Abstract
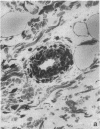
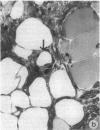
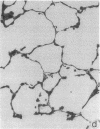
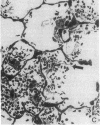
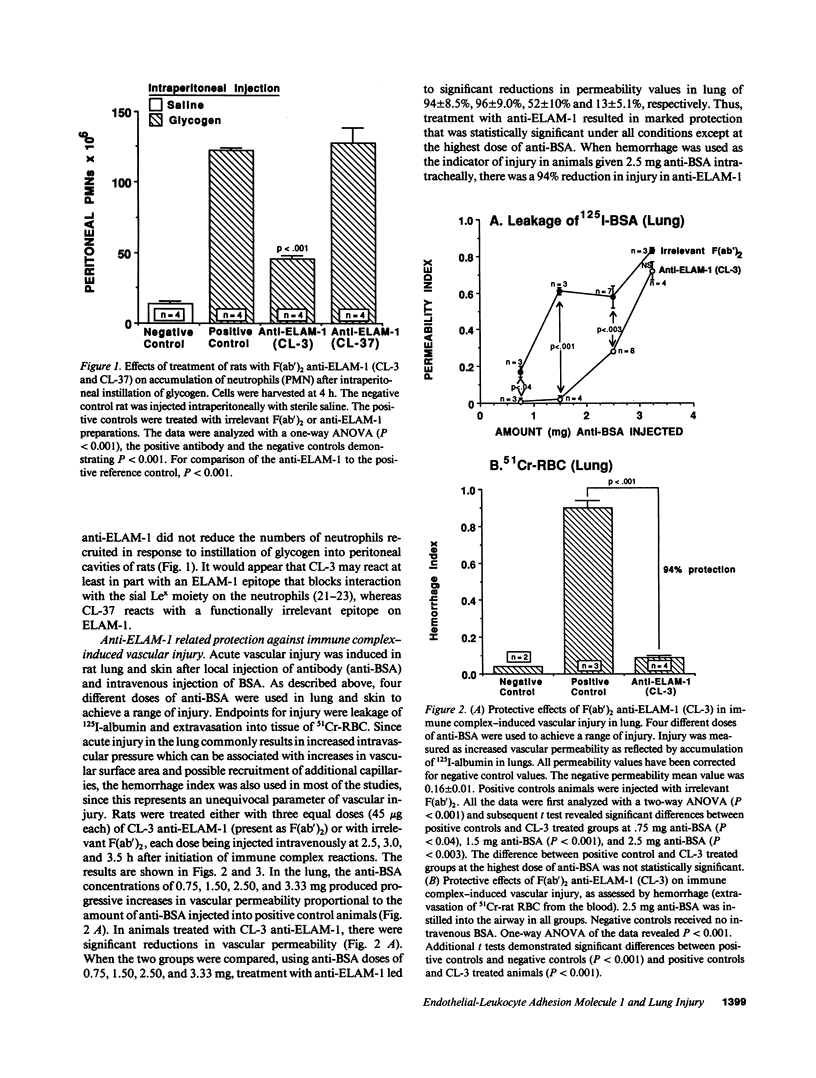
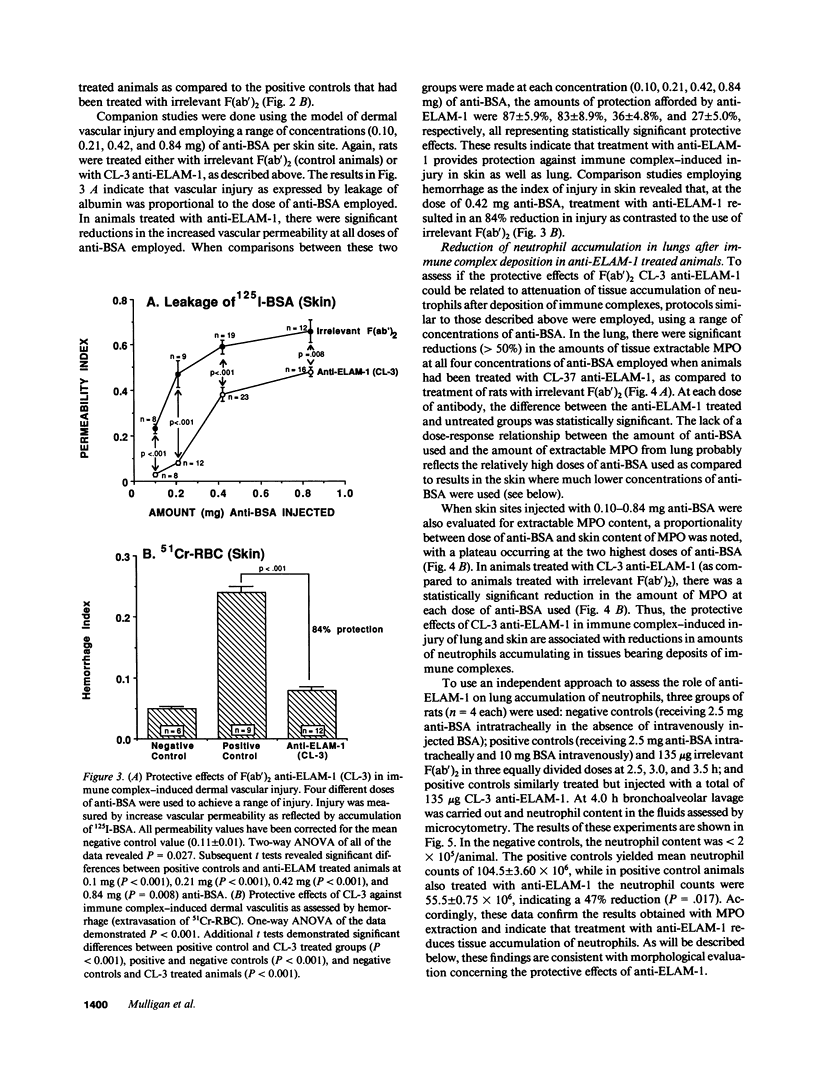
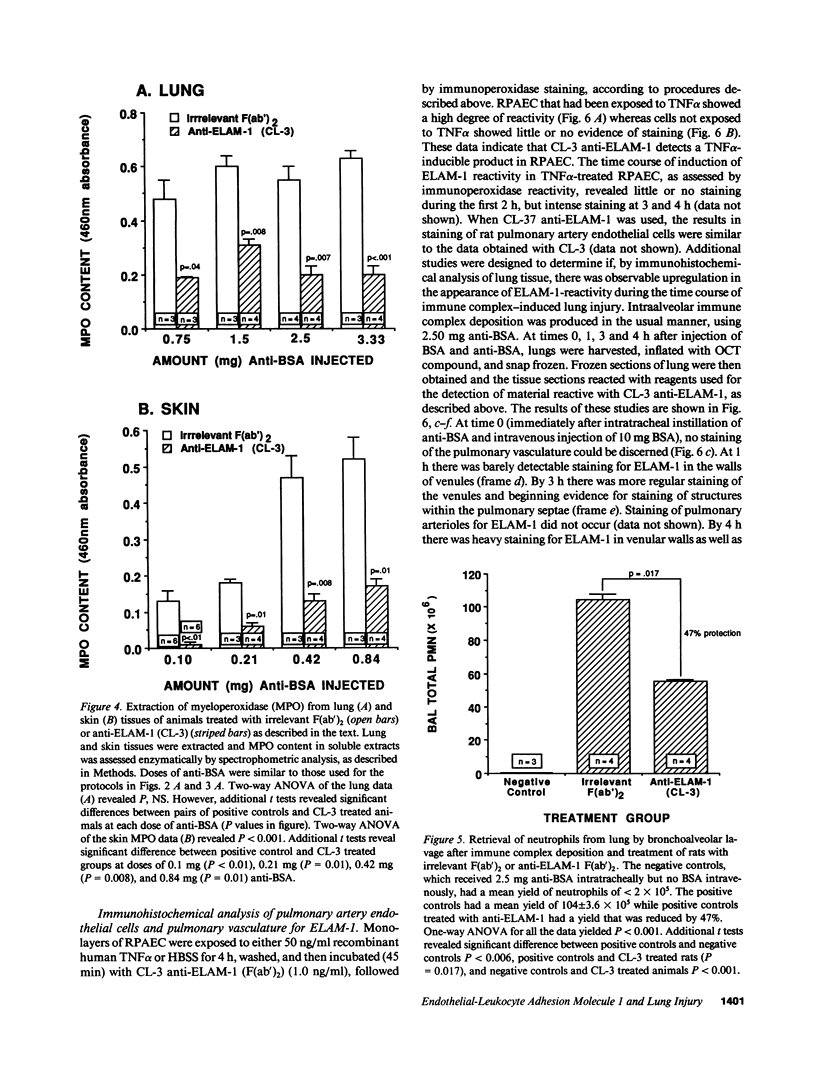
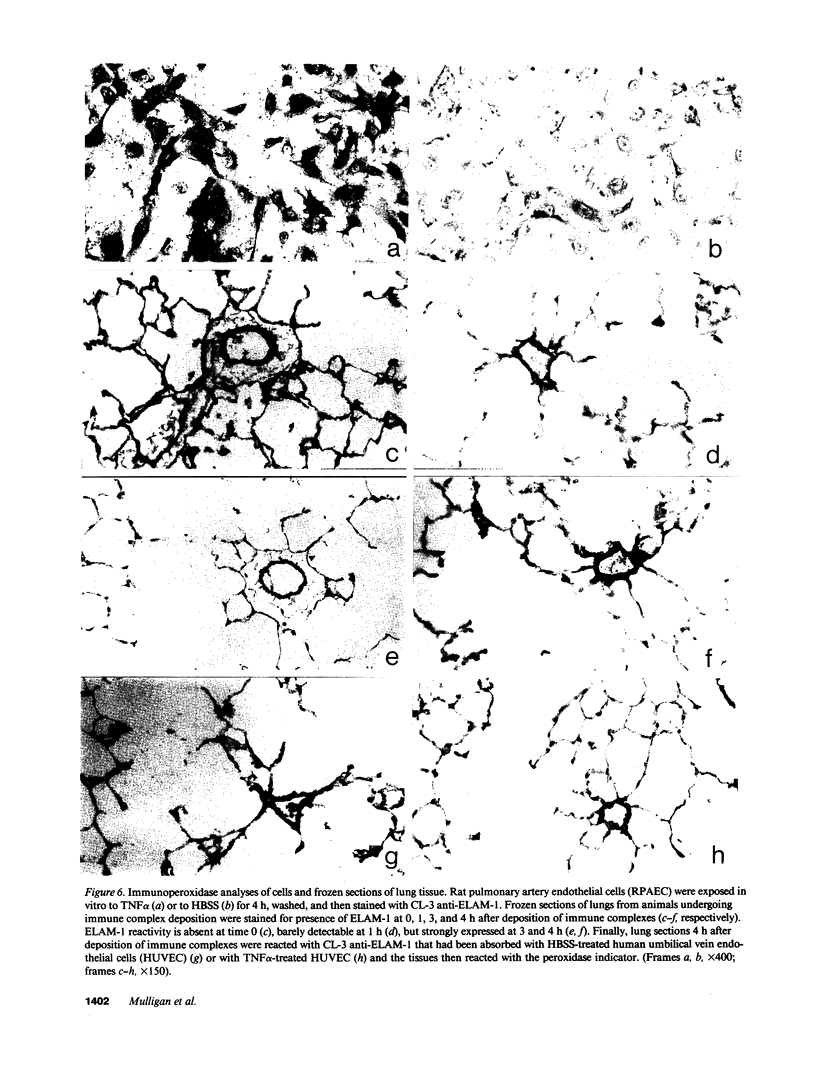
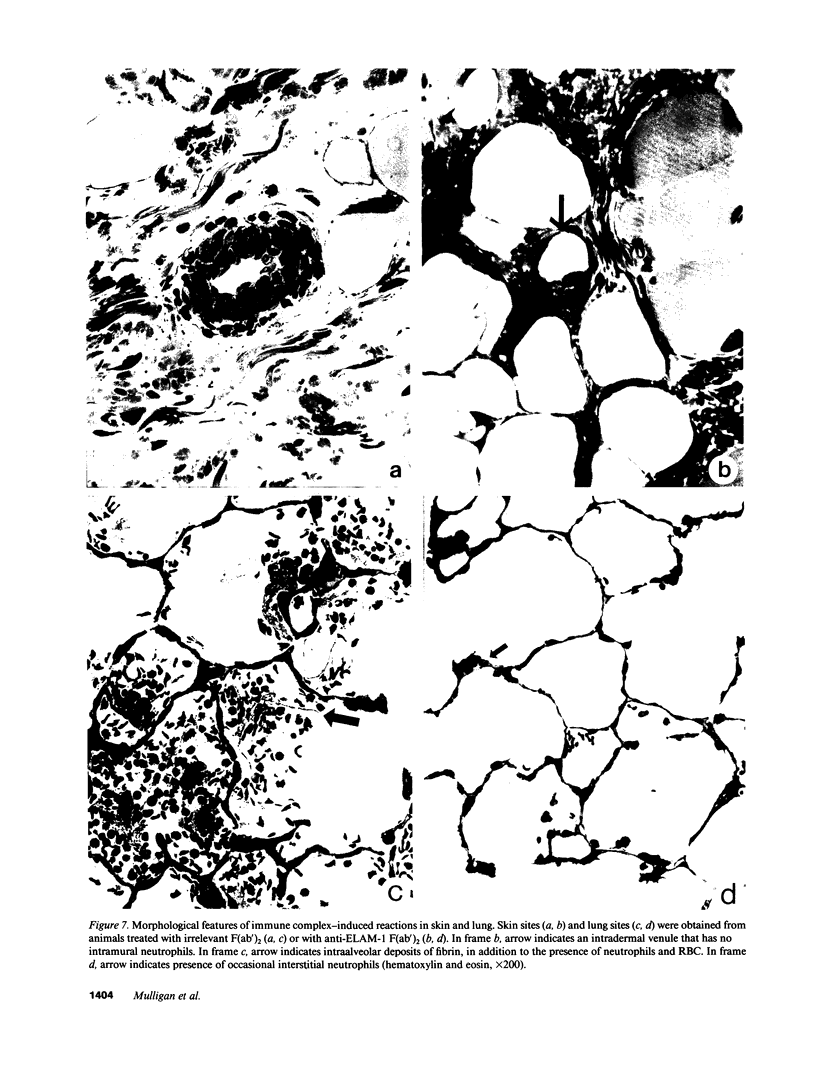
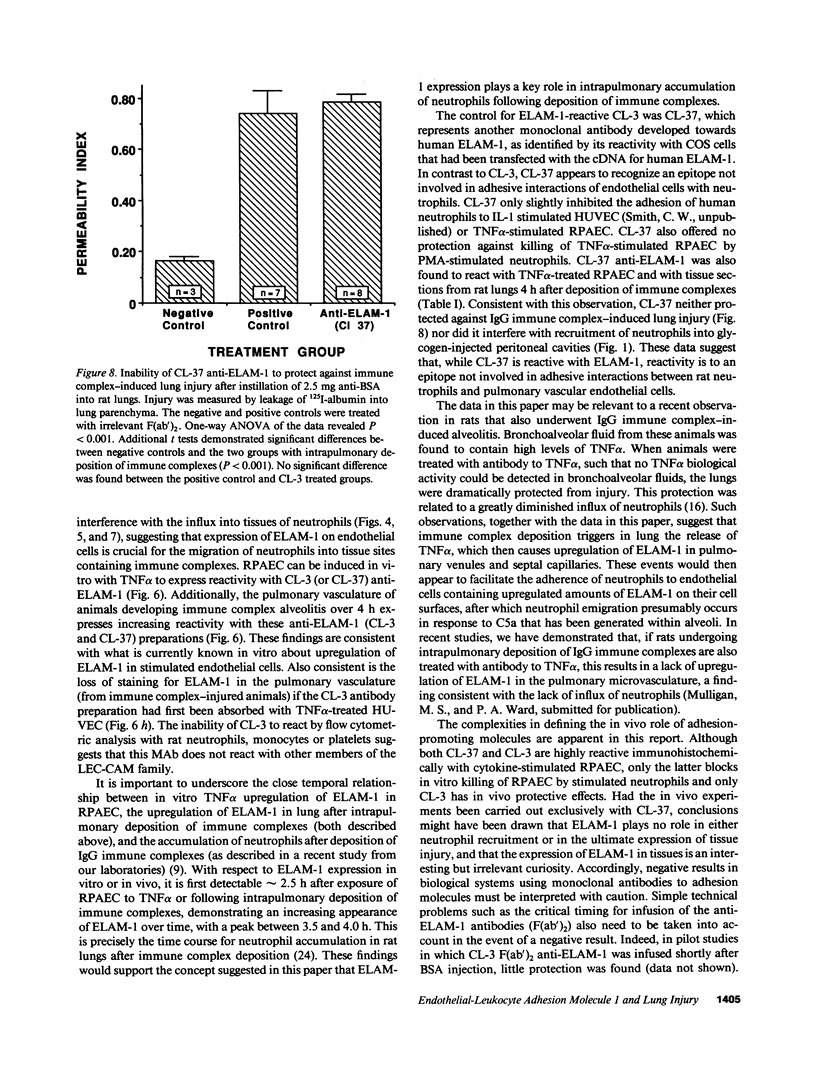
Two murine monoclonal antibodies (CL-3 and CL-37, both F(ab')2) to human endothelial-leukocyte adhesion molecule-1 (ELAM-1) were found to react immunohistochemically with rat pulmonary artery endothelial cells that had been pretreated with tumor necrosis factor (TNF alpha). CL-3, but not CL-37, blocked in vitro adherence of neutrophils to TNF alpha-treated endothelial cells and the killing of TNF alpha-treated rat endothelial cells by phorbol ester activated neutrophils. In rats treated systemically with CL-3, there was a 70% reduction in accumulation of neutrophils in glycogen-induced peritoneal exudates. Treatment of animals with CL-37 anti-ELAM-1 did not reduce neutrophil accumulation under the same conditions. When IgG immune complex deposition was induced in dermis and in lungs of rats, treatment with CL-3 anti-ELAM-1 markedly reduced vascular injury as measured by changes in