Abstract
OBJECTIVES
The objective of this study was to examine factors that are associated with aggression that is responsive versus refractory to individualized optimization of stimulant monotherapy among children with attention-deficit/hyperactivity disorder (ADHD).
METHODS
Children who were aged 6 to 13 years and had ADHD, either oppositional defiant disorder or conduct disorder, significant aggressive behavior, and a history of insufficient response to stimulants completed an open stimulant monotherapy optimization protocol. Stimulant titration with weekly assessments of behavior and tolerability identified an optimal regimen for each child. Families also received behavioral therapy. Parents completed the Retrospective-Modified Overt Aggression Scale (R-MOAS) at each visit. Children were classified as having stimulant-refractory aggression on the basis of R-MOAS ratings and clinician judgment. Differences that pertained to treatment, demographic, and psychopathology between groups with stimulant monotherapy–responsive and –refractory aggression were evaluated.
RESULTS
Aggression among 32 (49.3%) of 65 children was reduced sufficiently after stimulant dosage adjustment and behavioral therapy to preclude adjunctive medication. Those who responded to stimulant monotherapy were more likely to benefit from the protocol’s methylphenidate preparation (once-daily, triphasic release), showed a trend for lower average dosages, and received fewer behavioral therapy sessions than did children with stimulant-refractory aggression. Boys, especially those with higher ratings of baseline aggression and of depressive and manic symptoms, more often exhibited stimulant-refractory aggression.
CONCLUSIONS
Among children whose aggressive behavior develops in the context of ADHD and of oppositional defiant disorder or conduct disorder, and who had insufficient response to previous stimulant treatment in routine clinical care, systematic, well-monitored titration of stimulant monotherapy often culminates in reduced aggression that averts the need for additional agents.
Keywords: attention deficit and disruptive behavior disorders, aggression, drug therapy, clinical trial
Psychostimulant medication has long been the mainstay for the effective management of attention-deficit/hyperactivity disorder (ADHD).1,2 Stimulant therapy also often ameliorates comorbid disruptive behavior problems3,4; however, the use of antipsychotic agents for severe disruptive behaviors that frequently occur with ADHD, such as aggression, has proliferated.5–7 The high incidence and severity of antipsychotic-induced adverse effects among children have made such widespread use a growing concern.8–10
Pharmacotherapy guidelines for ADHD with aggression propose beginning stimulant and behavioral treatments and adding antipsychotic medication only when aggression persists.11 In practice, however, several factors may hasten the initiation of antipsychotic treatment and the premature conclusion or avoidance of stimulant monotherapy: (1) It is unclear how clinicians should establish that aggressive behavior is refractory to stimulant medication alone. (2) It is unknown how frequently stimulant monotherapy promotes remission of severe aggressive behavior among children with ADHD. This uncertainty may undermine the clinician’s confidence that protracted efforts to optimize stimulant treatment will prove fruitful. Previous studies that suggested stimulant efficacy for aggression emphasized reductions in rating scale scores of broad-spectrum disruptive symptoms rather than meaningful decreases in aggression per se.4 Similarly, previous stimulant trials did not select patients on the basis of severity of pretreatment aggressive behavior. (3) The irritability and brittle anger control that impulsive aggressive children typically show12–14 have drawn attention as possible heralds of mood disturbances, such as bipolar spectrum disorders, for which clinicians often prescribe antipsychotic medications and avoid stimulants.15–17
This study addressed these unresolved issues on appropriate stimulant treatment for ADHD that is accompanied by serious aggression among preadolescents. To furnish guidance on stimulant monotherapy that distinguishes responsive from refractory outcomes, we implemented a systematic titration protocol that endeavored to optimize each child’s stimulant regimen. To evaluate how frequently serious aggression abates to a clinically meaningful extent, (1) inclusion criteria required that children demonstrate marked, persistent aggressive behavior; (2) aggressive behavior assessment was unalloyed by other disruptive symptoms; and (3) the primary out come was diminution of aggression so that additional medication was unnecessary. To examine whether mood-relevant symptoms affected stimulant effectiveness, we evaluated whether baseline indices of mood disturbance portended insufficient response.
METHODS
Study Design
Patients participated in the stimulant-optimization lead-in phase to a controlled trial of adjunctive medications for aggressive children with ADHD.18 This phase endeavored to determine each child’s most effective, best tolerated stimulant regimen. Children whose aggression persisted were eligible for the controlled trial. The institutional review boards of the 2 clinical sites approved the study. Parents or legal guardians provided written informed consent, and children who were older than 8 years gave written assent.
Patients
Inclusion criteria required that boys and girls between the ages of 6 and 13 fulfill (1) diagnostic criteria for ADHD, (2) criteria for oppositional defiant disorder (ODD) or conduct disorder (CD), and (3) obtain parent-reported ratings of clinically significant aggression (per the Retrospective–Modified Overt Aggression Scale [R-MOAS], described in the next section). Eligibility required previous stimulant treatment at a minimum methylphenidate (MPH) dosage of 30 mg/day or equivalent with insufficient response. This criterion sought to exclude individuals with robust responses to initial treatment, for whom the issue of trial persistence versus alternative pharmacotherapy is moot. Stimulant-naive patients with ADHD are also more apt to show improvements with placebo treatment.19
Exclusion criteria comprised major depression, bipolar disorder, Tourette syndrome, psychotic disorders, pervasive developmental disorder, mental retardation, and aggressive behavior arising chiefly as a complication of an anxiety disorder (eg, behavioral dys-control only when others interfered with rituals for a child with obsessive-compulsive disorder), contraindications to stimulant treatment, seizure disorders, and pregnancy.
Evaluation Procedures and Measures
Diagnostic evaluation used the Schedule of Affective Disorders and Schizophrenia for School-Age Children (K[iddie]-SADS20), which a clinical child psychologist administered. A child and adolescent psychiatrist conducted a separate clinical diagnostic evaluation. The K-SADS interviewer and clinical assessor conferred to arrive at consensus diagnoses.
