Abstract
The present work provides a comprehensive overview of the recent progress of research work toward developing new one dimensional (1-D) ceria (CeO2) nanomaterials. The review has been classified into three parts: the preparation procedures with identification of the existing different dimensional ceria nanomaterials, the formation mechanisms, and an analysis of their applications. From literature survey, it is inaugurated that the fundamental structures of the ceria nanomaterials constructively dominate their properties and applications. In addition, this work will also provide a perspective on the future technical trends for the development of different dimensional CeO2 nanomaterials.
Keywords: cerium oxide, nanotube, nanomaterials, one dimensional nanostructure, formation mechanism
1. Introduction
In the recent years, development of lanthanide compounds (Z = 57–71) such as ceria nanomaterials have been paid much attention. Nanoscale ceria materials can rapidly formed redox Ce4+/Ce3+ sites into their 4f shell of ions; assisting industrial applications [1–3]. Since such small dimensions possess specific surface areas and have excellent fundamental technological consequences, the preparations of one dimensional (1-D) nanostructures are widely attractive. Fabrication of nanocrystalline with the desired dimension and shape to provide effective activity and efficiencies for catalytic purposes is still an ultimate challenge in modern material research. In the past few years, well-defined 1-D ceria nanostructures with various morphologies such as nanorods, nanowires, nanotubes, nanopolyhedrons, etc., have been successfully fabricated by a variety of methods [1–15].
Nanoscale zero-dimensional ceria clusters could effectively provide individuality and have led to substantial advances in particle size, including nanoscale catalysis. In addition, nanoscale ceria materials of 1-D structure are represented with surface morphologies, allowing attractive applications for catalytic reactions. Moreover, ceria nanomaterial research has focused on the scheme of the physical treatment, based on the controlling of the reaction time, temperature, pressure etc. In this perspective, preparations of ceria nanomaterials generally fall into four basic steps: synthesis of precursors, treatment of precursors before conversion to oxides, conversion of precursors to mixed oxides, and post treatment of mixed oxide material. These methods have been used for preparing not only pure ceria but also for doped and mixed ceria nanomaterials. Some of these methods are included with the precipitation, sol-gel, thermo-decomposition, etc. Several ways have also been used in colloidal systems to obtain ceria nanomaterial, such as emulsion and microemulsion. On the other hand, in the presence of surfactants and polymers aiming to enhance physical or chemical properties such as surface area, sintering resistance, activity towards a certain reaction, etc, there is a particular focus to synthesized different 1-D nanomaterial to amplify their potential applications, which are covered in this review. This typical procedure for preparation and characterization of the surface properties of 1-D ceria nanomaterials has been adversely associated with the preparation mechanism and this is also described in this review.
2. Recent Works on the Preparation of 1-D CeO2 Nanostructures
Ceria is associated with rich oxygen vacancies and higher redox ability between Ce3+ and Ce4+, therefore promoted as a higher capacity oxygen storage material. In addition, 1-D cerium oxide nanorod, nanowire or nanotube (Ce–NT) draw attention due to their novel properties for the fabrication of nanodevices and unique atomic efficiencies with rapid response to changing condition in the catalytic system.
2.1. 1-D CeO2 Nanorod
The solvent composition, surfactant, and the cerium source precursor are of importance in the final product morphology [1–5]. The reaction temperature, concentration of the cerium precursor, and reaction time have significant influence on the yield of CeO2 nanorods [1,2]. According to Ho et al. [2], ethylene glycol-mediated synthesis had been widely used owing to the impact of three significant physical properties: (1) a high dielectric constant, which enhances the solubility of inorganic salts; (2) a high boiling point (195 °C at atmospheric pressure), which makes it possible to carry out the preparation of inorganic compounds at relatively high temperatures; (3) its strong reducing power. Additionally, Ho et al. also observed that a higher precursor concentration with lower reaction time provides spherical shaped cerium oxide and increasing the reaction time consequently extended the spherical shape into 1-D rod structures [2]. In-addition, with similar experimental conditions and a lower precursor concentration, they obtained the spindle shape nanostructure. On the other hand, Thang and coworkers [3] successfully obtained needle shaped nanostructures at an environment with higher amount of oxidizing agent and a higher concentration of the precursor. The surface area of the 1-D cerium oxide was increased significantly with the calcination, attributable to the higher temperature treatment initiating the crystallization into the nanostructures. Surfactant plays an effective role for the preparation of 1-D nanophase compounds and has been adversely observed in the past decades [1–11]. Vantomme et al. [4] and Pan et al. [5] reported the formation of CeO2 nanorods with a diameter of 10–25 nm at 80–160 °C by the presence of cetyltrimethylammonium bromide (CTAB). Pan and coworkers [5] also synthesized the CeO2 nanoplates by hydrothermal reactions with CTAB. They controlled the conversion of nanoplates into nanotubes and nanorods by using changing CTAB/Ce3+ ratio values, reaction time, and temperature. Similarly, Zhou et al. [6] obtained the CeO2 nanorods of 15–30 nm in diameter and lengths of up to tens of micrometers by a precipitation method combined with the hydrothermal treatment.
Later on, Huang et al. [7] synthesized Au/CeO2 nanorods with the wet chemical reducing system in the presence of NaBH4 solution as a reducing agent. They also observed that hydrothermal temperatures influenced the nucleation and crystal growth of the CeO2 nanorod. Morphological transformation of the nanorod was not completed with hydrothermal temperatures below 150 °C at 5 or 10 M KOH solution. Consequently, higher alkaline concentration provides thicker nanorod structures. Therefore, it would be considered that higher alkaline concentration is involved in increasing the width of the nanostructures rather than the nucleation of length of the samples. Similar to this approach, it was also confirmed for the formation of different shape of ceria oxide 1-D nanostructures with the presence of different concentrations of alkali [8]. At lower precipitation concentrations, the shape of nanopolyhedra, and at higher concentrations, a mixture of rod and polyhedral shapes were provided, respectively. On the other hand, the precipitant mainly formed the cubic and rod shape structure at higher temperature and higher concentration, respectively.
The one-step synthesis of CeO2 nanorods is still a challenge. In this case, ultrasonication methods have been successfully used to prepare nanorods. In the previous reports, the synthesis methods of CeO2 nanorods were relatively complicated and always needed high-temperature, high-pressure or long-time treatments [9–12]. In addition, Qi et al. [9] synthesized the thicker CeO2 microrod (200–250 nm in diameter and 600–1200 nm in length) by an ultrasonication process then surfactant assisted hydrothermal method. Furthermore, Zhang et al. [11] prepared 1-D ceria nanorods at room temperature in a one-step process through polyethylene glycol (PEG) surfactant and alkali solution. They confirmed that vigorous agitation without ultrasound at various temperatures (25, 40, and 60 °C) would form only nanoparticles as the sole products, even with a longer reaction time. Moreover, the concentration of the surfactant (e.g., PEG or CTAB) significantly affects the formation of 1-D nanorods [4,5,11]. Recently, Feng et al. [12] approached the microwave-hydrothermal method for the facile, rapid synthesis of higher yields of 1-D CeO2 with average sizes of ~1.6 nm to ~20 nm. Compared with a conventional hydrothermal method, the microwave-assisted hydrothermal method shows advantages of rapidity, convenience, cost-effectiveness and could be potentially extended to the synthesis of other nanoparticles and nanorods.
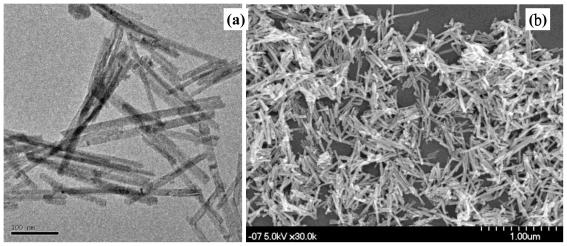
Recently, we have successively developed the CeO2 nanorod at a higher concentration of alkali (e.g., NaOH) solution and without surfactant with the well known hydrothermal method at 100 °C for 24 h. Morphology of the CeO2 nanorod is identified in the low-magnification transmission electron microscope (TEM) and field-emission scanning electron microscope (FE-SEM) images of Figure 1(a),(b), respectively. It was recognized that the 1-D CeO2 nanorod have a diameter of 20–40 nm and a length of 200–300 nm. Nanorod structure consisted of fluorite structure were confirmed with X-ray Diffraction (XRD) patterns after drying at 60 °C overnight and calcined at 300 °C for 3 h in the presence of air.
Figure 1.
(a) Transmission electron microscope (TEM) and (b) scanning electron microscope (SEM) images of ceria nanorods synthesized using a hydrothermal method.
