Abstract
Objective
Impaired functional capacity predicts morbidity and increased mortality in patients with PAD. We hypothesized that brachial-ankle pulse wave velocity (baPWV), a measure of arterial stiffness, is associated with functional capacity in patients undergoing noninvasive evaluation for peripheral arterial disease (PAD).
Methods
We studied 114 patients (age 68 ± 10 years) referred to Mayo Clinic’s noninvasive vascular laboratory. Functional capacity was estimated in terms of distance walked in 5 min on a treadmill at a speed of 1.0–2.0 mph. Ankle-brachial index (ABI) was obtained with Doppler method before and 1 min after exercise. baPWV was estimated noninvasively using an oscillometric device. The association of baPWV with walking distance was assessed using accelerated failure time and Cox proportional-hazards models.
Results
The mean baPWV was higher in patients who were unable to complete the walk test compared to those who successfully completed the test (P = 0.008). Higher baPWV was associated with a lower walking distance after adjustment for heart rate, mean arterial pressure, and cardiovascular risk factors (P = 0.017) and after additional adjustment for pulse pressure (P = 0.034) and ABI (P = 0.030). Higher baPWV was associated with failure to complete the treadmill walk test, after adjustment for heart rate, mean arterial pressure, and cardiovascular risk factors (P = 0.025) and after additional adjustment for pulse pressure (P = 0.041) and ABI (P = 0.039).
Conclusion
Increased baPWV, a measure of arterial stiffness, is associated with impaired functional capacity in patients undergoing evaluation for PAD.
Keywords: Arterial stiffness, Brachial-ankle pulse wave velocity, Functional capacity, Peripheral arterial disease
Atherosclerotic peripheral arterial disease (PAD) of lower extremities affects ~8 million Americans [1]. It is a marker of widespread atherosclerosis [2] and is associated with increased risk of myocardial infarction [3], stroke [4], and death [5]. In addition, patients with PAD often have reduced functional capacity that affects their activities of daily living [1,6]. Impaired functional capacity has been found to be predictive of poor quality of life and increased mortality, both in the presence and absence of PAD [7,8]. The determinants of functional capacity in patients with PAD are not clear. The ankle-brachial index (ABI), which is the ratio of systolic blood pressure (BP) at the ankle to simultaneously measured systolic BP at the brachial artery, is often used to identify patients with PAD as well as to express the anatomical severity of PAD. However, the correlation of ABI with functional capacity has been found to be modest [2,9].
Arterial stiffness represents the cumulative effect of cardiovascular risk factors, including aging, on the arterial wall. Increased arterial stiffness has been found to be predictive of adverse cardiovascular events and death [1]. Further, elevated arterial stiffness has been associated with impaired cardiorespiratory fitness in individuals free of cardiovascular disease [11]. Patients with PAD often have increased arterial stiffness [12]. We have previously demonstrated increased brachial pulse pressure (an indirect measure of arterial stiffness) and higher aortic augmentation index (a measure of arterial wave reflection that is affected by arterial stiffness) to be associated with impaired walking ability in patients undergoing evaluation for PAD [13]. The gold-standard measure of arterial stiffness – pulse wave velocity (PWV) – reflects the speed of pressure pulse transmission along the arterial wall. Recently, a method for easy estimation of PWV – the brachial-ankle PWV (baPWV) – has been proposed. We investigated whether baPWV is associated with functional capacity, assessed as the distance walked on the treadmill, in patients referred for noninvasive lower extremity arterial evaluation.
1. Methods
1.1. Patient characteristics
We studied 114 consecutive patients undergoing noninvasive lower extremity evaluation for suspected PAD. The Institutional Review Board of Mayo Clinic approved the study protocol, and verbal informed consent was obtained from all participants. The medical records were reviewed to obtain information about history of smoking and use of BP and lipid-lowering medications. Diabetes was considered present if a patient was being treated with insulin or oral agents or had a fasting glucose level of ≥126mg/dL (≥7 mmol/L). Weight was measured by an electronic scale, height by a stadiometer, and body mass index (BMI) was calculated in units of kg/m2.
1.2. Assessment of arterial stiffness
Arterial stiffness was assessed in terms of baPWV by a single observer (C.A.A.) using an automatic waveform analyzer (model BP-203RPE; Omron, Japan). The device uses the height of the subject to calculate path length from the aortic arch to the brachial sensor (d1) and that from the aortic arch to the ankle sensor (d2). The time interval (t) between the initial rise of the pulse pressure waveforms at the brachial and the tibial arteries is calculated and baPWV is calculated using the equation: baPWV (cm/s) = (d2 −d1)/t. The reproducibility and validity of the baPWV measurement using this method has been previously demonstrated [14,15].
The patient rested in the supine position for 5 min before BP cuffs were applied above the medial malleolus of both legs and above the elbows of both arms, and ECG electrodes were attached to both wrists. A microphone for phonocardiography was placed at the fourth intercostal space at the left margin of the sternum. Simultaneous recording of noninvasive BP, ECG lead I, noninvasive arterial pulse waveforms, and heart sounds was obtained. The waveform analyzer calculated baPWV separately for right and left sides. The higher of the two baPWV for each patient was used in the analyses.