Aggressive behavior was assessed by the parent-completed Modified Overt Aggression Scale (R-MOAS)21 adapted to acquire retrospective reports covering the preceding week. Parents rate the frequency of 16 aggressive behaviors in 4 areas: verbal aggression; physical aggression toward others; aggression toward oneself; and destruction or hostile misuse of property. Numeric weighting amplifies the seriousness of more harmful behaviors in the total score (eg, physical aggression items garner higher scores than verbal). Parents completed the R-MOAS at baseline and each study visit. Reliability and validity are summarized elsewhere.18 The R-MOAS, annotated to show scoring, is published as supporting information at Supplemental Information. A baseline score of 24 was required for enrollment.
ADHD symptom severity was quantified by parents’ completion of the Conners’ Global Index22 (ConnGI-P), which yielded age- and gender-referenced T scores for its restless-inattentive subscale. Because the ConnGI-P total score includes the emotional lability subscale, it would have confounded ADHD symptoms with aggression-related difficulties.
Two measures that were administered at baseline evaluated affective symptoms. The Child Depression Rating Scale–Revised (CDRS-R)23 generates an overall score that is based on parent and child interviews. The Young Mania Rating Scale (YMRS)24 yields clinician ratings for symptoms and associated features of mania. Although major depression and mania were exclusion criteria, these scales permitted analyses concerning the predictive value of subsyndromal affective symptoms for stimulant mono therapy response. Parents completed the Barkley Behavior and Adverse Events Questionnaire–Modified25 (BBAEQ-M) at baseline and each study visit, providing systematic assessment of adverse effects to augment open-ended inquiries.
Stimulant Titration and Treatment Protocol
Children discontinued nonstimulant psychopharmacotherapy with tapering as appropriate to the compound’s elimination time course. Children who were taking stimulant medications before enrollment also discontinued this treatment without tapering so that it could be supplanted by the stimulant titration protocol of this study.
Titration procedures resembled those used in other studies that undertook to optimize each child’s regimen.26–28 Key features were weekly dosage adjustments that took into account rating scale assessment of ADHD symptoms (ConnGI-P, restless-inattentive sub-scale), aggressive behavior (R-MOAS), and adverse effects (BBAEQ-M). When possible, we acquired behavioral ratings from teachers to aid in dosage selection, but inconsistent availability made teacher ratings unsuitable as a study outcome. Parents and children met with investigators weekly to review behavioral and health status and to obtain weight, height, blood pressure, and heart rate. These response and tolerability data guided decisions on adjustment of the child’s stimulant dosage for the succeeding week, as described next.
Stimulant monotherapy began with a triphasic-release MPH (MPH-TRI) preparation given once daily (Concerta [Ortho-McNeil-Janssen Pharmaceuticals, Titusville, NJ]). It is the MPH preparation with the longest duration of action, and, consequently, best resembles the regimens used in previous optimization protocols.26–28
Determination of optimal MPH-TRI dosage ordinarily involved increments from an 18-mg starting dose given shortly after waking but no later than 8:30 AM until (1) there was no room for additional symptomatic improvement, (2) unmanageable or intolerable adverse effects emerged, or (3) a ceiling dosage of 90 mg/day was attained. When 1 of these events occurred, the study physician and a child psychologist reviewed behavioral and tolerability data from preceding weeks to identify the best tolerated dosage associated with greatest overall improvement in ADHD symptoms and aggression. MPH-TRI dosage adjustments were ordinarilyin18-mgincrements, but clinicians had discretion to adjust MPH-TRI dosage by 9-mg increments (eg, for children with small stature; when previous history evoked tolerability concerns). When 2 dosage levels yielded similar results, the lower dosage was selected.
Children who experienced problems with sleep, nutrition, or other tolerability issues related to the long duration of action of MPH-TRI but who displayed symptomatic benefit during the day were switched to a biphasic-release MPH preparation (MPH-BI; Metadate CD [UCB, Smyrna, GA]). Starting MPH-BI dosages were ~60% of the effective MPH-TRI dosage. Subsequent dosage adjustments occurred in 10-mg increments/decrements each week on the basis of response and tolerability, to a maximum dosage of 60 mg/day. Candidate regimens for most effective, best tolerated treatment included MPH-TRI dosages the child had previously (viz, MPH-BI may have yielded substantially less benefit than a tolerated dosage of MPH-TRI).
Children who gained little or no symptomatic improvement of ADHD symptoms with MPH could be switched to mixed amphetamine salts, administered once daily as a biphasic-release preparation (Adderall XR [Shire Pharmaceuticals, Wayne, PA]). Starting dosage was approximately one-third of the MPH-TRI dosage or half of the MPH-BI dosage. Subsequent weekly adjustments were by 5-mg increments/decrements but did not exceeding a total of 35 mg/day.
The regimen identified as optimal was continued or reinstated for a replication week. When observation during that week confirmed it as the child’s optimal dosage, another week elapsed before the stimulant end-point assessment. When behavioral response during the replication week was worse than the response observed previously, additional adjustment to dosage or agent was permitted until optimal response was again evident and replicated, followed by the end-point assessment.
Concurrent Psychosocial Treatment
All families had behaviorally-oriented psychosocial treatment throughout the trial. Its purposes were (1) to reduce out-come variance from differences in participants’ previous exposure to psychosocial interventions, (2) to provide behavioral treatment so that children whose aggression diminished did not go on to additive pharmacotherapy, and (3) to offer individualized support to families. Treatment content was the Community Parent Education (COPE) program,29 modified for incorporation into clinical trials for children with ADHD.30 Advanced graduate students in clinical child psychology provided this treatment.