2.2. 1-D CeO2 Nanowire/Nanofiber
Surfactants were frequently used for the fabrication of cerium oxide 1-D nanowire/nanofibers. Qizheng et al. [13] were the first to demonstrate the electrospinning technique for the formation of PVP/Ce(NO3)3 composite fibers. They fabricated the cerium oxide hollow nanofibers with calcining the composite fibers at 600–800 °C for 10 h. According to the FE-SEM microphotographs, the diameters of CeO2 hollow nanofibers (300 nm at 600 °C and 600 nm at 800 °C, respectively) were smaller than those of PVP/Ce(NO3)3 composite fibers (1–2 μm), with the length of greater than 50 μm. They observed, through TG-DTA and FTIR data analysis, that the calcination temperatures largely influenced the formation of CeO2 hollow nanofibers. In a typical procedure, Gu et al. [14] successfully synthesized mesoporous ceria nanofibers, nanobelts, and rodlike nanoparticles using a reverse micelle method. In addition, BET surface area and pore volume of the nanobelts (114.9 m2g−1 and 0.1470 cm3 g−1, respectively), were about twice as high as those of the nanofibers (54.41 m2g−1 and 0.09051 cm3 g−1, respectively). On the other hand, Tang et al. [3] simply used the hydrothermal method to achieve nanowires without the presence of surfactant. They observed that the presence of acidic precipitant H2O2 with 0.1 M Ce(NO3)3 produces the nanowire and nanocubes, whereas lower concentration of the precursor (0.05 M Ce(NO3)3) formed only nanowire diameters of 20–70 nm and lengths up to 40 μm in the hydrothermal process at 250 °C for 3 h. Furthermore, aggregated nanoneedles have been formed when the oxidizing agent H2O2 was absence and thus act as a template agent in this experiment. Nanowires were structurally uniform and single crystalline. The interplane distance in this research was obtained as 0.28 nm, corresponding to the separation between the (200) lattice planes of cubic CeO2. The ordered CeO2 nanowire arrays embedded in anodic alumina membranes (AAM) fabrication are also a novel technique. La et al. [15] and Wu et al. [16] fabricated CeO2 nanowires with a diameter of 60–70 nm by using AAM as templates. As showed in Figure 2, anions and cations are conversely migrated into the hexagonally ordered nanochannels of the AAM and are reacted inside the channels to form 1-D nanostructures.
Figure 2.
A schematic image of CeO2 nanowires formed by using anodic alumina membranes (AAM) as templates.
Sun et al. [17] synthesized CeO2 nanowires, 30–120 nm in diameter, by a precipitation method combined with thermostatic treatment using sodium bis(2-ethylhexy) sulfosuccinate (AOT) as a template. By using a similar method, Yan et al. [18] and Vantomme et al. [4] carried out the ceria nanowire preparation with the presence of easily available CTAB. Yada et al. [19] prepared different types of 1-D nanowire structures with the presence of different order alcohol and AOT as anions at 700 °C or above. In the presence of AOT, adding lower order alcohol such as alkyl or butyl alcohol and higher order alcohol (octyl or dodecyl alcohol) only produced nanowire and the nanoring shape nanowire (diameter of ~280 nm and width of ~80 nm), respectively. In a typical reverse micelles procedure, Gu et al. [14] successfully synthesized mesoporous ceria nanofibers at the lower aging temperature at 30 °C with a diameter of 50–200 nm and length of more than 50 μm with the presence of nonionic surfactant Triton X-100. On the other hand, nanobelts materials with length of a few tens of μm, widths ranging from 0.5 to 5 μm, and the thicknesses ranging from 20 to 100 nm have been prepared at the slightly higher aging temperature at 40 °C and constant time of 48 h. In addition, Yang and Guo [20] also employed octadcylamine (C18H37NH2) (cationic surfactants) as the structure–directing agent to synthesize CeO2 nanowires with a diameter of 10–25 nm. Tuning the ammonium acetate concentration through the precipitation method, Bugayeva et al. [21] controlled the particle size, shape, and agglomeration of the 1-D nanowire. The hydrated CeO2 nanowires as thin as 5 nm in diameter and nanoneedles with various aspect ratios were obtained via a chemical precipitation technique in the presence of ammonium acetate.
2.3. 1-D CeO2 Nanotube
Generally, the tubular structure itself may consist of higher thermal, chemical, and structural stability [22–26]. Various preparation conditions have been employed to synthesize 1-D Ce-NT materials, such as the use of different surfactants and templates, ultrasound treatment, hydrothermal method with different temperatures, aging effect, and acidic treatment. The template synthesis method is an effective way for preparation of the nanomaterials in the presence of polymeric filtration membrane and similar materials [22–25]. Yang et al. [23] also synthesized the fluorite-type Ce-NT with an outer diameter of 10–20 nm and inner diameter of 5–6 nm. Ce(OH)CO3 was attained by a hydrothermal method using Ce(NH4)2(NO3)6 as the Ce source, octadecylamine as a surfactant template, and urea as a precipitation agent. In addition, a higher temperature and higher concentration of CTAB as a surfactant were used for the synthesis of Ce-NT in the two-step procedure by Pan and coworkers [5]. In the first step, a higher concentration of the CTAB led to an increase in the absorption force between the CTA+ and Ce3+/Ce4+ ion pairs and accelerated the formation of lamellar sheet. In the second steps, Ce-NT was formed as a result of rolling up the lamellar sheets. Chen et al. [24] studied three different ways for the formation of ceria nanotube on the basis of the Kirkendall effect (denoted as K-type), Template (T-type), and lamellar rolling (L-type). The K-type Ce-NT had been prepared by congregating Kirkendall voids and subsequent calcinations were acquired in the presence of air at 600 °C for 4 h. In addition, T-type and L-type nanotubes had been obtained without any calcination. Precipitant and the reaction temperature are implicated in the formation of the K-type ceria nanotube.
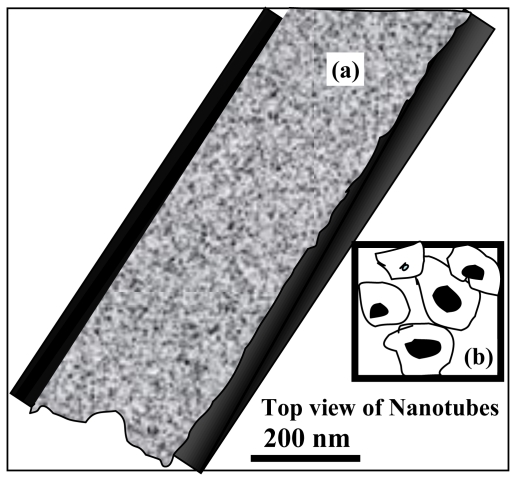
Carbon nanotube (CNT) as a template plays a significant role in the formation of 1-D ceria nanostructures. It was reported that the surface of the template was covered with ceria nanomaterials and possesses Ce-NT. In addition, higher temperature treatment was carried out for the removal of the templates [25–28]. The CNTs were refluxed in a mixture of concentrated KOH and NaOH at 450 °C and that could be coated with CeO2 for the formation of 1-D nanotubular structures [27,28]. The formation of Ce-NT is assisted with different methods just like ultrasonication, facile solvothermal method [25,26,29–31]. The preparation of Ce-NT is composed of several tiny interconnected nanocrystallites of about 10 nm in size. The pretreatment of CNTs and calcination temperature have been considered as crucial factors for determining the formation of Ce-NT. Metal ion doping is a promising technique to control the properties of material. Doping of metallic ion on the nanomaterials can influencethe surface morphology, nanocrystal shape, and growth in solution. Fuentes et al. [22] obtained the mixed Zr-Ce-NT oxide in the presence of polycarbonate film as a template through the microwave radiation at 800 W. Furthermore, Lu et al. [32] reported a route for the synthesis of Ce-NT within an AAM template (Figure 3).
Figure 3.
(a) Schematic microstructure of Ce-NT and (b) insert represented as the top-view of Ce-NT.
It is evident that complete and controlled conversion of CeO2 nanostructures through templates is not readily achievable. Additionally, fabrication and removal of the template have been achieved as very troublesome techniques for the Ce-NT synthesis process. Therefore, the formation of 1-D nanotubes with the absence of templates has been attractive owing to simple, quick, and economical considerations. On an important low-cost basis, Miao et al. [33] developed the procedure of ultrasound irradiations, in order to prepare Ce-NT from ceria nanoparticles at room temperature. In addition, Santos et al. [34] explained that the calcined temperature readily affected the crystallinity and morphology of the CeO2 nanostructure. Thus, the development of a facile and controllable formation of Ce-NT with proper crystalline structure is of great significance.
One of the most notable characterizations of the fluorite Ce-NT has been recently developed by a hydrothermal method. Han et al. [35] synthesized the yellowish CeO2−x nanotubes, nanowire, and nanoparticles in two steps. At the beginning, the samples were prepared at 100 °C in the presence of 7 mL of 5% ammonia hydroxide solution and then aged at 0 °C for 45 days. This procedure is time consuming. Tang et al. [36] proposed the lamellar rolling of the Ce(OH)3 crystal nanotubes through the alkali treatment of the trivalent ceria salt CeCl3 at 120 °C under an oxygen free environment with the hydrothermal method. They observed that 1-D Ce-NT was obtained from the annealing of Ce(OH)3 crystal nanotubes in the reducing atmosphere.
It was reported by Pan and coworkers [37] that cerium oxide nanorods are easily obtained under alkali treatment at room temperature. In addition, they explained that accumulation of the Ce3+ ion for 72 h on the cerium oxide nanorod surface would provide Ce-NT in the hydrothermal condition around 100 °C. As well, at increasing temperature the deposition of Ce3+ ion occurred at the tip of the nanorod and formed the nanowire and subsequently nanocubes. It was also shown that a larger surface area was achieved by the lower temperature nanorod preparation. However, this method is an effective way for the preparation of Ce-NT in the case of the template free controlled conversion system. Chen et al. [38] synthesized Ce-NT with a simple solid liquid interface reaction route in the absence of any surfactants by employing Ce(OH)CO3 nanorods as precursors.
