Abstract
Objective
Trauma influences on perinatal maternal-child interactions may affect the organization of offspring physiological systems involved in health outcomes. This study used a novel advanced system recently adapted for infants to examine associations of maternal lifetime trauma and related psychological symptoms in the perinatal period with infant cardiorespiratory reactivity and behavioral distress in response to a laboratory stressor.
Methods
Mothers self-reported lifetime exposure to trauma, perinatal traumatic stress, and current symptoms of posttraumatic stress disorder (PTSD) and depression. Heart rate and indices of respiratory volume, timing, and thoraco-abdominal coordination were continuously recorded using a non-invasive respiratory inductance plethysmography device from 23 infants during the Still-Face Paradigm, a videotaped mother-infant dyadic assessment that included baseline, stressor, and recovery phases. Infant behavioral distress during the procedure was also assessed.
Results
Infants of mothers with low exposure to trauma and perinatal traumatic stress showed expected increases in behavioral distress and cardiorespiratory activation from baseline to stressor and decreases in these parameters from stressor to recovery. Infants of mothers exposed to multiple traumas and with elevated perinatal traumatic stress showed similar patterns of activation from baseline to stressor but failed to show decreases during recovery. These patterns were maintained after controlling for current maternal PTSD and depressive symptoms.
Conclusions
Maternal lifetime trauma exposure and traumatic stress during the perinatal period were associated with disrupted infant cardiorespiratory regulation and behavioral distress during a stressor protocol. These results support the concept of perinatal programming and its potential role in physical and mental health outcomes.
Keywords: Perinatal Programming, Trauma, Stress, Autonomic Reactivity
A number of disorders, including hypertension, insulin resistance, and asthma, as well as vulnerability to mental health problems such as posttraumatic stress disorder (PTSD) and depression, are thought to have their origins in fetal and early postnatal development(1–3). Growing evidence suggests that environmental factors acting early in development may permanently organize key physiological systems involved in disease etiologies, a concept known as perinatal programming(4). However, little is known about the processes involved. Identifying specific programming agents and the mechanisms by which they exert their effects during developmentally sensitive periods may enhance our understanding of the origins of many disorders and suggest more efficacious preventative strategies.
Perinatal maternal stress has emerged as one important factor that may program relevant physiological systems(4, 5). While the nature of stressors likely to have a measurable impact is not fully elucidated, overlapping evidence suggests that maternal exposure to traumatic events—that is, events that involve threat of death or serious harm to self or others and that result in feelings of intense fear, horror, or helplessness [Criterion A traumatic events(6)] – may have particularly robust impact.
Stress is hypothesized to exert programming effects in utero via altered biobehavioral states in the mother that lead to disruptions in developing fetal autonomic and neuroendocrine systems(7). If these disruptions permanently organize fetal physiological systems, later regulatory responses involved in managing stress may be compromised(3). In addition, altered autonomic and neuroendocrine functioning modulates a host of related systems (e.g. immune functioning) that may increase vulnerability to later physical and mental health problems(7). Research with children and adults has demonstrated that trauma exposure frequently leads to such altered psychophysiological states, including abnormal patterns of reactivity of the sympathetic and parasympathetic nervous systems and the hypothalamic-pituitary-adrenal (HPA) axis(8), with alterations persisting even years following an event(9). Therefore, women exposed to traumatic events over their lifetime may manifest disrupted physiological states during pregnancy that could, in turn, disrupt the programming of their fetus’ developing regulatory systems.
Postnatal factors may have independent effects and/or modify prenatal programming effects. Numerous studies have shown that the quality of caregiving provided in the first years of life helps to shape stress regulatory systems (e.g. autonomic, HPA systems) (5). Specifically, parenting that is sensitive and responsive to the child’s physical and emotional needs has been associated with buffering of infants’ physiological responses to stress, whereas insensitive parenting is a risk factor for the development of extreme stress responses(5, 10). Caregiving influences on stress reactivity systems may be permanent(11). Therefore, factors that interfere with caregivers’ abilities to provide sensitive care in the first years of life may result in long-lasting programming effects. Stress in general has been demonstrated to compromise sensitive caregiving practices, and maternal trauma exposure in particular has been associated with poor parenting practices as well as with altered child behavioral and physiological stress regulation(3, 12, 13).
The autonomic nervous system has been identified as one of the key physiological systems involved in a range of physical and mental health problems, with cardiac and respiratory functioning receiving particular attention. For example, heart rate at rest and in response to challenge has been associated with stress tolerance and central nervous system integrity(14). Indices of cardiac activity assessed in infancy have been found to predict “stress vulnerability,” including altered physiologic and poor behavioral response to challenge, more time needed to recover from stress, and greater social problems later in development(15, 16). Altered balance between functional parasympathetic and sympathetic activity in response to stress has been implicated in the expression of allergic sensitization, early airway obstruction, and asthma(4) as well as anxiety, depression, and PTSD(17, 18). Respiratory dysregulation (e.g. breathing irregularities, overbreathing/hyperventilation) during baseline conditions and in response to stress has been documented among individuals with respiratory-related illnesses such as asthma and individuals with mental health disorders, particularly anxiety(17, 19). Other studies support a connection between an adverse intrauterine environment and alterations of autonomic functioning(20) (21). Existing studies in infants have ignored the impact of prenatal maternal stress on offspring respiratory functioning, due largely to unique challenges in infants (e.g., intrusiveness of existing recording techniques contributing to poor cooperation)(22). More recently adapted techniques that integrate state-of-the-art respiratory pattern sampling with electrocardiogram (ECG) recordings overcome many of these limitations(23).