vascular permeability (leakage of 125I-albumin) and hemorrhage (extravasation of 51Cr-red blood cells). The protective effects of CL-3 anti-ELAM-1 were related to greatly diminished recruitment of neutrophils (as assessed morphologically, by tissue extraction of myeloperoxidase, and by retrieval, via bronchoalveolar lavage, of neutrophils from lung). CL-37 had no protective effects in vivo after deposition of immune complexes in lung. Using either CL-3 or CL-37 anti-ELAM-1, immunohistochemical analysis of lungs undergoing IgG immune complex-induced injury revealed a striking upregulation of ELAM-1 in the lung vasculature (venules and interstitial capillaries), with a peak intensity developing between 3 and 4 h after deposition of immune complexes in lung. Vascular beds of spleen, liver, and kidney failed to show upregulation of ELAM-1 under these same conditions. The immunohistochemical reactivity of rat lung was abolished if the anti-ELAM-1 preparation was first absorbed with monolayers of human umbilical vein endothelial cells that had been pretreated with TNF alpha. Untreated human endothelial cells failed to cause loss of lung reactivity of the anti-ELAM-1 preparation. These data indicate that ELAM-1 is upregulated in the pulmonary vasculature of rats during deposition of immune complexes and that ELAM-1 appears to play an obligate role in the recruitment of neutrophils.