As a synthesis of hydrothermal method, Zhou et al. [39] converted CeO2 nanorods into nanotubes in an acidic treatment like H2O2 solution assisted by ultrasonication. The converted Ce-NT has higher reducible property, which was due to the higher activity of CeO2 surface (100) than that of common surface (111) [39,40]. In addition, CeO2 nanorods consisted of Ce4+ as a surface material and Ce3+ as inside [39]. On the other side, Han et al. [35] obtained the opposite phenomenon, since the fraction of Ce3+ is significantly larger than that of CeO2−x nanoparticles with the same diameter. Thus, Ce3+ ions remained on the surface of the 1-D Ce-NT. Chen and coworkers [41], through the Kirkendall effect, obtained 1-D Ce-NT in which Zr4+ ions may act as the catalyst to promote the diffusion rate of Ce3+/Ce4+ ions inside the nanorod. According to a partial oxidation of Ce3+ ions and differential rate of diffusion between Ce4+ and Ce3+ ions inside the material, the metal hydroxide nanorods gradually decompose to form ZrxCe1−xO2 nanotubes. Furthermore, Martin et al. [42] used the atomistic simulation techniques based on the Born model of solids to observe multilayer Ce-NT with a wall thickness of 5.5 nm and a lumen diameter of 4.8 nm. Besides, the 1-D ceria nanostructure was achieved with the electrochemically-synthesized route through change of the electric field, strength, and direction by Fu et al. [43]. They acquired the morphologies of ceria nanomaterials from nanoparticles and nanorods to nanowire by simply changing the potential direction and time of anodic oxidation.
2.4. Other Types of 1-D CeO2 Nanostructure
Reverse micelles provide spontaneous self-assemble of surfactants in solution for the formation of nanorods. Kuiry et al. [44] reported that the cylindrical supra-aggregates and their subsequent growth occurred by preferential assembling of ceria nanorods along the longitudinal direction with the addition of AOT/toluene/water and H2O2/AOT/toluene/water microemulsions after a few weeks of aging. Such nanorods have an aspect ratio of 6 with a diameter of approximately 40 nm. In addition, according to the TEM analysis, it was proposed that the abrupt change in surface free energy in the micelle might form the cone-shaped portions at both ends of the nanorods. However, Tang et al. [3] proposed and explained that the concentration of an oxidant such as H2O2 would significantly affect to impose the cone type or needle like phenomenon in the 1-D cerium structure. On the other hand, Ge et al. [45] successfully used the emulsion liquid membrane system to synthesize CeO2 sponge-like rods with diameters of 170–810 nm and lengths of 5–10 μm, which were successfully fabricated through a route of liquid emulsion membrane followed by heat treatment.
3. Formation Mechanism of 1-D CeO2 Nanostructures
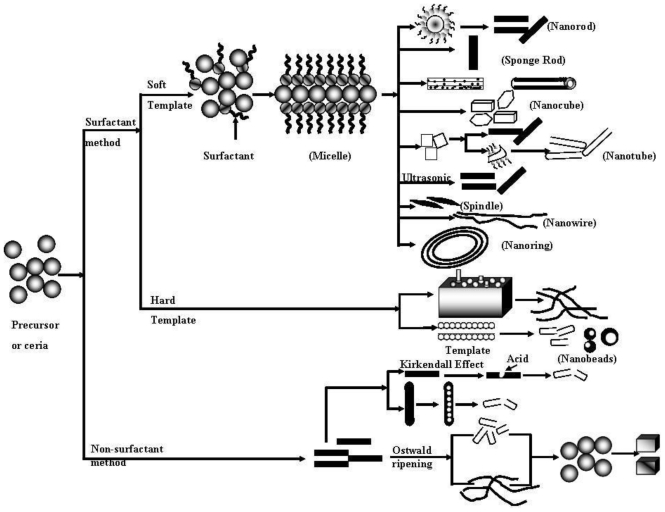
In recent years, literature data on the kinetics of 1-D ceria nanostructure has become plentiful. The preparation of the 1-D Ce-NT, nanorod or nanowire was thoroughly investigated by the surfactantassisted preparation and non-surfactant assisted Kirkendall coarsening; also known as the Ostwald ripening method as shown in Scheme 1. At the beginning, a famous paper by Terribile et al. [46], reported the first complete reaction mechanism of surfactant assisted 1-D Ce-NT nanostructure, and this is in essence still regarded to be valid. Afterwards, several fundamental preparation mechanisms assisted to obtain 1-D ceria nanostructure.
Scheme 1.
Details of the reaction mechanism pathways for the formation of ceria nanostructures.
3.1. Surfactant Assisted 1-D CeO2 Nanostructure Formation
Surfactant plays an important role in the preparation of ceria nanostructure. The reaction of cerium salts (either chloride or nitrate) under basic conditions with ammonia at room temperature results in the precipitation of gelatinous, hydrous cerium oxide. If the reaction is conducted in the presence of the “soft template” as cationic surfactants (i.e., alkyltrimethylammonium salts, CTAB, octadcylamine or ethylenediamine (C2H4(NH2)2)), hydrous cerium oxide can incorporate the organic molecule by exchange with surface OH− groups. This approach follows the observation that hydrous oxides can exchange either cations or anions, depending on the pH of the medium [19,46]. If the pH is higher than that of the isoelectric point of hydrous cerium oxide (6.75–8, depending on the environment) then incorporation of cationic surfactants takes place. The size and shape of the 1-D nanostructure is greatly influenced through the reaction time, reaction temperature and surfactant/Ce3+ ratio in the initial solution [5,17–23,46–47].
Scheme 1 shows the possible formation mechanism of CeO2 with different morphologies [5], in where surfactant (CTS+) is firstly absorbed on the surface of CeO2 nanoparticles (Equation (1)). The absorbed ligand molecules in the equation are likely to interact preferentially with the (111) surface plane to (100) at lower temperatures [4,40]. As pointed out here, Terribile et al. reported the first complete reaction scheme for 1-D ceria preparation in 1998 [46].
| (1) |
According to Terribile et al., the soluble isolated Ce3+ under basic conditions oxidizes to a hydrated Ce4+ formulated as Ce(H2O)x (OH)y (4−y)+ (Equation (2)), which then readily combines with the surfactant in accordance with reaction equation (3). This step can also be viewed as the two individual steps for the formation of polymeric hydrous oxide, which then reacts with the alkylammonium salt (Equations (3a) and (3b)) at a pH value well above that of the isoelectric point of ceria. Under these conditions, surfactant and the deprotonated hydroxy group form an inorganic/organic composite, which upon drying and calcination (Equations (4) and (5)) originates pure mesoporous cerium oxide with high surface area. According to reaction (3), they observed that the surfactant is able to promote oxidation of Ce3+ to Ce4+ and formation of hydrous oxide in solution, before drying. The presence of more surface Ce4+ atoms is a consequence of the smaller particles formed with the surfactants with a higher number of exposed Ce4+ atom.
| (2) |
| (3a) |
| (3b) |
| (4) |
| (5) |
Therefore, surfactant quantity easily affects the formation of the 1-D nanostructures and lower content of surfactants have been readily involved to produce smaller quantity of nanorods with a shorter length in size [4,18,20,40]. In addition, the exposed surfaces from the combination of the surfactant ceria surfaces tend to reduce the surface energy to form a cubic plane structure. A similar phenomenon for the formation of 1-D ceria nanomaterials was explained by Pan et al. [5] in 2008 (Scheme 1). They observed that the lower capping of the CTS+ forms the thicker ceria nanoplates and thus partially converts into nanorods. Furthermore, they considered a rolling strategy for the nanoplates to obtain nanotubes at higher capping of CTS+. The Cerium hydroxide, which combined by the hydrated Ce4+ ions with H2O molecules or OH− ions, polymerized at the micelles-solution interface and formed the nanowire structure clusters [20].
The distribution of the surfactant and template both significantly played an important role in the formation of nanowire and nanorod. According to Kuiry et al. [44], precursor is formed into nanoparticles by the process of nucleation through surfactant and growth occurs inside the cores of the micelles (Scheme 1). Hydroxyl ions are formed locally inside each micelle core as an intermittent product of the dissociation of hydrogen peroxide during the interaction (Equations (6) and (7)) [44].
| (6) |
| (7) |
The occurrence of this process is confirmed by color changes (colorless, violet, brown or yellow) during the reaction, which is consistent with a progressive variation of the redox state of cerium. The violet color is characteristic of poorly defined mixed valence hydrates intermediates, which finally give yellow Ce(IV) oxide. In the absence of surfactant, complete oxidation to Ce4+ occurs only at the drying stage when the precipitate turns yellow [46]. In addition, change of surface free energy in the micelle is readily accommodated to obtain the spindle shape nanostructures. The concentration of the oxidant such as H2O2 would have a significant effect to impose the cone type or needle like phenomenon in the 1-D cerium structures [2,3]. Dengsong et al. [11] explained the ultrasonication method for the formation of the ceria nanorod. In the presence of air and alkaline solution, Ce3+ oxidation state is unstable compared with the Ce4+ oxidation state, thus resulting in the formation of hydrated Ce4+ oxide (Equation (8)). Later on, CeO2 nanoparticles readily fuse with OH− ions through PEG. In addition, with the ultrasonication, the generated bubbles asymmetrically increase the collision between adjacent PEG adsorbed nanoparticles and as a result form the 1-D ceria nanorod in reaction Equation (9). It is well known that the strongly adsorbed stabilizer prevents the aggregation between colloidal nanoparticles due to its steric hindrance effect.
| (8) |
| (9) |
Recently, AAM (hard template) was fabricated by a novel technique in which anions and cations conversely migrated into the hexagonally ordered nanochannels of the AAM and reacted inside the channels to form 1-D nanostructures [15,16]. In the presence of a basic environment, Ce3+ ions are conversely transported into the nanochannels of the AAM by diffusion or convection. Additionally, precipitates with resultant morphology are obtained with the basis or conditions such like size, shape of the templates. Then, the precipitations with 1-D form decompose by oxidation (Equations (10) and (11)) and are translated into the single crystal CeO2 nanowires at 700 °C.
| (10) |
| (11) |
In 2009, Chen et al. explained the detailed reaction mechanisms for the formation of ceria nanotubes through the Ce(OH)CO3 nanorods using the reaction times [24]. They also extended the reaction with the appropriate amount of basic environment for Ce(OH)CO3 nanorods and that dissociated slowly to produce Ce3+, OH−, and CO32− ions in solution (Equation (12)).
| (12) |
| (13) |
| (14) |
| (15) |
The external surface of the Ce(OH)CO3 nanorods would lead to the formation of Ce(OH)3 (Equation (13)), due to the outward diffusion of Ce3+ in the Ce(OH)CO3 core being faster than inward diffusion of OH− ions in solution for different ionic radii and would prevent the diffusion of Ce3+ and OH− through the shell. In the presence of oxidizing agent, the Ce3+ ions present in the Ce(OH)3 nanostructures can be converted to a Ce4+ complex (Equation (14)). Thus, rod like ceria can be easily formed at the outer wall under the transformation of Ce(OH)22+ atoms in the solution. Additionally, Ce(OH)4 be dehydrated directly and simultaneously unreacted Ce(OH)CO3 completely decomposes during the afterward calcination process in the presence of air to form ceria (Equation (15)). Most of the outer wall is oxidized into ceria and 1-D Ce-NT would be formed. Owing to the early established voids that may lose surface atoms, thus the net inward flow of vacancies converges in a bigger space.
