Abstract
We tested the hypothesis that, in adults with essential hypertension, plasma levels of midregional proatrial natriuretic peptide (MR-proANP) are associated with target organ damage. MR-proANP is a newly described stable fragment of N-terminal proatrial natriuretic peptide. Participants included 1,919 adults with hypertension identified from the community (1,037 African-Americans, 65 ± 9 years of age, 72% women; 882 non-Hispanic whites, 61 ± 9 years of age, 55% women). We measured MR-proANP by an immunoluminometric assay. Measurements of target organ damage included the ankle–brachial index (ABI), urinary albumin–creatinine ratio (UACR), and left ventricular (LV) mass (available only in African-Americans). Generalized estimating equations were used to assess whether plasma MR-proANP was associated with measurements of target organ damage, independent of potential confounding variables. In African-Americans, higher MR-proANP was significantly associated with lower ABI (p < 0.0001), higher UACR (p<0.0001), and greater LV mass (indexed to height to the power of 2.7, p < 0.0001). After adjustment for age, gender, body mass index, systolic blood pressure, estimated glomerular filtration rate, smoking history, diabetes mellitus, total and high-density lipoprotein cholesterols, medication (blood pressure lowering, statin, and aspirin) use, and previous myocardial infarction or stroke, higher MR-proANP levels remained significantly associated with lower ABI (p = 0.01), higher UACR (p = 0.0007), and greater LV mass index (p<0.0001). In non-Hispanic whites, higher MR-proANP levels were significantly associated with lower ABI (p = 0.002) and greater UACR (p = 0.001), but not after adjustment for the covariates listed earlier. In conclusion, plasma MR-proANP may be a marker of target organ damage in the setting of hypertension, especially in African-Americans.
Atrial natriuretic peptide (ANP), a member of the natriuretic peptide family, regulates several physiologic parameters including diuresis and natriuresis, and lowers arterial blood pressure (BP). It is predominantly produced in the atrium of the heart and comprises 98% of natriuretic peptides in the circulation.1 ANP is derived from the cleavage of its precursor prohormone, which is significantly more stable in the circulation than the mature peptide. A midregional fragment of the precursor hormone (amino acids 53 to 90 of N-terminal pro-ANP), called midregional proANP (MR-proANP), may be relatively resistant to degradation by exoproteases, unlike epitopes in the N- or C-terminals of proANP used in previous immunoassays.2,3 We previously noted that, in adults with essential hypertension, higher plasma levels of MR-proANP were associated with higher systolic BP, greater arterial stiffness, and greater severity of hypertension.4 Based on these findings, we hypothesized that plasma MR-proANP may be associated with measurements of target organ damage in adults with hypertension. The 3 measurements of target organ damage available for this study were (1) ankle–brachial index (ABI), a measurement of peripheral arterial disease, (2) urinary albumin–creatinine ratio (UACR), a surrogate of glomerular endothelial function, and (3) left ventricular (LV) mass, a measurement of LV hypertrophy, available only in the African-American cohort. We sought to determine whether any detected association of plasma MR-proANP with these phenotypes was independent of potential confounding variables.
Methods
The study was part of the Proteomic Markers of Arteriosclerosis Study, which is investigating the association of markers in various etiologic pathways of vascular disease with several phenotypes of arteriosclerosis.5 Participants belonged to the Genetic Epidemiology Network of Arteriopathy Study, a multicenter, community-based study that aims to identify genetic variants influencing BP levels and the development of target organ damage due to hypertension. Recruitment and subject characteristics in the initial phase of this study have been previously described.6 The study was approved by the institutional review boards of the University of Mississippi Medical Center (Jackson, Mississippi) and the Mayo Clinic (Rochester, Minnesota). Written informed consent was obtained from each participant. The present study included 1,919 participants (1,037 African-Americans and 882 non-Hispanic whites) who had hypertension.
Height was measured by stadiometer, weight by electronic balance, and body mass index was calculated as weight in kilograms divided by the square of height in meters. Diabetes was considered present if the participant was being treated with insulin or oral agents or had a fasting glucose level ≥126 mg/dl. “Ever” smoking was defined as having smoked >100 cigarettes. Information about the use of medications was obtained from the participants at the time of the study visit. Each prescription drug recorded at the study visit was assigned a code number corresponding to the first 6 digits of the Medi-Span Generic Product Identifier (Medi-Span, Inc., Indianapolis, Indiana). This number identifies pharmacologically equivalent drug products and was used to categorize agents with a similar therapeutic action. BP-lowering medications were classified as diuretics, β blockers, calcium channel blockers, or renin-angiotensin-aldosterone system inhibitors.