Response Determination
At the end-point assessment, children who completed the stimulant-optimization phase with R-MOAS scores of < 16 (representing at least a 33% decrease from baseline) were categorized as stimulant monotherapy responders. Previous work with this measure indicated that scores of < 17 were associated with no more than minimal aggressive behavior medication.18 Those who scored > 16 were classified as refractory, reflecting the persistence of aggressive behavior even though reductions in both aggression and ADHD symptoms from baseline may have occurred.
Data Analysis
Analyses of variance and χ2 tests compared response groups on demographic and clinical variables. Because the distribution of R-MOAS scores was positively skewed, analyses of variance used the natural logarithm of R-MOAS raw scores. We determined effect sizes for each group’s change in aggression (R-MOAS) and ADHD symptom (ConnGI-P) severity by using Dunlap’s31 method to compute Cohen’s d for repeated-measures data (dcorr).
Logistic regression analyses evaluated child characteristics as predictors of response status. Bivariate associations were calculated between each predictor and outcome, followed by an adjusted model that incorporated all candidate predictors.
Tolerability and adverse effect analyses considered each item of the BBAEQ-M. Items were dichotomized whereby scores of >4, representing moderate severity, counted as positive. χ2 tests compared the outcome status groups on each with no correction for multiple tests.
RESULTS
Participant Sample
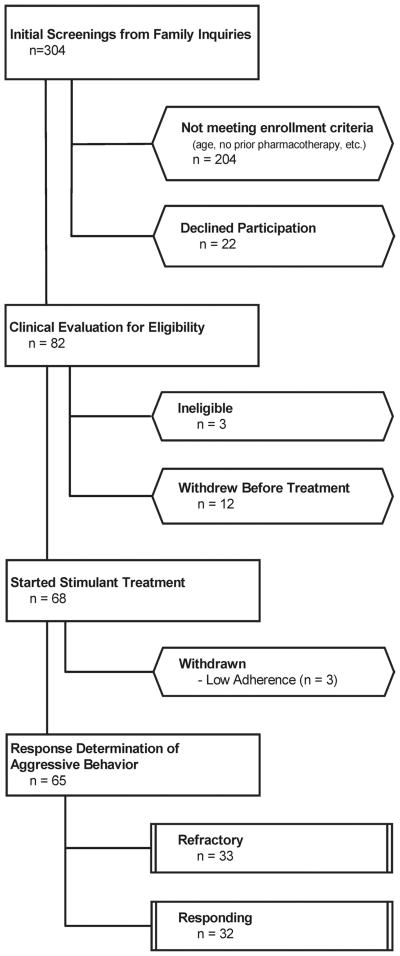
Figure 1 presents the disposition of all 304 initial screenings for this study. Table 1 contains demographic and clinical characteristics of the 65 children (50 boys, 15 girls; mean age: 8.95 years, [SD]: 2.48 years]) who started the trial and participated in subsequent assessments to determine their stimulant treatment response.
FIGURE 1.
Consolidated Standard of Reporting Trial (CONSORT) diagram showing disposition of all participants screened.
TABLE 1.
Participants’ Baseline Demographic and Clinical Characteristics
| Parameter | All Patients (n = 65) | Stimulant Responder Group (n = 32) | Stimulant Refractory Group (n = 33) | Tests for Group Differences |
|---|---|---|---|---|
| Age, mean (SD), y | 8.95 (2.48) | 9.52 (2.81) | 8.39 (2.00) | F1,63 = 3.40; P = .07 |
| Male gender, n (%) | 50 (77) | 25 (78) | 25 (76) | NSD |
| Race/ethnicity | NSD | |||
| White | 47 (72) | 22 (69) | 25 (76) | |
| Hispanic | 5 (8) | 4 (13) | 1 (3) | |
| Black | 8 (12) | 4 (13) | 4 (12) | |
| Other | 5 (8) | 2 (6) | 3 (9) | |
| Guardian’s relationship to child, n (%) | NSD | |||
| Biological parent | 56 (89) | 28 (90) | 28 (87) | |
| Adoptive parent | 6 (9) | 2 (7) | 4 (13) | |
| Grandparent | 1 (2) | 1 (3) | 0 (0) | |
| Diagnoses in addition to ADHD, n (%) | ||||
| ODD | 61 (94) | 31 (97) | 30 (91) | NSD |
| CD | 4 (6) | 1 (3) | 3 (9) | NSD |
| Anxiety disorder | 19 (29) | 12 (38) | 7 (21) | NSD |
| Depressive disorder, not otherwise specified | 4 (65) | 0 (0) | 4 (12) | ; P = .04 |
| Behavioral symptom severity, mean (SD) | ||||
| CBCL total, T | 74.61 (6.67) | 73.80 (6.90) | 75.91 (6.26) | NSD |
| CBCL externalizing, T | 72.49 (7.71) | 71.03 (8.08) | 73.91 (7.17) | NSD |
| CBCL internalizing, T | 66.62 (10.62) | 65.69 (11.70) | 67.52 (9.55) | NSD |
| ConnGI-P restless/inattentive, T | 79.08 (9.05) | 77.90 (10.68) | 80.15 (7.11) | NSD |
| R-MOAS total | 52.34 (32.53) | 44.16 (29.03) | 60.27 (34.18) | F1,63 = 4.19; P = .05 |
NSD indicates no significant difference; CBCL, Child Behavior Checklist.
Changes in Aggression and ADHD Symptoms During Stimulant Treatment
The period between baseline evaluation and final evaluation of stimulant monotherapy response averaged 63.26 days (SD: 23.98 days).