We test the hypothesis that maternal trauma and related symptoms in the perinatal period are associated with altered regulation of infant cardiac and respiratory systems and elevated behavioral distress in response to stressful challenge. This was accomplished by examining associations of maternal exposure to traumatic events and related psychological symptoms in the perinatal period with the coordinated assessment of infant heart rate and respiratory activity and behavioral indicators of distress in response to a laboratory stressor protocol, the Still-Face Paradigm (SFP)(24). Given that traumatic experiences are common in the general population and that low-income, urban, minority populations are at increased risk for both repeated trauma exposure and many chronic health problems(25), revealing perinatal programming effects of maternal trauma exposure may have significant public health implications.
Method
Participants
Participants were mothers and their 6-month-old infants enrolled in a pilot study of the intergenerational transmission of PTSD. Pregnant women receiving prenatal care at two major Boston hospitals and three urban community health centers and women attending Women, Infants and Children (WIC) programs associated with the health centers were recruited during the 1st or 2nd trimester between August 2006 and May 2007. Study inclusion criteria include (1) mother 18 years or older at child’s birth; (2) single gestation birth. Exclusion criteria include (1) mother not sufficiently fluent in English to complete study measures; (2) maternal endorsement of drinking ≥ 7 alcoholic drinks/week prior to pregnancy recognition or any alcohol following pregnancy recognition, and/or smoking ≥ 10 cigarettes/day during pregnancy, given potential effects on study outcome variables above these thresholds(26, 27); (3) high-risk infant (e.g., gestational age<32 weeks; birthweight<5.5 lbs; congenital abnormalities; neurological injury), as these factors are associated with increased risk for child neurodevelopmental problems that may affect regulatory systems(28). All procedures were approved by relevant Institutional Review Boards, and written informed consent was obtained.
Thirty-five mother-infant dyads completed the 6-month laboratory assessment, with 23 providing sufficient data for the current analyses. Dyads were excluded for the following reasons: infant too distressed during fitting to wear equipment (n=6); protocol terminated by mother or examiner due to infant distress (n=4) or sleepiness (n=1); excessive artifact (n=1). The demographic characteristics of the final sample are presented in Table 1. These dyads did not differ from excluded dyads based on demographic factors or on any of the maternal trauma or psychological variables included in the analyses.
Table 1.
Subject Demographic Characteristics (N = 23)
| N | % | M | SD | |
|---|---|---|---|---|
| Maternal Ethnicity/Race | ||||
| Hispanic or Latina/White | 5 | 22 | ||
| Not Hispanic or Latina/White | 10 | 43 | ||
| Not Hispanic or Latina/Black | 8 | 35 | ||
| Infant Gender: Female | 12 | 52 | ||
| Primiparous Birth | 11 | 48 | ||
| Infant Gestational Age | 39.0 weeks | 1.7 | ||
| Infant Birthweight | 3431.5 grams | 538.7 | ||
| Infant Age at Assessment | 27.6 weeks | 1.4 |
Measures
Trauma Exposure and Psychological Symptomatology
Life Stressor Checklist-Revised (LSC-R)(29)
Self-reported maternal lifetime exposure to traumatic events and the impact of exposure over the last year were assessed using the LSC-R, which includes experiences particularly relevant to women (e.g. rape, interpersonal violence) and has established reliability and validity in diverse populations(29). A “maternal lifetime trauma exposure” score (range 0–30) was derived from the number of endorsed events that met DSM-IV PTSD Criterion A(6). For each endorsed event, the mother was asked to rate on a 5-point scale how severely she had been affected by the event over the last year, and a total score for severity of impact was calculated (range 0–150). Because mothers were interviewed when the child was approximately 6 months old and were asked to report the impact of events over the last year, the period assessed covered the majority of the pregnancy and the entire postnatal period; the score is therefore labeled here as “perinatal trauma impact.”
Posttraumatic Stress Disorder Checklist-Civilian Version (PCL-C)(30)
Current PTSD symptomatology was assessed using the PCL-C, a 17-item self-report measure that reflects DSM-IV symptom criteria for PTSD(30). Mothers rated each symptom on a 5-point Likert scale in terms of how much it had bothered them in the past month, producing a single symptom score (range 17–85). The PCL-C has high internal consistency for the total scale (Cronbach’s α=0.89) and good test-retest reliability and convergent validity with a number of other PTSD scales and with the Clinician-Administered PTSD Scale (CAPS), a structured clinical interview for PTSD(31). Internal consistency in this sample was high, Cronbach’s α=0.93.
Edinburgh Postnatal Depression Scale (EPDS)(32)
Current maternal depressive symptoms were assessed using the 10-item EPDS (range=0–30), a measure specifically designed to address depressive symptoms in the postnatal period. The measure has been validated in childbearing women and has demonstrated high internal consistency (Cronbach’s α=0.87) and validity for detecting major depression in the perinatal period(33). Internal consistency in this sample was high, Cronbach’s α=0.91.
Still-Face Paradigm (SFP)(24)
The Still-Face Paradigm is a videotaped observational procedure used extensively to study infants’ behavioral and physiological responses to brief, moderate levels of induced stress (34). The procedure involves three 2-minute episodes during which the infant is seated in an infant seat across from the mother. Mothers were instructed to play with their infant for 2 minutes (“Play”=baseline), followed by a still-face episode when the mother was instructed to maintain a neutral facial expression and avoid touching or vocalizing at their infant (“Still-Face”=stressor). The Still-Face episode is hypothesized to be a stressor for infants because the mother is no longer providing behavioral cues needed to maintain an organized social and affective state. Mothers were then instructed to resume playing with the infant during a “reunion” episode (“Reunion”=recovery), which provided opportunity for recovery from the effects of the Still-Face episode.