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Barker J. N., Mitra R. S., Griffiths C. E., Dixit V. M., Nickoloff B. J. Keratinocytes as initiators of inflammation. Lancet. 1991 Jan 26;337(8735):211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- Barton R. W., Rothlein R., Ksiazek J., Kennedy C. The effect of anti-intercellular adhesion molecule-1 on phorbol-ester-induced rabbit lung inflammation. J Immunol. 1989 Aug 15;143(4):1278–1282. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Membrane proteins involved in phagocyte adherence to endothelium. Immunol Rev. 1990 Apr;114:5–28. doi: 10.1111/j.1600-065x.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Cotran R. S., Gimbrone M. A., Jr, Bevilacqua M. P., Mendrick D. L., Pober J. S. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986 Aug 1;164(2):661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerschuk C. M., Winn R. K., Coxson H. O., Harlan J. M. CD18-dependent and -independent mechanisms of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J Immunol. 1990 Mar 15;144(6):2327–2333. [PubMed] [Google Scholar]
- Geng J. G., Bevilacqua M. P., Moore K. L., McIntyre T. M., Prescott S. M., Kim J. M., Bliss G. A., Zimmerman G. A., McEver R. P. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990 Feb 22;343(6260):757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- Graber N., Gopal T. V., Wilson D., Beall L. D., Polte T., Newman W. T cells bind to cytokine-activated endothelial cells via a novel, inducible sialoglycoprotein and endothelial leukocyte adhesion molecule-1. J Immunol. 1990 Aug 1;145(3):819–830. [PubMed] [Google Scholar]
- Hallmann R., Jutila M. A., Smith C. W., Anderson D. C., Kishimoto T. K., Butcher E. C. The peripheral lymph node homing receptor, LECAM-1, is involved in CD18-independent adhesion of human neutrophils to endothelium. Biochem Biophys Res Commun. 1991 Jan 15;174(1):236–243. doi: 10.1016/0006-291x(91)90511-5. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- Jutila M. A., Rott L., Berg E. L., Butcher E. C. Function and regulation of the neutrophil MEL-14 antigen in vivo: comparison with LFA-1 and MAC-1. J Immunol. 1989 Nov 15;143(10):3318–3324. [PubMed] [Google Scholar]
- Lowe J. B., Stoolman L. M., Nair R. P., Larsen R. D., Berhend T. L., Marks R. M. ELAM-1--dependent cell adhesion to vascular endothelium determined by a transfected human fucosyltransferase cDNA. Cell. 1990 Nov 2;63(3):475–484. doi: 10.1016/0092-8674(90)90444-j. [DOI] [PubMed] [Google Scholar]