Similar to this approach, there is a template strategy with the presence of CNTs in the case of solvothermal and ultrasonic method [25–31]. During this process, CNTs are first treated by acid or pyridine, and then the surface acidic groups on the nanotubes can adsorb Ce3+ and form metal-oxygen bonds [25,30,31]. This phenomenon is observed due to the formation of noncovalent bond on to the surface of CNTs. In the case of the use of pyridine, an aromatic ring can be absorbed on CNTs by the π–π stacking interaction. Therefore, significant amount of water or alkaline solution (NaOH or KOH) may generate OH− around CNTs, and then OH− reacts with Ce3+ to produce nanoparticles [25–28,31]. CeO2 nanoparticles are absorbed on the CNTs to reduce the surface energy. With increasing time, more and more CeO2 nanoparticles absorbed on the CNTs form continuously the coating layer. The CeO2 nanoparticles fuse together for a steady structure under the solvothermal condition. Then, the as-prepared composites are heated at 450 °C in an air atmosphere for 30 min to remove CNTs. The diameter of ceria nanotubes was about 40–50 nm. According to the above carbon nanotube assisted results, the possible formation mechanism of the CeO2 nanotubes is proposed as shown in Scheme 1. It can be seen that the key steps involved in the formation of the CeO2 nanotubes are solvothermal modifications of CNTs and controlled calcinations. Considering the convenience of the procedure, this CNT template-assisted route is promising to extend to the preparation of CeO2 necklace-like hollow nanobeads [48]. CeO2 hollow nanobeads are 150–200 nm in outer diameter and 40–60 nm in inner diameter.
3.2. Non-Surfactant Assisted 1-D CeO2 Nanostructure Formation
A pronounced influence of nonsurfactant on the ceria nanostructure has been observed due to simple and economic concerns. Through thermal gravimetric analysis, Huang et al. [7] observed that in the basic environment with the presence of air, dehydrated ceria (CeO2·nH2O) nanostructure are deduced to be CeO2·1.1H2O (Equation (16)) and nanorods exhibit a light yellow color CeO2 due to the dehydration of the water (Equation (17)). The reaction steps are similar to those proposed by Dengsong et al. [11] in 2005 for the ultrasonication method.
| (16) |
| (17) |
Beside the above reaction, in 2008, Fu et al. [43] optimized the nanostructure morphology through changes of electric field and different potential sweep rates. Later on, Pan et al. [37] assisted the template free controlled conversion of ceria nanoparticles into 1-D nanostructures with changes in the reaction time and reaction temperature (Scheme 1). They explain the detailed mechanisms with coarsening; also know as Ostwald ripening through the particle size effect. A rate law for this process for the diffusion-limited particle growth in the solid and liquid state is developed by inserting the linearized Gibbs-Thompson equation into Fick’s first law as shown in equation (18). In the equation, r̄3 is the average particle size at time t, is the average initial particle size, and k is the rate constant.
| (18) |
In addition, ideal coarsening kinetics can be determined by equation (19), where, η is the viscosity of the solvent, a is the solvated ion radius, Vm is the molar volume, Cr=∞ is the equilibrium concentration, NA is Avogadro’s number, and γ is the surface energy.
| (19) |
The surface energy for the solid-vapor interface for metal oxides is of the order of 1 J m−2. On the other side, electrostatic and chemical interactions at the solid-liquid interface are expected to reduce the surface energy to values in the range 0.1–0.5 Jm−2. The difference in solubility, 5.23 × 10−12 mol L−1 for CeO2 and 4.85 × 10−6 mol L−1 for Ce(OH)3, greatly affects the rate constant and thus different nanostructures are obtained. They also observed that the change of precursor significantly affects the formation of the 1-D nanorods due to the effect of Ce3+ ions on the reaction mechanism. In addition, the change of rate constant for the oxidation of Ce3+ ions to Ce4+ ions readily increased the ceria nanotube formation. On the other hand, increases of the rate constant for the deposition of the Ce3+ ions on the tips of the nanorod results in the formation of CeO2 nanowire rather than nanotube structure and this phenomenon was previously confirmed by Huang et al. for the formation of nanorods [7]. Continuous rising of the temperature for the case of surface oxidation modifies the 1-D nanostructure into nanopowder with growth into nanocubes also reported. It is widely accepted that the Kirkendall effect controls experimental conditions for the formation of nanotube structures. Zhou et al. [39] carried out comparative experiments with the as-prepared 1-D Ce(OH)3 nanorod and H2O2 to clarify the mechanism for the formation of 1-D Ce-NT with the Kirkendall effect in 2007. They observed that both H2O2 and partial oxidation of Ce(OH)3 are essential for the formation of ceria tubular structure. In this, Ce3+ ions present in the Ce(OH)3 nanostructure can be converted to a Ce4+ complex (Ce(OH)22+) by hydroxyl free radicals in the H2O2 solution, thus Ce(OH)22+ transfers into the solution (Equation (20)). As the concentration of the Ce(OH)22+ increases, ceria can be formed easily under the Equation (21).
| (20) |
| (21) |
According to the simple hydrothermal method, Tang et al. [3] observed the precipitation mode together with the concentration of starting and acidic precipitant environment. This reaction environment played a significantly important role in the formation of the nanowire, confirmed by morphological analyses (Equation (20)). In addition, with the presence of an acid wash, the thickness of the shell and the interior space shrinks slightly, thus forming a hollow tubular nanostructure. Furthermore, Chen et al. [41] proposed that Zr4+ ions may act as the catalyst to promote the diffuse rate of Ce3+/Ce4+ ions inside the nanorods. Thus, the metal hydroxide nanorods gradually decompose to form ZrxCe1−xO2 nanotubes from ZrxCe1−x(OH)3 rod according to a partial oxidation of Ce3+ and differential rates of diffusion between Ce4+ and Ce3+ ions inside the material, see in Scheme 1.
4. Applications of Ceria Nanostructure Materials
The surface oxygen mobility through the lattice of the ceria is allowed to behave as an oxygen buffer and provides ceria an ultimate choice for an application based on the enhancement of the electrochemical phenomenon. In addition, enchantment of the oxygen mobility also varies with particle size and shape of the nanostructures. From the very beginning of nanomaterial research, it has been recognized that the size of the components is alterable. Additionally, shape and structure differences are attractive for several activities. The catalytic activity of many systems has been observed as structurally sensitive. Therefore, several industrial important reactions, including low-temperature CO oxidation, UV absorbing semiconductor materials, partial oxidation of hydrocarbons, hydrogenation of carbon oxides, and wastewater treatment, are exclusively affected by the structure of the nanomaterials.
4.1. UV-Vis Absorption
The optical property of absorbance of ceria in the UV region suggests that it can be used as a good candidate for UV absorbing semiconductor materials. To understand the correlation between the band gap energies and the grain size, the morphology of the ceria nanomaterials is important. The UV-Vis absorption spectra of 1-D like bulk nanomaterials, calcined CeO2 nanospheres, micro or nano rods, and spindle-like particles are recorded in several research works, represented in Table 1. In the past decade, film type ceria structure has been frequently investigated to understand optical properties in the UV-Vis region. The optical band gap Eg can be determined from the absorption coefficient according to the solid band theory for a semiconductor and is given by α(hν)n = constant (hν − Eg), where hυ is the photo energy, α is the absorption coefficient constant is relative to the material. The optical absorption coefficient α was calculated from k (extinction coefficient) value using α = (4πk/λ), according to the following equation: α = (2.303 × 103Aρ)/lc, where A is the absorbance of the sample, ρ is the real density of CeO2 (7.28 g cm−3), l is the path length of the quartz cell (1 cm), and c is the concentration of the ceria suspensions. The dependence of the absorption coefficient (α(hν)) relates to the energy of the incoming photons (hν) in the case of materials and n is either 2 for a direct transition [Ed] or 1/2 for an indirect transition with an indirect band gap [Ei].
Table 1.