1.3. Lower extremity arterial evaluation
ABI was measured before and 1-min after exercise in the noninvasive vascular laboratory (certified by the Intersocietal Commission for the Accreditation of Vascular Laboratories) by vascular technicians who were unaware of the baPWV results. With the patient lying supine, BP cuffs were placed over each brachial artery and above each malleolus and systolic BP measured using a handheld 5MHz Doppler probe (Meda Sonics, Fremont, CA). The higher of the two brachial systolic BP was used to calculate the ABI at the posterior tibial artery in each leg; the lower of the two ABI measurements from the two legs was used in the analyses. Patients with ABI> 1.5 or <0.5 are not exercised as per laboratory protocol.
1.4. Assessment of functional capacity
Functional capacity was assessed using a standardized exercise protocol with a motorized treadmill [2,16]. Patients walked on treadmill with ECG monitoring, at a speed of 1 mph (1.6 kph) for the first ~10 s and thereafter at a speed of 1.0 mph (1.6 kph), 1.5 mph (2.4 kph), or 2 mph (3.2 kph) at a fixed grade (10%), the speed being chosen as per the “usual” pace of the patient. The maximum duration of walking at completion of the protocol was 5 min, corresponding to 130m, 194 m, and 259mof distance walked for the treadmill speeds of 1.0, 1.5, and 2.0 mph, respectively. For patients developing exertional leg symptoms, dyspnea, or objective symptoms of myocardial ischemia, the test was terminated prior to 5 min.
1.5. Statistical analysis
Descriptive statistics are expressed as mean ± SD (1st quartile, 3rd quartile) for continuous data or as number (percent) for categorical data. For patients who walked the full 5 min (n = 75), the distance walked was censored at the maximum distance for the treadmill speed used. baPWV had skewed distribution and was log transformed. Survival analyses were used to investigate the association of baPWV with the distance walked on the treadmill in a univariable model and in a multivariable model after adjustment for heart rate, mean arterial pressure, and cardiovascular risk factors (age, sex, BMI, diabetes, and smoking history). Next, we included brachial pulse pressure in the multivariable regression model. Finally, we investigated whether the association of baPWV with walking distance was independent of the presence or severity or PAD. This was done by additional adjustment for ABI in the multivariable analyses and by repeating analyses in the subset of patients with PAD (n = 76) as defined by ABI <0.9 either at rest or after exercise. In separate analyses, we investigated whether baPWV was associated with the distance walked on the treadmill after adjustment for levels of total and HDL cholesterol and the use of hypertension and lipid-lowering medications, one-at-a-time.
The analyses for walking distance were performed using an accelerated failure time (AFT) model [17] using a log-normal distribution for walking distance. This model assumes that the predictor variables have a linear relationship to the expected log of the walking distance; when the observation is right-censored, the probability that the walking distance would exceed the lower bound is used in the analysis, rather than the probability density function at the observed value. Additionally, Cox proportional-hazards models [18] were used to assess the hazard of stopping during the treadmill test (the ‘event’), before and after adjustment for relevant covariates. We also used log-rank test to test the equality of two Kaplan–Meier survival curves comparing the ability to complete the treadmill walking test between patients with baPWV less than median (17.8 m/s) and those with baPWV equal to or greater than median.
A 2-sided P-value of <0.05 was taken as the criterion of statistical significance. Statistical analyses were carried out using SAS v 9.1 (SAS Institute, Cary, NC) software package.
2. Results
The characteristics of the study participants are shown in Table 1. Thirty-nine patients were unable to complete the exercise protocol—30 due to leg discomfort, 6 due to a combination of leg discomfort and dyspnea, 2 due to chest discomfort and leg pain, and 1 due to unsteadiness of gait.
Table 1.
Characteristics of the study sample (n = 114).
| Characteristic | Mean ± SD (Q1, Q3) or n (%) |
|---|---|
| Age, years | 68.4 ± 10.6 (33, 86) |
| Women | 48 (42.10) |
| Body mass index, kg/m2 | 28.5 ± 5.3 (16.4, 47.3) |
| Resting heart rate, bpm | 67.3 ± 11.0 (43, 97) |
| MAP, mmHg | 91.0 ± 10.2 (70.0, 116.7) |
| Diabetes | 23 (20.18) |
| Hypertension | 93 (81.58) |
| Smoking history | 88 (77.19) |
| Total cholesterol, mg/dL | 183.0 ± 46.0 (106, 376) |
| HDL cholesterol, mg/dL | 53.3 ± 16.0 (27, 101) |
| BP medication use | 87 (76.32) |
| Lipid-lowering medication use | 70 (61.40) |
| Systolic BP, mmHg | 132.4 ± 18.2 (96, 184) |
| Diastolic BP, mmHg | 70.3 ± 8.6 (50, 90) |
| Brachial PP, mmHg | 62 ± 15 (30, 112) |
| Ankle-brachial index | 0.97 ± 0.19 (0.41, 1.28) |
| Patients with PAD | 76 (66.67) |
| Walking distance, m | 180 ± 69.5 (11.9, 258.8) |
| Patients completed 5 min walk | 75 (65.79) |
| baPWV, m/s | 18.7 ± 4.64 (11.9, 37.2) |
Figures represent mean ± SD (first quartile [Q1], third quartile [Q3]) for quantitative variables and number (percentage) for categorical variables. bpm, beats per minute; MAP, mean arterial pressure; HDL, high-density lipoprotein; BP, blood pressure; PP, pulse pressure; PAD, peripheral arterial disease; baPWV, brachial ankle pulse wave velocity.