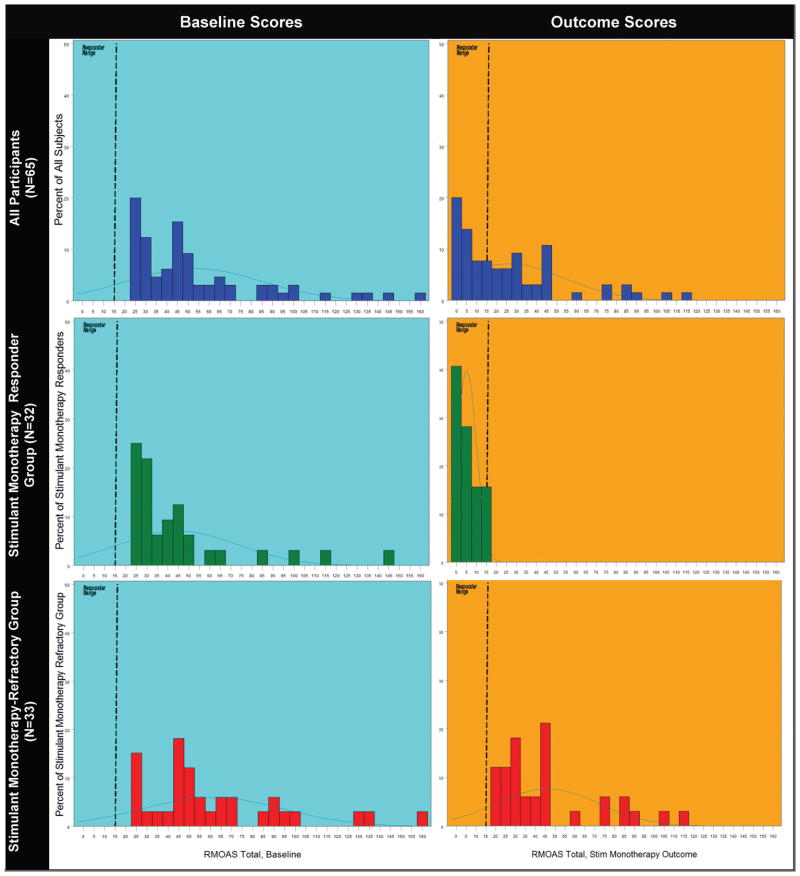
At the end of the stimulant-optimization procedures, 32 (49.3%) of 65 children (95% confidence interval: 37.3%–51.3%) fulfilled response criteria. Figure 2 presents the distributions of baseline and end-of-stimulant evaluation R-MOAS scores for the whole sample and for outcome status subgroups (responsive versus refractory aggression).
FIGURE 2.
Distributions of baseline and outcome R-MOAS scores for total sample and stimulant-responsive and stimulant-refractory subgroups. Left column presents baseline values, and right column shows scores at the end of stimulant optimization phase. Top row shows all subjects, middle row children whose aggression remitted with stimulant monotherapy, and the bottom row those whose aggression persisted.
Table 2 shows the means of behavioral measures for aggression response groups and the effect size for changes between baseline and post treatment assessments. The effect size for the reduction in mean R-MOAS scores during the stimulant treatment phase was >5 times as large among the responders as among children whose aggressive behavior was stimulant refractory. Nonetheless, the mean change over time for the latter group was statistically significant.
TABLE 2.
Behavioral Outcomes Open Stimulant Monotherapy
| Measure | Aggression Responders (n = 32) |
Aggression Refractory Group (n = 33) |
Group × Time Interaction | ||||
|---|---|---|---|---|---|---|---|
| Baseline | End Stimulant Lead-in | Effect | Baseline | End Stimulant Lead-in | Effect | ||
| R-MOAS total, mean (SD) | 44.16 (29.03) | 5.12 (2.05) | F1,31 = 163.78a | 60.27 (34.18) | 46.09 (26.07) | F1,32 = 5.81b | F1,63 = 101.46a |
| ES: dcorr = 2.58 | ES: dcorr = 0.46 | ||||||
| ConnGI (restless/impulsive scale) | 77.91 (10.68) | 56.47 (10.53) | F1,30 = 139.42a | 80.15 (7.11) | 70.39 (11.71) | F1,33 = 21.84a | F1,63 = 17.73a |
| T score, mean (SD) | ES: dcorr = 2.02 | ES: dcorr = 0.97 | |||||
ES indicates effect size.
P < .001.
P < .01.
Children in both response groups showed significant reductions on ADHD symptoms. The effect size for ADHD improvement (dcorr = 0.97) was considerably smaller among those with refractory aggression than among responders (dcorr = 2.02).
Treatment Characteristics of Remitter and Refractory Groups
Table 3 shows children’s optimized stimulant agents and dosages. Children whose aggression responded during stimulant optimization most often achieved stabilization with MPH-TRI: 84% compared with 49% of the refractory patients ( , P = .002). The average daily MPH-TRI dosage tended to be higher among patients with refractory than nonrefractory aggression (64 vs 52 mg; t41 = 1.83, P = .07). Families of children with stimulant-refractory aggression had significantly more behavioral therapy sessions than other families (3.06 vs 7.28 sessions; t63 = 4.85, P < .01).
TABLE 3.
Medication Treatments of Open Stimulant Monotherapy
| Pharmacotherapy | Aggression Responders |
Aggression Refractory Group |
||||
|---|---|---|---|---|---|---|
| n (%) | Mean (SD), mg | Range | n (%) | Mean (SD), mg | Range | |
| MPH-TRI | 27 (84.4) | 52.33 (18.00) | 18–90 | 16 (48.9) | 63.00 (19.16) | 36–108 |
| MPH-BI | 2 (6.3) | 40.00 (14.14) | 30–50 | 4 (12.1) | 27.50 (5.00) | 20–30 |
| Mixed amphetamine salts, extended release | 3 (9.4) | 26.67 (11.55) | 20–40 | 12 (36.4) | 25.83 (6.69) | 15–40 |
| Dextroamphetamine spansules | 0 (0) | NA | NA | 1 (3.0) | 10 (0) | 10 |
NA indicates not applicable.
