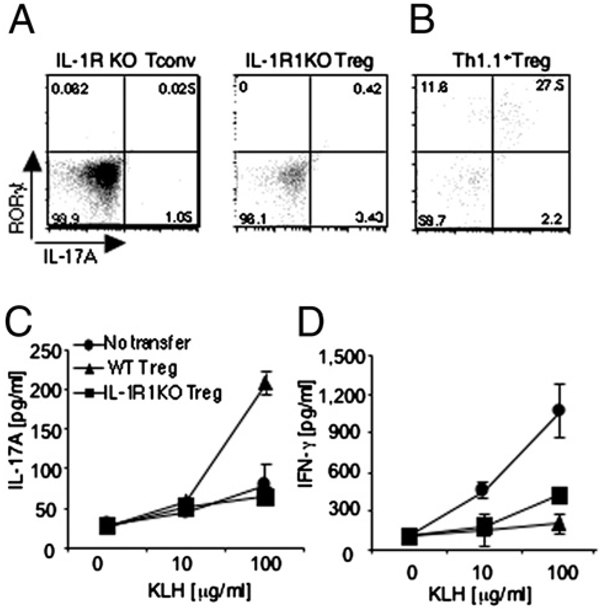
IL-1R1−/− mice fail to produce IL-17 after immunization (5). Based on our in vitro findings, we hypothesized that transferring Tregs into IL-1R1−/− mice would increase IL-17 production in response to Ag stimulation. To investigate this possibility, we adoptively transferred Tregs from either congenic B6.PL mice or IL-1R1−/− mice into IL-1R1−/− recipients that were subsequently immunized as previously described (5). Three days after immunization, IL-1R−/− Tregs and IL-1R−/− Tconvs were incapable of producing detectable levels of IL-17 or expressing RORγt (Fig. 4A, left and middle panels), consistent with previous studies (5) that have indicated that expression of IL-1R is required for IL-17 production in vivo. In contrast, a significant percentage of IL-17 and RORγt-positive cells were detected within the adoptively transferred Thy1.1+ Treg population (Fig. 4B) that also retained expression of Foxp3 (Supplemental Fig. 6). Furthermore, upon in vitro restimulation, increased levels of IL-17 were produced by T cells isolated from immunized mice that had received adoptively transferred wild type Tregs but not IL-1R1−/− Tregs (Fig. 4C). This effect was specific for IL-17 because IFN-γ production was suppressed at a comparable level upon adoptive transfer of either wild type Tregs or IL-1R−/− Tregs into IL-1R−/− mice (Fig. 4D). These results also indicate that attainment of IL-17 production capacity might not affect the suppressive effect of Tregs on Th1 cytokine production.
FIGURE 4.
Tregs are capable of producing IL-17A in vivo after immunization. IL-1R1−/− or B6.PL congenic (Thy1.1+) Tregs were transferred to IL-1R1−/− mice that were subsequently immunized with KLH in IFA. A and B, Three days later, IL-17A–producing cells in the draining lymph nodeswere assessed by intracellular staining. Plots represent three individual mice per group. C and D, Five days later, splenocytes were harvested and restimulated in vitro, and levels of IL-17A and IFN-γ were assessed by ELISA.
Differential functional and signaling responses of Tregs and Tconvs to IL-1β
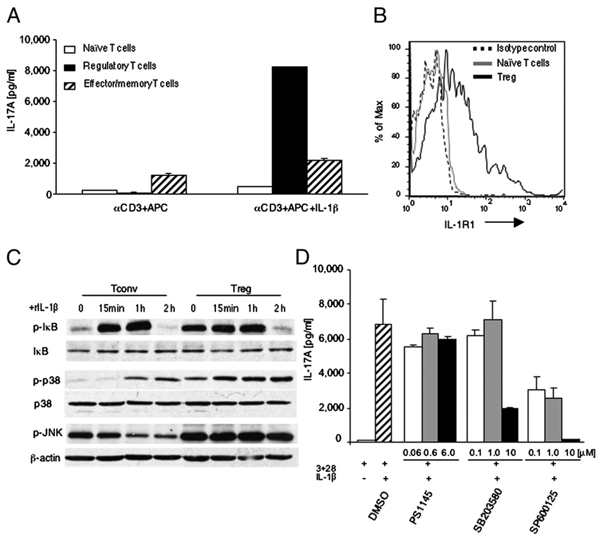
In our system, naive Tconvs and Tregs were under the same influence of endogenous IL-1β, but only Tregs produced IL-17, suggesting that Tregs might be more sensitive to IL-1β–mediated signals. To investigate this issue, we further subfractionated Tconvs into naive (CD25−CD62L+) and effector memory (CD44+CD62L−) CD4+ T cell populations and cultured the individual cell subsets with exogenous IL-1β. Under these conditions, Tregs produced a substantial amount of IL-17, whereas IL-17 production by naive Tconvs was comparable to baseline (Fig. 5A). A similar pattern of responses were observed at least until day 5 of culture (data not shown). Addition of IL-1β increased IL-17 production by effector memory cells, but to a much lesser extent compared with Tregs. These results indicate that IL-1β preferentially drives differentiation of Tregs but not Tconvs into IL-17 producing cells.