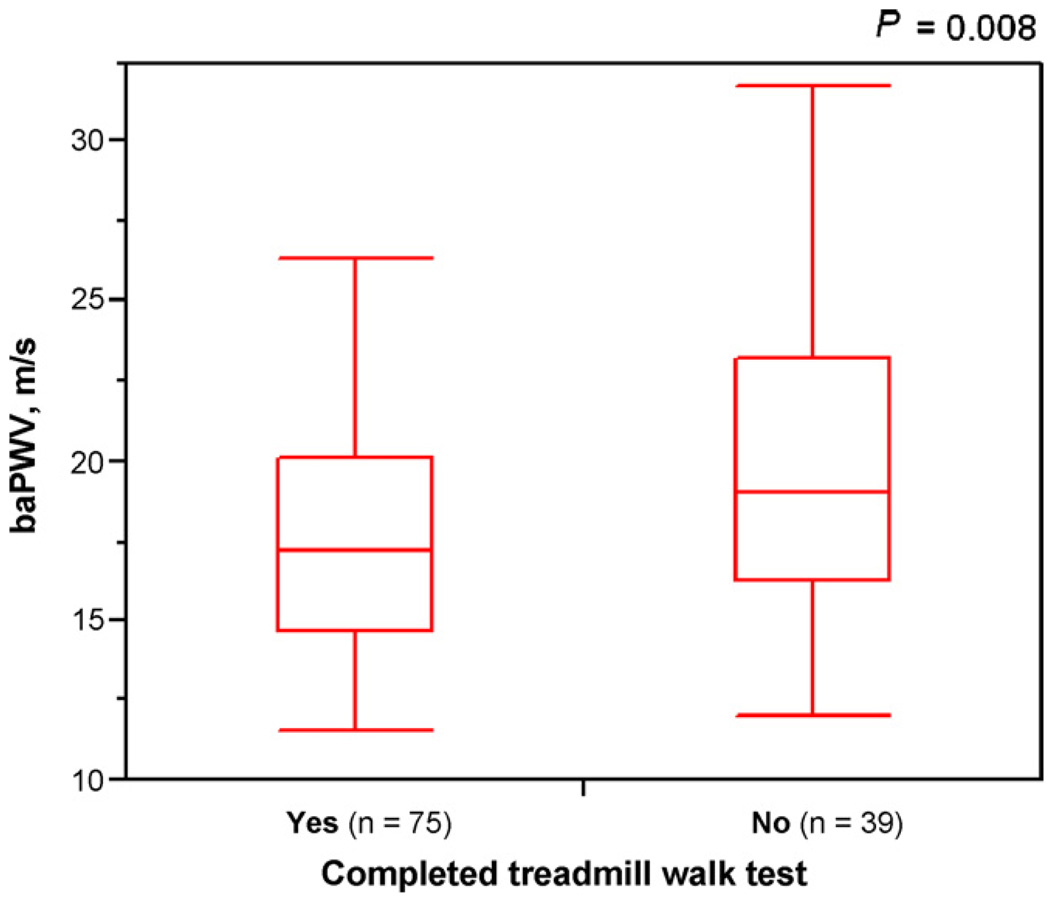
The mean baPWV was 20.36 ± 5.54 m/s in patients stopping during the treadmill walk test compared to 17.65 ± 3.59 m/s in patients able to complete the test (P = 0.008) (Fig. 1).
Fig. 1.
Box plots showing brachial-ankle pulse wave velocity (baPWV) in patients with respect to their ability to complete the treadmill walk test. Patients who stopped before the completion of the test had significantly higher baPWV compared to the patients who were able to complete the test (P = 0.008).
In univariable analysis, higher baPWV was associated with a shorter walking distance (P = 0.0004). Other variables associated with a shorter walking distance were: greater age, higher BMI, smoking history, use of hypertension medications, and greater brachial pulse pressure (Table 2). In multivariable analyses, higher baPWV was associated with a shorter walking distance, independent of resting heart rate, and mean arterial pressure, and conventional risk factors (P = 0.017); baPWV remained a significant predictor of walking distance after additional adjustment for pulse pressure (P = 0.034) and ABI (P = 0.030) (Table 3). ABI and pulse pressure were not independently associated with walking distance in multivariable models. In separate analyses (not shown), baPWV was associated with walking distance even after adjustment for total cholesterol, HDL cholesterol, and the use of hypertension and lipid-lowering medications.