Blood was drawn by venipuncture after an overnight fast. Serum total cholesterol and high-density lipoprotein cholesterol were measured by standard enzymatic methods. Glomerular filtration rate was estimated using the Modification of Diet in Renal Disease equation as previously described.7 Systolic BP and diastolic BP at rest were measured by random 0 sphygmomanometer (Hawskley and Sons, London, United Kingdom) after participants had rested for ≥10 minutes in the supine position. Three measurements ≥2 minutes apart were taken and the average of the second and third measurements was used. Diagnosis of hypertension was established based on BP levels measured at the study visit (≥140/90 mm Hg) or a previous diagnosis of hypertension and current treatment with antihypertensive medications.
Plasma was collected at the time of blood sampling in plastic vials containing ethylenediaminetetra-acetic acid. These were placed on ice and then centrifuged at 3,000g and frozen at −80°C until assayed. MR-proANP was detected using a novel commercial sandwich immunoassay in the chemiluminescence-coated tube format (MR-proANP LIA, BRAHMS, Hennigsdorf/Berlin, Germany) as previously described.3 Briefly, patient samples (1:40 dilution of plasma 5 μl in incubation buffer) or standards were added in duplicate to antibody-coated tubes (affinity-purified sheep poly-clonal antibodies directed against proANP peptides 73 to 90) and incubated for 30 min at room temperature. After washes with washing buffer 1 ml, tracer 200 μl was added, containing acridinium ester–labeled anti-proANP antibody (affinity-purified sheep polyclonal antibodies directed against proANP peptides 53 to 72), followed by 30-minute incubation at room temperature. Tubes were washed with washing buffer 1 ml, and detection was performed in a LB952T luminometer (Berthold, Bad Wildbad, Germany; 1-second detection time per sample). Relative light units of the chemiluminescence assay were expressed in picomoles of MR-proANP per liter, as calculated from a calibration curve (4 to 1,800 pmol/L) that was included in every analytical run. The lower detection limit of the assay is 4.3 pmol/L and the functional sensitivity of the assay is MR-proANP 11 pmol/L. The interassay coefficient of variation within the range of plasma measurements was <10% (8.0% at 100 pmol/L, 6.5% at 400 pmol/L). Participants (n = 13) with MR-proANP levels >400 pmol/L were excluded from the analyses because such levels may be due to LV dysfunction.
At each center, the ABI was measured by examiners who had undergone training in Mayo Clinic’s noninvasive vascular laboratory in Rochester. An identical, standardized protocol was used at the 2 centers. After a 5-minute rest, subjects were evaluated in the supine position. Appropriately sized BP cuffs were placed on each arm and ankle, and Doppler ultrasonic instrument (Medisonics, Minneapolis, Minnesota) was used to detect arterial signals. The cuff was inflated to 10 mm Hg above systolic BP and deflated at 2 mm Hg/s. The first reappearance of the arterial signal was taken as the systolic BP. To calculate the ABI, the systolic BP at each ankle site (posterior tibial and dorsalis pedis arteries) was divided by the higher of the 2 brachial pressures. The lower of the average ABIs from the 2 legs was used in the analyses. Subjects with an ABI >1.3 (n = 142) were excluded from the analyses because they may have noncompressible arteries due to medial arterial calcification.8
Albuminuria was assessed by UACR. The first voided urine was collected on the morning of the study visit and stored at −80°C until analyzed. Urine albumin, urine creatinine, and serum creatinine concentrations were measured by standard methods on a Hitachi 911 Clinical Chemistry Analyzer (Roche Diagnostics, Indianapolis, Indiana), and UACR was expressed as milligrams of albumin per gram of creatinine. Participants with a creatinine level >2.5 mg/dl (n = 9) or a UACR >3,000 mg/g (n = 6) were excluded from the analyses.