Infant Autonomic Reactivity
Infant physiological responses were assessed using the LifeShirt® System (VivoMetrics, Inc., Ventura, CA), a non-invasive ambulatory respiratory inductance plethysmography device(23) recently adapted for infants as young as 6 months that collects continuous measures of multiple physiological parameters, with a particular focus on detailed analysis of cardiac and respiratory functioning. The system has the integrated capability of measuring the timing, flow, and volume parameters of respiratory patterns by inductance bands sewn into a sleeveless Velcro undergarment at rib cage and abdomen levels. It also measures a basic three-lead ECG with electrodes attached to the sternum, the lower left rib, and the left clavicle. The breath waveforms are sampled with a rate of 50 Hz that allows valid detection of breaths in a range of 0–150 breaths per minute (i.e., physiologically relevant frequencies). During the course of the study, the system was modified to increase the ECG sampling rate from 200 Hz (range detection 30–250 beats per minute) to 1000 Hz (range detection 0–350 beats per minute) for greater accuracy of R-wave detection, given infants’ higher heart rates relative to adults. Fifteen (65%) of the infants in the current study were assessed with the 200 Hz system and 8 (35%) with the 1000 Hz system. The specific parameters assessed and analyzed in the current study are described below under Data Reduction and Analysis.
Infant Distress Behavior
Videotaped recordings of the SFP were scored by two trained coders. For each of the three SFP episodes, infant level of behavioral distress was rated on a 5-point scale: 0=no distress, 1=minimal distress (e.g. negative facial expression, distress sounds); 2=fussing<25% of episode, no crying; 3=hard crying<10 seconds; 4=hard crying ≥10 seconds. All instances in which there was disagreement were conferenced until consensus was reached.
Procedures
Laboratory Protocol
A licensed clinical psychologist (1st author) administered the study protocol. After fitting of the respiratory inductance plethysmography device, the infant was placed in a car seat on a table 3 feet from the mother, who was seated facing the infant at the infant’s eye level. Following a 3-minute period when the mother read the child a story or blew bubbles, the dyad participated in the SFP. Prior to or following the SFP, the mother was administered study questionnaires.
Data Reduction and Analysis
Heart Rate
Continuous ECG signals were examined for artifacts, which were removed using the VivoLogic software for analysis of the LifeShirt® System data. Means and standard deviations of heart rate (HR) were then extracted for each of the three SFP episodes. Rates of edited artifacts were slightly higher with the 1000 Hz system [M=12.2%(10.5%)] than with the 200 Hz system [M=2.5%(2.7%)].
Respiratory Measures
A Qualitative Diagnostic Calibration procedure(35) was applied to derive the proportionality constant between rib cage and abdominal amplifiers of the respiratory inductive plethysmograph. The QDC procedure is based on equations of the isovolume maneuver calibration and is carried out during a 5-minute period of natural breathing without use of a mouthpiece or mask. Following calibration, the data were edited for respiratory artifacts by exporting the data cut by breath and removing any segments that did not fall within upper and lower limits for the various respiratory parameters: inspiratory or expiratory tidal volume <20 ml or > 400 ml; inspiratory or expiratory duration <.3 s or > 5 s; total breath duration <.8 s or > 6 s; percent rib cage contribution to tidal volume negative and visual inspection of raw waveform suggestive of artifact. The determination of limits was guided by literature on infant respiratory functioning(36, 37). Following artifact removal, timing, volume and flow parameters and parameters of thoraco-abdominal coordination were scored breath-by-breath. This included tidal volume (VT), minute ventilation (V’min), inspiratory duration (TI), expiratory duration (TE), total breath duration (TTOT), respiratory timing (TI/TTOT), percent rib cage contribution to VT (%RC), and inspiratory and expiratory thoraco-abdominal asynchrony (PhRIB and PhREB, respectively). Means and standard deviations for each of the respiratory parameters were then calculated for each of the three SFP episodes. Rates of artifact detected and edited were 18.7% (6.7%) for the 200 Hz version and 17.8% (4.4%) for the 1000 Hz version.
Data Analyses
Descriptive and correlational analyses were run to determine associations among maternal lifetime trauma exposure, perinatal trauma impact, and current PTSD and depressive symptoms. Linear mixed models were then used to examine whether the maternal trauma scores (lifetime trauma exposure, perinatal trauma impact) were associated with different infant response patterns on the physiological and behavioral distress measures during the SFP. Specifically, for each maternal trauma variable, a linear mixed model was used to relate the infants’ physiological/behavioral scores to the phase in the SFP, the trauma score, and the SFP phase x trauma score interaction. Current maternal symptoms of PTSD and depression were included in analyses as control variables. Maternal trauma variables were centered so that main effects of SFP episode on infant behavioral and physiological responding could be interpreted. Likelihood ratio tests were used to test the null hypothesis that the pattern of infant physiological/behavioral scores during the SFP was unrelated to the maternal trauma scores. One-way univariate ANOVAs followed by post-hoc Scheffé tests explored whether infant behavioral distress responses were associated with HR and respiratory parameters. For this purpose, the behavioral distress variable was collapsed into three categories: “minimal to no distress” (scores=0–1), “moderate distress”(score=2), and “high distress” (scores=3–4). We examined the data for outliers and influential observations using standard regression diagnostics including standardized residuals and Cook's D(38).
Results
Mothers reported experiencing a range of Criterion A traumatic events [M=3.3(2.6), range=0–10] and significant variability in how much impact events had on their lives over the past year [M=21.8(11.8), range=5–50]. There was also significant variability in maternal report of current symptoms of PTSD [M=33.7(14.1), range=19–63], with 30% of participants meeting criteria for a likely diagnosis of PTSD, 35% falling in the subclinical range, and 35% in the nonclinical range according to DSM-IV-guided scoring criteria. Similar variability in depression scores were found [M =8.5(6.0), range=0–23], with 30% surpassing the measure cutoff score suggestive of a diagnosis of major depression. Lifetime exposure to traumatic events was highly correlated with the perinatal trauma impact score (rs=.80, p<.0005), and both the lifetime exposure to traumatic events and perinatal trauma impact scores were moderately correlated with current PTSD symptoms (rs=.40, p=.06 and rs=.41, p=.06, respectively) but not significantly associated with current depressive symptoms (rs=.16, p=.48 and rs=.05, p=.83, respectively). Current PTSD and depressive symptoms were moderately correlated (rs=.44, p=.04). None of the infant physical characteristic variables—birthweight, gestational age, age at the time of assessment, gender—were associated with the maternal trauma or psychological variables.