Details of the ceria nanomaterials UV-Vis absorption analyses.
| References | Preparation Procedure | Sample | Band Gap a(eV) | |
|---|---|---|---|---|
| Ed | Ei | |||
| e[2] | Polyol | Polycrystalline CeO2 | 3.19 | N.A. |
| CeO2 nanospheres (80–100 nm), | 3.46 | |||
| Microrods (d WD several100 nm; d L 15 to 20 μm, d AR 25 to 33), | 3.62 | |||
| Spindle-like (d WD several 100 nm, d L 2 to 4 μm, d AR 4-8) | 3.36 | |||
| [49] | Hydrothermal | Spindle like (d WD 800 nm and d L 5 μm) | 3.55 | N.A. |
| [50] | Hydrothermal | CeO2 prism-like mesocrystal Bulk CeO2 |
3.02 3.19 |
N.A. |
| [51] | Spray pyrolysis | CeO2 films (cerium chloride) (cerium nitrate) | 3.6 3.53 |
N.A. |
| [52] | Electron beam evaporation; Ion beam assisted deposition | Nanostructured CeO2−x | 3.48 | 3.18 |
| e[11] | Ultrasonication | CeO2 nanorods (d AR 10 to 15:1, d L 50–150 nm) | 2.9 | 2.67 |
| [53] | Microemulsion | Ceria ultrafine nanostructure | 3.44 2.6 |
2.87 2.73 |
| [54] | Pulsed electron beam | CeO2 nanocrystalline films | N.A. | 2.58 |
| [56–58] | Physical vapor-deposited | CeO2 films | N.A. | 3.15–3.5 |
| [59] | Spray deposition | CeO2 films | N.A. | 3.06–3.08 |
| [60] | Sol-gel method | CeO2 films | N.A. | 3.03–3.07 |
Notes:
According to the solid band theory for a semiconductor (hν)n = constant(hν – Eg), where hυ is the photo energy, α is the absorption coefficient, constant is relative to the material, Eg is the band gap;
Ed: Band gap energy for direct transitions in where n = 2; Ei: Band gap energy for indirect transitions in where n = 1/2;
AR = aspect ratio; L = length; N.A. = not available; WD = width;
Surfactant method.
The optical direct band gap energy for different 1-D nanostructures was observed by Ho et al. [2] through UV-Vis spectroscopy to understand the morphological applications of CeO2 nanostructures. Compared to the no-noriented polycrystalline CeO2 (Ed = 3.19 eV), CeO2 nanospheres (80–100 nm), microrods (width several 100 nm; length 15 and 20 μm, aspect ratio (AR) = 25–33), and spindle-like structures (width several 100 nm, length 2–4 μm, AR = 4–8) were prepared through polyol process and showed an increase in Ed by a value exceeding 0.27, 0.43, and 0.17 eV, respectively. According to Zhang et al. [49], in the hydrothermal method, spindle-like 1-D nanostructures with particle diameters of about 800 nm and lengths up to five micrometers have a direct bandgap of 3.55 eV. The absorption spectrum indicates that the CeO2 spindle is allocated with fairly larger band in different preparation methods for the implication of the particle sizes [2,10,49]. In addition, hydrothermally synthesized prism-like mesocrystal [50] CeO2 sample exhibit the direct band gap of 3.02 eV, which is smaller than the value for the bulk CeO2 (Eg = 3.19 eV). It could be ascribed to the coexistence of abundant defects in such prism-like mesocrystal CeO2.
Furthermore, Elidrissi, and co workers [51] prepared the CeO2 films by using two different kind of solution precursors (cerium chloride and cerium nitrate as the sources of cerium ions) to reveal the optical transmission properties in the spray pyrolysis procedure. The optical properties of CeO2 thin films are determined from transmission and reflection measurements in the range of 0.3–2.5 μm. Both films exhibit a transmittance above 80% in the visible and near-infrared region with a sharp absorption edge to approximately 350 nm. The direct band gaps of the films prepared by cerium chloride and cerium nitrate are 3.6 and 3.53 eV, respectively. The difference observed in the band gap resulted from the two different sources of cerium ions. Moreover, Charitidis et al. [52] grew the nanostructured CeO2−x films through electron beam evaporation (EBE) and ion beam assisted deposition (IBAD) consisting of grain sizes of 9–28 nm. They kept the nanoscale voids to enhance the surface and quantum-size effect. The optical properties of the CeO2−x sample locate at least ~0.3 eV difference between indirect and direct band gaps. According to Zhang et al. [11], 1-D CeO2 nanorods were readily synthesized through ultrasonication procedure in the presence of polyethylene glycol. The aspect ratio of the CeO2 nanorods were 10 to 15:1, and the length of the nanorods were 50–150 nm in length, with (111) and (220) lattice fringes of 0.31 and 0.19 nm, respectively. In addition, the direct transition band energy (Ed) of 2.90 eV and the indirect band gap energy (Ei) of 2.67 eV for CeO2 nanorods were observed. Additionally, the 1-D CeO2 nanoparticles synthesized using the microemulsion method were consistent with (Ed is 3.44 eV for 2.6 nm and 3.38 eV for 4.1 nm, while Ei is 2.87 eV for 2.6 nm and 2.73 eV for 4.1 nm, respectively) several particle size effects for the band gap analyses [53].
According to Tatar et al. [54], the refractive index was determined as 1.8–2.7 in the photon energy interval from 3.5 to 1.25 eV with the optical model. In addition, the optical indirect band gap (Ei) of CeO2 nanocrystalline films was calculated as 2.58 eV. The calculated indirect band gap values were lower than the band gap values of other physical vapor-deposited CeO2 films (3.15–3.5 eV) [55–57]. However optical band gap values for the spray deposited ceria films are smaller than those of the films prepared by magnetron sputtering at about 800 °C (Eg ~3.30 eV) [58]. The differences observed in the band gap values of nano- and micro-crystalline ceria were attributed to the presence of increased oxygen vacancies in the nanocrystalline structure of the ceria, which led to a distortion of the local symmetry. According to Gallage et al. [59], ceria films on glass substrates showed high transparency with more than 70% transmittance (85% with respect to the glass substrate) in both visible and infrared regions. The values of the optical band gap for all ceria films are ~3.06–3.08 eV. These values are comparable to the values of ceria films prepared by the sol-gel method at 450 °C (3.03–3.07 eV) [60]. Both sol-gel deposited films and spray deposited films at low temperatures have a smaller grain size with random orientation. Therefore, it can be suggested that the higher concentration of grain boundaries is responsible for the broadening of absorption edge and apparent shift towards the lower energy of the optical band gap. Patsalas et al. [61] observed the correlation of the indirect optical band gap with their microstructures and composition of nanocrystalline (grain of 8–40 nm) ceria film prepared by EBE at room temperature and 950 °C. Furthermore, they showed that Eg was decreased by increasing Ce3+ ion content in EBE film. Several data have revealed that Ed and Ei decrease with the increasing size of CeO2 nanoparticles owing to the quantum confinement effect [49,52]. In addition, Zhang et al. [10] explained that the band gap decreased from 3.95 to 3.86 eV as the reaction temperature increases from 500 to 800 °C. Therefore, reaction temperatures significantly affect the band gap for the 1-D nanostructures. Furthermore, precursor as the sources of cerium ions may affect the band gap properties. Although the detailed reasons are not clear for the increases of the band gap in the nanoparticles, it would be concerned with the size effects [10,11,49–60].
4.2. UV-Vis Absorption Shift Phenomenon
Absorption and emission spectra of nanomaterials are readily assisted to ensure an overview of particle size and internal morphology. Theoretically, absorbance band edge shifts towards the shorter wavelength is demonstrated as a blue-shift [2]. On the other side, red-shift is characteristic of the electron–phonon coupling phenomenon [62]. The size of the particle is readily influenced by the quantum confinement consequences [2]. It is well known that decreasing size of materials increases with the electron-phonon-coupling coefficients. In certain systems, electron-phonon coupling could be strong enough to overcome the spatial confinement to determine the energy of excitons. It determines or modifies the effective mass of carriers and the style of carrier scattering by the lattice, leading to a red-shift of the emission band.
The blue-shifting phenomenon in the UV absorption spectra of CeO2 nanocrystals has attracted the interest of many researchers in recent years [2,11]. Generally, the absorption of ceria in the UV region originates from the charge-transfer transition between the O 2p and Ce 4f states in O2− and Ce4+. This spectral profile indicates that charge-transfer transition of Ce4+ overlaps with the 4f1 → 5d1 transition of Ce3+, which overrun the well-known f to f spin-orbit splitting of the Ce 4f state [2,11,52,63]. According to Guo et al. [64], ultraviolet blocking materials, CeO2 single/multiwall hollow microspheres have strong absorption properties in the ultraviolet range. As the shell thickness increases from 20 to 50 nm, the absorbing boundary of CeO2 hollow microspheres is blue-shifted from 450 to 430 nm. A clear blue-shifting of the absorption threshold edge can be observed for the CeO2 nanospheres and microrods, contrasting with the bulk powder, due to the decrease of particle sizes [2,49] and can also be affected by temperature [10]. Normally, nanophase crystallinity is expected to lead to blue-shift effects because of quantum confinement. However, the red-shift of the absorption bands of CeO2 nanorods, nanoneedles, prism-like mesocrystal and single/multiwall hollow microspheres have specific oriented aggregation of individual nanostructures contributing to the existence of a large number of defects [1,50,62]. In addition, the red-shift effect observed in the nanocrystalline ceria would be explained by the formation of localized states within the band gap owing to oxygen vacancies and increase Ce3+ ion concentration. This phenomenon is due to the shift of absorbance band shift towards the longer wavelength [50,52].
4.3. Carbon-monoxide Oxidation Phenomenon
In recent years, oxidation catalysts have received considerable attention because of their potential role in the environmentally important fuel cell technologies. As an important component in catalysts, ceria promotes high oxygen storage capacity (OSC) and high oxygen ion conductivity. Several morphological structures of CeO2 such as nanorod, nano-sponge single or multiwall, hollow structure, mesoporous, spindle etc., have been investigated widely for the selective oxidation of mainly carbonmonoxide, nitrogen oxides, sulfur oxide, and so on, due to OSC of ceria. In addition, surface area, structural defects, and oxygen vacancy have a positive effect on CO oxidation [2]. The formation of oxygen vacancy can be expressed by the following equation (22):
| (22) |
where V(O,s) represents an empty position (anion-vacant site) originating from the removal of O2− from the lattice. Charge balance is maintained by the reduction of two cerium cations from +4 to +3. The radius of the Ce3+ ion (1.14 Å) is larger than that of Ce4+ (0.97Å) and hence the lattice expansion is a consequence of the reduction of Ce4+ ions to Ce3+. There is a gradual decrease in the concentration of oxygen vacancies extended from the surface to the bulk. Such gradient enables the outward diffusion of lattice oxygen to the surface. Therefore, the reduction of Ce4+ to Ce3+ by oxygen ion leads to the generation of surface oxygen vacancy. These oxygen vacancies can act as promoting sites for NO and CO conversion [62–70].