LV mass was measured in African-Americans by echocardiography as previously described.9 In brief, the parasternal acoustic window was used to record ≥10 consecutive beats of 2-dimensional and M-mode recordings of the LV internal diameter and wall thicknesses at, or just below, the tips of the anterior mitral leaflet in long- and short-axis views. Correct orientation of planes for imaging and Doppler recordings was verified using standardized protocols. LV internal dimension and interventricular septal and posterior wall thicknesses were measured at end-diastole and end-systole in ≥3 cardiac cycles according to the recommendations of the American Society of Echocardiography.10 The correlation between repeated measurements of LV mass was 0.93 between paired echocardiograms in hypertensive adults. LV mass was indexed by height (to the power of 2.7) to normalize heart size to body size.11
Statistical analyses were carried out using SAS 9.1 (SAS Institute, Cary, North Carolina).
Because of the presence of sibships in the sample, regression analyses were performed using generalized estimating equations.12 Continuous data were summarized as means ± SDs or medians and interquartile range and categorical data were expressed as percentages. Because of significant differences in age and the proportion of women between the 2 ethnic groups, ethnic differences in participant characteristics were compared after adjustment for age and gender. UACR, LV mass index, and estimated glomerular filtration rate were log-transformed to decrease skewness. In each ethnic group, to evaluate the association of MR-proANP with measurements of target organ damage, we divided the participants according to quartiles of MR-proANP, with participants in lowest quartile considered the referent group. In each ethnic group, we constructed multiple regression models including age, gender, body mass index, systolic BP, smoking history, diabetes, total and high-density lipoprotein cholesterols, estimated glomerular filtration rate, medication (BP-lowering, statin, and aspirin) use, previous myocardial infarction or stroke, and MR-proANP. Age and gender were forced into all multivariable regression models. Backward elimination was performed to identify the set of variables independently associated with each measurement of target organ damage. In each model, means for each measurement of target organ damage in quartiles of plasma levels of MR-proANP were estimated using least-squares means. A 2-sided p value <0.05 was deemed statistically significant.
Results
African-Americans were older and there was a larger proportion of women in the African-American and non-Hispanic white cohorts (Table 1). After adjustment for age and gender, African-Americans had a higher prevalence of diabetes, lower use of statins, and higher estimated glomerular filtration rate, systolic BP, and diastolic BP than their non-Hispanic white counterparts. MR-proANP levels were similar in the 2 ethnic groups (Table 1). After adjustment for age and gender, African-Americans had lower ABI and greater UACR than their non-Hispanic white counterparts. LV mass index was measured only in African-Americans (Table 2).
Table 1.
Participant characteristics
| Variables | African-Americans (n = 1,037) | Non-Hispanic Whites (n = 882) | p Value* |
|---|---|---|---|
| Age (yrs) | 64.8 ± 8.6 | 61.1 ± 9.3 | <0.001 |
| Women | 751 (72%) | 492 (56%) | <0.001 |
| Body mass index (kg/m2) | 32.0 ± 6.6 | 31.4 ± 6.3 | 0.008 |
| Total cholesterol (mg/dl) | 201.5 ± 41.7 | 196.6 ± 33.6 | 0.004 |
| HDL cholesterol (mg/dl) | 57.6 ± 17.8 | 50.7 ± 14.7 | <0.001 |
| Plasma glucose level (mg/dl) | 115.6 ± 51.0 | 107.9 ± 26.3 | <0.001 |
| Systolic BP (mm Hg) | 142.3 ± 20.8 | 135.3 ± 17.1 | <0.001 |
| Diastolic BP (mm Hg) | 79.9 ± 11.4 | 74.6 ± 9.7 | <0.001 |
| Heart rate (beats/min) | 68 ± 12 | 65 ± 11 | 0.001 |
| Serum creatinine (mg/dl) | 0.91 ± 0.33 | 0.91 ± 0.26 | NS |
| eGFR (ml/min) | 97.9 ± 31.2 | 83.3 ± 22.8 | <0.001 |
| Ever-smoker | 420 (40%) | 431 (49%) | <0.001 |
| Diabetes mellitus | 348 (34%) | 160 (18%) | <0.001 |
| Previous myocardial infarction or stroke | 138 (13%) | 122 (14%) | NS |
| β blocker | 213 (20%) | 375 (42%) | <0.001 |
| Calcium channel blocker | 367 (35%) | 174 (20%) | <0.001 |
| Diuretic | 587 (57%) | 447 (51%) | 0.004 |
| Renin-angiotensin-aldosterone system inhibitors | 508 (49%) | 420 (48%) | NS |
| Statin | 222 (21%) | 307 (35%) | <0.001 |
| Aspirin | 371 (36%) | 432 (49%) | 0.003 |
| MR-proANP (pmol/L) | 62.5 (43.8–93.1) | 63.1 (44.0–95.0) | 0.35 |
Continuous variables are presented as mean ± SD or median (interquartile range), and categorical variables are presented as numbers (percentages).