Table 2.
Variables associated with walking distance: univariable analyses based on accelerated failure time models.
| Variable | β ± SE | P |
|---|---|---|
| Age, years | −0.027 ± 0.013 | 0.039 |
| Female sex | 0.245 ± 0.233 | 0.29 |
| Body mass index, kg/m2 | −0.032 ± 0.021 | 0.13 |
| Resting heart rate, bpm | −0.014 ± 0.010 | 0.16 |
| MAP, mmHg | −0.012 ± 0.008 | 0.13 |
| Brachial PP, mmHg | −0.023 ± 0.009 | 0.01 |
| Diabetes | −0.027 ± 0.276 | 0.92 |
| Smoking history | −0.660 ± 0.332 | 0.047 |
| Smoking current | −0.288 ± 0.255 | 0.26 |
| Total cholesterol, mg/dL | −0.003 ± 0.002 | 0.28 |
| HDL cholesterol, mg/dL | −0.001 ± 0.009 | 0.92 |
| BP medication use | −0.797 ± 0.371 | 0.032 |
| Lipid-lowering medication use | −0.442 ± 0.245 | 0.072 |
| Ankle-brachial index | 0.226 ± 0.563 | 0.69 |
| Log baPWV, m/s | −0.082 ± 0.023 | 0.0004 |
Abbreviation as in Table 1.
Table 3.
Variables associated with walking distance: multivariable analyses based on accelerated failure time models.
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β ± SE | P | β ± SE | P | β ± SE | P | |
| Age, years | −0.015 ± 0.015 | 0.33 | −0.013 ± 0.016 | 0.43 | −0.011 ± 0.016 | 0.48 |
| Female sex | −0.075 ± 0.231 | 0.74 | 0.071 ± 0.251 | 0.78 | 0.072 ± 0.249 | 0.77 |
| Body mass index, kg/m2 | −0.051 ± 0.023 | 0.023 | −0.041 ± 0.023 | 0.07 | −0.044 ± 0.024 | 0.063 |
| Resting heart rate, bpm | −0.008 ± 0.010 | 0.43 | −0.008 ± 0.009 | 0.4 | −0.007 ± 0.009 | 0.45 |
| MAP, mmHg | 0.006 ± 0.009 | 0.47 | −0.015 ± 0.011 | 0.17 | 0.015 ± 0.011 | 0.17 |
| Smoking history | −0.504 ± 0.325 | 0.12 | −0.497 ± 0.323 | 0.12 | −0.487 ± 0.321 | 0.13 |
| Diabetes | 0.302 ± 0.259 | 0.28 | 0.008 ± 0.015 | 0.24 | 0.320 ± 0.264 | 0.23 |
| Log baPWV, m/s | −3.801 ± 1.597 | 0.017 | −3.440 ± 1.618 | 0.034 | −3.550 ± 1.632 | 0.03 |
| Brachial PP, mmHg | – | −0.019 ± 0.012 | 0.13 | −0.019 ± 0.012 | 0.13 | |
| Ankle-brachial index | – | 0.227 ± 0.580 | 0.7 | |||
| R2 | 0.17 | 0.18 | 0.18 | |||
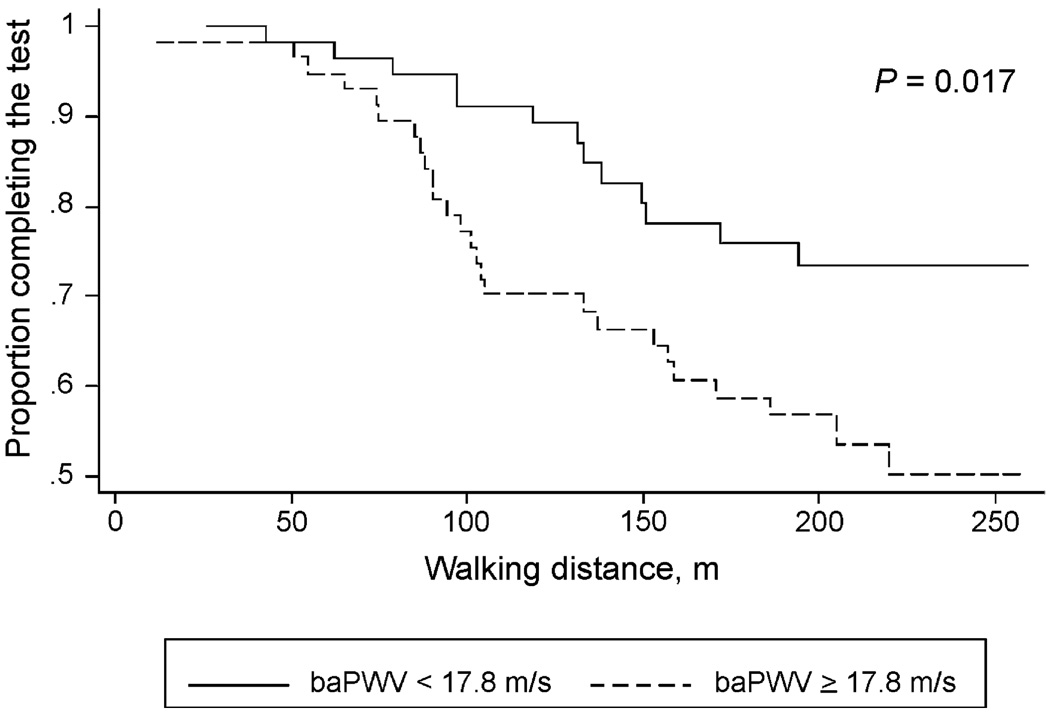
In the Cox proportional hazards models, higher baPWV was associated with a greater likelihood of stopping during the treadmill walk test, independent of heart rate, mean arterial pressure, and conventional cardiovascular risk factors; the association remained significant after additional adjustment for brachial pulse pressure (P = 0.041) and ABI (P = 0.039) (Table 4). ABI and pulse pressure were not independently associated with the ability to complete the walk test in multivariable models. In separate analyses (not shown), baPWV was a significant predictor of the ability to complete the walk test even after adjustment for total cholesterol, HDL cholesterol, and hypertension and lipid-lowering medications. Patients with baPWV <median were significantly more likely to complete the walk test compared to patients with baPWV ≥median (P = 0.017; Fig. 2).
Table 4.