Child Factors Associated With Aggression Outcome
Table 4 contains baseline clinical and demographic variables that were examined through logistic regression for their associations with outcome. Odds ratios when response status was regressed onto each predictor singly are presented. Higher baseline R-MOAS, CDRS-R, and YMRS scores predicted a lower likelihood that aggression would remit. Although few in number (n=4), all children who had a diagnosis of depressive disorder, not otherwise specified, were in the stimulant-refractory group (Table 1), although none had a history of manic or hypomanic episodes.
TABLE 4.
Predictors of Aggressive Behavior Remission After Stimulant Trial
| Baseline Predictor | Bivariate Associations: Single Predictors with Odds of Response |
Combined Predictors Adjusted Model for Odds of Response |
Gender-Stratified Combined Models for Odds of Response |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | χ2 | P | OR (95% CI) | χ2 | P | OR (95% CI) | χ2 | P | |
| Age, y | 1.210 (0.981–1.502) | 3.39 | .07 | 1.032 (0.759–1.400) | 0.04 | .840 | |||
| Male (n = 50) | 1.020 (0.710–1.460) | 0.01 | .92 | ||||||
| Female (n = 15) | 0.770 (0.320–1.810) | 0.37 | .54 | ||||||
| Gender (female vs male) | 0.875 (0.275–2.780) | 0.05 | .82 | 8.992 (1.326–60.981) | 5.06 | .020 | |||
| ConnGI, restless/impulsive T score | 0.972 (0.920–1.027) | 1.02 | .32 | 0.990 (0.910–1.076) | 0.06 | .810 | |||
| Male (n = 50) | 0.957 (0.857–1.070) | 0.61 | .44 | ||||||
| Female (n = 15) | 1.028 (0.085–1.240) | 0.08 | .77 | ||||||
| R-MOAS | 0.983 (0.966–1.000) | 4.29 | .06 | 0.971 (0.947–0.994) | 5.97 | .010 | |||
| Male (n = 50) | 0.985 (0.960–1.010) | 1.14 | .26 | ||||||
| Female (n = 15) | 0.941 (0.870–1.010) | 2.55 | .11 | ||||||
| Child Depression Inventory–Revised, total | 0.929 (0.846–1.020) | 2.85 | .09 | 0.830 (0.657–0.956) | 5.87 | .010 | |||
| Male (n = 50) | 0.788 (0.640–0.970) | 5.22 | .02 | ||||||
| Female (n = 15) | 0.778 (0.370–1.620) | 0.45 | .50 | ||||||
| YMRS, total | 0.839 (0.722–0.974) | 6.24 | .01 | 0.630 (0.526–0.886) | 8.21 | .004 | |||
| Male (n = 50) | 0.611 (0.430–0.880) | 7.15 | .01 | ||||||
| Female (n = 15) | 0.849 (0.400–1.820) | 0.18 | .67 | ||||||
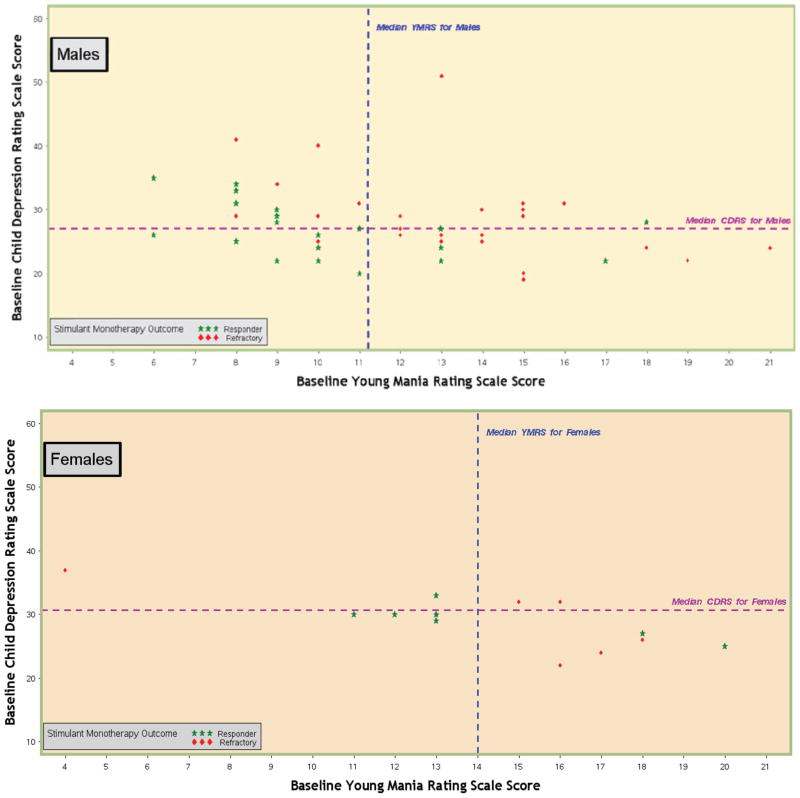
In a combined-variable model (Table 4), higher baseline R-MOAS, CDRS-R, and YMRS ratings made independent contributions toward predicting a lower likelihood of response. However, gender emerged as a significant predictor in the combined model (odds ratio: 8.99 [95% confidence interval: 1.33–60.98]; χ2 = 5.06, P = .02). That is, adjusting for the effects of baseline aggression, depression, and manic symptoms, girls were far more likely to experience remission of aggressive behavior with stimulant medication.
Separate computations of parameters for the combined variable model examined boys and girls. Table 4 presents these gender-stratified results. Higher baseline YMRS and CDRS-R total scores jointly predicted less favorable stimulant response status only for boys. Figure 3 illustrates these associations. Two (20%) of the 10 boys with above-median scores on both the YMRS and the CDRS-R fulfilled response criteria, compared with 7 (87.5%) of the 8 boys who were below the median on both. Children whose aggression did not fulfill response criteria nonetheless demonstrated relative improvements on both aggressive behavior ratings and ADHD symptoms (Tables 2 and 3). No child experienced worsening of mood-relevant symptoms on his or her optimized stimulant regimen.