For ethnic differences after adjustment for age and gender.
eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein.
Table 2.
Measurements of target organ damage
| No. of Subjects | African Americans | No. of Subjects | Non-Hispanic Whites | p Value* | |
|---|---|---|---|---|---|
| ABI | 1,009 | 0.98 ± 0.14 | 743 | 1.11 ± 0.13 | <0.001 |
| UACR (mg/g) | 1,028 | 6.9 (3.1–20.6) | 834 | 3.3 (1.7–6.7) | <0.001 |
| LV mass index (g/m2.7) | 1,010 | 39.0 (33.6–47.1) | — | — | — |
Based on distributional properties, variables are presented as mean ± SD or median (interquartile range).
For ethnic differences after adjustment for age and gender.
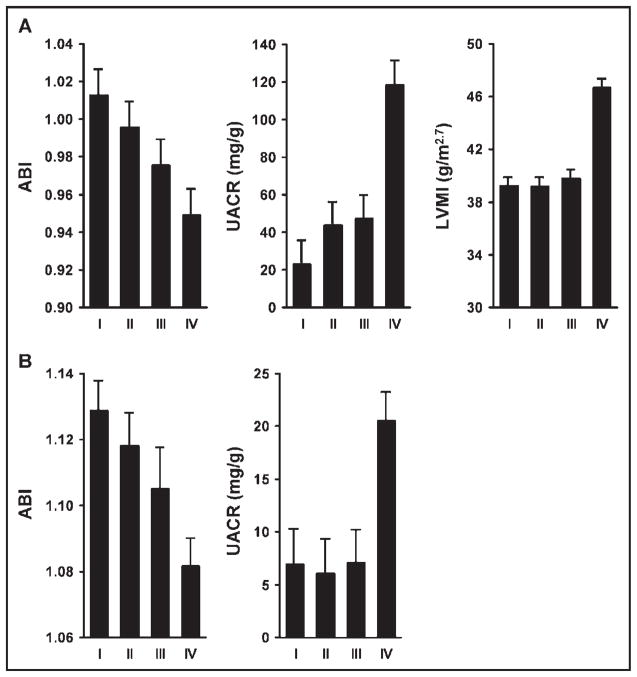
In African-Americans, higher MR-proANP levels were significantly associated with lower ABI (p < 0.0001; Figure 1). After adjustment for age and gender, African-Americans in the highest quartile for MR-proANP levels had a significantly lower ABI compared with those in the lowest quartiles (mean ABI 0.96 vs 1.00, p = 0.001). These associations remained significant after additional adjustment for conventional risk factors, medication use, and estimated glomerular filtration rate (Table 3). In addition, older age, higher total cholesterol, history of smoking, previous myocardial infarction or stroke, diabetes, and statin and diuretic use were associated with a lower ABI, whereas higher body mass index and higher high-density lipoprotein cholesterol were associated with a higher ABI.
Figure 1.
Association of MR-proANP with measurements of target organ damage. (A) Mean ± SE of ABI, UACR, and LV mass index (LVMI) in quartiles of plasma levels of MR-proANP in African-Americans. Cut-off values for MR-proANP quartiles were <44 pmol/L for quartile I, 44 to 62 pmol/L for II, 62 to 93 pmol/L for III, and >93 pmol/L for IV. (B) Mean ± SE of ABI and UACR in quartiles of plasma levels of MR-proANP in non-Hispanic whites. Cut-off values for MR-proANP quartiles were <44 pmol/L for quartile I, 44 to 63 pmol/L for II, 62 to 95 pmol/L for III, and >95 pmol/L for IV.
Table 3.