Variables associated with the hazard of stopping walking: multivariable regression analyses based on Cox proportional-hazards models.
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β ± SE | P | β ± SE | P | β ± SE | P | |
| Age, years | 0.021 ± 0.024 | 0.40 | 0.017 ± 0.025 | 0.50 | −0.015 ± 0.026 | 0.56 |
| Female sex | 0.111 ± 0.368 | 0.76 | −0.104 ± 0.407 | 0.80 | −0.110 ± 0.406 | 0.79 |
| Body mass index, kg/m2 | 0.070 ± 0.035 | 0.043 | 0.057 ± 0.036 | 0.11 | 0.060 ± 0.037 | 0.11 |
| Resting heart rate, bpm | 0.012 ± 0.015 | 0.45 | 0.012 ± 0.015 | 0.43 | 0.011 ± 0.015 | 0.47 |
| MAP, mmHg | −0.008 ± 0.014 | 0.55 | −0.021 ± 0.017 | 0.23 | −0.021 ± 0.017 | 0.22 |
| Smoking history | −0.756 ± 0.509 | 0.14 | 0.765 ± 0.511 | 0.14 | 0.755 ± 0.501 | 0.14 |
| Diabetes | −0.415 ± 0.413 | 0.32 | −0.450 ± 0.416 | 0.28 | −0.474 ± 0.425 | 0.26 |
| Log baPWV, m/s | 5.678 ± 2.527 | 0.025 | 5.246 ± 2.567 | 0.041 | 5.410 ± 2.616 | 0.039 |
| Brachial PP, mmHg | – | 0.027 ± 0.020 | 0.17 | 0.027 ± 0.020 | 0.17 | |
| Ankle-brachial index | – | – | −0.292 ± 0.951 | 0.76 | ||
| R2 | 0.15 | 0.16 | 0.16 | |||
Fig. 2.
Kaplan–Meier survival curves comparing ability to complete the 5-min treadmill walk test between patients with baPWV less than median (<17.8m/s) and those with baPWV equal to or above median. Patients with baPWV below median (n=57) were significantly more likely to complete the test compared to patients with baPWV equal to or above median (n = 58) (hazard ratio, 2.199; 95% confidence interval, 1.129–4.281).
In subgroup analyses limited to patients with PAD (n = 75), higher baPWV was associated with shorter walking distance during the treadmill walk test after adjustment for heart rate, mean arterial pressure, conventional risk factors, and pulse pressure (β ± SE = −1.987 ± 0.753, P = 0.008); the association of baPWV with walking distance remained significant after adjustment for ABI (β ± SE = −1.945 ± 0.757, P = 0.01). In the Cox proportional hazards models, higher baPWV was associated with a greater likelihood of stopping during the walk test, independent of heart rate, mean arterial pressure, conventional risk factors, and pulse pressure (β ± SE = 3.039 ± 1.202, P = 0.012); the association remained significant after adjustment for ABI (β ± SE = 2.951 ± 1.211, P = 0.015).
3. Discussion
Increased arterial stiffness is associated with cardiovascular disease and increased risk of adverse cardiovascular events including myocardial infarction and stroke [10]. In the present study, we demonstrate that baPWV, a measure of arterial stiffness, predicts walking distance in patients undergoing lower extremity arterial evaluation. A higher baPWV was associated with a reduced walking distance, even after adjustment for ABI and conventional risk factors for cardiovascular disease. These findings indicate that arterial stiffness is a determinant of functional capacity in patients with suspected PAD.