FIGURE 5.
Tregs display increased IL-1β signaling and IL-17 production. A, Naive Tconvs (CD4+CD62L+), effector memory Tconvs (CD4+CD62L−), and Tregs (CD4+ CD25+Foxp3−GFP+) were cultured with anti-CD3 mAb and APCs with or without IL-1β, and IL-17A was measured by ELISA. B, Expression of IL-1R1 the same cell populations as in A was assessed by flow cytometry. C, Naive Tconvs and Tregs were cultured with IL-1β and phosphorylated IκB; p38 and JNK were examined in cell lysates by SDS-PAGE and immunoblot analysis. D, Tregs were cultured for 3 d with anti-CD3 and CD28 mAb in the presence of IL-1β (10 ng/ml) alone or with the indicated concentrations of inhibitors, and IL-17A was measured by ELISA.
To determine the mechanisms of the distinct effects of IL-1β on Tregs and Tconvs, we examined expression of IL-1R1 and IL-1β–mediated signaling. Expression of IL-1R1 in freshly isolated Tregs was significantly increased compared with naive Tconvs (Fig. 5B); it remained higher during culture in vitro (data not shown), whereas expression of IL-1R1 in effector memory Tconv cells was only slightly higher compared with naive Tconv cells (Supplemental Fig. 7). These results indicate that the different effects of IL-1β on Tregs might be due to lineage characteristics. Based on these findings, we hypothesized that the distinct levels of IL-1R1 expression might lead to differential activation of signal pathways mediated by IL-1β in Tconvs and Tregs. IL-1β binding to IL-1R triggers activation of NF-κB and MAPKs (20). To determine the activation status of these pathways, we examined the phosphorylation of IκB, p38, and JNK MAPKs in freshly isolated naive Tconvs and Tregs as well as IL-1β stimulated Tconvs and Tregs. Increased levels of phosphorylated IκB, p38, and JNK were observed in freshly isolated Tregs (Fig. 5C), indicating that IL-1β-induced signals were constitutively activated in vivo. Incubation with IL-1β upregulated IκB phosphorylation to comparable maximum levels in Tconvs and Tregs (Fig. 5C). IL-1β induced robust and sustained activation of p38 in Treg that was detected as early as 15 min. In contrast, in Tconv activation of p38 was delayed and never reached the levels observed in Tregs. Moreover, IL-1β sustained high JNK phosphorylation in Tregs but not in Tconvs (Fig. 5C).
We used pharmacologic inhibitors specific for NF-κB (21), p38 (22), and JNK (23) to investigate the role of these pathways on IL-17 production by Treg. Addition of the NF-κB inhibitor PS1145 had no effect, whereas the p38 inhibitor SB20358 significantly reduced IL-17 production at the concentration of 10 µM. More potently, the JNK inhibitor SP600125 significantly reduced IL-17 production at all concentrations tested and completely abrogated production of IL-17 at the concentration of 10 µM (Fig. 5D). Collectively, our results indicate that IL-1R expression and signaling are differentially regulated in Tconvs and Tregs and that IL-1β–mediated activation of p38 and JNK is involved in the conversion of Tregs into IL-17–producing cells.
Discussion
This study reveals that coculture of naive Tconvs and Tregs in the presence of APCs during T cell activation promotes the differentiation of Tregs but not naive Tconvs into IL-17–producing cells. This study confirms and extends the previously proposed view that the Treg phenotype is not an end stage (24). In addition, our findings indicate that Tregs are subjected to regulation mediated through interactions with activated Tconvs and APCs. Moreover, although Tregs are known to mediate immunosuppressive functions, our studies provide evidence that Tregs may also facilitate proinflammatory responses by producing IL-17 during physiologic stimulation. Using both in vitro culture and in vivo genetic approaches, we demonstrate that IL-1β plays an essential role in controlling the differentiation of Tregs into IL-17–producing cells and that activation of p38 and JNK has an active role in this differentiation process.