Mean ankle–brachial index, log (urine albumin–creatinine ratio + 1), and log (left ventricular mass index) in quartiles of plasma midregional proatrial natriuretic peptide levels in African-Americans
| Quartiles of MR-proANP (pmol/L) |
p Value for Trend | |||||||
|---|---|---|---|---|---|---|---|---|
| I (<44) | II (44–62) | p Value | III (62–93) | p Value | IV (>93) | p Value | ||
| Age- and gender-adjusted | ||||||||
| ABI | 1.00 ± 0.01 | 0.99 ± 0.01 | 0.50 | 0.98 ± 0.01 | 0.031 | 0.96 ± 0.01 | 0.001 | 0.005 |
| Log (UACR + 1) (mg/g) | 2.21 ± 0.08 | 2.27 ± 0.09 | 0.59 | 2.35 ± 0.10 | 0.27 | 2.93 ± 0.12 | <0.0001 | <0.0001 |
| Log LV mass index (g/m2.7) | 3.65 ± 0.02 | 3.64 ± 0.02 | 0.46 | 3.64 ± 0.02 | 0.68 | 3.78 ± 0.02 | <0.0001 | <0.0001 |
| Adjusted for age, sex, conventional risk factors, medication use, and eGFR | ||||||||
| ABI | 1.00 ± 0.01 | 0.99 ± 0.01 | 0.44 | 0.97 ± 0.01 | 0.011 | 0.96 ± 0.01 | 0.006 | 0.01 |
| Log (UACR + 1) (mg/g) | 2.51 ± 0.11 | 2.48 ± 0.11 | 0.72 | 2.61 ± 0.12 | 0.41 | 2.93 ± 0.13 | 0.003 | 0.0007 |
| Log LV mass index (g/m2.7) | 3.64 ± 0.02 | 3.63 ± 0.02 | 0.81 | 3.65 ± 0.02 | 0.68 | 3.80 ± 0.02 | <0.0001 | <0.0001 |
Abbreviation as in Table 1.
In non-Hispanic whites, higher MR-proANP levels were also significantly associated with a lower ABI (p = 0.002; Figure 1). However, after adjustment for age and gender, plasma MR-proANP was not significantly different between participants in the highest quartiles for MR-proANP and those in the lowest quartile (analyses not shown). Further adjustment for other covariates did not change the associations. Among conventional risk factors, older age, female gender, smoking, and previous myocardial infarction or stroke were associated with a lower ABI.
In African-Americans, higher MR-proANP levels were significantly associated with higher UACR (p < 0.0001; Figure 1). After adjustment for age and gender, African-Americans in the highest quartile for MR-proANP levels had significantly higher UACR compared with those in the lowest quartiles (mean log [UACR + 1] 2.93 vs 2.21 mg/g, p < 0.0001). These associations remained significant after additional adjustment for conventional risk factors, medication use, and estimated glomerular filtration rate (Table 3). In addition, male gender, higher systolic BP, diabetes, previous myocardial infarction or stroke, higher total cholesterol, and calcium blocker use were associated with higher UACR, whereas a higher estimated glomerular filtration rate was associated with lower UACR.
In non-Hispanic whites, higher MR-proANP levels were also significantly associated with higher UACR (p = 0.001; Figure 1). However, after adjustment for age and gender, plasma MR-proANP was not significantly different between participants in the highest quartiles for MR-proANP and those in the lowest quartile (analyses not shown). Further adjustment for other covariates did not change the associations. Among conventional risk factors, higher systolic BP, diabetes, lower high-density lipoprotein, and previous myocardial infarction or stroke were associated with higher UACR.
In African-Americans, higher MR-proANP levels were significantly associated with a greater LV mass index (p < 0.0001; Figure 1). After adjustment for age and gender, African-Americans in the highest quartile for MR-proANP levels had a significantly greater LV mass index compared with those in the lowest quartiles (mean log LV mass index 3.78 vs 3.65 g/m2.7, p < 0.0001). These associations remained significant after additional adjustment for conventional risk factors, medication use, and estimated glomerular filtration rate (Table 3). In addition, older age, male gender, higher systolic BP, higher body mass index, previous myocardial infarction or stroke, diabetes, β-blocker and calcium channel blocker use were associated with a higher LV mass index.