The catalytic performance of the 1-D CeO2 nanomaterials is affected by the structure and surface area as shown in Table 2. Zhang et al. compared the two different kinds of 1-D nanomaterials to exhibit CO oxidation, where they derived that CeO2 single/multiwall hollow microspheres may provide CO total conversion at 230 °C and for bulk CeO2 at 500 °C [62]. Hollow microspheres afford more available oxygen and oxygen deficiency for CO oxidation [48,62,66]. In addition, high catalytic activity on CO oxidation obtained for CeO2 single/multiwall hollow microspheres was consistent with similar activity at 240 °C for T100 in the second and the third runs, which revealed its excellent thermal stability and recycling performance [62]. Similar tendency of the CO oxidation was followed for the hollow nanobeads and hollow nanocubes [48,67]. CNT templates in the CeO2 hollow nanobeads may be formed of CeO2−xC and thus increase the catalytic activity [48]. According to Chen et al. [68], the CO conversion of CeO2 hollow nanocubes is 56% and almost 3.5 times higher than that of the CeO2 powder at 270 °C. They explained that the interconnected hollow structure enables better contact with the gas molecule owing to the existence of interior spaces and penetrable shells, therefore exhibit better performance. The stability and recycling performance of CeO2 catalysts are important factors for the practical applications.
Table 2.
Carbon-monoxide oxidation effect on several ceria nanostructures.
| References | Sample | T50 [°C] | T100 [°C] | BET [m2g−1] | Remarks |
|---|---|---|---|---|---|
| [54] | CeO2/Al2O3 | 270 | N.A.d | 165 | Microemulsion method provides higher catalytical activity |
| Microemulsion | N.A. | N.A. | 73 | ||
| CeO2/Al2O3 | 320 | N.A. | 167 | ||
| Coprecipitation | N.A. | N.A. | 73 | ||
| [62] | CeO2/single multiwall | 210 | 230 | 44.9 | In the second and third run, provides 100% conversion at 240 °C. |
| [67] | CeO2 hollow | 265 | N.A. | N.A. | Similar conversion provided at the second run. |
| Commercial | >300 | N.A. | N.A. | ||
| [69] | Mesoporous CeO2 with | N.A. | 220 | N.A. | Higher content of the CuO may alter the surface to volume ratio of the catalyst and affect the gas transfer. |
| [70] | CuO | N.A. | N.A. | N.A. | |
| Bulk CeO2 | N.A. | 500 | 141 | ||
| Nano CeO2(NC) | 435 | N.A. | N.A. | ||
| 2%Cu-NC | 166 | N.A. | 107 | ||
| 10%Cu-NC | 148 | N.A. | 131 | ||
| 20%Cu-NC | 150 | N.A. | 118 | ||
| [26] | Bulk CeO2 | >300 | N.A. | 5.67 | Ceria nanotubes are more active than the ceria nanoparticles and bulk ceria due to large surface area. |
| CeO2 nanoparticle | 298 | N.A. | 30.33 | ||
| CeO2 nanotube | 205 | 275 | 83.15 | ||
| e[5] | Nanoplate | 215 | >300 | 37.2 | Crystal plane (100) greatly affects the oxidation. |
| Nanorod | 273 | >340 | 52.5 | ||
| Nanotube | 264 | >325 | 80.1 | ||
| e[2] | CeO2-nanoparticle | 295 | 380 | N.A. | BET surface area increases after the calcination at 400 °C and that may influence the conversion. |
| Spherical | 284 | 315 | 40.3 | ||
| Rods | 265 | 315 | 67.8 | ||
| Spindle | 250 | 300 | 67.4 | ||
| [6] | CeO2 nanorod | N.A. | 275 | 50.1 | N.A. |
| [45] | CeO2 nanoparticle | N.A. | 300 | 62.4 | |
| CeO2 sponge rod | 190 | 205 | N.A. | ||
| [70] | Au/CeO2 nanorod | N.A. | >220 | N.A. | Au-supported nanoparticle provides better conversion due to the thermal stability. |
| CeO2 nano particle | >220 | N.A. | N.A. | ||
| CeO2 nanorod | >220 | N.A. | N.A. | ||
| Au/CeO2 nano particle | N.A. | 160 | N.A. | ||
| [48] | Ceria nanobead | 240 | 300 | 87.5 | CNT templates in the CeO2 hollow nanobeads may be formed from CeO2− xC. |
| Ceria nanoparticle | >300 | N.A. | 5.7 | ||
| f[37] | Nanoroda | 290 | N.A. | 128.2 | Possesses enough aging time to increase BET surface area and consequently affect oxidation process. |
| Nanorodb | 224 | N.A. | 115.9 | ||
| Nanoparticle | 305 | N.A. | 105.1 | ||
| Nanowire | 245 | N.A. | 79.8 | ||
| Nanotube | 223 | N.A. | 98.2 | ||
| Nanocubec | 315 | N.A. | 3.5 | ||
Notes:
CeO2 nanorods synthesized at 20 °C for 24 h;
CeO2 nanorods synthesized at 20 °C for 9 d;
CeO2 nanocubes synthesized at 180 °C for 24h;
“N.A.” denotes “not available”;
Surfactant method;
Nonsurfactant method.
According to TEM analyses, they demonstrated that the hollow structure does not collapse at high temperature (300 °C) and the catalytic operation was conducted after the reactor cooled down to room temperature, which demonstrated its excellent stability and recycling performance [66,68]. On the other hand, the overall catalytic activity and the BET specific surface area are affected by the preparation method of the catalyst [5,53]. Masui et al. [53] reported that the CeO2/Al2O3 catalyst prepared by the microemulsion method shows higher activity for carbon monoxide oxidation, despite the fact that the CeO2/Al2O3 catalyst surface area is as low as that prepared by the co-precipitation method. Pan and coworkers [5] explained that nanomaterials consisting of similar BET specific area greatly influence the crystal surface to represent the catalytic activity. They also observed that CeO2 nanorods, nanoplate, and nanotubes exhibit higher BET surface area: 52.5, 37.2, and 80.1 m2g−1 respectively, as well nanoplates, consistent with higher crystal surface (100), and that contributes to create enormous oxygen vacancies, thus favors the higher catalytic performance.
Regarding the several types of nanostructures such as spindle, rod etc., the effect of carbon monoxide oxidization of CeO2 was revealed by Ho et al. [2]. The spindle-like sample shows the highest CO conversion rates 0.861 μmol g−1 s−1, which is almost 4.5 times that of CeO2 particles (as referred), 0.189 μmol g−1 s−1. According to XRD analysis, they observed the order of the lattice cell volume was strongly related to the degree of Ce4+ reduction and the extent of oxygen vacancy. Interestingly, the surface area and pore volume of the samples significantly increased after calcinations and affected the CO oxidation. The same result for the effects of surface area was also demonstrated for the ceria nanorod and sponge nanorod [6,45]. Zhou et al. [6] attained that CeO2 nanorods are three-times more active than CeO2 nanoparticles for CO oxidation and found that the T100 (the temperature at which the CO conversion is 100%) for the CeO2 nanorods and CeO2 nanoparticles catalysts approach to 275 and 300 °C, respectively. In addition, using CeO2 sponge-like rods as a catalyst, the T100 is only 205 °C, which shows that catalytic property of CeO2 sponge-like rods has an advantage over that of CeO2 nanorods and CeO2 nanoparticles [45]. The sponge nanorod may provide a larger percentage of atoms onto the surface and would create structural defects and generate pronounced oxygen vacancies than nanorod or nanoparticles [6,45,68]. Similar tendency is also observed for the case of ceria nanotube [26], therefore it could provide the three times higher catalytical activity than bulk ceria and ceria nanoparticle.
Recently, Pan et al. [37] explained that 1-D ceria nanorods synthesized at low temperature with enough aging time can possess a large BET specific area and thus provide a perfect crystalline form and have high performance for CO oxidation. The physical and chemical properties of ceria can be tuned by doping with different metals to obtain low-temperature reducibility (Au, Cu, Pr and Sn). Metallic doping with tetravalent cations, (such as Zr and Hf) onto the ceria nanostructures may enhance the OSC and consequently archive high ionic conductivities with trivalent cations (such as La, Sm, Gd, and Y) [69–71]. Sunder et al. [69] observed that the catalytic activity of the CO oxidation with Cu-CeO nanocomposite can significantly increase due to the addition of CuO. Similar research was also conducted by Sun et al. [70], who observed that the quickly accelerated CO conversion starts below 120 °C, and complete CO oxidation is achieved at about 220 °C over the catalysts containing more than 10 wt% CuO on to the 3-D flower-type CeO2 nanomaterials. The performance of the flowerlike CeO2 microspheres loaded with 20 wt% CuO became worse and the 15 wt% CuO sample had the best catalytic activity for CO oxidation. The activity may be affected by higher CuO content or the surface-volume ratio of the catalyst [69,70].