In multivariable models that adjusted for heart rate, mean arterial pressure, and conventional cardiovascular risk factors, baPWV was a significant predictor of the distance walked during treadmill test and elevated baPWV was associated with a greater hazard of inability to complete the test. Previously, in a similar cohort of patients, we have shown that increased aortic augmentation index (a measure of arterial wave reflection) and reduced time to arterial wave reflection (a surrogate for increased arterial stiffness) are associated with impaired walking ability [13]. A recent study has shown that improvement in arterial stiffness is associated with improvement in walking ability in patients with PAD [19]. Taken together, these findings suggest that assessment of arterial stiffness such as by measuring baPWV may provide useful information about functional capacity in the settings of PAD.
Several potential mechanisms could be invoked to explain the association of baPWV with functional capacity. Increased arterial stiffness has been associated with microvascular and conduit artery dysfunction, including abnormalities in endothelial function, which could potentially influence the functional capacity of the lower extremities [20]. Alterations in the gradient of stiffness along the arterial tree that occurs with stiffening of central elastic arteries may favor increased distal transmission of pressure pulse energy with potential deleterious consequences for microvasculature [21]. Such vascular abnormalities could affect capillary perfusion and nutritive flow in the exercising extremity. The hemodynamic consequences of increased arterial stiffness (increased systolic workload with reduced diastolic perfusion of the left ventricle) may per se adversely influence cardiac performance, limiting exercise capacity. In the study by Enko et al. [22], increased baPWV was associated with reduced functional capacity, independent of left ventricular function and severity of coronary obstruction. Furthermore, a higher baPWV was associated with hyperventilatory response to exercise and a lower threshold for exercise-induced myocardial ischemia, suggesting that increased arterial stiffness could predispose to left ventricular diastolic dysfunction as well as myocardial ischemia, both of which could limit functional capacity. Finally, limitation of functional capacity in patients with PAD may be a direct manifestation of the arterial obstruction. Arterial stiffness is known to be increased in the presence of risk factors that predispose to coronary atherosclerosis which could limit functional capacity on a cardiogenic basis [23]. Further, patients with PAD often reduce their physical activity which, in turn, could promote arterial stiffening. In the present study, the association of baPWV with walking ability remained significant even after adjustment for conventional risk factors and ABI and the association was seen even when the analyses were restricted to the subset of patients with PAD (ABI <0.90).
ABI was not an independent predictor of walking ability, either in the entire sample or in the subset of patients with PAD. In earlier studies, an association between severity of lower extremity arterial obstruction and functional capacity has not been consistently noted. While some studies have reported ABI to be a predictor of functional capacity [24] and its decline over time [25] other studies have shown poor correlation between ABI and walking distance in patients with PAD [2,9,26]. In addition, some studies have demonstrated that patients can improve walking distance independent of changes in the severity of PAD [27]. In our prior study [13], the association of ABI with functional capacity was weak and was not statistically significant in the subset of patients with PAD, while measures of arterial stiffness (augmentation index and time to wave reflection) were independently associated with functional capacity in patients with and without PAD. The findings of the present study add support to the hypothesis that arterial stiffness influences functional capacity in patients with PAD, independent of the severity of arterial obstruction.
Epidemiological studies have so far mostly relied on the use of carotid-femoral PWV as a measure of arterial stiffness as it includes the segment of arterial tree predominantly affected by arterial stiffening. On the other hand, baPWV incorporates both central and peripheral segments of the arterial tree. Previous studies have demonstrated a significant correlation between baPWV and carotid-femoral PWV [28] as well as invasively measured aortic PWV [14]. In a study of 409 healthy adults, aortic PWV explained 58% of the variance in baPWV while leg PWV explained additional 23% of the variance [28]. baPWV has been found to be associated with cardiovascular risk factors in healthy adolescents [29], coronary artery disease in men [30], carotid atherosclerosis in patients with end-stage kidney disease [31], and white matter damage in elderly individuals [32]. Increased baPWV has also been shown to be an independent predictor of adverse prognosis after an acute coronary event [33]. One study demonstrated baPWV to be more closely associated with left ventricular mass and diastolic function than carotid-femoral PWV [34]. The main advantage of baPWV is that its measurement is technically simple, does not need exposure of the groin, and may be more time-efficient.