Discussion
This study is the first to report an association between plasma MR-proANP and measurements of target organ damage in adults with hypertension. In African-Americans, higher MR-proANP was significantly associated with lower ABI, higher UACR, and greater LV mass index. These associations were independent of age, gender, conventional risk factors, estimated glomerular filtration rate, and medication use. In non-Hispanic whites, higher MR-proANP levels were significantly associated with lower ABI and greater UACR. However, after adjustment for relevant covariates, the associations were no longer statistically significant. Our findings suggest that plasma MR-proANP may be a marker of target organ damage in adults with hypertension, particularly in African-Americans.
We found that higher natriuretic peptide levels are associated with lower ABI, even after accounting for potential confounders. This observation suggests that systemic atherosclerosis leads to increased secretion and release of MR-proANP. Increased arterial stiffness in the setting of atherosclerosis could increase cardiac afterload and impair coronary blood flow due to lower diastolic BP, thereby increasing vulnerability to ischemia. This may in turn result in ventricular stiffness and increased intracardiac filling pressures. These LV anatomic and hemodynamic disturbances are transmitted to the left atrium, promoting atrial stretch and dilatation that result in increased ANP secretion.
In patients with essential hypertension, LV hypertrophy is considered a major risk factor, and its presence is an indication for more aggressive BP lowering. Furthermore, a decrease in LV mass results in a decreased risk of subsequent cardiovascular adverse events, independent of changes in BP.13 In the present study, plasma levels of MR-proANP were independently associated with LV mass in African-Americans. Although we were not able to study the association in whites, previous studies in whites have also shown natriuretic peptide levels to be correlated with LV mass,14 independent of conventional risk factors.15 Mechanical strain in the cardiac chambers is a potent stimulus for expression of the ANP gene.16 Therefore, plasma MR-proANP level may be useful for early detection of LV hypertrophy in hypertensive adults. Renal filtration is an important determinant of circulating ANP levels.17 In our study, however, the observed association between MR-proANP and LV mass index was independent of estimated glomerular filtration rate. Because LV function may influence MR-proANP levels, we also adjusted for LV ejection fraction, but this did not change the association between MR-proANP and LV mass index (analyses not shown).
MR-proANP was independently associated with UACR in African-Americans. Previous studies have demonstrated that increased urinary albumin excretion is a manifestation of hypertension-related target organ damage18 and a risk marker for renal damage and cardiovascular morbidity/mortality.19 Proposed mechanisms for albuminuria in hypertension include increased glomerular filtration of albumin because of hypertension-related intrarenal hemodynamic abnormalities leading to increased intraglomerular pressure20 and defective proximal tubular handling of albumin, possibly mediated by cytokines.21 Natriuretic peptides are known to affect renal hemodynamics22 and microvascular permeability to macromolecules23 and could thus affect albumin excretion. In addition, it has been shown that infusion of ANP increases albumin excretion in diabetics likely due to increased glomerular permeability.24 Whether an increased level of MR-proANP precedes the development of albuminuria in hypertension needs further investigation.
In the present study, plasma MR-proANP was not independently associated with ABI and UACR in non-Hispanic whites. It is not clear what accounts for ethnicity-specific associations between MR-proANP and measurements of target organ damage. The 2 ethnic groups may vary substantially in extent of target organ damage. We and others have previously described ethnic differences in ABI and peripheral arterial disease.25,26 African-Americans had significantly lower ABI and higher UACR than non-Hispanic whites and the prevalence of peripheral arterial disease (ABI < 0.9) and microalbuminuria (UACR ≥30 mg/g) were 3 to 4 times higher in African-Americans than in non-Hispanic whites. Therefore, lack of an independent association of MR-proANP with target organ damage in non-Hispanic whites does not rule out a pathophysiologic role of the natriuretic peptides in this ethnic group. Further research at the molecular and population levels is essential to clarify the role of natriuretic peptides as markers of target organ damage.
Although plasma concentrations of MR-proANP showed significant associations with indexes of target organ damage, its ability to discriminate between normal and “abnormal” ABI, UACR, and LV mass index was relatively modest. The area under the receiver operating characteristics curve when using MR-proANP to predict target organ damage (ABI < 0.9, UACR ≥30 mg/g, and LV mass index >51 g/m2.7) was 0.60 to 0.66 (analyses not shown). However, MR-proANP may have clinical utility in monitoring the progress of target organ damage in adults with hypertension, once an individual, steady state of MR-proANP level is determined.