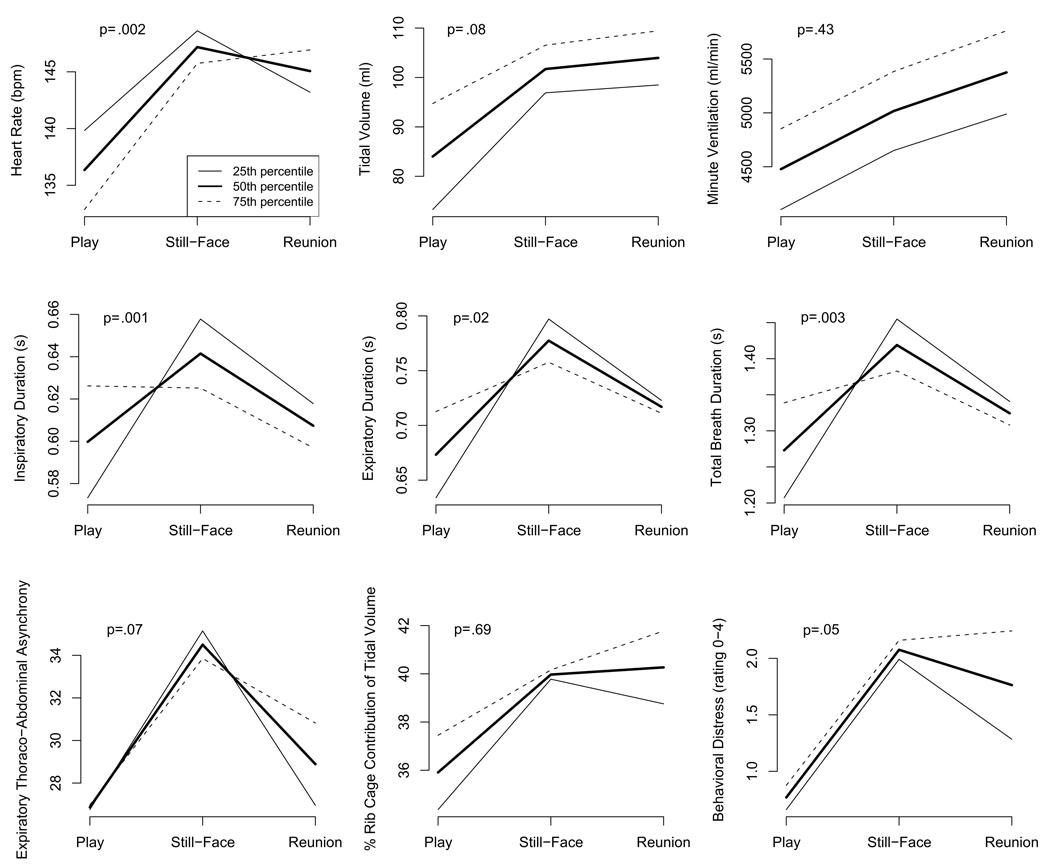
Mixed model analyses revealed that both maternal lifetime trauma exposure and perinatal trauma impact were associated with infant physiological functioning during the baseline Play and during the overall time course of the SFP. During Play, elevated maternal trauma exposure and perinatal trauma impact were associated with higher TI, TE, TTOT, and VT and tended to be associated with lower HR. The pattern of infants’ responses in the sample as a whole included significant increases in behavioral distress, HR, VT, V’min, TI, TE, TTOT, PhRIB, and PhREB from Play to Still-Face and decreases in most of these parameters from Still-Face to Reunion (Table 2). Likelihood ratio scores indicated that maternal lifetime trauma exposure and perinatal trauma impact were significantly related to the overall time course of HR, TI, TE, and TTOT and tended to be related to the overall time course of behavioral distress, VT, and PhREB across the SFP. Higher maternal trauma scores were associated with weaker or no increases in timing parameters, in particular in TI, from Play to Still-Face, and delayed or failed recovery during the Reunion in terms of behavioral distress, HR, PhRIB, and PhREB. As results for both maternal lifetime trauma exposure and perinatal trauma impact were nearly identical, only the mixed model results for maternal lifetime trauma exposure are shown in Table 3 and Figure 1. These divergent patterns by maternal lifetime trauma exposure and perinatal trauma impact were maintained across parameters even after controlling for current maternal PTSD and depressive symptoms.
Table 2.
Means and Standard Deviations of Infant Responses to Still-Face Paradigm
| Play | Still-Facea | Reunionb | |
|---|---|---|---|
| HR | 135.9 (11.5) | 147.0 (12.4)# | 145.3 (10.7)# |
| VT | 85.4 (25.2) | 102.3 (24.0)# | 104.7 (34.2)# |
| V’min | 4526.5 (1480.8) | 5065.0 (1362.7)** | 5425.9 (1846.6)# |
| TI | 0.60 (0.07) | 0.64 (.05)* | 0.61 (0.05) |
| TE | 0.68 (0.11) | 0.77 (0.10)# | 0.72 (0.13) |
| TTOT | 1.28 (0.16) | 1.41 (0.13)# | 1.32 (.16) |
| TI/TTOT | 0.48 (0.02) | 0.47 (.02) | 0.47 (.03) |
| PhRIB | 27.3 (7.1) | 33.7 (6.4)# | 30.0 (7.5) |
| PhREB | 26.9 (7.1) | 34.4 (6.1)# | 29.1 (6.3) |
| %RC | 36.1 (9.3) | 40.0 (10.4)+ | 40.5 (14.2)* |
| Behavioral Distress | 0.78 (.90) | 2.09 (1.20)# | 1.83 (1.53)# |
Significance level for difference in infant response between Still-Face and Play episodes.