The present study has several limitations. Patients with or at high risk of atherosclerotic arterial disease are likely to have increased arterial stiffness and the incremental predictive value of baPWV may be difficult to determine; although we adjusted for conventional atherosclerotic risk factors, a potential residual confounding influence of risk factors cannot be ruled out. Measurement of baPWV is closely dependent upon BP and delay in the transmission of arterial pressure wave across an area of stenosis could diminish the accuracy of baPWV measurement in presence of PAD [35]. In a prior study in diabetic subjects, patients with PAD were shown to have reduced rather than elevated baPWV despite the presence of atherosclerosis; relief of arterial obstruction through angioplasty was associated with increase in baPWV [15].We believe that the presence of PAD would more likely bias the association between baPWV with functional capacity towards null. Nevertheless, we tried to minimize the effect of this limitation by excluding patients who had resting ABIs ≤0.90 in both legs; patients with PAD have been shown to have different baPWV values in the two limbs [15] and it is likely that baPWV in the unaffected limb may more be a accurate measure of arterial stiffness in these patients. For patients who walked the full 5 min (n = 75), the lower bound, but not the actual distance that the patient could have walked is known. We used survival analyses to account for these right-censored observations since discarding them or simply treating them as the actual walking distance could lead to biased results. We did not analyze the relationship of baPWV with ischemic leg pain, the primary limiting factor in the ability to complete the walk test. About two-thirds of the patients were taking different types of antihypertensive medications which could have general and specific effects on measures of arterial stiffness as well as on functional capacity; although we adjusted for the use of antihypertensive medication, we did not adjust for the type of the medication. Finally, our results need to be confirmed in larger studies and in other ethnic groups.
4. Conclusions
The present study demonstrates that in patients being evaluated for PAD, baPWV is a predictor of functional capacity, independent of cardiovascular risk factors and independent of the severity of PAD. Since functional capacity predicts future morbidity and mortality in patients with PAD, baPWV could provide a useful tool in the evaluation of patients with, or being evaluated for, PAD. Further investigation is needed to assess whether reducing arterial stiffness will improve functional capacity in such patients.
Acknowledgments
The authors would like to thank the study participants and acknowledge the staff of the Mayo Clinic Vascular Laboratory for their help with the study. Comfort A. Amoh-Tonto was supported by NIH Grant T35 HL07766-14. Dr. Malik was supported by an unrestricted grant by Omron Healthcare Inc.
References
- 1.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic) Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 3.Saw J, Bhatt DL, Moliterno DJ, et al. The influence of peripheral arterial disease on outcomes: a pooled analysis of mortality in eight large randomized percutaneous coronary intervention trials. J AmColl Cardiol. 2006;48:1567–1572. doi: 10.1016/j.jacc.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 4.Feringa HH, Karagiannis SE, Schouten O, et al. Prognostic significance of declining ankle-brachial index values in patients with suspected or known peripheral arterial disease. Eur J Vasc Endovasc Surg. 2007;34:206–213. doi: 10.1016/j.ejvs.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 6.Breek JC, Hamming JF, De Vries J, van Berge Henegouwen DP, van Heck GL. The impact of walking impairment, cardiovascular risk factors, and comorbidity on quality of life in patients with intermittent claudication. J Vasc Surg. 2002;36:94–99. doi: 10.1067/mva.2002.124369. [DOI] [PubMed] [Google Scholar]
- 7.McDermott MM, Guralnik JM, Tian L, et al. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol. 2007;50:974–982. doi: 10.1016/j.jacc.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott MM, Tian L, Liu K, et al. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J AmColl Cardiol. 2008;51:1482–1489. doi: 10.1016/j.jacc.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinglass J, McCarthy WJ, Slavensky R, Manheim LM, Martin GJ The Chicago Claudication Outcomes Research Group. Effect of lower extremity blood pressure on physical functioning in patients who have intermittent claudication. J Vasc Surg. 1996;24:503–511. doi: 10.1016/s0741-5214(96)70066-6. [discussion 511-2] [DOI] [PubMed] [Google Scholar]
- 10.Kullo IJ, Malik AR. Arterial ultrasonography and tonometry as adjuncts to cardiovascular risk stratification. J Am Coll Cardiol. 2007;49:1413–1426. doi: 10.1016/j.jacc.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira I, Twisk JW, Van Mechelen W, Kemper HC, Stehouwer CD. Current and adolescent levels of cardiopulmonary fitness are related to large artery properties at age 36: the Amsterdam Growth and Health Longitudinal Study. Eur J Clin Invest. 2002;32:723–731. doi: 10.1046/j.1365-2362.2002.01066.x. [DOI] [PubMed] [Google Scholar]