FIGURE 3.
Association between stimulant monotherapy aggression-response status and total baseline scores on the YMRS and the CDRS-R.
Tolerability and Adverse Effects
We counted a BBAEQ-M item present when the parent rated it for at least moderate intensity. Table 5 displays adverse effect frequencies that were reported for at least 1 child at either baseline or trial end. Group differences in prevalence were assessed at each assessment time via χ2, with P set liberally at .10. Two sleep-related difficulties, initial insomnia and early waking, were more prevalent at base-line among children who would subsequently show remission of aggression.
TABLE 5.
Adverse Effect Rates
| Adverse Effect | Baseline |
Stimulant Monotherapy End Point |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aggression Responder Group |
Aggression Refractory Group |
χ2 (P) | Aggression Responder Group |
Aggression Refractory Group |
χ2 (P) | |||||
| n | % | n | % | n | % | n | % | |||
| Abdominal pain | 1 | 1 | 3 | 9 | 0 | 0 | 1 | 3 | ||
| Anxiety or nervousness | 4 | 15 | 7 | 22 | 4 | 13 | 8 | 26 | ||
| Enuresis | 2 | 6 | 1 | 3 | 1 | 3 | 1 | 3 | ||
| Bite fingernails | 4 | 15 | 8 | 25 | 4 | 13 | 5 | 16 | ||
| Bruises easily | 1 | 3 | 1 | 3 | 1 | 3 | 0 | 0 | ||
| Crying | 3 | 11 | 8 | 25 | 3 | 10 | 5 | 16 | ||
| Insomnia, initial | 6 | 22 | 15 | 47 | 3.88 (.05) | 6 | 20 | 11 | 38 | |
| Decrease appetite | 6 | 22 | 5 | 16 | 3 | 20 | 10 | 32 | ||
| Diarrhea | 1 | 3 | 0 | 0 | 1 | 3 | 1 | 3 | ||
| Insomnia, middle | 2 | 7 | 2 | 9 | 1 | 3 | 3 | 9 | ||
| Dizzy | 1 | 3 | 2 | 6 | 0 | 0 | 1 | 3 | ||
| Dry mouth | 1 | 4 | 3 | 10 | 1 | 3 | 1 | 3 | ||
| Headache | 5 | 19 | 1 | 3 | 3.80 (.05) | 1 | 3 | 0 | 0 | |
| Increase appetite | 2 | 7 | 4 | 13 | 0 | 0 | 1 | 3 | ||
| Irritability | 10 | 37 | 16 | 50 | 4 | 13 | 12 | 39 | 5.07 (.03) | |
| Low energy | 3 | 11 | 2 | 7 | 1 | 3 | 1 | 3 | ||
| Nightmares | 1 | 2 | 6 | 0 | 0 | 2 | 6 | |||
| Rash | 1 | 3 | 0 | 0 | 0 | 0 | 1 | 3 | ||
| Restlessness | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 9 | 3.05 (.09) | |
| Sadness | 3 | 11 | 7 | 22 | 3 | 10 | 6 | 19 | ||
| Shaking | 1 | 3 | 0 | 0 | 1 | 3 | 1 | 3 | ||
| Stares into space | 2 | 7 | 3 | 9 | 1 | 3 | 2 | 6 | ||
| Stomachache | 2 | 7 | 2 | 7 | 2 | 7 | 3 | 10 | ||
| Heart races | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 3 | ||
| Less talkative | 3 | 11 | 2 | 6 | 2 | 6 | 2 | 6 | ||
| Overly talkative | 10 | 37 | 8 | 25 | 3 | 10 | 5 | 16 | ||
| Tics | 2 | 6 | 2 | 6 | 2 | 7 | 1 | 3 | ||
| Tired | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 3 | ||
| Trouble waking | 1 | 3 | 0 | 0 | 1 | 3 | 0 | 0 | ||
| Tremors | 1 | 3 | 0 | 0 | 0 | 0 | 2 | 6 | ||
| Lack of interest | 2 | 6 | 2 | 6 | 1 | 3 | 5 | 16 | 2.81 (.10) | |
| Unusually happy | 3 | 9 | 3 | 9 | 6 | 7 | 6 | 6 | ||
| Early waking | 3 | 11 | 7 | 22 | 3 | 10 | 3 | 9 | ||
DISCUSSION
Within a group of children whose chronic aggressive behavior developed in the context of ADHD with comorbid ODD or CD and whose previous treatment history indicated insufficient response to stimulant medication, nearly half attained remission or near-remission of aggressive behavior by the conclusion of rigorously titrated stimulant monotherapy accompanied by psychosocial treatment. Treatment-refractory aggression did not result from less potent therapy. Stimulant responders tended to have more robust response to the first stimulant agent prescribed and at somewhat lower dosages. The stimulant-refractory group also had more family behavioral therapy sessions. Children whose aggression responded adequately had a mean MPH-TRI dosage of 52.33 mg/day, which approaches the labeled maximum dosage of 54 mg for preadolescents.32 Thislabeling may deter clinicians from prescribing MPH dosages that this and previous research have shown to be efficacious for this patient group.28
Boys were more likely to have aggression that would prove refractory to stimulant monotherapy, particularly those with higher baseline scores of depressive and manic symptom severity. However, optimized stimulant monotherapy did not induce mania or worsen other symptoms of mood disturbance and, notwithstanding inadequate response of aggression, still culminated in improved ADHD symptoms and relative decreases in aggression.