Significance level for difference in infant response between Reunion and Play episodes.
p<.10.
p<.05.
p <.01.
p <.001.
Table 3.
Standardized Betas and Standard Errors from Mixed Model Results for Maternal Lifetime Trauma Exposure Effects on Infant Responses
| Play – Still-Facea | Play – Reunionb | Still-Face – Reunionc | |
|---|---|---|---|
| HR | 1.02 (.70) | 2.67 (.70)# | 1.65 (.70)* |
| VT | −2.95 (1.78) | −2.62 (1.78) | .33 (1.78) |
| V’min | −3.98 (75.50) | 5.83 (75.50) | 9.81 (75.50) |
| TI | −.02 (.006)# | −.02 (.006)** | .003 (.006) |
| TE | −.03 (.01)** | −.02 (.01)* | .007 (.01) |
| TTOT | −.05 (.01)# | −.04 (.01)** | .01 (.01) |
| TI/TTOT | .002 (.002) | .0004 (.002) | −.002 (.002) |
| PhRIB | −.03 (.64) | 1.11 (.64)+ | 1.14 (.64)+ |
| PhREB | −.38 (.52) | .91 (.52)+ | 1.29 (.52)* |
| %RC | −.68 (.81) | −.01 (.81) | .66 (.81) |
| Behavioral Distress | −.01 (.10) | .19 (.10)+ | .20 (.10)* |
Change in infant response from Play to Still-Face associated with one unit change in maternal lifetime trauma exposure.
Difference in infant response between Play and Reunion associated with one unit change in maternal lifetime trauma exposure.
Change in infant response from Still-Face to Reunion associated with one unit change in maternal lifetime trauma exposure.
p<.10.
p<.05.
p <.01.
p <.001.
Figure 1.
Infant responses to Still-Face Paradigm by maternal lifetime trauma exposure.
Note. p values are for likelihood ratio tests used to test the null hypothesis that infant responding during the SFP was unrelated to maternal lifetime trauma exposure.
Analyses of the association between infant behavioral distress and physiological responses revealed differences on several parameters, as displayed in Table 4. Greater infant distress during the Still-Face was associated with greater increases in HR, VT, V’min, and %RC and tended to be associated with greater increases in PhRIB from the Play to the Still-Face. High infant distress during the Reunion was associated with increases in HR and TE and decreases in TI/TTOT and tended to be associated with increases in %RC from Still-Face to Reunion, whereas minimal to moderate distress during the Reunion was associated with decreases in HR and TE and increases in TI/TTOT and tended to be associated with decreases in %RC. Furthermore, all of the infants who were highly distressed during the Reunion (26%) failed to return to a non-distressed state (i.e. no longer fussing or crying) by the end of the episode, and these infants had mothers with significantly higher lifetime trauma exposure and perinatal trauma impact scores [M=5.3(3.3) and M=31.8(10.9), respectively] than infants who recovered during the Reunion [M=2.5(2.0) and M =18.2(10.2), respectively], Mann-Whitney U test, Z=−1.98, p=.048 and Z=−2.35, p=.02, respectively.
Table 4.
Infant Physiology Response Change Scores by Behavioral Distress Level during Still-Face Paradigm
| Minimally Distressed | Moderately Distressed | Highly Distressed | ANOVA F | p | |
|---|---|---|---|---|---|
| Δ Play to Still-Face | |||||
| HR | 5.3 (5.7)a | 10.3 (3.7) | 16.8 (6.7)a | 8.60 | .002 |
| VT | 1.8 (9.7)a | 16.3 (14.1) | 30.8 (15.1)a | 10.31 | .001 |
| V’min | −58.1 (499.9)a | 552.6 (686.0) | 1059.2 (295.5)a | 11.02 | .001 |
| PhRIB | 1.6 (3.5) | 7.1 (8.1) | 10.4 (8.4) | 3.37 | .055 |
| %RC | −.81 (6.3)a | 3.6 (6.2) | 8.2 (8.1)a | 3.52 | .049 |
| Δ Still-Face to Reunion | |||||
| HR | −6.4 (7.2)a | −2.8 (9.0) | 7.5 (9.7)a | 5.14 | .016 |
| TE | −.12 (.11) | −.05 (.09) | .03 (.14) | 3.50 | .05 |
| TI/TTOT | .01 (.02)a | .006 (.02) | −.02 (.03)a | 5.07 | .017 |
| %RC | −2.5 (5.0) | −1.2 (6.3) | 7.4 (14.2) | 2.74 | .089 |
Groups significantly different in follow-up Scheffé test.
Discussion
This is the first study to examine associations among maternal trauma exposure and related psychological symptoms in the perinatal period and infant cardiorespiratory reactivity in response to a laboratory stressor paradigm. Results indicated that maternal lifetime trauma exposure and elevated perinatal trauma impact were associated with diminished infant recovery from a mild laboratory stressor, as reflected in higher heart rate, respiratory dysregulation, and distress behaviors during the recovery phase of the paradigm. These results were maintained even after controlling for current maternal PTSD and depressive symptoms, suggesting that infant responses to the laboratory stressor were not solely due to current maternal functioning.
Consistent with previous studies, the Still-Face episode led to an increase in heart rate and behavioral distress in the sample as a whole, suggesting that the Still-Face acts as a stressor for infants at this age(39). Lower levels of maternal trauma and perinatal trauma impact were associated with decreases in heart rate and behavioral distress, though not necessarily back to baseline levels, during the recovery episode. Higher levels of maternal trauma and perinatal trauma impact, however, were associated with lack of decrease in heart rate or behavioral distress; in fact, the highest levels of maternal trauma and perinatal trauma impact were associated with increases in heart rate and behavioral distress from the stressor to the recovery episode. At the highest level of distress, which involved crying, heart rate was particularly elevated, and a number of respiratory parameters suggested pronounced increases in ventilation and/or thoracic breathing with expiratory emphasis. Infants categorized as “highly distressed” during the Reunion failed to return to a non-distressed state by the end of the episode and had mothers with significantly higher lifetime trauma and perinatal impact scores than infants who did not exhibit high distress during the Reunion.