IL-17–producing cells are the newest addition to the growing family of Th cell subsets. However, the molecular basis of Th17 cell differentiation is not well understood. For example, the role of TGF-β in the development of IL-17–producing cells is still controversial. Differentiation of Th17 cells is guided by transcription factors RORα and RORγt (14, 19), and TGF-β is one of the stimuli that can upregulate RORγt expression (25). Interestingly, TGF-β also induces Foxp3 expression, which inhibits Th17 cell differentiation by antagonizing RORγt function (26). In fact, it is clear that TGF-β alone cannot induce the production of IL-17. Recent studies, however, revealed that the role of TGF-β in IL-17 production by Tconvs was due to its inhibitory function on both Th1 and Th2 cytokine production. As a consequence, TGF-β maximized IL-17 production (27), suggesting that TGF-β was not directly required for the generation of Th17 cells.
Regardless of the mechanisms by which TGF-β regulates IL-17 production, most of the studies agree that TGF-β synergizing with proinflammatory cytokines promotes IL-17 production by Tconvs (4). It is worth noting that activated T cells, including Tregs, can be the source of TGF-β. Our studies showed that addition of TGF-β neutralizing Ab in the culture during T cell activation resulted in significantly reduced IL-17A production, suggesting that TGF-β was involved in regulation of IL-17A production. However, IL-17A was not completely abrogated and significant amounts of IL-17 were still detected in the presence of the highest concentration of TGF-β neutralizing Ab. Two possible explanations can be entertained. The first possibility is that Tregs had been subjected to the influence of endogenous TGF-β in vivo; therefore, TGF-β neutralizing Ab could not alter TGF-β signals that had been already mediated during Treg development. Indeed, assessment of TGF-β signaling in freshly isolated Tconvs and Tregs revealed a constitutively increased level of Smad3 phosphorylation in Tregs compared with naive Tconv cells (L. Li and V.A. Boussiotis, unpublished data). At this point, it is unclear whether such signals might program the ability of Tregs to produce IL-17 under permissive conditions. The second possibility is that dominant factors other than TGF-β are involved in the process of Treg conversion into IL-17–producing cells.
We tested whether IL-6 was a key factor, because it is known that IL-6 has a critical role in the commitment of naive T cells toward Th17 development. Moreover, a number of studies have demonstrated that murine Tregs are converted predominantly to Th17 cells under the influence of IL-6 (13, 19, 28). Although we observed detectable IL-6 levels, IL-6–neutralizing assays failed to inhibit IL-17 production by Tregs, suggesting that IL-6 was not a dominant factor driving Treg conversion into IL-17–producing cells in our system. Previous studies have determined that IL-1β is a critical player in driving several cell types into IL-17 producers (5, 29, 30). Recent studies, particularly in human cells, have underlined the dominant role of IL-1β over IL-6 in generating Treg-producing IL-17 (10, 11, 17), although the mechanism of IL-1β–mediated expression of IL-17 has not been determined. Interestingly, converted IL-17–producing peripheral Tregs retain Foxp3 expression and their suppressive function (31). Our studies indicated that IL-1β/IL-1R was an essential pathway for induction of IL-17 production by Tregs in our experimental system. Furthermore, this effect of IL-1β on Tregs was far more profound than on Tconvs. Mechanistic analysis revealed that resting Tregs express higher levels of IL-1R1 compared with their non-Treg CD4+ T cell counterparts. In addition, the signals delivered through IL-1R1, particularly via p38 and JNK MAPK activation, were significantly more prominent in Tregs. Interestingly, a study published during the revision of this manuscript reported that JNK and p38 are also involved in IL-6–induced IL-17 production by Tconvs (32). Whether these pathways are also involved in the conversion of Tregs by IL-6 in vivo in other experimental models (33) remains to be determined. Identification of the signaling pathways that link MAPKs and IL-17 production may provide strategies to control the conversion of Tregs and Tconvs into IL-17–producing cells.