Determining each child’s optimal stimulant regimen involved weekly assessments at varying dosages and switches among preparations as needed, an approach that seems uncommon in primary care settings.33 A medical claims analysis indicated that 44.27% of children who began stimulant treatment experienced no dosage change in 3 months, and the remainder experienced only a modest average increase that was well below regimens that have been shown to be effective for ADHD symptoms.33 More intensive and methodical approaches to optimal stimulant agent and dosage identification may reduce the need to resort to antipsychotic treatment for severe aggression and behavioral dys-control among children with ADHD. Current pediatric guidelines articulate clearly the overall principle of stimulant dosage escalation as tolerated to achieve optimal response.34 The results of this study illustrate the viability of tactics to do so for children with ADHD, comorbid ODD or CD, and aggressive behavior.
Controlled trials for childhood ADHD show superiority of stimulants over placebo, but placebo response among an estimated 15% to 20% of patients2,19 precludes attribution of benefit solely to direct treatment effects. The eligibility criteria of this study included characteristics that have been shown to reduce placebo response, namely previous stimulant treatment (37% less likely), ADHD subtypes that feature hyperactive/impulsive symptoms (57% less likely), and preadolescent age (36% less).19 In addition, placebo response among children is generally short-lived,27,28 and our classification scheme required at least 3 weeks of subthreshold aggression.
CONCLUSIONS
Stimulant medication is the most common pharmacotherapy for children’s mental health problems in primary care. Pediatric providers are well-situated to leverage this treatment to affect the course of not only ADHD but also associated conduct disturbances, including severe aggression. Overcoming systemic barriers to optimize ADHD treatment in primary care35 may yield faster improvements and obviate the need for additional pharmacotherapy with greater health liabilities.
These findings suggest that impulsivity and affective instability are distinct influences on childhood aggression.14 Youth with greater affective disturbance were more likely to experience incomplete response to stimulant monotherapy. Understanding the high emotional reactivity that impulsive aggressive youth often display remains a high research priority to improve care of this vulnerable patient population.
WHAT’S KNOWN ON THIS SUBJECT
Although stimulant medications frequently alleviate symptoms of ADHD and related behavioral problems, it is unclear how often and with which regimens stimulant treatment culminates in remission of severe aggressive behavior.
WHAT THIS STUDY ADDS
Among aggressive 6- to 13-year-olds with ADHD and previous insufficient stimulant response, individually optimized stimulant monotherapy and behavioral intervention were associated with reductions in aggression that precluded need for additional medications. Boys with higher affect-related disturbances gained less robust benefit.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants K23MH064975 (principal investigator: Dr Blader) and M01RR10710 (Stony Brook General Clinical Research Center), a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression, and a grant for investigator-initiated research from Abbott Laboratories.
We thank Jennifer Puig, Marcela Torres, and Alisa Jahns, who served as study coordinators; Carmel Foley, MD, Deyan Budimirovich, MD, Jeffrey Sverd, MD, Josefina Antonio, MD, Malgorzata Relja, MD, and Mira Sachdev, MD, for serving as study psychiatrists; Frank Albergo, RPh, and Wayne Patterson, RPh, for assistance with preparing study medications; and psychosocial therapists Esme Londahl-Shaller, Eve-lyn Flaherty, Alisa Hurwitz, and Erica Chin for providing behavioral therapy to families.
ABBREVIATIONS
- ADHD
attention-deficit/hyperactivity disorder
- ODD
oppositional defiant disorder
- CD
conduct disorder
- R-MOAS
Retrospective–Modified Overt Aggression Scale
- MPH
methylphenidate
- K-SADS
Schedule for Schizophrenia and Affective Disorders for School-Age Children
- ConnGI-P
Conners Global Index, Parent Version
- CDRS-R
Child Depression Rating Scale–Revised
- YMRS
Young Mania Rating Scale
- BBAEQ-M
Barkley Behavior and Adverse Events Questionnaire–Modified
- MPH-TRI
triphasic-release MPH
- MPH-BI
biphasic-release MPH
Footnotes
This trial has been registered at www.clinicaltrials.gov (identifier NCT00228046).
FINANCIAL DISCLOSURE: Dr Blader has received research support from Abbott Laboratories and consultant fees from Otsuka America Pharmaceuticals and Validus Pharmaceuticals; Dr Pliszka has received research support from Ortho-McNeil Janssen Scientific Affairs, Shire, Eli Lilly, AstraZeneca, and Cephalon and consulting fees or honoraria from Shire and McNeil Pediatrics; Dr Schooler has received research support from Ortho-McNeil Janssen Scientific Affairs and consultant fees or honoraria from Organon, Ortho-McNeil, and Schering Plough; Dr Jensen has received speaking fees or consulting honoraria from Johnson & Johnson, Janssen-Ortho, UC Pharma, NEI, CME Outfitters, and CMED; and Dr Kafantaris has received research support from Eli Lilly and Pfizer and donation of study medication and matching placebo from AstraZeneca, GlaxoSmithKline, Janssen, Eli Lilly, and Pfizer.