A strength of the present study is the inclusion of a large biethnic cohort of adults with hypertension and the use of uniform protocols including questionnaires and anthropometric and laboratory measurements. In addition, plasma levels of MR-proANP were measured using a novel immunoassay covering midregional epitopes, allowing a more reliable assessment of ANP release. Most patients in this study were on BP-lowering medications at the time of measurement of plasma MR-proANP levels. However, this would tend to lessen the ability to find associations between ANP and measurements of target organ damage. Limitations of this study include its cross-sectional nature. We speculated that, because natriuretic peptides are secreted from cardiomyocytes in response to atrial and ventricular wall stretch27 and have a fundamental role in cardiovascular remodeling, volume homeostasis, and response to ischemia,28,29 higher MR-proANP levels in the setting of target organ damage in adults with hypertension may indicate a neurohormonal response to limit target organ damage.
Acknowledgments
This work was supported by Grant HL-81331 from the National Institutes of Health, Bethesda, Maryland.
The authors have no financial or other relationships that might lead to a conflict of interest regarding this work.
Footnotes
Dr. Morganthaler, Dr. Struck, and Dr. Bergmann are employed by BRAHMS AG, which developed the assay used for the measurement of MR-proANP in this study.
References
- 1.Vesely DL. Atrial natriuretic peptide prohormone gene expression: hormones and diseases that upregulate its expression. IUBMB Life. 2002;53:153–159. doi: 10.1080/15216540212336. [DOI] [PubMed] [Google Scholar]
- 2.Ala-Kopsala M, Magga J, Peuhkurinen K, Leipala J, Ruskoaho H, Leppaluoto J, Vuolteenaho O. Molecular heterogeneity has a major impact on the measurement of circulating N-terminal fragments of A-and B-type natriuretic peptides. Clin Chem. 2004;50:1576–1588. doi: 10.1373/clinchem.2004.032490. [DOI] [PubMed] [Google Scholar]
- 3.Morgenthaler NG, Struck J, Thomas B, Bergmann A. Immunoluminometric assay for the midregion of pro-atrial natriuretic peptide in human plasma. Clin Chem. 2004;50:234–236. doi: 10.1373/clinchem.2003.021204. [DOI] [PubMed] [Google Scholar]
- 4.Khaleghi M, Saleem U, Morgenthaler NG, Turner ST, Bergmann A, Struck J, Mosley TH, Kullo IJ. Plasma midregional proatrial natriuretic peptide is associated with blood pressure indices and hypertension severity in adults with hypertension. Am J Hypertens. 2009 doi: 10.1038/ajh.2009.8. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granger CB, Van Eyk JE, Mockrin SC, Anderson NL. National Heart, Lung, and Blood Institute clinical proteomics working group report. Circulation. 2004;109:1697–1703. doi: 10.1161/01.CIR.0000121563.47232.2A. [DOI] [PubMed] [Google Scholar]
- 6.O’Meara JG, Kardia SL, Armon JJ, Brown CA, Boerwinkle E, Turner ST. Ethnic and sex differences in the prevalence, treatment, and control of dyslipidemia among hypertensive adults in the GENOA study. Arch Intern Med. 2004;164:1313–1318. doi: 10.1001/archinte.164.12.1313. [DOI] [PubMed] [Google Scholar]
- 7.Ellington AA, Malik AR, Klee GG, Turner ST, Rule AD, Mosley TH, Jr, Kullo IJ. Association of plasma resistin with glomerular filtration rate and albuminuria in hypertensive adults. Hypertension. 2007;50:708–714. doi: 10.1161/HYPERTENSIONAHA.107.095257. [DOI] [PubMed] [Google Scholar]
- 8.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 9.Skelton TN, Andrew ME, Arnett DK, Burchfiel CM, Garrison RJ, Samdarshi TE, Taylor HA, Hutchinson RG. Echocardiographic left ventricular mass in African-Americans: the Jackson cohort of the Atherosclerosis Risk in Communities Study. Echocardiography. 2003;20:111–120. doi: 10.1046/j.1540-8175.2003.03000.x. [DOI] [PubMed] [Google Scholar]