- Luscinskas F. W., Brock A. F., Arnaout M. A., Gimbrone M. A., Jr Endothelial-leukocyte adhesion molecule-1-dependent and leukocyte (CD11/CD18)-dependent mechanisms contribute to polymorphonuclear leukocyte adhesion to cytokine-activated human vascular endothelium. J Immunol. 1989 Apr 1;142(7):2257–2263. [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989 Jul;135(1):121–133. [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Pober J. S. Warner-Lambert/Parke-Davis award lecture. Cytokine-mediated activation of vascular endothelium. Physiology and pathology. Am J Pathol. 1988 Dec;133(3):426–433. [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Kishimoto T. K., Abbassi O., Hughes B., Rothlein R., McIntire L. V., Butcher E., Anderson D. C., Abbass O. Chemotactic factors regulate lectin adhesion molecule 1 (LECAM-1)-dependent neutrophil adhesion to cytokine-stimulated endothelial cells in vitro. J Clin Invest. 1991 Feb;87(2):609–618. doi: 10.1172/JCI115037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolman L. M. Adhesion molecules controlling lymphocyte migration. Cell. 1989 Mar 24;56(6):907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Varani J., Bendelow M. J., Sealey D. E., Kunkel S. L., Gannon D. E., Ryan U. S., Ward P. A. Tumor necrosis factor enhances susceptibility of vascular endothelial cells to neutrophil-mediated killing. Lab Invest. 