- 12.Cheng KS, Tiwari A, Baker CR, Morris R, Hamilton G, Seifalian AM. Impaired carotid and femoral viscoelastic properties and elevated intima-media thickness in peripheral vascular disease. Atherosclerosis. 2002;164:113–120. doi: 10.1016/s0021-9150(02)00042-4. [DOI] [PubMed] [Google Scholar]
- 13.Brewer LC, Chai HS, Bailey KR, Kullo IJ. Measures of arterial stiffness and wave reflection are associated with walking distance in patients with peripheral arterial disease. Atherosclerosis. 2007;191:384–390. doi: 10.1016/j.atherosclerosis.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 14.Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–364. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama H, Shoji T, Kimoto E, et al. Pulse wave velocity in lower-limb arteries among diabetic patients with peripheral arterial disease. J Atheroscler Thromb. 2003;10:253–258. doi: 10.5551/jat.10.253. [DOI] [PubMed] [Google Scholar]
- 16.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23:402–408. [PubMed] [Google Scholar]
- 17.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley & Sons, Inc; 1980. [Google Scholar]
- 18.Cox DR. Regression models and life-tables (with discussions) J R Stat Soc. 1972;(34):187–220. Series B. [Google Scholar]
- 19.Ahimastos AA, Dart AM, Lawler A, Blombery PA, Kingwell BA. Reduced arterial stiffness may contribute to angiotensin-converting enzyme inhibitor induced improvements in walking time in peripheral arterial disease patients. J Hypertens. 2008;26:1037–1042. doi: 10.1097/HJH.0b013e3282f8e3b6. [DOI] [PubMed] [Google Scholar]
- 20.Malik AR, Kondragunta V, Kullo IJ, et al. Forearm vascular reactivity and arterial stiffness in asymptomatic adults from the community. Hypertension. 2008;51(6):1512–1518. doi: 10.1161/HYPERTENSIONAHA.107.106088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell GF, Vita JA, Larson MG, et al. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 22.Enko K, Sakuragi S, Kakishita M, et al. Arterial stiffening is associated with exercise intolerance and hyperventilatory response in patients with coronary artery disease. Clin Med: Cardiol. 2008;2:41–48. [Google Scholar]
- 23.O’Rourke MF, Hayward CS, Lehmann ED. Arterial stiffness. In: Oparil S, Weber MA, editors. Hypertension: a companion to Brenner and Rector’s the kidney. Philadelphia: Elsevier Mosby: 2005. [Google Scholar]
- 24.McDermott MM, Liu K, Guralnik JM, et al. The ankle brachial index independently predicts walking velocity and walking endurance in peripheral arterial disease. J Am Geriatr Soc. 1998;46:1355–1362. doi: 10.1111/j.1532-5415.1998.tb06001.x. [DOI] [PubMed] [Google Scholar]
- 25.McDermott MM, Liu K, Greenland P. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 26.McDermott MM, Mehta S, Liu K, et al. Leg symptoms, the ankle-brachial index, and walking ability in patients with peripheral arterial disease. J Gen Intern Med. 1999;14:173–181. doi: 10.1046/j.1525-1497.1999.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiatt WR, Regensteiner JG, Hargarten ME, Wolfel EE, Brass EP. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation. 1990;81:602–609. doi: 10.1161/01.cir.81.2.602. [DOI] [PubMed] [Google Scholar]
- 28.Sugawara J, Hayashi K, Yokoi T, et al. Brachial-ankle pulse wave velocity: an index of central arterial stiffness? J Hum Hypertens. 2005;19:401–406. doi: 10.1038/sj.jhh.1001838. [DOI] [PubMed] [Google Scholar]
- 29.Im JA, Lee JW, Shim JY, Lee HR, Lee DC. Association between brachial-ankle pulse wave velocity and cardiovascular risk factors in healthy adolescents. J Pediatr. 2007;150:247–251. doi: 10.1016/j.jpeds.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 30.Imanishi R, Seto S, Toda G, et al. High brachial-ankle pulse wave velocity is an independent predictor of the presence of coronary artery disease in men. Hypertens Res. 2004;27:71–78. doi: 10.1291/hypres.27.71. [DOI] [PubMed] [Google Scholar]
- 31.Munakata M, Sakuraba J, Tayama J, et al. Higher brachial-ankle pulse wave velocity is associated with more advanced carotid atherosclerosis in end-stage renal disease. Hypertens Res. 2005;28:9–14. doi: 10.1291/hypres.28.9. [DOI] [PubMed] [Google Scholar]
- 32.Ohmine T, Miwa Y, Yao H, et al. Association between arterial stiffness and cerebral white matter lesions in community-dwelling elderly subjects. Hypertens Res. 2008;31:75–81. doi: 10.1291/hypres.31.75. [DOI] [PubMed] [Google Scholar]
- 33.Tomiyama H, Koji Y, Yambe M, et al. Brachial-ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circ J. 2005;69:815–822. doi: 10.1253/circj.69.815. [DOI] [PubMed] [Google Scholar]
- 34.Yu WC, Chuang SY, Lin YP, Chen CH. Brachial-ankle vs carotid-femoral pulse wave velocity as a determinant of cardiovascular structure and function. J Hum Hypertens. 2008;22:24–31. doi: 10.1038/sj.jhh.1002259. [DOI] [PubMed] [Google Scholar]
- 35.Motobe K, Tomiyama H, Koji Y, et al. Cut-off value of the ankle-brachial pressure index at which the accuracy of brachial-ankle pulse wave velocity measurement is diminished. Circ J. 2005;69:55–60. doi: 10.1253/circj.69.55. [DOI] [PubMed] [Google Scholar]