- 10.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 11.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 12.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 13.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, Reboldi G, Porcellati C. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation. 1998;97:48–54. doi: 10.1161/01.cir.97.1.48. [DOI] [PubMed] [Google Scholar]
- 14.Vasan RS, Benjamin EJ, Larson MG, Leip EP, Wang TJ, Wilson PW, Levy D. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA. 2002;288:1252–1259. doi: 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 15.Schirmer H, Omland T. Circulating N-terminal proatrial natriuretic peptide is an independent predictor of left ventricular hypertrophy in the general population. The Tromso Study. Eur Heart J. 1999;20:755–763. doi: 10.1053/euhj.1998.1396. [DOI] [PubMed] [Google Scholar]
- 16.Kinnunen P, Vuolteenaho O, Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132:1961–1970. doi: 10.1210/endo.132.5.8477647. [DOI] [PubMed] [Google Scholar]
- 17.Omland T, Aakvaag A, Vik-Mo H. Plasma cardiac natriuretic peptide determination as a screening test for the detection of patients with mild left ventricular impairment. Heart. 1996;76:232–237. doi: 10.1136/hrt.76.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedrinelli R, Dell’Omo G, Di Bello V, Pontremoli R, Mariani M. Microalbuminuria, an integrated marker of cardiovascular risk in essential hypertension. J Hum Hypertens. 2002;16:79–89. doi: 10.1038/sj.jhh.1001316. [DOI] [PubMed] [Google Scholar]
- 19.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 20.Rosa TT, Palatini P. Clinical value of microalbuminuria in hypertension. J Hypertens. 2000;18:645–654. doi: 10.1097/00004872-200018060-00001. [DOI] [PubMed] [Google Scholar]
- 21.Russo LM, Bakris GL, Comper WD. Renal handling of albumin: a critical review of basic concepts and perspective. Am J Kidney Dis. 2002;39:899–919. doi: 10.1053/ajkd.2002.32764. [DOI] [PubMed] [Google Scholar]
- 22.Awazu M, Ichikawa I. Biological significance of atrial natriuretic peptide in the kidney. Nephron. 1993;63:1–14. doi: 10.1159/000187137. [DOI] [PubMed] [Google Scholar]
- 23.Zietse R, Derkx FH, Weimar W, Schalekamp MA. Effect of atrial natriuretic peptide on renal and vascular permeability in diabetes mellitus. J Am Soc Nephrol. 1995;5:2057–2066. doi: 10.1681/ASN.V5122057. [DOI] [PubMed] [Google Scholar]
- 24.Vervoort G, Wetzels JF, Lutterman JA, Bravenboer B, Berden JH, Smits P. Atrial natriuretic peptide-induced microalbuminuria is associated with endothelial dysfunction in noncomplicated type 1 diabetes patients. Am J Kidney Dis. 2002;40:9–15. doi: 10.1053/ajkd.2002.33907. [DOI] [PubMed] [Google Scholar]
- 25.Kullo IJ, Bailey KR, Kardia SL, Mosley TH, Jr, Boerwinkle E, Turner ST. Ethnic differences in peripheral arterial disease in the NHLBI Genetic Epidemiology Network of Arteriopathy (GENOA) study. Vasc Med. 2003;8:237–242. doi: 10.1191/1358863x03vm511oa. [DOI] [PubMed] [Google Scholar]
- 26.Kuller L, Fisher L, McClelland R, Fried L, Cushman M, Jackson S, Manolio T. Differences in prevalence of and risk factors for subclinical vascular disease among black and white participants in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1998;18:283–293. doi: 10.1161/01.atv.18.2.283. [DOI] [PubMed] [Google Scholar]
- 27.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 28.Kook H, Itoh H, Choi BS, Sawada N, Doi K, Hwang TJ, Kim KK, Arai H, Baik YH, Nakao K. Physiological concentration of atrial natriuretic peptide induces endothelial regeneration in vitro. Am J Physiol Heart Circ Physiol. 2003;284:H1388–H1397. doi: 10.1152/ajpheart.00414.2002. [DOI] [PubMed] [Google Scholar]
- 29.Molkentin JD. A friend within the heart: natriuretic peptide receptor signaling. J Clin Invest. 2003;111:1275–1277. doi: 10.1172/JCI18389. [DOI] [PMC free article] [PubMed] [Google Scholar]