Infants of mothers with higher trauma scores were also less responsive in respiratory timing parameters and generally breathed at a higher volume than infants of mothers with lower trauma scores. Furthermore, during the baseline Play episode, infants of mothers with higher trauma scores were breathing slower than infants of mother with lower trauma scores, but under challenge infants of mothers with lower trauma scores slowed their breathing markedly to values below those of infants of mothers with higher trauma scores. At the same time, infants across the range of maternal trauma increased their VT equally. The combination of comparably faster breathing with equal volume increases would theoretically lead to stronger ventilation in infants of mothers with higher trauma scores. However, although V’min was generally elevated in these infants, it did not increase disproportionately during the Still-Face challenge. Future research with larger samples should examine whether the observed pattern translates into a tendency for infants of mothers with high levels of trauma exposure to hyperventilate in response to stress. Notably, hypocapnia accompanying hyperventilation has been linked to a variety of acute and chronic illnesses(40). The overall higher VT in infants of mothers with higher trauma scores could also be a by-product of perinatal HPA-axis overactivity. Trials of perinatal corticosteroid therapy have been demonstrated to subsequently increase lung function and VT in infants(41), and the same may apply to endogenous hormones elevated secondary to maternal trauma. While no measures of infant anthropometry were available at the time of testing to determine whether larger body mass may have contributed to the association between high VT and elevated maternal trauma, perinatal corticosteroid exposure has been linked more frequently with restricted growth (41). Notably, birthweight, gestational age, and age at the time of assessment were not associated with the maternal trauma scores.
Several mechanisms may account for the noted associations. Previous research suggests that maternal stress may exert programming effects on offspring physiology and later health via prenatal undernutrition(1); however, this is an unlikely explanation for the current findings, given that birthweight less than 5.5 pounds was an exclusionary criterion. A more likely prenatal mechanism is disruption of the maternal/fetal HPA system and fetal exposure to nonoptimal levels of circulating glucocorticoids(8). Research into the effects of trauma exposure during pregnancy suggests that the development of PTSD in pregnant women is associated with changes in reactivity of both maternal and infant HPA rhythms(3). Such alterations are hypothesized to disrupt infant HPA system functioning through the stress-induced stimulation of placental secretion of corticotrophin-releasing hormone, which in turn is elevated in the neonatal circulation(2). This process may stimulate the fetal HPA axis to amplify glucocorticoid excess as well as activate additional elements of the fetal stress response, influencing the developing autonomic nervous system(42). Disturbed regulation of the autonomic system and HPA axis, in turn, are hypothesized to modulate offspring immune functioning, setting the stage for the development of various diseases later in life. Changes in normal HPA axis activity are also known to affect infant lung function(41), but much remains to be learned about perinatal interactions between the respiratory and endocrine systems.
These findings may also be related to postnatal effects, including the impact of trauma on maternal caregiving behaviors, particularly distress regulation behaviors. Trauma exposure and related psychopathology are associated with physiological, affective, and behavioral regulation difficulties that are hypothesized to cause serious disruptions to the formation and maintenance of social relationships, including the parent-child relationship(8). Infant crying may be especially challenging for traumatized mothers with limited self-regulation abilities. As a consequence, traumatized mothers may demonstrate particular difficulties in helping their infants recover from distress, and their infants, in turn, may fail to develop the skills necessary to regulate their own arousal to stressors. Through repeated experiences, these infants may develop expectations that their mothers will be ineffectual in assisting them in recovering from distress and, as a result, develop more extreme responses to stress (e.g. hypervigilance, rapid and intense crying)(43). Moreover, findings suggest that postnatal caregiving behaviors modify the effects of prenatal stress exposure in animals, though similar research with humans remains limited(44).
Other factors that may account for the observed effects include genetics, other unmeasured environmental exposures, and recall bias. Recent data suggest a genetic vulnerability to PTSD in a gene involved in the regulation of the HPA axis(45). Therefore, children of mothers susceptible to the impact of trauma may share an underlying genetic polymorphism involved in physiological stress regulation and health outcomes. Other negative environmental factors (e.g. financial strain, poor social support) may contribute both to mothers’ experience of trauma and infants’ stress reactivity . Finally, having a more reactive infant may lead to increased parenting stress for mothers which, in turn, may negatively bias their recall of trauma exposure and its impact.
Study Strengths and Limitations
While a number of studies link maternal trauma exposure to disrupted offspring HPA axis functioning, this is the first to document associations with altered infant cardiorespiratory reactivity. This is also the first study documenting feasibility of assessing infant cardiorespiratory reactivity in response to a psychological stressor using a respiratory inductance plethysmography device that provides a broad range of indicators to characterize respiration.
Despite the small sample size, observed effects emerged consistently. Testing for outliers and influential points did not change the inferences drawn from the fitted models. In the current analyses, no differences were found between dyads who did and did not complete the procedure. However, the failure of some dyads to complete the procedure may have been due to highly impaired stress regulatory abilities. Determining whether such a self-selection bias is present and, if so, whether membership in this group is related to maternal trauma may be more definitively examined in a larger sample. Also, though the current sample endorsed a variety of types of trauma exposure (e.g. accidents, disasters, interpersonal violence) across a range of developmental periods (early childhood, adolescence, adulthood), the current sample size did not allow for an examination of the potential effects of exposure type and timing in modifying the magnitude of impact on infant outcome.