The role of the support and the oxygen supply for the catalytic reaction remain controversial. Although it is accepted that factors, such as gold particle size, synthesis method, pretreatment conditions, and support, influence the reactivity of the supported gold catalysts, the nature of the active sites and the reaction mechanism for CO oxidation are still subjects of debate. According to Raman spectroscopic analyses, Guzman et al. [71] indicated that nanocrystalline CeO2, in the presence of gold catalyst, supplies reactive oxygen in the form of surface η1 superoxide species and peroxide adspecies. The conventionally precipitated CeO2 tends to stabilize O2δ− (0 < δ < 1) adspecies and molecular O2 on to the surface. Thus, both cationic and metallic gold are attributed in nanocrystalline CeO2 to accelerate CO oxidation at low temperatures. The formation of the surface chemisorbed oxygen species can be facilitated by defects in the catalyst structure. Therefore, Sun et al. [70] demonstrated that 2.77 wt% Au-loaded flower-like CeO2 microsphere catalysts highly active with CO gas conversion into CO2 above 80% at room temperature and T100 is observed at 130 °C. On the other hand, around 81% CO conversion is achieved at 220 °C for Au/CeO2 nanorods as a catalyst, while only 20–22% CO conversion is obtained at the same temperature for pure CeO2 nanorods and nanoparticles as a catalyst [7]. The catalytic activities of Au/CeO2 flower, nanorods, and nanoparticles are much higher than that of pure CeO2 nanorods and nanoparticles, consequently [7,70].
5. Conclusions
Ceria nanomaterials have received attraction in the past decade due to their effective applications in the fields of environmental protection and in semiconductor industries. One-dimensional ceria nanostructures have reached such potential owing to their size, shapes and crystallographic behaviors. Due to their preparation procedure and preparation mechanism, different 1-D ceria nanostructures can be accomplished. To improve the properties of the ceria nanomaterials in terms of environmental and other issues, an enormous amount of reaction mechanisms and preparation procedures have been developed. So far, correlations between the details of ceria nanomaterial preparation and the mechanisms of the 1-D nanomaterial have not been established. Therefore, an overview of several 1-D ceria nanomaterials like nanorod, nanowire/nanofiber, nanotube etc., and the preparation mechanisms and applications are provided in the present work, and should facilitate the choice of the right type of ceria for a specific application, as well as to provide a better understanding for designing new ceriabased materials with the desired properties.
Acknowledgements
The financial supports of National Science Council (Contract No.: NSC–97–2221–E–155–005) of Taiwan are gratefully acknowledged.
References
- 1.Sun C, Li H, Zhang H, Wang Z, Chen L. Controlled synthesis of CeO2 nanorods by a solvothermal method. Nanotechnology. 2005;16:1454–1463. [Google Scholar]
- 2.Ho C, Yu JC, Kwong T, Mak AC, Lai S. Morphology-controllable synthesis of mesoporous CeO2 nano-and microstructures. Chem. Mater. 2005;17:4514–4522. [Google Scholar]
- 3.Tang B, Zhuo L, Ge J, Wang G, Shi Z, Niu J. A surfactant-free route to single-crystalline CeO2 nanowires. Chem. Commun. 2005;28:3565–3567. doi: 10.1039/b500708a. [DOI] [PubMed] [Google Scholar]
- 4.Vantomme A, Yuan ZY, Du G, Su BL. Surfactant-assisted large-scale preparation of crystalline CeO2 nanorods. Langmuir. 2005;21:1132–1135. doi: 10.1021/la047751p. [DOI] [PubMed] [Google Scholar]
- 5.Pan C, Zhang D, Shi L. CTAB assisted hydrothermal synthesis, controlled conversion and CO oxidation properties of CeO2 nanoplates, nanotubes, and nanorods. J. Solid State Chem. 2008;181:1298–1306. [Google Scholar]
- 6.Zhou K, Wang X, Sun X, Peng Q, Li Y. Enhanced catalytic activity of ceria nanorods from well-defined reactive crystal planes. J. Catal. 2005;229:206–212. [Google Scholar]
- 7.Huang PX, Wu F, Zhu BL, Gao XP, Zhu HY, Yan TY, Huang WP, Wu SH, Song DY. CeO2 nanorods and gold nanocrystals supported on CeO2 nanorods as catalyst. J. Phys. Chem. B. 2005;109:19169–19174. doi: 10.1021/jp052978u. [DOI] [PubMed] [Google Scholar]
- 8.Mai HX, Sun LD, Zhang YW, Si R, Feng W, Zhang HP, Liu HC, Yan CH. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes. J. Phys. Chem. B. 2005;109:24380–24385. doi: 10.1021/jp055584b. [DOI] [PubMed] [Google Scholar]
- 9.Qi RJ, Zhu YJ, Cheng GF, Huang YH. Sonochemical synthesis of single-crystalline CeOHCO3 rods and their thermal conversion to CeO2 rods. Nanotechnology. 2005;16:2502–2506. [Google Scholar]
- 10.Zhang DE, Ni XM, Zheng HG, Zhang XJ, Song JM. Fabrication of rod-like CeO2: Characterization, optical and electrochemical properties. Solid State Sci. 2006;8:1290–1293. [Google Scholar]
- 11.Zhang D, Fu H, Shi L, Pan C, Li Q, Chu Y, Yu W. Synthesis of CeO2 nanorods via ultrasonication assisted by polyethylene glycol. Inorg. Chem. 2007;46:2446–2451. doi: 10.1021/ic061697d. [DOI] [PubMed] [Google Scholar]
- 12.Gao F, Lu Q, Komarneni S. Fast synthesis of cerium oxide nanoparticles and nanorods. J. Nanosci. Nanotechnol. 2006;6:3812–3819. doi: 10.1166/jnn.2006.609. [DOI] [PubMed] [Google Scholar]
- 13.Cui Q, Dong X, Wang J, Li M. Direct fabrication of cerium oxide hollow nanofibers by electrospinning. J. Rare Earths. 2008;26:664–669. [Google Scholar]
- 14.Gu F, Wang Z, Han D, Shi C, Guo G. Reverse micelles directed synthesis of mesoporous ceria nanostructures. Mater. Sci. Eng. B. 2007;139:62–68. [Google Scholar]
- 15.La RJ, Hu ZA, Li HL, Shang XL, Yang YY. Template synthesis of CeO2 ordered nanowire arrays. Mater. Sci. Eng. A. 2004;368:145–148. [Google Scholar]
- 16.Wu GS, Xie T, Yuan XY, Cheng BC, Zhang LD. An improved sol-gel template synthetic route to large-scale CeO2 nanowires. Mater. Res. Bull. 2004;39:1023–1028. [Google Scholar]
- 17.Sun C, Li H, Wang Z, Chen L, Huang X. Synthesis and characterization of polycrystalline CeO2 nanowires. Chem. Lett. 2004;33:662–663. [Google Scholar]
- 18.Yan L, Xing X, Yu R, Deng J, Chen J, Liu G. Facile alcohothermal synthesis of large-scale ceria nanowires with organic surfactant assistance. J. Phys. B: Condens. Matter. 2007;390:59–64. [Google Scholar]
- 19.Yada M, Sakai S, Torikai T, Watari T, Furuta S, Katsuki H. Cerium compound nanowires and nanorings templated by mixed organic molecules. Adv. Mater. 2004;16:1222–1226. [Google Scholar]
- 20.Yang R, Guo L. Synthesis of cubic fluorite CeO2 nanowires. J. Mater. Sci. 2005;40:1305–1307. [Google Scholar]
- 21.Bugayeva N, Robinson J. Synthesis of hydrated CeO2 nanowires and nanoneedles. Mater. Sci. Technol. 2007;23:237–241. [Google Scholar]
- 22.Fuentes RO, Acuña LM, Zimicz; MG, Lamas DG, Sacanell JG, Gabriela Leyva A, Baker RT. Formation and structural properties of Ce–Zr mixed oxide nanotubes. Chem. Mater. 2008;20:7356–7363. [Google Scholar]
- 23.Yang R, Guo L. Synthesis of the nanotublar cubic fluorite CeO2. Chin. J. Inorg. Chem. 2004;20:152–158. [Google Scholar]
- 24.Chen G, Sun S, Sun X, Fan W, You T. Formation of CeO2 nanotubes from Ce(OH)CO3 nanorods through kirkendall diffusion. Inorg. Chem. 2009;48:1334–1338. doi: 10.1021/ic801714z. [DOI] [PubMed] [Google Scholar]
- 25.Zhang D, Fu H, Shi L, Fang J, Li Q. Carbon nanotube assisted synthesis of CeO2 nanotubes. J. Solid State Chem. 2007;180:654–660. [Google Scholar]
- 26.Fang J, Cao Z, Zhang D, Shen X, Ding W, Shi L. Preparation and CO conversion activity of ceria nanotubes by carbon nanotubes templating method. J. Rare Earths. 2008;26:153–157. [Google Scholar]
- 27.Li Y, Ding J, Chen J, Xu C, Wei B, Liang J, Wu D. Preparation of ceria nanoparticles supported on carbon nanotubes. Mater. Res. Bull. 2002;37:313–318. [Google Scholar]
- 28.Wei J, Ding J, Zhang X, Wu D, Wang Z, Luo J, Wang K. Coated double-walled carbon nanotubes with ceria nanoparticles. Mater. Lett. 2005;59:322–325. [Google Scholar]
- 29.Zhang D, Shi L, Fu H, Fang J. Ultrasonic-assisted preparation of carbon nanotube/cerium oxide composites. Carbon. 2006;44:2849–2867. [Google Scholar]