1988 Aug;59(2):292–295. [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Till G. O., Kunkel R. G., Ryan U. S., Ward P. A. Pulmonary endothelial cell killing by human neutrophils. Possible involvement of hydroxyl radical. Lab Invest. 1985 Dec;53(6):656–663. [PubMed] [Google Scholar]
- Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990 Nov 23;250(4984):1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Cunningham T. W., Johnson K. J. Signal transduction events in stimulated rat neutrophils: effects of adenine nucleotides. Clin Immunol Immunopathol. 1989 Jan;50(1 Pt 1):30–41. doi: 10.1016/0090-1229(89)90219-5. [DOI] [PubMed] [Google Scholar]
- Warren J. S., Yabroff K. R., Mandel D. M., Johnson K. J., Ward P. A. Role of O2- in neutrophil recruitment into sites of dermal and pulmonary vasculitis. Free Radic Biol Med. 1990;8(2):163–172. doi: 10.1016/0891-5849(90)90089-2. [DOI] [PubMed] [Google Scholar]
- Warren J. S., Yabroff K. R., Remick D. G., Kunkel S. L., Chensue S. W., Kunkel R. G., Johnson K. J., Ward P. A. Tumor necrosis factor participates in the pathogenesis of acute immune complex alveolitis in the rat. J Clin Invest. 1989 Dec;84(6):1873–1882. doi: 10.1172/JCI114374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]