Another significant finding of our studies was that the conversion of Tregs into IL-17–producing cells in the absence of an inflammatory cytokine milieu required simultaneous activation of Tregs and Tconvs. The mechanistic contribution of activated CD4+ T in this process remains unclear. It is possible that activated CD4+ Tconvs produce IL-2, which is necessary for the survival of Tregs. Supporting a role of IL-2 in this process, addition of IL-2 in cultures of Tregs without Tconvs, resulted in IL-17 production (L. Li and V.A. Boussiotis, unpublished data). Thus, IL-2 may promote development of IL-17–producing Tregs or may be a precondition for the differentiation of Tregs into IL-17–producing cells under the effect of different factors (11). Interestingly, IL-2 inhibits differentiation of Tconvs into Th17 cells, possibly owing to an inhibitory effect of STAT5 binding to the Il17a promoter (34). Thus, the influence of IL-2 in the Th17 cell differentiation program may be more complex than anticipated. In addition, activated CD4+ T cells might also indirectly affect the fate and function of Tregs by increasing production of cytokines, including IL-1β, by APCs via CD40–CD40L interactions (16).
In conclusion, this study reveals that although Tregs have heretofore been associated primarily with immunosuppressive functions in T cell immunity, they may also facilitate proinflammatory responses by promoting IL-17 production during physiologic Ag stimulation. These findings could have significant implications for clinical strategies that use the administration of Tregs for the treatment of autoimmune diseases or GVHD, in which Treg administration could lead to enhanced rather than diminished inflammation. Identification of factors and signaling pathways that control development of IL-17–producing Tregs will be highly important.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants HL087870 (to L.L.) and CA123855, AI43552, and CA104596 (to V.A.B.).
Abbreviations used in this paper
- KLH
keyhole limpet hemocyanin
- MOG
myelin oligodendrocyte glycoprotein
- Tconv
conventional T cell
- TcKO
Tconv from IL-1R1−/− mice
- TcWT
Tconv from wild type mice
- Treg
regulatory T cell
- TrKO
Treg from IL-1R1−/−
- TrWT
Treg from wild type mice
Footnotes
Disclosures
The authors have no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 3.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 4.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu D, Liu H, Komai-Koma M, Campbell C, McSharry C, Alexander J, Liew FY. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J. Immunol. 2003;170:394–399. doi: 10.4049/jimmunol.170.1.394. [DOI] [PubMed] [Google Scholar]
- 7.Stummvoll GH, DiPaolo RJ, Huter EN, Davidson TS, Glass D, Ward JM, Shevach EM. Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J. Immunol. 2008;181:1908–1916. doi: 10.4049/jimmunol.181.3.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J. Exp. Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deknuydt F, Bioley G, Valmori D, Ayyoub M. IL-1beta and IL-2 convert human Treg into T(H)17 cells. Clin. Immunol. 2009;131:298–307. doi: 10.1016/j.clim.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 12.Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, Reis e Sousa C. DC activated via dectin-1 convert Treg into IL-17 producers. Eur. J. Immunol. 2008;38:3274–3281. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J. Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 14.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4 +CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 16.Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M. CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc. Natl. Acad. Sci. USA. 2009;106:876–881. doi: 10.1073/pnas.0810769106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc. Natl. Acad. Sci. USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 21.O’Shaughnessy MJ, Vogtenhuber C, Sun K, Sitcheran R, Baldwin AS, Murphy WJ, Dang L, Jaffee B, Palmer E, Serody JS, Blazar BR. Ex vivo inhibition of NF-kappaB signaling in alloreactive T-cells prevents graft-versus-host disease. Am. J. Transplant. 2009;9:452–462. doi: 10.1111/j.1600-6143.2008.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber S, Schrader J, Fritz G, Presser K, Schmitt S, Waisman A, Lüth S, Blessing M, Herkel J, Schramm C. P38 MAP kinase signaling is required for the conversion of CD4+CD25− T cells into iTreg. PLoS ONE. 2008;3:e3302. doi: 10.1371/journal.pone.0003302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das J, Ren G, Zhang L, Roberts AI, Zhao X, Bothwell AL, Van Kaer L, Shi Y, Das G. Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. J. Exp. Med. 2009;206:2407–2416. doi: 10.1084/jem.20082286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4 +CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J. Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 29.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, Hafler DA. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu L, Wang J, Zhang F, Chai Y, Brand D, Wang X, Horwitz DA, Shi W, Zheng SG. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J. Immunol. 2010;184:4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J. Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.