- 30.Fu HX, Zhang DS, Shi LY, Fang JH. Synthesis and characterization of cerium oxide nanotubes based on carbon nanotubes. Chem. J. Chin. Univ. 2007;28:617–620. [Google Scholar]
- 31.Zhang D, Pan C, Shi L, Huang L, Fang J, Fu H. A highly reactive catalyst for CO oxidation: CeO2 nanotubes synthesized using carbon nanotubes as removable templates. Microporous Mesoporous Mater. 2009;117:193–200. [Google Scholar]
- 32.Yu KL, Ruan GL, Ben YH, Zou JJ. Convenient synthesis of CeO2 nanotubes. Mater. Sci. Eng. B. 2007;139:197–200. [Google Scholar]
- 33.Miao JJ, Wang H, Li YR, Zhu JM, Zhu JJ. Ultrasonic-induced synthesis of CeO2 nanotubes. J. Cryst. Growth. 2005;281:525–529. [Google Scholar]
- 34.Dos Santos ML, Lima RC, Riccardi CS, Tranquilin RL, Bueno PR, Varela JA, Longo E. Preparation and characterization of ceria nanospheres by microwave-hydrothermal method. Mater. Lett. 2008;62:4509–4511. [Google Scholar]
- 35.Han WQ, Wu L, Zhu Y. Formation and oxidation state of CeO2-x nanotubes. J. Am. Chem. Soc. 2005;127:12814–12815. doi: 10.1021/ja054533p. [DOI] [PubMed] [Google Scholar]
- 36.Tang C, Bando Y, Liu B, Golberg D. Cerium oxide nanotubes prepared from cerium hydroxide nanotubes. Adv. Mater. 2005;17:3005–3009. [Google Scholar]
- 37.Pan C, Zhang D, Shi L, Fang J. Template-free synthesis, controlled conversion, and CO oxidation properties of CeO2 nanorods, nanotubes, nanowires, and nanocubes. Eur. J. Inorg. Chem. 2008;15:2429–2436. [Google Scholar]
- 38.Chen G, Xu C, Song X, Zhao W, Ding Y, Sun S. Interface reaction route to two different kinds of CeO2 nanotubes. Inorg. Chem. 2008;47:723–728. doi: 10.1021/ic701867f. [DOI] [PubMed] [Google Scholar]
- 39.Zhou K, Yang Z, Yang S. Highly reducible CeO2 nanotubes. Chem. Mater. 2007;19:1215–1217. [Google Scholar]
- 40.Wang ZL, Feng X. Polyhedral shapes of CeO2 nanoparticles. J. Phys. Chem. B. 2003;107:13563–13566. [Google Scholar]
- 41.Chen YC, Chen KB, Lee CS, Lin MC. Direct synthesis of Zr-doped ceria nanotubes. J. Phys. Chem. C. 2009;113:5031–5034. [Google Scholar]
- 42.Martin P, Parker SC, Sayle DC, Watson GW. Atomistic modeling of multilayered ceria nanotubes. Nano Lett. 2007;7:543–546. doi: 10.1021/nl0626737. [DOI] [PubMed] [Google Scholar]
- 43.Fu Y, Wei ZD, Ji MB, Li L, Shen PK, Zhang J. Morphology-controllable synthesis of CeO2 on a Pt electrode. Nanoscale Res. Lett. 2008;3:431–434. [Google Scholar]
- 44.Kuiry SC, Patil SD, Deshpande S, Seal S. Spontaneous self-assembly of cerium oxide nanoparticles to nanorods through supraaggregate formation. J. Phys. Chem. B. 2005;109:6936–6939. doi: 10.1021/jp050675u. [DOI] [PubMed] [Google Scholar]
- 45.Ge M, Guo C, Li L, Zhang B, Feng Y, Wang Y. Preparation of CeO2 novel sponge-like rods by emulsion liquid membrane system and its catalytic oxidation property. Mater. Lett. 2009;63:1269–1271. [Google Scholar]
- 46.Terribile D, Trovarelli A, Llorca J, De Leitenburg C, Dolcetti G. The synthesis and characterization of mesoporous high-surface area ceria prepared using a hybrid organic/inorganic route. J. Catal. 1998;178:299–308. [Google Scholar]
- 47.Wang H-C, Lu C-H. Synthesis of cerium hydroxycarbonate powders via a hydrothermal technique. Mater. Res. Bull. 2002;37:783–792. [Google Scholar]
- 48.Zhang D, Yan T, Pan C, Shi L, Zhang J. Carbon nanotube–assisted synthesis and high catalytic activity of CeO2 hollow nanobeads. Mater. Chem. Phys. 2009;113:527–530. [Google Scholar]
- 49.Zhang D-E, Zhang XJ, Ni XM, Song JM, Zheng HG. Optical and electrochemical properties of CeO2 spindles. Chem. Phys. Chem. 2006;7:2468–2470. doi: 10.1002/cphc.200600388. [DOI] [PubMed] [Google Scholar]
- 50.Lu X, Li X, Chen F, Ni C, Chen Z. Hydrothermal synthesis of prism-like mesocrystal CeO2. J. Alloys Compd. 2009;476:958–962. [Google Scholar]
- 51.Elidrissi B, Addou M, Regragui M, Monty C, Bougrine A, Kachouane A. Structural and optical properties of CeO2 thin films prepared by spray pyrolysis. Thin Solid Films. 2000;379:23–27. [Google Scholar]
- 52.Charitidis C, Patsalas P, Logothetidis S. Optical and mechanical performance of nanostructured cerium oxides for applications in optical devices. J. Phys. Conf. Ser. 2005;10:226–229. [Google Scholar]
- 53.Masui T, Fujiwara K, Machida K, Adachi G. Characterization of cerium(IV) oxide ultrafine particles prepared using reversed micelles. Chem. Mater. 1997;9:2197–2204. [Google Scholar]
- 54.Tatar B, Sam ED, Kutlu K, Ürgen M. Synthesis and optical properties of CeO2 nanocrystalline films grown by pulsed electron beam. J. Mater. Sci. 2008;43:5102–5108. [Google Scholar]
- 55.Izu N, Murayama N, Shin W, Matsubara I, Kanzaki S. Resistive oxygen sensors using cerium oxide thin films prepared by metal organic chemical vapor deposition and sputtering. Jpn. J. Appl. Phys.: Part 1. 2004;43:6920–6924. [Google Scholar]
- 56.Porqueras I, Person C, Corbella C, Vives M, Pinyol A, Bertran E. Characteristics of e-beam deposited electrochromic CeO2 thin films. Solid State Ionics. 2003;165:131–137. [Google Scholar]
- 57.Kanakaraju S, Mohan S, Sood AK. Optical and structural properties of reactive ion beam sputter deposited CeO2 films. Thin Solid Films. 1997;305:191–195. [Google Scholar]
- 58.Guo S, Arwin H, Jacobsen SN, Järrendahl K, Helmersson U. A spectroscopic ellipsometry study of cerium dioxide thin films grown on sapphire by rf magnetron sputtering. J. Appl. Phys. 1995;77:5369–5376. [Google Scholar]
- 59.Gallage R, Matsuo A, Watanabe T, Matsushita N, Yoshimura M. Fabrication of transparent ceria films by spray deposition without post firing. J. Electroceram. 2009;22:33–39. [Google Scholar]
- 60.Özer N. Optical properties and electrochromic characterization of sol–gel deposited ceria films. Sol. Energ. Mat. Sol. C. 2001;68:391–400. [Google Scholar]
- 61.Patsalas P, Logothetidis S, Metaxa C. Optical performance of nanocrystalline transparent ceria films. Appl. Phys. Lett. 2002;81:466–468. [Google Scholar]
- 62.Zhang Y, Cheng T, Hu Q, Fang Z, Han K. Study of the preparation and properties of CeO2 single/multiwall hollow microspheres. J. Mater. Res. Soc. 2007;22:1472–1478. [Google Scholar]
- 63.Cui MY, He JX, Lu NP, Zheng YY, Dong WJ, Tang WH, Chen BY, Li CR. Morphology and size control of cerium carbonate hydroxide and ceria micro/nanostructures by hydrothermal technology. Mater. Chem. Phys. 2010;121:314–319. [Google Scholar]
- 64.Sun Z, Zhang H, An G, Yang G, Liu Z. Supercritical CO2-facilitating large-scale synthesis of CeO2 nanowires and their application for solvent-free selective hydrogenation of nitroarenes. J. Mater. Chem. 2010;20:1947–1952. [Google Scholar]
- 65.Guo Z, Jian F, Du F. A simple method to controlled synthesis of CeO2 hollow microspheres. Scr. Mater. 2009;61:48–51. [Google Scholar]
- 66.Deshmukh SS, Zhang M, Kovalchuk VI, D’Itri JL. Effect of SO2 on CO and C3H6 oxidation over CeO2 and Ce0.75Zr0.25O2. Appl. Catal. B: Environ. 2003;45:135–145. [Google Scholar]
- 67.Chen G, Xu C, Song X, Xu S, Ding Y, Sun S. Template-free synthesis of single-crystalline- like CeO2 hollow nanocubes. Cryst. Growth Des. 2008;8:4449–4453. [Google Scholar]
- 68.Fornasiero P, Dimonte R, Rao GR, Kaspar J, Meriani S, Trovarelli A, Graziani M. Rh-Loaded CeO2-ZrO2 solid-solutions as highly efficient oxygen exchangers: Dependence of the reduction behavior and the oxygen storage capacity on the structural-properties. J. Catal. 1995;151:168–177. [Google Scholar]
- 69.Sundar RS, Deevi S. CO oxidation activity of Cu-CeO nano-composite catalysts prepared by laser vaporization and controlled condensation. J. Nanopart. Res. 2006;8:497–509. [Google Scholar]
- 70.Sun C, Li H, Chen L. Study of flowerlike CeO2 microspheres used as catalyst supports for CO oxidation reaction. J. Phys. Chem. Solids. 2007;68:1785–1790. [Google Scholar]
- 71.Guzman J, Carrettin S, Corma A. Spectroscopic evidence for the supply of reactive oxygen during co oxidation catalyzed by gold supported on nanocrystalline CeO2. J. Am. Chem. Soc. 2005;127:3286–3287. doi: 10.1021/ja043752s. [DOI] [PubMed] [Google Scholar]