References
- 1.Brown RT, Amler RW, Freeman WS, et al. Treatment of attention-deficit/hyperactivity disorder: overview of the evidence. Pediatrics. 2005;115(6) doi: 10.1542/peds.2004-2560. Available at: www.pediatrics.org/cgi/content/full/115/6/e749. [DOI] [PubMed]
- 2.Greenhill LL, Halperin JM, Abikoff H. Stimulant medications. J Am Acad Child Adolesc Psychiatry. 1999;38(5):503–512. doi: 10.1097/00004583-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Klein RG, Abikoff H, Klass E, Ganeles D, Seese LM, Pollack S. Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry. 1997;54(12):1073–1080. doi: 10.1001/archpsyc.1997.01830240023003. [DOI] [PubMed] [Google Scholar]
- 4.Connor D, Glatt S, Lopez I, Jackson D, Melloni R. Psychopharmacology and aggression: I—a meta-analysis of stimulant effects on overt/covert aggression: related behaviors in ADHD. J Am Acad Child Adolesc Psychiatry. 2002;41(3):253–261. doi: 10.1097/00004583-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Olfson M, Blanco C, Liu L, Moreno C, Laje G. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry. 2006;63(6):679–685. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- 6.Patel NC, Crismon ML, Hoagwood K, et al. Trends in the use of typical and atypical antipsychotics in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2005;44(6):548–556. doi: 10.1097/01.chi.0000157543.74509.c8. [DOI] [PubMed] [Google Scholar]
- 7.Crystal S, Olfson M, Huang C, Pincus H, Gerhard T. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood) 2009;28(5):w770–w781. doi: 10.1377/hlthaff.28.5.w770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sikich L, Frazier JA, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) Study. Am J Psychiatry. 2008;165(11):1420–1431. doi: 10.1176/appi.ajp.2008.08050756. [DOI] [PubMed] [Google Scholar]
- 10.Cheng-Shannon J, McGough JJ, Pataki C, McCracken JT. Second-generation antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol. 2004;14(3):372–394. doi: 10.1089/cap.2004.14.372. [DOI] [PubMed] [Google Scholar]
- 11.Pliszka SR, Crismon ML, Hughes CW, et al. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder (ADHD) J Am Acad Child Adolesc Psychiatry. 2006;45(6):642–657. doi: 10.1097/01.chi.0000215326.51175.eb. [DOI] [PubMed] [Google Scholar]
- 12.Connor DF, Carlson GA, Chang KD, et al. Juvenile maladaptive aggression: a review of prevention, treatment, and service configuration and a proposed research agenda. J Clin Psychiatry. 2006;67(5):808–820. [PubMed] [Google Scholar]
- 13.Jensen PS, Youngstrom EA, Steiner H, et al. Consensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: implications for medication studies. J Am Acad Child Adolesc Psychiatry. 2007;46(3):309–322. doi: 10.1097/chi.0b013e31802f1454. [DOI] [PubMed] [Google Scholar]
- 14.Blader JC, Jensen PS. Aggression in children: an integrative approach. In: Martin A, Volkmar FR, editors. Lewis’ Textbook of Child and Adolescent Psychiatry. 4. Baltimore, MD: Lippincott Williams & Wilkins; 2007. pp. 467–483. [Google Scholar]
- 15.Dubovsky SL. How to reduce mania risk when prescribing stimulants: evidence-based hierarchy helps when bipolar and ADHD symptoms overlap. Curr Psychiatry. 2005;4(10):36–54. [Google Scholar]
- 16.Mick E, Spencer T, Wozniak J, Biederman J. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol Psychiatry. 2005;58(7):576–582. doi: 10.1016/j.biopsych.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 17.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160(3):430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 18.Blader JC, Schooler NR, Jensen PS, Pliszka SR, Kafantaris V. Adjunctive divalproex sodium vs. placebo for children with ADHD and aggression refractory to stimulant monotherapy. Am J Psychiatry. 2009;166(12):1392–1401. doi: 10.1176/appi.ajp.2009.09020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newcorn JH, Sutton VK, Zhang S, et al. Characteristics of placebo responders in pediatric clinical trials of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(12):1165–1172. doi: 10.1097/CHI.0b013e3181bc730d. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Donovan SJ, Stewart JW, Nunes EV, et al. Divalproex treatment for youth with explosive temper and mood lability: a double-blind, placebo-controlled crossover design. Am J Psychiatry. 2000;157(5):818–820. doi: 10.1176/appi.ajp.157.5.818. [DOI] [PubMed] [Google Scholar]
- 22.Conners CK. Technical Manual. North Tonawanda, NY: Multi-Health Systems; 1997. Conners’ Rating Scales–Revised. [Google Scholar]
- 23.Poznanski EO, Mokros HB. Children’s Depression Rating Scale–Revised. Los Angeles, CA: Western Psychological Services; 1995. [Google Scholar]
- 24.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 25.Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics. 1990;86(2):184–192. [PubMed] [Google Scholar]
- 26.Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA study: Relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry. 1996;35(10):1304–1313. doi: 10.1097/00004583-199610000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Abikoff H, Hechtman L, Klein R, et al. Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43(7):802–811. doi: 10.1097/01.chi.0000128791.10014.ac. [DOI] [PubMed] [Google Scholar]
- 28.MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56(12):1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham CE, Bremner RB, Secord-Gilbert M. COPE: The Community Parent Education Program: A School Based Family Systems Oriented Workshop for Parents of Children With Disruptive Behavior Disorders; Hamilton, Ontario, Canada: COPE Works; 2000. [Google Scholar]
- 30.Pelham WE, Gnagy EM, Burrows-Maclean L, et al. Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics. 2001;107(6) doi: 10.1542/peds.107.6.e105. Available at: “ www.pediatrics.org/cgi/content/full/107/6/e105”. [DOI] [PubMed]
- 31.Dunlap WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol Methods. 1996;1(2):170–177. [Google Scholar]
- 32.McNeil Pediatrics. Concerta® (Methylphenidate HCl) Extended-Release Tablets: Full Prescribing Information. Titusville, NJ: Mc-Neil Pediatrics; Jun, 2009. [Google Scholar]
- 33.Olfson M, Marcus S, Wan G. Stimulant dosing for children with ADHD: a medical claims analysis. J Am Acad Child Adolesc Psychiatry. 2009;48(1):51–59. doi: 10.1097/CHI.0b013e31818b1c8f. [DOI] [PubMed] [Google Scholar]
- 34.American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder and Committee on Quality Improvement. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108(4):1033–1044. doi: 10.1542/peds.108.4.1033. [DOI] [PubMed] [Google Scholar]
- 35.American Academy of Child and Adolescent Psychiatry Committee on Health Care Access and Economics, American Academy of Pediatrics Task Force on Mental Health. . Improving mental health services in primary care: reducing administrative and financial barriers to access and collaboration. Pediatrics. 2009;123(4):1248–1251. doi: 10.1542/peds.2009-0048. [published correction appears in Pediatrics. 2009;123(6):1611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.