Future research should explore the various prenatal and postnatal mechanisms that may account for the associations and identify the developmental windows when the fetus/infant is most vulnerable. Though maternal trauma may exert influence as early as the prenatal period, effects may not be stably observable until 6 months of age or later, when the prefrontal cortex begins to exert regulatory control over autonomic reactivity(46) and physiological and behavioral systems become increasingly organized and integrated. The influence of maternal behaviors may intensify during late infancy as the attachment relationship strengthens. Understanding whether, how, and when maternal trauma may influence infant outcomes will be critical to designing perinatal interventions that may help diminish vulnerability to developing a variety of physical and mental health disorders throughout the lifespan. In light of the high rate of trauma exposure in the general population and among childbearing women in particular, these results may have significant public health relevance(25).
Acknowledgments
The authors thank the parents and infants who participated in this study and the research staff who have made data collection possible. During preparation of this manuscript, the authors were supported by K08MH074588 (Bosquet Enlow) and R01 HL080674 (Wright). Laboratory assessments were made possible through use of the Harvard School of Public Health, Department of Society, Human Development, and Health’s Psychophysiology Laboratory, funded by the Taplin Foundation (PIs Laura Kubzansky, Ph.D. and Karestan Koenen, Ph.D.). The authors would like to thank Lauren Philbrook and Jessie Kang for assistance in data coding and Karestan Koenen, Laura Kubzansky, David Rosenfield, and Glenn Saxe for useful comments on earlier drafts.
Abbreviations
- PTSD
Posttraumatic Stress Disorder
- HPA
hypothalamic-pituitary-adrenal
- ECG
Electrocardiogram
- SFP
Still-Face Paradigm
- LSC-R
Life Stressor Checklist-Revised
- EPDS
Edinburgh Postnatal Depression Scale
- PCL-C
Posttraumatic Stress Disorder Checklist-Civilian Version
- HR
heart rate
- VT
tidal volume
- V’min
minute ventilation
- TI
inspiratory duration
- TE
expiratory duration
- TTOT
total breath duration
- TI/TTOT
respiratory timing
- %RC
percentage of rib cage contribution to tidal volume
- PhRIB
inspiratory thoraco-abdominal asynchrony
- PhREB
expiratory thoraco-abdominal asynchrony
- QDC
Qualitative Diagnostic Calibration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker DJ. A new model for the origins of chronic disease. Medicine, Health Care & Philosophy. 2001;4:31–35. doi: 10.1023/a:1009934412988. [DOI] [PubMed] [Google Scholar]
- 2.Seckl JR. Glucocorticoid programming of the fetus: adult phenotypes and molecular mechanisms. Molecular and Cellular Endocrinology. 2001;185:61–71. doi: 10.1016/s0303-7207(01)00633-5. [DOI] [PubMed] [Google Scholar]
- 3.Yehuda R, Bierer LM. Transgenerational transmission of cortisol and PTSD risk. Progress in Brain Research. 2008;167:121–134. doi: 10.1016/S0079-6123(07)67009-5. [DOI] [PubMed] [Google Scholar]
- 4.Wright RJ. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatric and Perinatal Epidemiology. 2007;21:8–14. doi: 10.1111/j.1365-3016.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 5.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 6.American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders, text revision (DSSM-IV-TR) 4th edition. Washington DC: American Psychiatric Press; 2000. [Google Scholar]
- 7.Cacioppo JT, Berntson CG, Malarkey WB, Kiecolt-Glaser JK, Sheridan JF, Poehlmann KM, Burleson MH, Ernst JM, Hawkley LC, Glaser R. Autonomic, neuroendocrine, and immune responses to psychological stress: the reactivity hypothesis. Annals of the New York Academy of Sciences. 1998;840:664–673. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- 8.Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Archives of General Psychiatry. 1993;50:295–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- 9.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood: A convergence of evidence for neurobiology and epidemiology. European Archives Psychiatry Clinics Neuroscience. 2006:3. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spangler G, Grossmann KE. Biobehavioral organization in securely and insecurely attached infants. Child Development. 1993;64:1439–1450. doi: 10.1111/j.1467-8624.1993.tb02962.x. [DOI] [PubMed] [Google Scholar]
- 11.Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biological Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- 12.Levendosky AA, Huth-Bocks AC, Shapiro DL, Semel MA. The impact of domestic violence on the maternal-child relationship and preschool-age children's functioning. Journal of Family Psychology. 2003;17:275–287. doi: 10.1037/0893-3200.17.3.275. [DOI] [PubMed] [Google Scholar]
- 13.Schechter DS, Zeanah CH, Myers MM, Brunelli SA, Liebowitz MR, Marshall RD, Coates SW, Trabka KA, Baca P, Hofer MA. Psychobiological dysregulation in violence-exposed mothers: Salivary cortisol of mothers with very young children pre- and post-separation stress. Bulletin of the Menninger Clinic. 2004;68:319–336. doi: 10.1521/bumc.68.4.319.56642. [DOI] [PubMed] [Google Scholar]
- 14.Hsu H, Porter CL. Young infants' behavioral reactivity to mild perturbation: Developmental continuity, stability, and organization. Infancy. 2004;6:95–120. [Google Scholar]
- 15.Doussard-Roosevelt JA, McClenny BD, Porges SW. Neonatal cardiac vagal tone and school-age developmental outcome in very low birth weight infants. Developmental Psychobiology. 2001;38:56–66. [PubMed] [Google Scholar]
- 16.Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal "brake" predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 17.Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosomatic Medicine. 2007;69:935–943. doi: 10.1097/PSY.0b013e31815a8f6b. [DOI] [PubMed] [Google Scholar]
- 18.Tulen HM, Bruijn JA, de Man KJ, van der Velden E, Pepplinkhuizen L, Man in 't Veld AJ. Anxiety and autonomic regulation in major depressive disorder: an explorative study. Journal of Affective Disorders. 1996;40:61–71. doi: 10.1016/0165-0327(96)00042-0. [DOI] [PubMed] [Google Scholar]
- 19.Ritz T, Thons M, Fahrenkrug S, Dahme B. The airways, respiration, and respiratory sinus arrhythmia during picture viewing. Psychophysiology. 2005;42:568–578. doi: 10.1111/j.1469-8986.2005.00312.x. [DOI] [PubMed] [Google Scholar]
- 20.Card JP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. Journal of Neuroscience. 2005;25:9102–9111. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward AM, Moore VM, Steptoe A, Cockington RA, Robinson JS, Phillips DI. Size at birth and cardiovascular responses to psychological stressors: evidence for prenatal programming in women. Journal of Hypertension. 2004;22:2295–2301. doi: 10.1097/00004872-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Ritz T, Dahme B, DuBois AB, Folgering H, Fritz GK, Harver AR, Kotses H, Lehrer PM, Ring C, Steptoe A, Van de Woestijne KP. Guidelines for mechanical lung function measurements in psychophysiology. Psychophysiology. 2002;39:546–567. doi: 10.1017.S0048577202010715. [DOI] [PubMed] [Google Scholar]
- 23.Grossman P. The LifeShirt: a multi-function ambulatory system monitoring health, disease, and medical intervention in the real world. Student in Health Technology & Infomatics. 2004;108:133–141. [PubMed] [Google Scholar]
- 24.Tronick EZ, Als H, Adamson L, Wise S, Brazelton TB. The infant's response to entrapment between contradictory messages in face-to-face interaction. Journal of the American Academy of Child & Adolescent Psychiatry. 1978;17:1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- 25.Breslau N, Davis G, Andreski P, Petersen E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48:216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 26.Wakschlag LS, Pickett KE, Cook EJ, Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. American Journal of Public Health. 2002;92:966–974. doi: 10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Testa M, Quigley BM, Eiden RD. The effects of prenatal alcohol exposure on infant mental development: A meta-analytic review. Alcohol and Alcoholism. 2003;38:295–304. doi: 10.1093/alcalc/agg087. [DOI] [PubMed] [Google Scholar]
- 28.Minde K. Prematurity and serious medical conditions in infancy: Implications for development, behavior, and intervention. In: Zeanah CHJ, editor. Handbook of infant mental health. 2nd ed. New York: Guilford; 2000. pp. 176–194. [Google Scholar]
- 29.Wolfe J, Kimerling R. Gender issues in the assessment of posttraumatic stress disorder. In: Wilson JP, Keane TM, editors. Assessing psychological trauma and PTSD. New York: Guildford; 1997. pp. 192–238. [Google Scholar]
- 30.Weathers FW, Huska JA, Keane TM. The PTSD Checklist - Civilian Version (PCL - C) 150 S. Huntington Avenue, Boston, MA 02130: F.W. Weathers, National Center for PTSD, Boston Veterans Affairs Medical Center; 1991. Available from. [Google Scholar]
- 31.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depression & Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 32.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 33.Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Research Triangle Park, NC: RTI-University of North Carolina Evidence-Based Practice Center; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adamson LB, Frick JE. The still face: A history of a shared experimental paradigm. Infancy. 2003;4:451–473. [Google Scholar]
- 35.Sackner MA, Watson H, Belsito AS, Feinerman D, Sauarez M, Gonzalez G, Bizousky F, Krieger B. Calibration of respiratory inductive plethysmograph during natural breathing. Journal of Applied Physiology. 1989;66:410–420. doi: 10.1152/jappl.1989.66.1.410. [DOI] [PubMed] [Google Scholar]
- 36.Dundas I, Beardsmore C, Wellman T, Stocks J. A collaborative study of infant respiratory function testing. European Respiratory Journal. 1998;12:944–953. doi: 10.1183/09031936.98.12040944. [DOI] [PubMed] [Google Scholar]
- 37.Sovik S. Quantifying infant respiratory variability: how to capture complexity. Acta Paediatrics. 2000;89:1401–1407. doi: 10.1080/080352500456525. [DOI] [PubMed] [Google Scholar]
- 38.Belsely DA, Kuh E, Welsch RE. Regression diagnostics: identifying influential data and sources of collinearity. Canada: John Wiley & Sons, Inc; 2004. [Google Scholar]
- 39.Haley DW, Stansbury K. Infant stress and parent responsiveness: Regulation of physiology and behavior during still-face and reunion. Child Development. 2003;74:1534–1546. doi: 10.1111/1467-8624.00621. [DOI] [PubMed] [Google Scholar]
- 40.Laffey JG, Kavanagh BP. Carbon dioxide and the critically ill-too little of a good thing? Lancet. 1999;354:1283–1286. doi: 10.1016/S0140-6736(99)02388-0. [DOI] [PubMed] [Google Scholar]
- 41.Durand M, Mendoza ME, Tantivit P, Kugelman A, McEvoy C. A randomized trial of moderately early low-dose dexamethasone therapy in very low birth weight infants: dynamic pulmonary mechanics, oxygenation, and ventilation. Pediatrics. 2002;109:262–268. doi: 10.1542/peds.109.2.262. [DOI] [PubMed] [Google Scholar]
- 42.Arck PC, Knackstedt MK, Blois SM. Current insights and future perspectives on neuro-endocrine-immune circuitry challenging pregnancy maintenance and fetal health. Journal Reproduktionsmed Endokrinol. 2006;3:98–102. [Google Scholar]
- 43.Warren SL, Huston L, Egeland B, Sroufe LA. Child and adolescent anxiety disorders and early attachment. Journal American Academy Child & Adolescent Psychiatry. 1997;36:637–644. doi: 10.1097/00004583-199705000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. Journal of Neuroscience. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koenen KC, Saxe G, Purcell S, Smoller JW, Bartholomew D, Miller A, Hall E, Kaplow J, Bosquet M, Moulton S, Baldwin C. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Molecular Psychiatry. 2005;10:1058–1059. doi: 10.1038/sj.mp.4001727. [DOI] [PubMed] [Google Scholar]
- 46.Chugani HT. Developmental aspects of regional brain glucose metabolism, behavior and plasticity. In: Dawson G, Fisher K, editors. Human behavior and the developing brain. New York: Guilford; 1994. [Google Scholar]